Abstract
Purpose
MRI targeted prostate biopsy has been shown to find many high-grade prostate cancers in men with concurrent negative transrectal ultrasound (TRUS) systematic biopsy. The oncologic risk of such tumors can be explored by looking at long-term outcomes of men with negative TRUS-biopsy followed without MRI. The aim was to analyze the mortality after initial and second negative TRUS-biopsy.
Materials and methods
All men who underwent initial TRUS-biopsies between January 1st, 1995 and December 31st, 2016 in Denmark were included. A total of 37,214 men had a negative initial TRUS-biopsy and 6,389 underwent a re-biopsy. Risk of cause-specific mortality was analyzed with competing risks. Diagnosis of Gleason score ≥7 prostate cancer following negative biopsies was analyzed with multivariable logistic regression including time to re-biopsy, PSA, age and digital rectal examination.
Results
The 15-year prostate cancer-specific mortality was 1.9% (95% CI: 1.7 – 2.1). Prostate cancer-specific mortality was 1.3% (95% CI: 0.9–1.6) and 4.6% (95% CI: 3.4 – 5.8) for men with PSA < 10 ng/ml and > 20 ng/ml, respectively. 12% of the TRUS re-biopsies were Gleason score ≥7 and risk of Gleason score ≥7 increased with longer time to re-biopsy (p<0.001). Mortality after re-biopsy was similar to after initial biopsy.
Conclusions
Men with negative TRUS-biopsies have a very low prostate cancer-specific mortality, especially with PSA <10. This raises serious questions about the routine use of MRI-targeting for initial prostate biopsy and suggests that MRI-targeting should only be recommended for men with PSA >10 ng/ml after negative biopsy.
Keywords: Magnetic Resonance Imaging, Prostatic Neoplasm, Biopsy, Mortality, Epidemiology
Introduction
For many years, standard management for a man with elevated prostate-specific antigen (PSA) involved systematic transrectal ultrasound-guided (TRUS) biopsy. Systematic sampling is not 100% sensitive and TRUS re-biopsy diagnoses an additional 10–12% of cancers1–3. These observations led to the development of targeted biopsy, where several biopsy cores are directed towards lesions mostly identified by magnetic resonance imaging (MRI). Numerous studies have shown that significant proportions of men with negative TRUS biopsy have prostate cancer detected on MRI-targeted biopsy4. For instance, the Trio study included 999 men with negative TRUS biopsy of whom 208 (21%) had cancer on MRI-targeted biopsy, with 134 Gleason score ≥ 7 cancers and 37 Gleason score 8 – 10 cancers5.
There are reasons to believe that Gleason grading will be higher in MRI targeted biopsies compared to systematic TRUS biopsies6. In brief, taking multiple cores at an MRI lesion and selecting the core with the highest grade will, on average, lead to a higher grading than a single needle placed at a location that is systematic for the prostate, yet random with respect to the tumor6. Thus, it is important to understand which patients with negative TRUS biopsy are at high risk of a missed lethal prostate cancer and, moreover, determine how TRUS re-biopsy performs in a population-based setting7–9. Here, we build on our previously published result, including additional analysis of men with re-biopsies and extended follow-up8. We are particularly interested in mortality at 10 – 15 years as it seems unlikely, even given the prolonged lead time of prostate cancer, that failing to identify a cancer on TRUS biopsy would mean a missed opportunity for cure, if mortality occurred more than 10 – 15 years subsequently. As a second aim, we assessed the risk of a cancer diagnosis following a negative initial biopsy and mortality in men with a later re-biopsy for suspicion of prostate cancer.
Methods
Data acquisition
The Danish Prostate Cancer Registry (DaPCaR) is a population-based database including information on all men who underwent histopathological assessment of prostate tissue merged with national registries on patient-level10. The inclusion period was January 1st, 1995 to December 31st, 2016. The registry is approved by the Danish Data Protection Agency (file number: 2012–41-0390), the Research Ethics Committee of the Capital Region of Denmark (local journal number: VD-2019–38), and the Danish Patient Safety Authority with reference 3–3013-2814/1. Data included the clinical assessment at initial TRUS-biopsies, findings at the initial TRUS-biopsies and the first TRUS re-biopsy, age at biopsy, PSA-values, histopathological assessments, method of sampling, and causes of death (prostate cancer or other causes). Danish guidelines recommended six biopsies cores taken until early 2000 and since have recommended 10–12 biopsy cores. Cause of death was obtained from the Danish Cause of Death Registry, and vital status from the Central Personal Registry. For all men with an initial negative biopsy and registered prostate cancer-specific death the medical records were reviewed from time of cancer suspicion until death, by two experienced urologists. The latest PSA-values were included if taken within two years before the TRUS-biopsies. In DaPCaR all digital rectal examinations (DRE) have been classified according to the TNM definition. Here we define cT1 and a negative biopsy as “normal DRE”. Non-unique personal identification numbers (e.g. non-Danish residents), men with MRI of the prostate prior to TRUS and men who emigrated were excluded. The histological assessment followed the guidelines from the International Society of Urological Pathology (ISUP) at the time of biopsy. Benign findings were defined as negative biopsies. Follow-up was from time of initial biopsy until death or censoring date (e.g. last known date of vital status, 31–12-2019). As a population-based registry, there was no standardized follow-up subsequent to a negative biopsy.
Statistical analysis
Median follow-up was calculated as the time from negative biopsy until censoring by reverse Kaplan Meier estimate. Overall mortality was estimated using cumulative incidence. For mortality analysis, a competing risk model, with prostate cancer-specific and other cause death as competing events, was used, including univariate analysis with PSA as stratification. To determine the relation of PSA with mortality in conjunction with cT-category, a non-parametric regression model with locally estimated scatterplot smoothing (LOESS) on PSA for men with normal DRE was used. The risk of detecting Gleason score ≥7 in re-biopsy was estimated using a multivariable logistic regression model including time between the initial biopsy and the first re-biopsy, with restricted cubic splines, PSA, DRE, and age at the time of initial biopsy. Statistical analyses were performed with R version 4.0.4 and p-values < 0.05 were considered significant11. The dataset is available upon request to the authors and the Danish Data Protection authorities.
Results
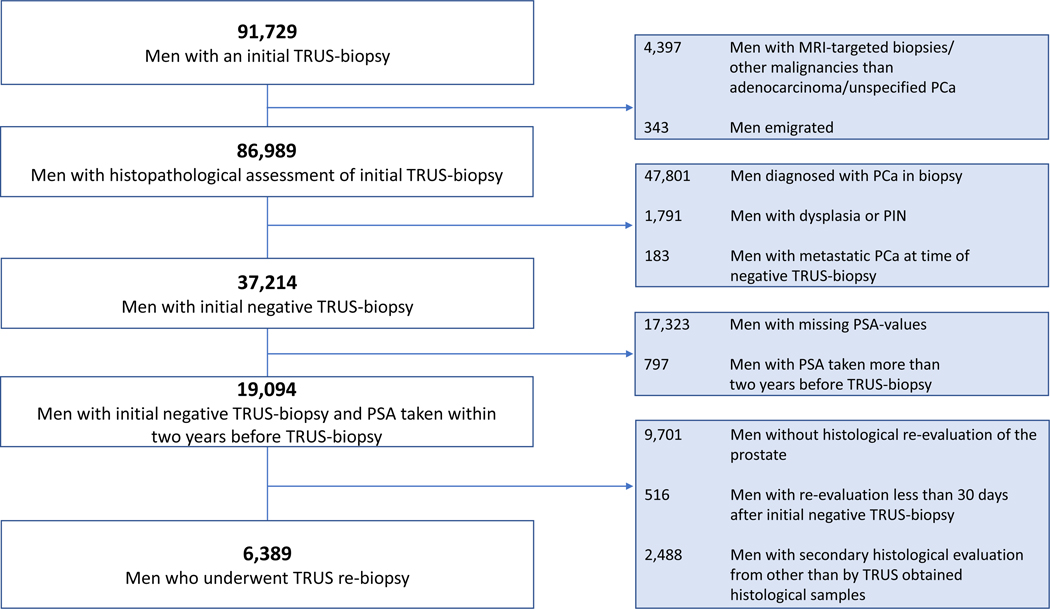
A flow-chart is depicted in Figure 1, a total of 86,989 men with an initial TRUS-biopsy set were eligible for analysis. The median follow-up was 10 years [IQR: 7 – 13] and 6040 men were followed longer than 15 years. Clinical characteristics of each specific cohort is shown in Table 1.
Figure 1:
Flow chart for the cohort.
Abbreviations: MRI: magnetic resonance imaging, PCa: prostate cancer, PIN: prostatic intraepithelial neoplasia, PSA: prostate specific-antigen, TRUS: transrectal ultrasound-guided.
Table 1.
Clinical characteristics for the cohorts of men with an initial TRUS-biopsy
| Initial TRUS-biopsy (N=86,989) | Initial negative TRUS-biopsy, all men (N=37,214) | Initial negative TRUS-biopsy and PSA-values (N=19,094) | TRUS re-biopsy (N=6,389) | ||
|---|---|---|---|---|---|
| Age, years | Median [IQR] | 68.4 [62.8 – 74.3] | 66.1 [60.8 – 71.4] | 65.8 [60.7 – 70.9] | 64.1 [59.7 – 68.4] |
|
| |||||
| PSA before biopsies, ng/ml | Median [IQR] | 9.6 [6.1 – 23.3] (N=39,187) | - | 7.3 [5.4 – 11.0] | 9.1 [6.6 – 13.9] |
|
| |||||
| GS-score in biopsies, N (%) | GS ≥ 7 | 34,784 (40%) | - | - | 751 (12%) |
| GS < 7 | 13,017 (15%) | - | - | 803 (13%) | |
| Without malignancy | 39,188 (45%) | - | - | 4,835 (76 %) | |
|
| |||||
| DRE, N (%) | T1 | 52,578 (60%) | 33,826 (90.9 %) | 17,353 (90.9 %) | - |
| T2 | 12,651 (15%) | 1,423 (3.8 %) | 771 (4.0 %) | - | |
| T3+4 | 12,855 (15%) | 693 (1.9 %) | 358 (1.9 %) | - | |
| Unknown | 8,905 (10%) | 1,272 (3.4 %) | 612 (3.2 %) | - | |
|
| |||||
| Number of deaths in entire follow-up (%) | 34,765 | 10,076 | 4,380 | 1,138 | |
|
| |||||
| Number of PCa deaths in entire follow-up (%) | 14,123 | 428 | 191 | 122 | |
|
| |||||
| Number of PCa deaths (%) | 10 years | 12,895 | 238 | 111 | 69 |
| 15 years | 13,949 | 380 | 176 | 114 | |
| 20 years | 14,110 | 420 | 190 | 122 | |
|
| |||||
| Age at PCa death, years | Median [IQR] | 78.2 [71.6 – 84.0] | 79.9 [74.4 – 85.3] | 79.6 [73.6 – 84.4] | 78.1 [72.7 – 83.7] |
|
| |||||
| Days from initial TRUS-biopsy to first re-biopsy | Median [IQR] | - | - | - | 392 [151 – 1,121] |
Abbreviations: DRE: digital rectal examination, GS: Gleason score, PCa: prostate cancer, PSA: prostate-specific antigen, TRUS: transrectal ultrasound-guided.
Men with initial TRUS-biopsy
Prostate cancer was diagnosed in 47,801 men at initial biopsy, of which 34,784 with Gleason score ≥ 7 (Table 1). The 15-year prostate cancer-specific and other-cause mortality following initial TRUS-biopsies was 20.6% (95% confidence interval (95% CI): 20.2 – 20.9) and 35% (95% CI: 34.6 – 35.5), respectively (Supplementary Figure A & Supplementary Table 2). An association between the PSA-level, at the time of biopsy, and mortality was found (p<0.001, by cause-specific Cox regression), with increased other-cause and prostate cancer-specific mortality for men with a higher PSA (Supplementary Figure B).
Men with negative initial TRUS-biopsy
37,214 men had a benign histopathological evaluation of the initial TRUS-biopsy. During follow-up, 10,076 men died following an initial negative TRUS-biopsy, with 428 prostate cancer deaths. The 15-year prostate cancer-specific and other cause mortality was 1.9% (95% CI: 1.7 – 2.1) and 37.6% (95% CI: 36.8 – 38.3), respectively (Supplementary Figure C & Supplementary Table 3). The median age at prostate cancer-specific death was 80 years [IQR: 74 – 85] (Table 1).
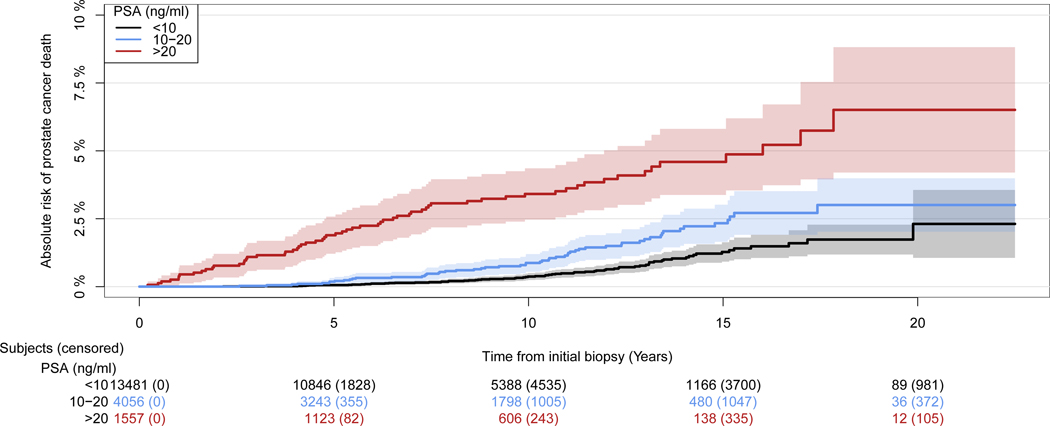
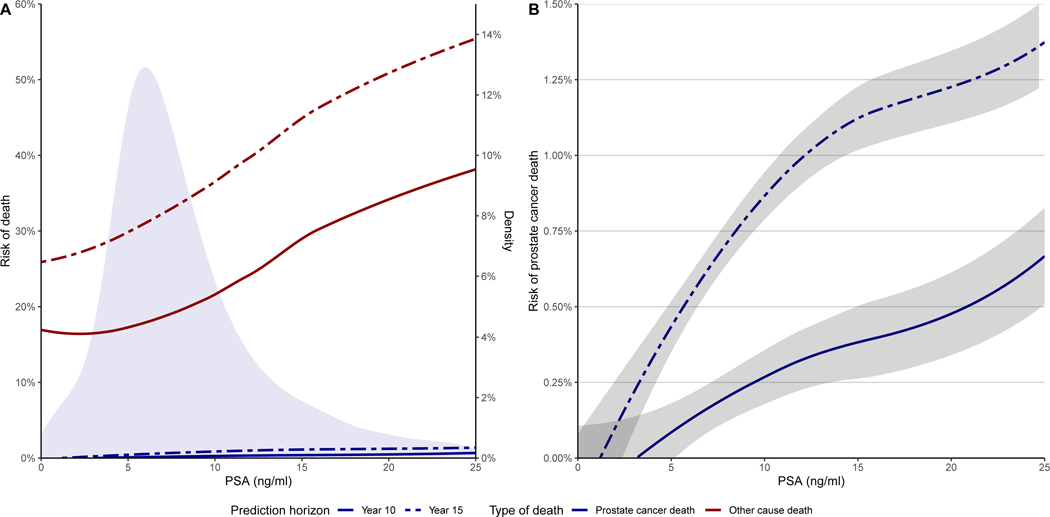
There was a strong association between PSA and prostate cancer-specific mortality (p<0.001 by Gray’s test; Figure 2 & Supplementary Table 1). Prostate cancer-specific mortality at year 15 was 1.3% (95% CI: 0.9 – 1.6), 2.3% (95% CI: 1.6 – 3.0) and 4.6% (95% CI: 3.4 – 5.8) for men with PSA < 10 ng/ml, PSA 10 – 20 ng/ml and PSA > 20 ng/ml, respectively. An association between PSA and mortality of both prostate cancer-specific and other-cause was found in men with normal DRE, e.g. men with higher PSA had higher mortality (p<0.001, by cause-specific Cox regression; Figure 3).
Figure 2:
Cumulative incidence of prostate cancer-specific mortality after initial negative transrectal ultrasound-guided biopsies, stratified on prostate-specific antigen (PSA) values (N=19,094).
Figure 3:
Non-parametric regression model with locally estimated scatterplot smoothing (LOESS) for prostate-specific antigen (PSA) of men with cT1 at the time of initial biopsy for men with benign histopathological evaluation. Figures illustrate the risk of prostate cancer-specific death (Blue) and other cause death (Red) at 10 years (solid line) or 15 years (dashed line) following an initial negative transrectal ultrasound-guided biopsy (A) and a magnification for prostate cancer-specific death with 95% confidence interval of the fitted line (B). The density plot shows the distribution of the PSA-values for the cause of death (A). Note that there is a second y-axis in plot A on the right illustrating the scale of the density plot and that the y-axes between plot A and B are different.
Men with TRUS re-biopsy
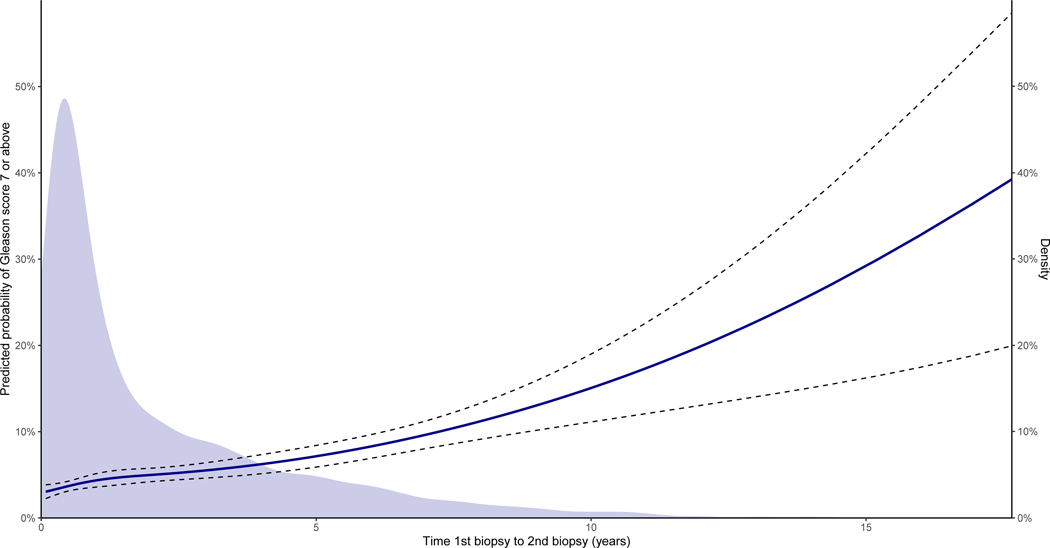
A total of 6,389 men with an initial negative biopsy and PSA-values underwent TRUS re-biopsies more than 30 days after their initial TRUS-biopsy (Table 1). The median time from the initial biopsy to the re-biopsy was 392 days [IQR: 151 – 1,121]. The number of re-biopsies was 3,527 out of 8,731 and 2,862 out of 10,363 for men below and above 70 years of age, respectively. The median PSA-level before re-biopsies was higher than before the initial negative biopsy (Table 1). More than 75% of the re-biopsies were negative and only 12% of the re-biopsies were found to have Gleason score ≥7 (Table 1). The risk of subsequently being diagnosed with Gleason score ≥7 increased with the time between biopsies (p<0.001, Figure 4). The relation between PSA and prostate cancer-specific and other-cause mortality after negative secondary biopsy closely resembled that of the relation shown after initial negative biopsy in men with normal DRE (Supplementary Figure D).
Figure 4:
Risk of Gleason score 7 or above prostate cancer in transrectal ultrasound-guided (TRUS) re-biopsy depending on the time between initial biopsy and first re-biopsy modelled for mean age, median prostate-specific antigen (PSA) and a normal digital rectal examination. at time of initial TRUS-biopsies. The 95% confidence interval is depicted with dotted lines (N=6,389). The density plot shows the distribution of time between initial biopsy and first re-biopsy. Note that there is a second y-axis on the right illustrating the scale of the density plot.
Discussion
Here we confirm the previously reported low prostate cancer-specific mortality after initial negative TRUS-biopsy with extended follow-up. We show that PSA has a strong relation with prostate cancer-specific mortality, with a much higher mortality in men with PSA above 10 ng/ml. This casts doubt on efforts to improve the sensitivity of prostate biopsy for lethal disease. Hence, while our data raises serious questions as to the value of routine MRI-targeting at initial biopsy, they also suggest a natural role for MRI-targeting as a reflex option in men with negative systematic biopsy and high PSA. We should also emphasize that our results pertain specifically to MRI-targeting, not to MRI in general. It remains plausible, for instance, that avoiding biopsy in men with negative MRI could reduce overdiagnosis and overtreatment.
Our findings indicate that systematic biopsies, in men at average risk undergoing initial biopsy, are sufficiently sensitive for lethal cancer, challenging recent guideline recommendations12. MRI-targeting finds many Gleason score ≥7 cancers in men with negative systematic biopsy. Our results suggest that few of these lesions will develop into significant disease in terms of cancer-specific mortality. Accordingly, our findings support the contention that MRI-targeting leads to a high risk of overdiagnosis and overtreatment.
Several other studies have found similarly low prostate cancer-specific mortality following negative biopsy. In the Gothenburg PSA Screening Trial, there were five deaths by 20 years in 452 men with initial negative biopsy, of which three occurred in men with high PSA (12.5, 33.5, 73.1 ng/ml). Both of the men with PSA < 10 ng/ml who died ceased followed-up at 70 due to the study protocol, an approach that would not be taken in contemporary practice; even including these men, 20-year mortality for PSA < 10 ng/ml was 0.6%9. The Rotterdam arm of the ERSPC reported a mortality of 0.2%, 11 years after initial negative biopsy, but results did not specify PSA-levels13. Our estimates are modestly higher, and the most plausible explanation is the lack of standardized follow-up in our population-based data and that many men in this historic cohort also only underwent sextant biopsies. During the study period, there were no Danish clinical guidelines for men with previous negative biopsies, with the decision to perform new biopsies or other investigations following the initial negative biopsy in the hands of the treating physician. In contrast, patients with negative biopsy in the screening trials had regular repeat PSAs, with follow-up biopsy if PSA remained elevated. It seems likely that some of the deaths in our study would have been prevented by more careful monitoring following negative biopsy.
This study is an updated version of a previous Danish study by Klemann et al. criticized for a short follow-up and a low number of men included8,14. Additionally, the historic nature of the cohort has been criticized for not resembling the contemporary management of men referred for biopsy. Recently, two prospective MRI studies including systematic TRUS-biopsy have reported diagnostic hit rates for the initial biopsies comparable to the current study. In the BIDOC-study, the detection rate was 34% in a cohort with median age of 67 years (IQR: 61–71) and median PSA of 8 ng/ml (IQR: 5.7 – 13.0)15. The PRECISION trial, A selected cohort of younger men with lower PSA and fewer clinical suspicious findings compared to our study, reported a 26% detection rate by TRUS-biopsies16. If we approximated the same inclusion criteria in our cohort, the diagnostic hit rate for Gleason score ≥7 prostate cancer is 22 – 30%.
There are some differences in estimates reported here in comparison with our former publication. In the first analysis in men with PSA < 10 ng/ml, Klemann et al. reported prostate cancer-specific mortality of 0.7% compared to 1.3% in here at 15 years. This difference could reflect a chance finding, but also reflect the fact that we identified more PSAs from the first cohort from 1995–2011 now included here and added significantly more men and follow-up. Secondly we did a more in-depth analysis of cause of death due to changes in our cause of death registry8 and assessed medical history from time of cancer suspicion of all prostate cancer-specific deaths Therefore, we also find that a higher prostate cancer-specific mortality in patients with PSA > 20 ng/ml.
In our study, the low, 12% rate of Gleason score ≥7 is comparable to the finding in Drost et al.17. The median PSA in the re-biopsy setting was higher than at the initial biopsy, indicating a selection towards men with persistent elevated or rising PSA. The probability of finding a Gleason score ≥7 in the TRUS re-biopsies was low for the first five years and about 30% after 15 years. Prostate cancer-specific mortality was only twice as high compared to the initial negative biopsy. This reflects that lethal lesions are difficult to diagnose early and perhaps lethal cancers are newly emerging, fast-growing tumors that occur by chance late in life because the patient has survived long enough18. This is substantiated by the median age of 80 years at death from prostate cancer. If not all prostate cancer-specific deaths after negative TRUS biopsy could have been prevented, had the initial biopsy involved MRI-targeting, then the effects of MRI-targeting of mortality would be weakened.
A relationship between PSA and other-cause mortality was observed. Only a few studies have investigated this relationship, but results of other population-based analyses lead to speculate that rising PSA is also associated with other cause mortality8,9,19–22. The explanation for this relationship is unclear, but there are several potential explanations. The most plausible explanation is a referral artifact: there is a lower propensity to PSA testing and taking of a biopsy in men who have comorbidities; such men are only likely to undergo a PSA test or biopsy if they represent themselves with clear clinical symptoms. Another explanation is that a high PSA could be associated with other medical conditions, such as inflammatory diseases, independently related to an increased mortality. Further research is warranted to explore the association between PSA and other-cause mortality.
The main strength of this study is related to the large population-based cohort including all men referred for TRUS-biopsies and re-biopsy in Denmark, and the long follow-up. The study is based on national registries ensuring complete follow-up. A major limitation is the lack of information regarding PSA-values for many men as well as information on reasons for referral. Future studies must address if other markers, such as prostate volume, PSA density, PSA kinetics, or serum/urine biomarkers, can further aid in defining who needs additional follow-up in the presence of initial negative biopsies. We excluded men with a re-biopsy within 30 days of the initial biopsies to avoid the likelihood of the second assessment to be either a revision of the initial biopsy or re-biopsies in men with overt prostate cancer undergoing biopsies immediately following false negative examination. This is rare in practice, and so is unlikely to importantly affect our results.
Conclusion
In men with negative biopsies, later prostate cancer-specific mortality is low, especially in men with PSA < 10 ng/ml. Prognosis was similar for men who undergo re-biopsies for persistently elevated PSA after previous negative biopsies. Our findings raise serious questions about the routine use of MRI-targeting for initial prostate biopsy compared to reflex use for men with high PSA after negative biopsy. Further investigation of the association between PSA and other cause mortality is planned to determine the mechanism.
Supplementary Material
Funding:
This work was supported in part by the National Institutes of Health/National Cancer Institute (NIH/NCI) with a Cancer Center Support Grant to Memorial Sloan Kettering Cancer Center [P30 CA008748], a SPORE grant in Prostate Cancer to Dr. H. Scher [P50-CA92629], the Sidney Kimmel Center for Prostate and Urologic Cancers and David H. Koch through the Prostate Cancer Foundation.
Abbreviations:
- 95% CI
95% confidence interval
- DaPCaR
Danish Prostate Cancer Registry
- DRE
Digital Rectal Examination
- LOESS
Locally estimated scatterplot smoothing
- MRI
Magnetic resonance imaging
- PSA
Prostate-specific Antigen
- TRUS
Transrectal ultrasound
Footnotes
Conflict of interest. Andrew Vickers is named on a patent for a statistical method to detect prostate cancer, the 4Kscore, that has been commercialized by OPKO Health. Andrew Vickers receives royalties from sales of the test and has stock options in OPKO Health.
References
- 1.Nafie S, Wanis M and Khan M: The efficacy of transrectal ultrasound guided biopsy versus transperineal template biopsy of the prostate in diagnosing prostate cancer in men with previous negative transrectal ultrasound guided biopsy. Urol. J 2017; 14. [PubMed] [Google Scholar]
- 2.Abraham NE, Mendhiratta N and Taneja SS: Patterns of repeat prostate biopsy in contemporary clinical practice. J. Urol 2015; 193. [DOI] [PubMed] [Google Scholar]
- 3.Djavan B, Ravery V, Zlotta A, et al. : Prospective evaluation of prostate cancer detected on biopsies 1, 2, 3 and 4: When should we stop? J. Urol 2001; 166. [PubMed] [Google Scholar]
- 4.Fütterer JJ, Briganti A, De Visschere P, et al. : Can Clinically Significant Prostate Cancer Be Detected with Multiparametric Magnetic Resonance Imaging? A Systematic Review of the Literature. Eur. Urol 2015; 68. [DOI] [PubMed] [Google Scholar]
- 5.Ahdoot M, Wilbur AR, Reese SE, et al. : MRI-Targeted, Systematic, and Combined Biopsy for Prostate Cancer Diagnosis. N. Engl. J. Med 2020; 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vickers AJ and Fine SW: Three Things About Gleason Grading That Just About Everyone Believes But That Are Almost Certainly Wrong. Urology 2020. [DOI] [PubMed] [Google Scholar]
- 7.Kawa SM, Benzon Larsen S, Helgstrand JT, et al. : What is the risk of prostate cancer mortality following negative systematic TRUS-guided biopsies? A systematic review. BMJ Open 2020; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klemann N, Røder MA, Helgstrand JT, et al. : Risk of prostate cancer diagnosis and mortality in men with a benign initial transrectal ultrasound-guided biopsy set: a population-based study. Lancet Oncol. 2017; 18. [DOI] [PubMed] [Google Scholar]
- 9.Palmstedt E, Månsson M, Frånlund M, et al. : Long-term Outcomes for Men in a Prostate Screening Trial with an Initial Benign Prostate Biopsy: A Population-based Cohort. Eur. Urol. Oncol 2019; 2. [DOI] [PubMed] [Google Scholar]
- 10.Helgstrand JT, Klemann N, Røder MA, et al. : Danish Prostate Cancer Registry – Methodology and early results from a novel national database. Clin. Epidemiol 2016; 8: 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Assel M, Sjoberg D, Elders A, et al. : Guidelines for reporting of statistics for clinical research in urology. J. Urol 2019; 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mottet N, Bellmunt J, Briers E, et al. : EAU – ESTRO – ESUR – SIOG Guidelines on Prostate Cancer. Edn. presented at the EAU Annual Congress Milan 2021 Arnhem, The Netherlands.: EAU Guidelines Office.; 2021. [Google Scholar]
- 13.Schröder FH, van den Bergh RCN, Wolters T, et al. : Eleven-Year Outcome of Patients with Prostate Cancers Diagnosed During Screening After Initial Negative Sextant Biopsies. Eur. Urol 2010; 57. [DOI] [PubMed] [Google Scholar]
- 14.Emberton M: Is a negative prostate biopsy a risk factor for a prostate cancer related death? Lancet Oncol. 2017; 18. [DOI] [PubMed] [Google Scholar]
- 15.Boesen L, Nørgaard N, Løgager V, et al. : Assessment of the Diagnostic Accuracy of Biparametric Magnetic Resonance Imaging for Prostate Cancer in Biopsy-Naive Men: The Biparametric MRI for Detection of Prostate Cancer (BIDOC) Study. JAMA Netw. Open 2018; 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasivisvanathan V, Rannikko AS, Borghi M, et al. : MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl. J. Med 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drost FJH, Osses D, Nieboer D, et al. : Prostate Magnetic Resonance Imaging, with or Without Magnetic Resonance Imaging-targeted Biopsy, and Systematic Biopsy for Detecting Prostate Cancer: A Cochrane Systematic Review and Meta-analysis. Eur. Urol 2020; 77. [DOI] [PubMed] [Google Scholar]
- 18.Bechis SK, Carroll PR and Cooperberg MR: Impact of age at diagnosis on prostate cancer treatment and survival. J. Clin. Oncol 2011; 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ørsted DD, Nordestgaard BG, Jensen GB, et al. : Prostate-specific antigen and long-term prediction of prostate cancer incidence and mortality in the general population. Eur. Urol 2012; 61. [DOI] [PubMed] [Google Scholar]
- 20.Wu X, Lv D, Eftekhar M, et al. : Cause-specific mortality of low and selective intermediate-risk prostate cancer patients with active surveillance or watchful waiting. Transl. Androl. Urol 2021; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang P, Sun L, Uhlman MA, et al. : Baseline PSA as a predictor of prostate cancer-specific mortality over the past 2 decades: Duke university experience. Cancer 2010; 116. [DOI] [PubMed] [Google Scholar]
- 22.Carter HB, Metter EJ, Wright J, et al. : Prostate-specific antigen and all-cause mortality: Results from the Baltimore longitudinal study on aging [3]. J. Natl. Cancer Inst 2004; 96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.