Abstract
The normal cornea has no blood vessels but has abundant innervation. There is emerging evidence that sensory nerves, originated from the trigeminal ganglion (TG) neurons, play a key role in corneal angiogenesis. In the current study, we examined the role of TG sensory neuron-derived calcitonin gene-related peptide (CGRP) in promoting corneal neovascularization (CNV). We found that CGRP was expressed in the TG and cultured TG neurons. In the cornea, minimal CGRP mRNA was detected and CGRP immunohistochemical staining was exclusively co-localized with corneal nerves, suggesting corneal nerves are likely the source of CGRP in the cornea. In response to intrastromal suture placement and neovascularization in the cornea, CGRP expression was increased in the TG. In addition, we showed that CGRP was potently pro-angiogenic, leading to vascular endothelial cell (VEC) proliferation, migration, and tube formation in vitro and corneal hemangiogenesis and lymphangiogenesis in vivo. In a co-culture system of TG neurons and VEC, blocking CGRP signaling in the conditioned media of TG neurons led to decreased VEC migration and tube formation. More importantly, subconjunctival injection of a CGRP antagonist CGRP8-37 reduced suture-induced corneal hemangiogenesis and lymphangiogenesis in vivo. Taken together, our data suggest that TG sensory neuron and corneal nerve-derived CGRP promotes corneal angiogenesis.
Keywords: Corneal angiogenesis, Neovascularization, Calcitonin gene-related peptide, Neuropeptide, Trigeminal ganglion neuron, Corneal innervation
1. Introduction
Angiogenesis is a process during which new blood vessels develop from existing ones and involves vascular endothelial cell (VEC) migration, proliferation and tube formation (Rajabi and Mousa, 2017). The normal cornea is transparent and avascular. Corneal neovascularization (CNV) is a sight-threatening condition that can result from various pathological insults such as loss of limbal stem cell barrier, corneal inflammation, infection, hypoxia and trauma (Nicholas and Mysore, 2021). Abnormal new vessel can invade the corneal stroma from the limbus and result in corneal opacification and decrease in vision. Moreover, presence of CNV greatly reduces the survival of corneal transplants (Di Zazzo et al., 2017; Zhong et al., 2018). One study estimated that 1.4 million people per year suffer from CNV (Sharif and Sharif, 2019). In another study, 4.14% of all patients presented in a comprehensive ophthalmology clinic were diagnosed with CNV and 12% of them showed visual impairment (Lee et al., 1998). Vascular endothelial growth factor-A (VEGF-A), a potent pro-angiogenic factor, has been found to play a key role in physiological and pathological angiogenesis (Melincovici et al., 2018).
In healthy individuals, the cornea is avascular but densely innervated, possessing the highest sensory nerve density in the human body (Shaheen et al., 2014; Eghrari et al., 2015). Corneal sensory nerves are derived from the ophthalmic branch of the trigeminal nerve (cranial nerve V1) (Müller et al., 2003; Shaheen et al., 2014). After entering the cornea from the limbus, the large nerve trunks branch and form a radial arrangement, resulting in a swirl pattern at cornea apex (Müller et al., 2003; He and Bazan, 2016). There is emerging evidence that the corneal sensory nerves play a role in regulating corneal angiogenesis. For instance, one study reported that corneal denervation leads to spontaneous development of blood vessels in the cornea (Ferrari et al., 2013). This phenomenon is also observed clinically in a patient developing CNV after trigeminal nerve block for neuralgia (Kodama-Takahashi et al., 2018). However, the direct interaction between blood vessels and nerves in the cornea and the underlying mechanisms have not been studied. We hypothesize that sensory nerves directly regulate corneal angiogenesis via secreted neuropeptides. We previously developed a co-culture system of trigeminal ganglion (TG) sensory neurons and VEC to investigate their interactions ex vivo and demonstrated that substance P, a neuropeptide secreted by TG neurons, potently promotes CNV in response to corneal inflammation (Liu et al., 2020). In this study, we sought to investigate the role of sensory neuron-derived calcitonin gene-related peptide (CGRP) in regulating corneal angiogenesis.
CGRP, a 37-amino acid neuropeptide, is widely distributed in the central and peripheral nervous systems (Rosenfeld et al., 1983). The expression of CGRP in TG and the cornea has been reported. About 31% of TG neurons are CGRP positive and 70% of the total sub-basal nerve fibers in the central cornea are CGRP positive, more abundant than the expression of substance P in the cornea (He and Bazan, 2016). CGRP signals through a heterodimeric receptor composed of two parts: calcitonin-like receptor (CLR, a G protein-coupled receptor) and receptor activity-modifying protein (RAMP) (Clark et al., 2021). There are three variants of RAMP: RAMP1, RAMP2, RAMP3. CGRP has the highest binding affinity to CLR-RAMP1, however it was reported to bind to CLR-RAMP2 and CLR-RAMP3 as well (Roehrkasse et al., 2018). Many studies have shown the role of CGRP in immunoregulation (Holzmann, 2013), cardiovascular homeostasis (Favoni et al., 2019), and pain modulation (Iyengar et al., 2017). Its proangiogenic effects in wound healing and tumor development have also been reported (Toda et al., 2008; Toda et al., 2008). However, the role of sensory neuron and corneal nerve-derived CGRP in regulating corneal angiogenesis has not been explored.
Herein, we employed a suture-induced CNV mouse model in vivo and the co-culture system of TG neurons and VEC ex vivo to determine the expression and role of sensory neuron-derived CGRP in promoting corneal angiogenesis.
2. Materials and methods
2.1. Animals
BALB/c and C57BL/6 mice of both sexes and aged from six to eight weeks were purchased from Charles River Laboratories, Wilmington, MA, USA. All animal experiments were approved by the Schepens Eye Research Institute Animal Care and Use Committee and adhered to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Mice were anesthetized for surgical procedures using intraperitoneal injections of 120 mg/kg Ketamine and 20 mg/kg Xylazine. Post-operative pain management and care were provided.
2.2. Corneal neovascularization (CNV) model in vivo
Corneal neovascularization was induced as described previously (Inomata et al., 2017; Satitpitakul et al., 2018). In brief, one intrastromal figure-eight suture knot was placed on the temporal side of cornea of BALB/c mice using 11–0 nylon suture (AB-0550S, MANI, Tochigi, Japan). After suture placement, subcutaneous injection of buprenorphine and topical application of triple antibiotic ointment were given to ease suture-induced pain and prevent infection, respectively. Postoperatively, CGRP8-37 (BACHEM, Torrance, CA, USA Cat.4034544), a CGRP antagonist, was given (200 μg/ml, diluted in 1 × phosphate-buffered saline, PBS) subconjunctivally three times weekly and the animals were observed for 2 weeks. In addition, CGRP (BACHEM, Cat. 4025897) diluted in 1 × PBS was applied topically three times daily with PBS as a control treatment. CNV was photographed at day 0 and day 14. Area covered by neovascularization on the whole cornea was photographed and analyzed by Photoshop CS2 and ImageJ (Amparo et al., 2013). On day 14, sutures were removed, and corneas were collected for immunohistochemical and molecular analysis.
2.3. Vascular endothelial cell (VEC) culture
Mouse VEC (MILE SVEN1 cell line) was purchased from the American Type Culture Collection (Manassas, VA, USA). After digesting VEC with trypsin-EDTA, the cells were incubated in complete medium DMEM (Thermo Fisher Scientific, Waltham, MA, USA) containing 10% fetal bovine serum (FBS, Atlanta Biologicals, Flower Branch, GA, USA) and 1% penicillin-streptomycin (Millipore Sigma, Burlington, MA, USA) at 37 °C and in 5% CO2.
2.4. Primary culture of trigeminal ganglion (TG) neurons
TG neurons were isolated from C57BL/6 mice and cultured as described in previous publication (Liu et al., 2020) (Malin et al., 2007). Briefly, TG tissue were dissected and placed in ice-cold Hank’s balanced salt solution (HBSS). Following this, TGs were digested with papain (Worthington, Lakewood, NJ, USA) and collagenase type II/dispase (Thermo Fisher Scientific). TG suspensions were then centrifuged at 1300g for 10 min in Percoll (VWR, Radnor, PA, USA) gradient. After washing twice with complete L15 (L15 with 5% FCS, penicillin/streptomycin, HEPES) and Neurobasal A medium supplemented with 2% B27 supplement (Thermo Fisher Scientific) and 1% penicillin-streptomycin, TG neurons at the bottom were counted and planted on coverslips precoated with poly-d-lysine and laminin. Each coverslip had 3000–4000 neurons. These isolated TG neurons were maintained in Neurobasal A medium supplemented with 2% B27 supplement, 1% penicillin-streptomycin, l-glutamine (500 μM), nerve growth factor (NGF; 50 ng/ml), glial-cell-derived neurotrophic factor (GDNF; 50 ng/ml), and mitotic inhibitor fluorodeoxyuridine (60 μM).
2.5. Co-culture of TG neurons and VEC
TG neurons were cultured for 3 days as described above, and the culture medium was changed with fresh 1% FBS DMEM and incubated for 24 h. The conditioned medium (CM) was then collected and used to incubate VEC in different conditions. 1% FBS DMEM medium was used as the control. VEC activities were then assessed as described below.
2.6. VEC proliferation assay
VECs were placed in 12-well-plate (5 × 104 cells/well) with or without CGRP (100 nM) or CGRP8-37 (100 nM). After 24 h of incubation, the VEC numbers were counted directly using a hemacytometer under a 20 × objective phase contrast microscope field.
2.7. VEC migration assay
VECs were placed in 12-well-plate (1 × 105 cells/well), cultured for 24 h in complete medium and then serum-started with 1% FBS DMEM overnight. A 10-μl micropipette tip was used to make a liner scratch in the VEC monolayer. VECs were then cultured with conditioned media, CGRP, CGRP8-37 or controls for 24 h. VEC migration was captured and analyzed using Image J software.
2.8. VEC tube formation assay
A Matrigel-based tube formation assay was performed as described previously (Francescone et al., 2011). In brief, 60 μl Matrigel (Corning, Cat. 354230; NY, USA) in a 96-well plate was incubated at 37 °C for 50 min to allow adequate polymerization. VECs (3.0 × 104 cells/well) cultured in various conditions were then placed on the Matrigel surface for 1.5 h. The tube formation was photographed with an inverted microscope and the results were calculated by Angiogenesis Analyze plugin of Image J software.
2.9. Immunofluorescence staining and imaging
To determine the expression of CGRP in TG, double immunofluorescence was used. Briefly, mice were euthanized, the chest were opened immediately, following infusion through the left ventricle of the heart with 50 ml PBS and 50 ml 4% paraformaldehyde. The whole TG was collected and fixed in 4% paraformaldehyde overnight and incubated in 30% sucrose at 4 °C for 1 day. TG tissue was then embedded in OCT compound and cryostat-sectioned (10-μm thickness). After dried at room temperature, the slides were washed with PBS and then blocked with 2% BSA supplemented with 0.1% Triton X-100 for 1 h at room temperature. Coverslip with cultured TG neurons were fixed in 4% paraformaldehyde for 15 min and blocked with 2% BSA for 1 h at room temperature. These slides (with either TG tissue or cultured TG neurons) were incubated with anti-beta III Tubulin (Millipore Sigma, Cat. AB15708A4) and anti-CGRP antibodies (Santa Cruz, Cat. sc-57053) in 1% BSA. Following washing with PBS, the slides were incubated with corresponding Cy3-conjugated secondary antibody (Jackson ImmunoResearch, Cat. 115-165-062) for 1 h at room temperature and mounted with VECTASHIELD (Vector Laboratories, Burlingame, CA, USA).
Whole corneas were also stained to determine the expression of CGRP. In brief, corneas with limbus were dissected and fixed in 4% paraformaldehyde for 1 h, followed by three washes with PBS. The corneas were then blocked in 5% BSA with 0.5% Triton X-100 for 2 h at room temperature. After blocking, the tissue was incubated with Alexa Fluor 488 conjugated anti-beta III Tubulin and anti-CGRP for 72 h at 4 °C. The corneas were then incubated with corresponding Cy3-conjugated secondary antibody for 24 h at 4 °C and mounted by VECTASHIELD with epithelial side up.
To assess corneal hemangiogenesis and lymphangiogenesis, corneas were isolated and fixed in 4% paraformaldehyde for 1 h. After incubated in 20 mM pre-warmed EDTA for 30 min at 37 °C, corneas were blocked with 2% BSA+0.2% solution of Triton X-100 for 2 h at room temperature. Corneas were then incubated with anti-CD31 antibody (BD Biosciences, Cat. 550274) and anti-LYVE-1 antibody (Angiobio, Cat. 11–034) overnight at 4 °C, followed by Alexa Fluor 488 conjugated secondary antibody (Jackson ImmunoResearch, Cat.712-545-150) and anti-rabbit Alexa Fluor 594 conjugated secondary antibody (Invitrogen, Cat. A-11012). Tissues were then washed and mounted as described above. All immunofluorescence staining slides were examined using TCS-SP8 confocal microscopy and images were analyzed with Image J software.
2.10. Real-time PCR
RNeasy® Micro kit (Qiagen, Valencia, CA, USA) was used to isolate total RNA in TG tissue and the cornea, and SuperScript™ III kit (Thermo Fisher Scientific) was used to reverse RNA to cDNA according to the manufacturer’s instructions. We used a LightCycler® 480 II System (Roche Applied Science) to perform RT-PCR cycle. The following primers were used: CGRP (Mm00801463_g1), VEGF-A (Mm00437306_m1), RAMP1 (Mm00489796_m1), RAMP2 (Mm00490256_g1), RAMP3 (Mm00840142_m1), CLR (Mm00516986_m1) and GAPDH (Mm99999915_g1) (Thermo Fisher Scientific). All assays were performed in duplicate. The results were analyzed by the comparative threshold cycle method and normalized to GAPDH as an internal control.
2.11. Statistical analysis
One-way ANOVA was used to assess differences among 3 groups or more, and unpaired two-tailed t-test was applied to compare the difference between two groups. Significant difference was set at P values less than 0.05 and all data shown are representative of at least two independent experiments, presenting as means ± SEM.
3. Results
3.1. Expression of CGRP in the trigeminal ganglion and cornea
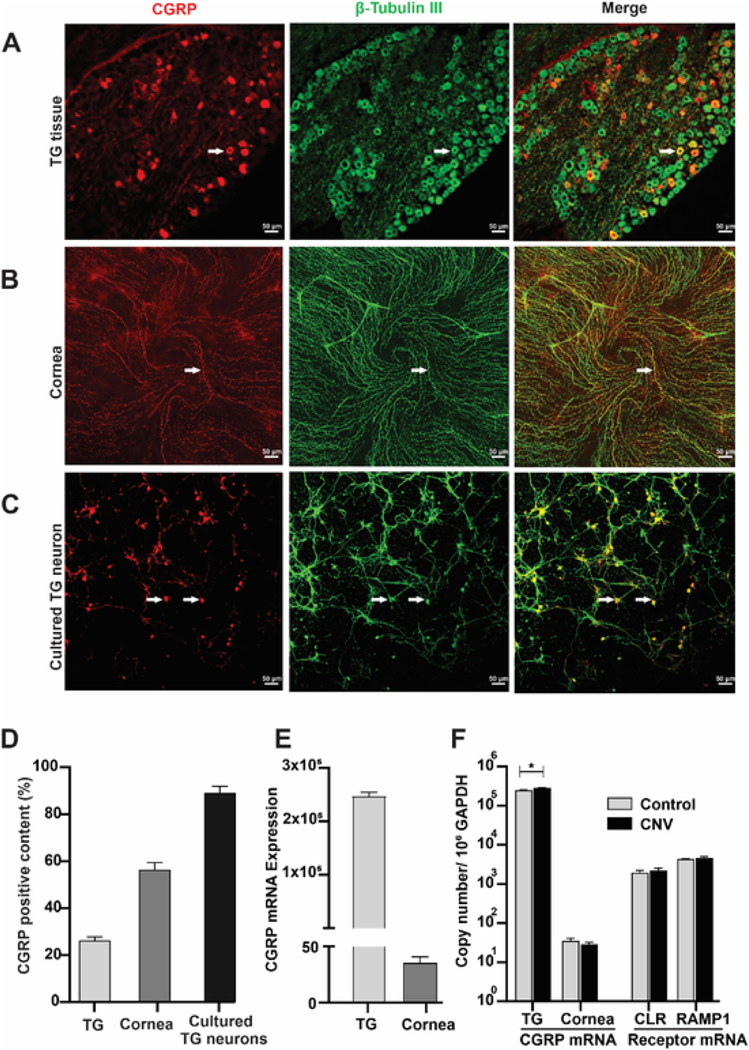
To explore the function of CGRP in corneal angiogenesis, we firstly determined the expression of CGRP in the trigeminal ganglion (TG) and cornea. Consistent with previous report (He and Bazan, 2016), double immunofluorescence with CGRP and β-tubulin III (sensory nerve marker) antibodies showed that 26.3% TG neurons were CGRP positive (Fig. 1A&D) and 56.5% of corneal nerves were CGRP positive (Fig. 1B&D). It is worth noting that in the cornea, all CGRP-positive staining coincided with β-tubulin III staining, and was not found in the corneal epithelium, keratocytes, or endothelium. We then cultured TG neurons and found that almost 90% of cultured neurons were CGRP-positive (Fig. 1C&D). Interestingly, while there were high copy numbers of CGRP mRNA in the TG, minimal mRNA level of CGRP was found in the cornea (Fig. 1E, fewer than 40 copies). The lack of CGRP mRNA in the cornea and the co-staining of CGRP exclusively with β-tubulin III suggest that nerves are likely the only source of CGRP in the cornea.
Fig. 1. Expression of CGRP and its receptor.
(A–C) Representative images of CGRP (red) and β-tubulin III (nerve marker, green) double immunofluorescence showing the distribution of CGRP in the trigeminal ganglion (TG, A), cornea (B) and cultured TG neurons (C); arrows showing representative double-positive neurons/nerves. (D) CGRP positivity was calculated as percentage of CGRP-positive versus total β-tubulin III-positive neurons or nerves (n = 3). (E) CGRP mRNA expression levels in TG and cornea, (n = 3). (F) Corneal intrastromal suture was placed in the mouse cornea to induce corneal neovascularization (CNV) for 1 week. The expression of CGRP in TG and cornea and the expression of CGRP receptor CLR and RAMP1 in the cornea were determined using RT-PCR (n = 6) and expressed as number of copies in log scale per 106 GAPDH (internal control). *p < 0.05 unpaired t-test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2. Expression of CGRP and its receptors in response to suture-induced corneal neovascularization
Intrastromal suture placement is a classic method to induce corneal neovascularization (CNV) and evaluate pro- and anti-angiogenic factors in the cornea (Cho et al., 2012; Montezuma et al., 2009). Seven days after suture placement, the TG and cornea were collected to assess the levels of CGRP. CGRP mRNA level was significantly elevated in the TG after suture placement (249,114 ± 8077 in control group vs 284,707 ± 6988 in CNV group copies per 106 GAPDH, P = 0.029, Fig. 1F). As mentioned above, the copy number of CGRP mRNA in the cornea was very low with and without suture placement (Fig. 1E). We then determined the mRNA expression of CGRP receptors CLR, and RAMP1-3 in the cornea. While cornea expressed CLR, RAMP1, RAMP2, it did not express RAMP3 (data not shown). Since CGRP binds to RAMP1 with the highest affinity, we then assessed CLR and RAMP1 levels after suture placement and found them unchanged (Fig. 1F). These findings indicate that in response to corneal suture placement, TG neurons expressed higher levels of CGRP while CGRP receptor levels in the cornea are unchanged, suggesting that CGRP may play a role in CNV.
3.3. CGRP promotes vascular endothelial cell activities in vitro
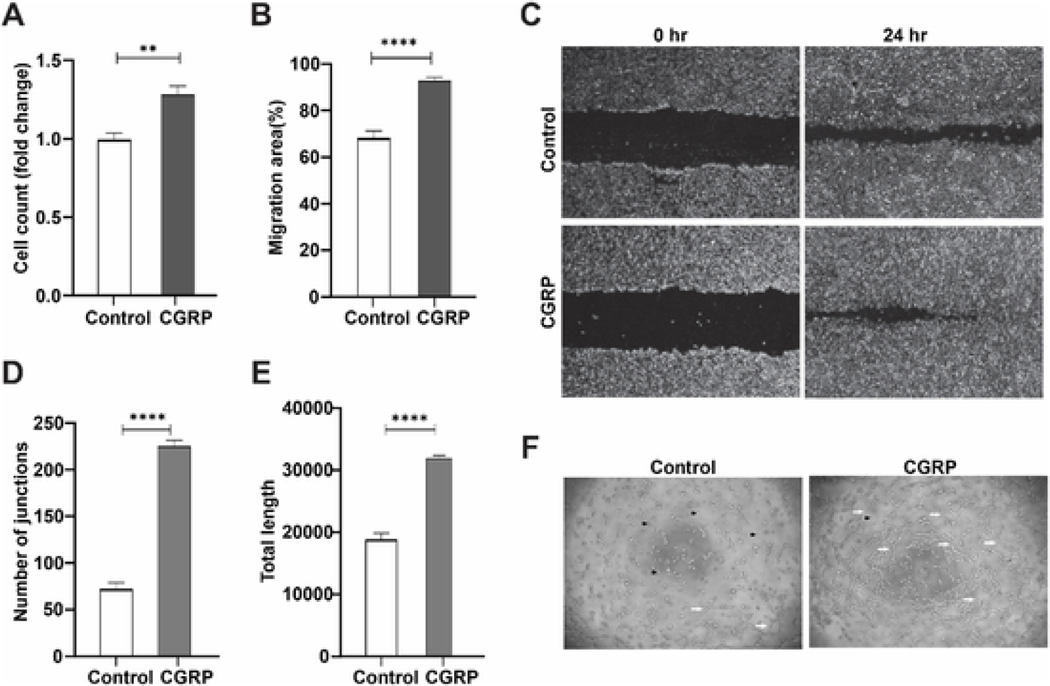
To determine CGRP’s function in angiogenesis, we used exogenous CGRP to stimulate vascular endothelial cells (VEC) in vitro. VEC treated with CGRP (100 nM) showed higher proliferation (P = 0.0015, Fig. 2A) and faster migration (P < 0.0001, Fig. 2B&C), compared to the control group. Similarly, CGRP greatly promoted VEC tube formation (Fig. 2F), increasing the number of junctions (P < 0.0001, Fig. 2D) and length (P < 0.0001, Fig. 2E) of the tubes formed. These data demonstrate that CGRP is potently pro-angiogenic in vitro.
Fig. 2. CGRP promotes vascular endothelial cell activities in vitro.
VEC were treated with or without exogenous CGRP (100 nM, n = 5). (A) VEC proliferation was determined after 24hr incubation. (B, C) Migration of VEC was quantified and photographed at 0hr and 24hr. (D-F) Tube formation in the Matrigel (F), was quantified as the number of junctions (D), and length of the tubes formed (E); black arrowheads indicating non-tube VEC, while the white arrows showing VEC tube formation. **p < 0.01, ****p < 0.0001, unpaired t-test.
3.4. CGRP promotes corneal hemangiogenesis and lymphangiogenesis in vivo
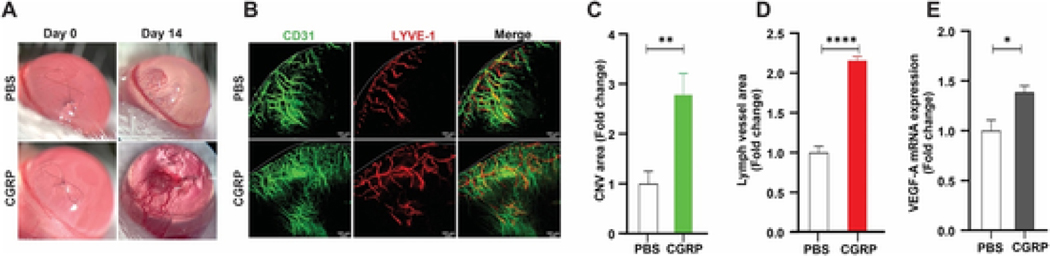
To determine CGRP’s pro-angiogenic function in vivo, we applied exogenous CGRP topically to sutured mouse corneas. After 14 days, CGRP-treated corneas had larger and more active neovascularization compared to control ones, as demonstrated by slit lamp photography in Fig. 3A. Hemangiogenesis and lymphangiogenesis were determined with CD31 and LYVE-1 immunostaining, respectively. CGRP treatment led to robust formation of blood and lymphatic vessels, approximately 3- and 2-fold over controls. (Fig. 3B–D). Similarly, CGRP treatment led to significantly elevated VEGF-A levels in the cornea (Fig. 3E). Collectively, these data demonstrate the CGRP promotes corneal neovascularization in vivo.
Fig. 3. CGRP promotes corneal hemangiogenesis and lymphangiogenesis in vivo.
A single figure-eight intrastromal suture was placed on mouse cornea and CGRP (50μΜ) or PBS control was given topically three times daily for 14 days (n = 5). (A) Representative images of slit lamp photography. (B) Representative images of corneal whole mount stained with CD31 (Blood vessels, green) and LYVE-1 (Lymph vessels, red), × 100 magnification, dashed line indicates the limbus. (C, D) Quantification of corneal neovascularization (CNV) area (CD31, C) and Lymph vessel area (LYVE-1, D) at day 14 after. (E) mRNA level of VEGF-A in the cornea was assessed. *p < 0.05, **p < 0.01, ****p < 0.0001, unpaired t-test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.5. Blocking CGRP signaling in the conditioned media of TG neurons suppresses VEC activities in vitro
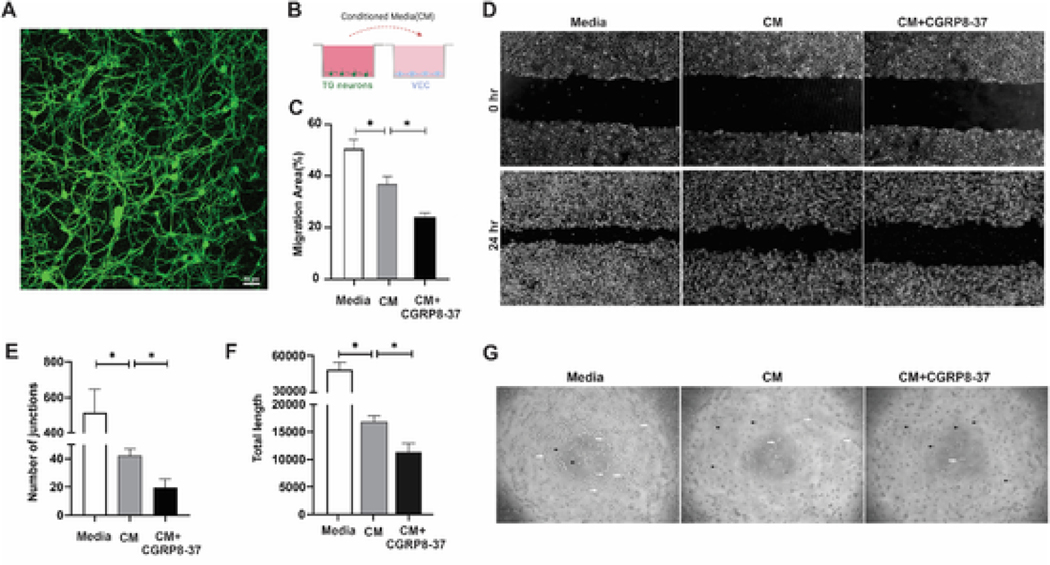
Having demonstrated the expression of CGRP in TG neurons and the cornea, as well as the pro-angiogenic function of CGRP, we next sought to determine the role of TG neuron-derived CGRP in regulating VEC activities in an ex vivo co-culture system. We first cultured TG neurons, whose viability and purity can be maintained up to seven days (Fig. 4A). Conditioned media from the cultured TG neurons were then collected and used to culture VEC with or without CGRP8-37, a CGRP receptor antagonist (Fig. 4B). Conditioned media alone led to a decrease in VEC migration and tube formation, suggesting that it is anti-angiogenic. Blocking CGRP signaling with CGRP8-37 in the conditioned media further reduced VEC migration (Fig. 4C&D, P = 0.004). Similarly, presence of CGRP8-37 in the conditioned media resulted in decreased VEC tube formation (Fig. 4G), both in the number of junctions (Fig. 4E, P = 0.029) and length (Fig. 4F, P = 0.027) of tubes formed. These data suggest that CGRP secreted by TG neurons promoted VEC activities in vitro.
Fig. 4. Blocking CGRP signaling in the conditioned media (CM) of trigeminal ganglion (TG) neurons suppresses VEC activities in vitro.
(A) Representative image of TG neurons cultured for 3 days then stained with β-tubulin III. (B) Schematic of the co-culture system, in which CM from cultured TG neurons were collected and applied to VEC culture. CGRP antagonist CGRP8-37 (100 nM) was added to VEC culture (n = 4). (C, D) Migration of VEC was quantified and photographed at 0hr and 24hr. (E–G) Tube formation in the Matrigel was photographed (G), and quantified as the number of junctions (E), and length of the tube formed (F); black arrowheads indicating non-tube VEC, while the white arrows showing VEC tube formation. *p < 0.05, one-way ANOVA followed by Tukey’s multiple comparisons test.
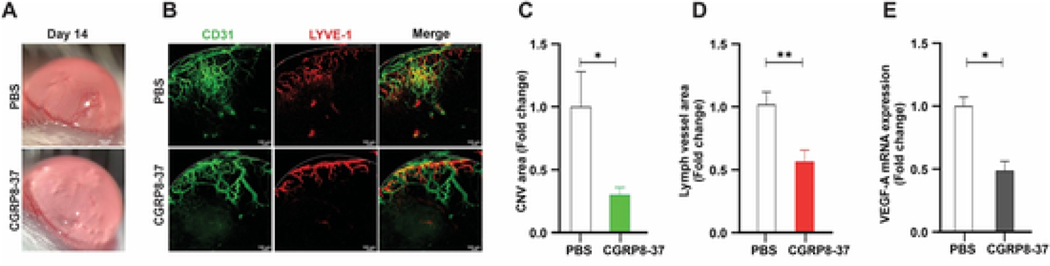
3.6. Blocking CGRP signaling inhibits corneal hemangiogenesis and lymphangiogenesis in vivo
We next applied CGRP8-37 subconjunctivally to sutured mouse corneas in vivo. After 14-day treatment, application of CGRP8-37 significantly reduced CNV as demonstrated by slit lamp photography in Fig. 5A, compared to the PBS-treated eye. Similarly, hemangiogenesis (CD31) and lymphangiogenesis (LYVE-1) were reduced approximately 70% and 50% by CGRP8-37 treatment (Fig. 5B–D). mRNA expression of VEGF-A was also decreased in the CGRP8-37-treated corneas (Fig. 5E). Together, these results show that blocking CGRP signaling in the cornea reduces hemangiogenesis and lymphangiogenesis with therapeutic potential in treating CNV.
Fig. 5. Blocking CGRP signaling inhibits corneal hemangiogenesis and lymphangiogenesis in vivo.
A single figure-eight intrastromal suture was placed on mouse cornea and CGRP8-37 (200 μg/ml) or PBS control was administered via subconjunctival injection three times/week for two weeks (n = 5). (A) Representative images of corneal neovascularization (CNV) with slit-lamp photography. (B) Representative images of corneal whole mount stained with CD31 (Blood vessels, green) and LYVE-1 (Lymph vessels, red), × 100 magnification, dashed line indicates the limbus. (C, D) Quantification of CNV area (CD31, C) and Lymph vessel area (LYVE-1, D) at day 14. (E) mRNA level of VEGF-A in cornea was assessed. *p < 0.05, **p < 0.01, unpaired t-test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
We have previously published that in response to ocular surface desiccating stress, corneal nerves, derived from trigeminal ganglion (TG) sensory neurons, secrete higher levels of neuropeptide substance P, which directly promotes corneal neovascularization (CNV) (Liu et al., 2020). In the current study, we investigated the role of TG neuron and corneal nerve-derived calcitonin gene-related peptide (CGRP) in promoting corneal angiogenesis. We found that CGRP in the cornea was exclusively from nerves and its expression in the TG was increased in response to corneal intrastromal suture placement and neovascularization. Our results showed that CGRP was potently pro-angiogenic in vitro and in vivo. In addition, we showed that in a TG neuron and vascular endothelial cell (VEC) co-culture system, blocking CGRP signaling in the neuron-conditioned media led to decreased VEC migration and tube formation. More importantly, subconjunctival injection of a CGRP antagonist CGRP8-37 reduced suture-induced hemangiogenesis and lym-phangiogenesis in vivo. Collectively, our data demonstrate that CGRP secreted by TG neurons is transported to the cornea via sensory nerves and plays a key role in promoting corneal angiogenesis.
Recently, there has been increasing evidence showing the vital role of nerve in regulating angiogenesis. In cancer, noradrenaline released from adrenergic nerves has been shown to promote angiogenesis via VEGF signaling (Kamiya et al., 2021; Silverman et al., 2021). Similarly, nerve growth factor, a neurotrophin essential to nerve development and growth, was found to stimulate VEGF production and subsequently promote angiogenesis in epithelial ovarian cancer and limb ischemia (Campos et al., 2007; Diao et al., 2016). In a sciatic nerve transection model, angiogenesis is activated after nerve injury (Wang et al., 2017), which is similar to the observation in the cornea that neovascularization develops after corneal denervation (Ferrari et al., 2013). Our laboratory explores the neuro-regulation of corneal angiogenesis and recently found that in homeostasis corneal nerves directly contribute to the angiogenetic privilege (avascularity) of the cornea via secreted neuropeptides (Yin et al., 2021). Indeed, in the current study, we found that conditioned media from cultured TG sensory neurons isolated from naïve mice reduces VEC migration and tube formation. More importantly we found that in response to ocular surface inflammation, TG neurons and corneal nerves change their secretion profile including an increase in substance P that is pro-angiogenic and promotes CNV. Similar to substance P, CGRP has been shown to be a crucial neuropeptide secreted by corneal nerves (He and Bazan, 2016). We observed that after intrastromal suture placement and the development of CNV, CGRP levels in the TG were increased. We therefore sought to determine its role in the neuro-regulation of corneal angiogenesis in the current study.
CGRP is a 37-amino acid neuropeptide and widely found in the nervous system (Rosenfeld et al., 1983). CGRP has been shown as one of the key neuropeptides in the cornea (Jones and Marfurt, 1991; He and Bazan, 2016). Our data are consistent with previous reports that roughly one third of TG neurons and two thirds of corneal nerves are positive for CGRP staining (He and Bazan, 2016). Moreover, we showed that almost 90% of cultured TG neurons are CGRP positive. The abundance of CGRP in corneal innervation argues for its importance in corneal pathophysiology. By detecting the levels of CGRP mRNA (via RT-PCR) and protein (via immunohistochemistry) in the TG and cornea, we found that there is minimal CGRP mRNA in the cornea while its expression in TG neurons is abundant. In addition, we noted that CGRP immunohistochemical positivity in the cornea is exclusively costained with nerve marker β tubulin III, and not found in (epithelial, stromal, endothelial, or immune) cellular components. These data suggest that CGRP transcription and translation occur in TG neurons and the protein is then transported via the trigeminal nerve and its branches to the cornea. It can also be deduced from these data that CGRP in the cornea exclusively originates from the nerves in vivo.
In addition to its roles in immunoregulation (Holzmann, 2013), cardiovascular homeostasis (Favoni et al., 2019), and pain modulation (Iyengar et al., 2017), CGRP has been shown to be pro-angiogenic in distraction osteogenesis (Mi et al., 2021), ischemia (Zheng et al., 2010; Mishima et al., 2011), and tumor growth (Toda et al., 2008). CGRP has been noted to promote endothelial progenitor cell proliferation and inhibit its apoptosis (Wu et al., 2018). Toda et al. showed that angiogenesis induced by full-thickness skin wound is significantly suppressed in CGRP knockout mice compared with wild-type C57BL/6 mice of the same age (Toda et al., 2008). While these data endorse the pro-angiogenic function of CGRP, others have shown that it may suppress angiogenesis in different conditions. For instance, in a high glucose-induced cerebral damage model, overexpression of CGRP suppresses hyperglycemia-induced cerebral microvascular endothelial tube formation (Guo et al., 2019). In a laser-induced choroidal neovascularization model, CGRP knockout mice presented with more severe choroidal neovascularization lesions, compared to the wild-type mice (Toriyama et al., 2015). The exact role of CGRP in angiogenesis, therefore, varies depending on the tissues, diseases, and pathological settings.
In our study, we found CGRP to promote VEC proliferation, migration, and tube formation in vitro. More importantly, we showed that exogenous CGRP promotes suture-induced CNV when given topically. Noting that lymphangiogenesis usually runs in parallel with hemangiogenesis (Patel and Dana, 2009) and that the lymph vessel development in corneal graft marks a high risk for rejection (Lee et al., 2021), we also studied the effect of the CGRP on the lymphangiogenesis. Similar to hemangiogenesis, we found that CGRP treatment increases lymph vessel formation and branching in the cornea. CNV is a complex process involving not only blood and lymphatic vessel formation, but also inflammatory response (Krishnamoorthy and Honn, 2006). CGRP has been reported to play an anti-inflammatory role in septic shock, autoimmune diabetes, and inflammatory bowel disease (Holzmann, 2013); and immune cells such as lymphocytes, dendritic cells, mast cells, macrophages have been shown to express receptors for CGRP (Assas et al., 2014). In our unpublished data, we found that CGRP reduced the maturation of bone marrow derived dendritic cells in vitro and that topical treatment with CGRP reduced CD45+ leukocyte infiltration and the production of inflammatory cytokines TNF-α and IL-1β in the cornea after mechanical injury in vivo. These data demonstrate the anti-inflammatory role of CGRP in the cornea. But despite reducing corneal inflammation, CGRP still potently promotes CNV in vivo. This suggests that there is divergence in the pro-angiogenic and anti-inflammatory functions of CGRP in the cornea. Using a co-culture system of TG neurons and VEC, we showed that blocking CGRP signaling using its antagonist CGRP8-37 in the conditioned media of TG neurons reduces VEC tube formation and migration, confirming the pro-angiogenic function of TG neuron-secreted CGRP. In suture-induced CNV mouse model, we showed subconjunctival injection of CGRP8-37 leads to a reduction in hemangiogenesis, lymphangiogenesis, and VEGF-A production in the cornea. Collectively, these data prove that the TG sensory neuron-derived CGRP plays a key role in promoting corneal angiogenesis.
In our study, we established the expression of CGRP receptor components CLR, RAMP1, and RAMP2, but not RAMP3, in the cornea. It has been previously reported that CLR and RAMP1 are expressed by vascular endothelial cells (Toda et al., 2008). In response to corneal suture placement and neovascularization, their mRNA levels are not significantly changed, suggesting that it is the increase of CGRP secretion by TG neurons, but not its receptor levels in the cornea, that contributes to CNV in our model. Throughout the study, we used a competitive CGRP antagonist CGRP8-37 (Chiba et al., 1989), which is a N-terminally truncated peptide that has high affinity for RAMP1, less so for RAMP3, and none for RAMP2 (Roehrkasse et al., 2018). CGRP binding to its receptors (CLR-RAMP1 and CLR-RAMP2) initiates signaling through either adenylyl cyclase activation generating cAMP and mobilizing the intracellular Ca2+ or through extracellular signal-regulated kinase 1/2 (ERK1/2) pathway. This signaling cascade in endothelial cells was reported to be involved in nitric oxide generation and cell proliferation leading to angiogenesis (Hay et al., 2018; Watkins et al., 2013). Several reports have shown that VEGF is a downstream signaling molecule of CGRP (Toda et al., 2008; Du et al., 2018; Majima et al., 2019). The elevation of intracellular cAMP may enhance VEGF production (Zheng et al., 2010). Interestingly, we also found that changes in VEGF-A expression in the cornea correspond to the changes in CGRP signaling in vivo. Whether the changes in VEGF-A levels in our model is dependent on CGRP signaling requires additional study.
In addition to CGRP investigated in the current study, we have previously demonstrated a key role of substance P in the promotion of corneal angiogenesis by inflamed sensory nerves (Liu et al., 2020). While both neuropeptides have been shown to be pro-angiogenic in our model, it is worth noting that the conditioned media from normal TG sensory neurons is overall anti-angiogenic, leading to less VEC activation in vitro and CNV in vivo when given as a topical treatment (Yin et al., 2021). This suggests that there are soluble anti-angiogenic factors secreted by TG sensory neurons and their effect on angiogenesis over-powers that of pro-angiogenic ones such as CGRP and substance P in homeostasis. Our preliminary exploration points toward alpha melanocyte-stimulating hormone as a potential anti-angiogenic corneal nerve-derived neuropeptide that plays a key role in this process (data not shown). On the other hand, in response to inflammation, such balance of neuropeptides is disrupted with an increase in pro-angiogenic factor such as substance P, leading to an overall pro-angiogenic profile of neuron secretion and more CNV (Liu et al., 2020).
CGRP and substance P are both key neuropeptides in the cornea with prominent angiogenic function, but their interplay in the neuroregulation of corneal angiogenesis is scarcely studied. In an older study with cultured pulmonary artery endothelial cells, a synergistic effect on cell growth was found between substance P and CGRP, although no mechanism was offered (Kaibara et al., 1994). In our co-culture system with normal, non-inflamed TG neurons, blocking CGRP, but not substance P (Liu et al., 2020), signaling in the conditioned media leads to a decrease in VEC activities. This suggests that between these two peptides, CGRP, but not substance P, is the key pro-angiogenic factor in homeostasis in our system. Interestingly, we found that exogenous substance P is able to overcome the anti-angiogenic effect of CGRP antagonist CGRP8-37 (Supplementary Fig. S1A), suggesting that substance P signaling is independent of CGRP signaling in this system. Conversely, when substance P signaling is blocked with neurokinin 1 receptor (NK1R) antagonist Spantide I, exogenous CGRP is no longer able to promote VEC tube formation (Supplementary Fig. S1B). This suggests that the pro-angiogenic effect triggered by high doses of CGRP may at least partially signal through NK1R. Indeed, it has been reported that CGRP increases NK1R mRNA levels in spinal neurons, leading to increased substance P binding (Allen et al., 1976). It is with noting that there may be other neuropeptides secreted by the TG sensory neurons that play critical roles it their regulation of corneal angiogenesis in homeostasis and disease state. In addition, the cornea has been shown to be innervated not only by sensory nerves derived from the trigeminal ganglion but also by autonomic nerves (sympathetic and parasympathetic) from the superior cervical ganglion (Shaheen et al., 2014). Therefore, roles of other neuropeptides released by sensory neurons, and whether and how autonomic nerves regulate corneal angiogenesis warrant further studies.
In summary, our study provides new evidence that trigeminal ganglion sensory neurons and corneal nerves secrete neuropeptide CGRP, which promotes vascular endothelial cell activities in vitro, and corneal hemangiogenesis and lymphangiogenesis in vivo. Blocking CGRP signaling in the cornea may have therapeutic potential in treating diseases associated with corneal neovascularization.
Supplementary Material
Acknowledgements
The authors wish to thank Dr. Patricia A. D’Amore, Schepens Eye Research Institute of Massachusetts Eye and Ear, Harvard Medical School, for her scientific and technical support. The current research is supported by NIH 5K08EY031340 (JY), Alcon Research Institute Young Investigator Award (JY), the Mass General Hospital Claflin Distinguished Scholar Award (JY), NIH P30 EY003790 (core), and the Chinese Scholarship Council (SZ).
Footnotes
Disclosure/conflict of interest statement
JY is a consultant for Claris Biotherapeutics and reports equity in Kera Therapeutics. For the remaining authors, none were declared.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.exer.2022.109125.
References
- Allen GS, Gross CJ, Henderson LM, Chou SN, 1976. Cerebral arterial spasm. Part 4: in vitro effects of temperature, serotonin analogues, large nonphysiological concentrations of serotonin, and extracellular calcium and magnesium on serotonin-induced contractions of the canine basilar artery. J. Neurosurg. 44 (5), 585–593. 10.3171/jns.1976.44.5.0585. [DOI] [PubMed] [Google Scholar]
- Amparo F, Sadrai Z, Jin Y, Alfonso-Bartolozzi B, Wang H, Shikari H, Ciolino JB, Chodosh J, Jurkunas U, Schaumberg DA, Dana R, 2013. Safety and efficacy of the multitargeted receptor kinase inhibitor pazopanib in the treatment of corneal neovascularization. Investig. Ophthalmol. Vis. Sci. 54 (1), 537–544. 10.1167/iovs.12-11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assas BM, Pennock JI, Miyan JA, 2014. Calcitonin gene-related peptide is a key neurotransmitter in the neuro-immune axis. Front. Neurosci. 8 (8 FEB), 1–9. 10.3389/fnins.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos X, Muñoz Y, Selman A, Yazigi R, Moyano L, Weinstein-Oppenheimer C, Lara HE, Romero C, 2007. Nerve growth factor and its high-affinity receptor trkA participate in the control of vascular endothelial growth factor expression in epithelial ovarian cancer. Gynecol. Oncol. 104 (1), 168–175. 10.1016/j.ygyno.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Chiba T, Yamaguchi A, Yamatani T, Nakamura A, Morishita T, Inui T, Fukase M, Noda T, Medicine I, 1989. Calcitonin antagonist gene-related peptide receptor. Am. J. Physiol. 331–335. [DOI] [PubMed] [Google Scholar]
- Cho YK, Uehara H, Young JR, Archer B, Zhang X, Ambati BK, 2012. Vascular endothelial growth factor receptor 1 morpholino decreases angiogenesis in a murine corneal suture model. Investig. Ophthalmol. Vis. Sci. 53 (2), 685–692. 10.1167/iovs.11-8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AJ, Mullooly N, Safitri D, Harris M, de Vries T, MaassenVanDenBrink A, Poyner DR, Gianni D, Wigglesworth M, Ladds G, 2021. CGRP, adrenomedullin and adrenomedullin 2 display endogenous GPCR agonist bias in primary human cardiovascular cells. Commun. Biol. 4 (1), 1–12. 10.1038/s42003-021-02293-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Zazzo A, Kheirkhah A, Abud TB, Goyal S, Dana R, 2017. Management of high-risk corneal transplantation. Surv. Ophthalmol. 62 (6), 816–827. 10.1016/j.survophthal.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao YP, Cui FK, Yan S, Chen ZG, Lian LS, Guo LL, Li YJ, 2016. Nerve growth factor promotes angiogenesis and skeletal muscle fiber remodeling in a murine model of hindlimb ischemia. Chinese Med J 129 (3), 313–319. 10.4103/0366-6999.174496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghrari AO, Riazuddin SA, Gottsch JD, 2015. Overview of the cornea: structure, function, and development. In: Progress in Molecular Biology and Translational Science, first ed., vol. 134. Elsevier Inc. 10.1016/bs.pmbts.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Favoni V, Giani L, Al-Hassany L, Asioli GM, Butera C, De Boer I, Guglielmetti M, Koniari C, Mavridis T, Vaikjärv M, Verhagen I, Verzina A, Zick B, Martelletti P, Sacco S, 2019. CGRP and migraine from a cardiovascular point of view: what do we expect from blocking CGRP? J. Headache Pain 20 (1). 10.1186/s10194-019-0979-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari G, Hajrasouliha AR, Sadrai Z, Ueno H, Chauhan SK, Dana R, 2013. Nerves and neovessels inhibit each other in the cornea. Investig. Ophthalmol. Vis. Sci. 54 (1), 813–820. 10.1167/iovs.11-8379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francescone RA, Faibish M, Shao R, 2011. A matrigel-based tube formation assay to assess the vasculogenic activity of tumor cells. JoVE 55, 2–5. 10.3791/3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Zhang Q, Chen H, Jiang Y, Gong P, 2019. Overexpression of calcitonin gene-related peptide protects mouse cerebral microvascular endothelial cells from high-glucose-induced damage via ERK/HIF-1/VEGF signaling. J. Physiol. Sci. 69 (6), 939–952. 10.1007/s12576-019-00708-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay DL, Garelja ML, Poyner DR, Walker CS, 2018. Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR Review 25. Br. J. Pharmacol. 175 (1), 3–17. 10.1111/bph.14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Bazan HEP, 2016. Neuroanatomy and neurochemistry of mouse cornea. Investig.Ophthalmol. Vis. Sci. 57 (2), 664–674. 10.1167/iovs.15-18019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzmann B, 2013. Modulation of immune responses by the neuropeptide CGRP. Amino Acids 45 (1), 1–7. 10.1007/s00726-011-1161-2. [DOI] [PubMed] [Google Scholar]
- Inomata T, Mashaghi A, Di Zazzo A, Lee SM, Chiang H, Dana R, 2017. Kinetics of angiogenic responses in corneal transplantation. Cornea 36 (4), 491–496. 10.1097/ICO.0000000000001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar S, Ossipov MH, Johnson KW, 2017. The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. Pain 158 (4), 543–559. 10.1097/j.pain.0000000000000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MA, Marfurt CF, 1991. Calcitonin gene-related peptide and corneal innervation: a developmental study in the rat. J. Comp. Neurol. 313 (1), 132–150. 10.1002/cne.903130110. [DOI] [PubMed] [Google Scholar]
- Kaibara M, Mitarai S, Yano K, Kameyama M, 1994. Involvement of Na+-H+ antiporter in regulation of L-type Ca2+ channel current by angiotensin II in rabbit ventricular myocytes. Circ. Res. 75 (6), 1121–1125. 10.1161/01.RES.75.6.1121. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Hiyama T, Fujimura A, Yoshikawa S, 2021. Sympathetic and parasympathetic innervation in cancer: therapeutic implications. Clin. Auton. Res. 31 (2), 165–178. 10.1007/s10286-020-00724-y. [DOI] [PubMed] [Google Scholar]
- Kodama-Takahashi A, Sugioka K, Sato T, Nishida K, Aomatsu K, Fukuda M, Shimomura Y, 2018. Neurotrophic keratopathy after trigeminal nerve block for treatment of postherpetic neuralgia. Case Rep. Ophthalmol. Med. 2018 (December 2016), 1–5. 10.1155/2018/6815407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy S, Honn KV, 2006. Inflammation and disease progression. Cancer Metastasis Rev. 25 (3), 481–491. 10.1007/s10555-006-9016-0. [DOI] [PubMed] [Google Scholar]
- Lee HK, Lee SM, Lee DI, 2021. Corneal lymphangiogenesis: current pathophysiological understandings and its functional role in ocular surface disease. Int. J. Mol. Sci. 22 (21). 10.3390/ijms222111628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Wang CC, Adamis AP, 1998. Ocular neovascularization: an epidemiologic review. Surv. Ophthalmol. 43 (3), 245–269. 10.1016/S0039-6257(98)00035-6. [DOI] [PubMed] [Google Scholar]
- Liu L, Dana R, Yin J, 2020. Sensory neurons directly promote angiogenesis in response to inflammation via substance P signaling. FASEB (Fed. Am. Soc. Exp. Biol.) J. 34 (5), 6229–6243. 10.1096/fj.201903236R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majima M, Ito Y, Hosono K, Amano H, 2019. CGRP/CGRP receptor antibodies: potential adverse effects due to blockade of neovascularization? Trends Pharmacol. Sci. 40 (1), 11–21. 10.1016/j.tips.2018.11.003. [DOI] [PubMed] [Google Scholar]
- Malin SA, Davis BM, Molliver DC, 2007. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat. Protoc. 2 (1), 152–160. 10.1038/nprot.2006.461. [DOI] [PubMed] [Google Scholar]
- Melincovici CS, Boşca AB, Şuşman S, Mărginean M, Mihu C, Istrate M, Moldovan IM, Roman AL, Mihu CM, 2018. Vascular endothelial growth factor (VEGF) – key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 59 (2), 455–467. [PubMed] [Google Scholar]
- Mi J, Xu J, Yao H, Li X, Tong W, Li Y, Dai B, He X, Chow DHK, Li G, Lui KO, Zhao J, Qin L, 2021. Calcitonin gene-related peptide enhances distraction osteogenesis by increasing angiogenesis. Tissue Eng. 27 (1–2), 87–102. 10.1089/ten.tea.2020.0009. [DOI] [PubMed] [Google Scholar]
- Mishima T, Ito Y, Hosono K, Tamura Y, Uchida Y, Hirata M, Suzsuki T, Amano H, Kato S, Kurihara Y, Kurihara H, Hayashi I, Watanabe M, Majima M, 2011. Calcitonin gene-related peptide facilitates revascularization during hindlimb ischemia in mice. Am. J. Physiol. Heart Circ. Physiol. 300 (2), 431–439. 10.1152/ajpheart.00466.2010. [DOI] [PubMed] [Google Scholar]
- Montezuma SR, Vavvas D, Miller JW, 2009. Review of the ocular angiogenesis animal models. Semin. Ophthalmol. 24 (2), 52–61. 10.1080/08820530902800017. [DOI] [PubMed] [Google Scholar]
- Müller LJ, Marfurt CF, Kruse F, Tervo TMT, 2003. Corneal nerves: structure, contents and function. Exp. Eye Res. 76 (5), 521–542. 10.1016/S0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- Nicholas MP, Mysore N, 2021. Corneal neovascularization. Exp. Eye Res. 202 (June 2020), 108363. 10.1016/j.exer.2020.108363. [DOI] [PubMed] [Google Scholar]
- Patel SP, Dana R, 2009. Corneal lymphangiogenesis: implications in immunity. Semin.Ophthalmol. 24 (3), 135–138. 10.1080/08820530902801320. [DOI] [PubMed] [Google Scholar]
- Rajabi M, Mousa SA, 2017. The Role of Angiogenesis in Cancer Treatment. 10.3390/biomedicines5020034. [DOI] [PMC free article] [PubMed]
- Roehrkasse AM, Booe JM, Lee SM, Warner ML, Pioszak AA, 2018.Structure–function analyses reveal a triple-turn receptor-bound conformation of adrenomedullin 2/intermedin and enable peptide antagonist design. J. Biol. Chem. 293 (41), 15840–15854. 10.1074/jbc.RA118.005062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld MG, Mermod JJ, Amara SG, Swanson LW, Sawchenko PE, Rivier J, Vale WW, Evans RM, 1983. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature 304 (5922), 129–135. 10.1038/304129a0. [DOI] [PubMed] [Google Scholar]
- Satitpitakul V, Sun Z, Suri K, Amouzegar A, Katikireddy KR, Jurkunas UV, Kheirkhah A, Dana R, 2018. Vasoactive intestinal peptide promotes corneal allograft survival. Am. J. Pathol. 188 (9), 2016–2024. 10.1016/j.ajpath.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen BS, Bakir M, Jain S, 2014. Corneal nerves in health and disease. Surv.Ophthalmol. 59 (3), 263–285. 10.1016/j.survophthal.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif Z, Sharif W, 2019. Corneal neovascularization: updates on pathophysiology,investigations & management. Rom. J. Ophthalmology 63 (1), 15–22. 10.22336/rjo.2019.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman DA, Martinez VK, Dougherty PM, Myers JN, Calin GA, Amit M, 2021. Cancer-associated neurogenesis and nerve-cancer cross-talk. Cancer Res. 81 (6), 1431–1440. 10.1158/0008-5472.CAN-20-2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda M, Suzuki T, Hosono K, Hayashi I, Hashiba S, Onuma Y, Amano H, Kurihara Y, Kurihara H, Okamoto H, Hoka S, Majima M, 2008a. Neuronal system-dependent facilitation of tumor angiogenesis and tumor growth by calcitonin gene-related peptide. Proc. Natl. Acad. Sci. U.S.A. 105 (36), 13550–13555. 10.1073/pnas.0800767105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda M, Suzuki T, Hosono K, Kurihara Y, Kurihara H, Hayashi I, Kitasato H, Hoka S, Majima M, 2008b. Roles of calcitonin gene-related peptide in facilitation of wound healing and angiogenesis. Biomed. Pharmacother. 62 (6), 352–359. 10.1016/j.biopha.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Toriyama Y, Iesato Y, Imai A, Sakurai T, Kamiyoshi A, Ichikawa-Shindo Y, Kawate H, Yamauchi A, Igarashi K, Tanaka M, Liu T, Xian X, Zhai L, Owa S, Murata T, Shindo T, 2015. Pathophysiological function of endogenous calcitonin gene-related peptide in ocular vascular diseases. Am. J. Pathol. 185 (6), 1783–1794. 10.1016/j.ajpath.2015.02.017. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhu H, Guo Q, Qian T, Zhang P, Li S, Xue C, Gu X, 2017. Overlapping mechanisms of peripheral nerve regeneration and angiogenesis following sciatic nerve transection. Front. Cell. Neurosci. 11 (October), 1–13. 10.3389/fncel.2017.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins HA, Rathbone DL, Barwell J, Hay DL, Poyner DR, 2013. Structure-activity relationships for α-calcitonin gene-related peptide. Br. J. Pharmacol. 170 (7), 1308–1322. 10.1111/bph.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Liu S, Wang Z, Ma S, Meng H, Hu J, 2018. Calcitonin gene-related peptide promotes proliferation and inhibits apoptosis in endothelial progenitor cells via inhibiting MAPK signaling. Proteome Sci. 16 (1), 1–12. 10.1186/s12953-018-0146-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, zhu shuyan, Liu L, Pang K, 2021. Corneal nerves modulate angiogenesis via secreted neuropeptides. Investig. Ophthalmol. Vis. Sci. 62 (8), 881. [Google Scholar]
- Zheng S, Li W, Xu M, Bai X, Zhou Z, Han J, Shyy JYJ, Wang X, 2010. Calcitonin gene-related peptide promotes angiogenesis via AMP-activated protein kinase. Am. J. Physiol. Cell Physiol. 299 (6), 1485–1492. 10.1152/ajpcell.00173.2010. [DOI] [PubMed] [Google Scholar]
- Zhong W, Montana M, Santosa SM, Isjwara ID, Huang YH, Han KY, O’Neil C,Wang A, Cortina MS, de la Cruz J, Zhou Q, Rosenblatt MI, Chang JH, Azar DT, 2018. Angiogenesis and lymphangiogenesis in corneal transplantation–A review. Surv. Ophthalmol. 63 (4), 453–479. 10.1016/j.survophthal.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.