Despite the advances made over the past decades, the known loci for coronary artery disease (CAD) still only explain <20% of the genetic variation in risk [1,2]. The known loci with the strongest effects usually confer a 20%–37% increased CAD risk and the most loci modulate risk by ≤10% [1]. While most of the current data are from European populations, the use of trans-ethnic analyses could be helpful to identify additional loci. Genetic inheritance may impose a high burden on early-onset coronary artery disease (EOCAD). In addition, most of the CAD genetic studies have been conducted in early-onset myocardial infarction [3,4], whose underlying pathophysiological mechanisms might be variable [5]. The gold standard involving angiography for the diagnosis of coronary atherosclerosis would minimize the etiological heterogeneity and enhance the statistical power in the genetic study of EOCAD.
Here, we conducted a prospective, multicenter, coronary angiography-based GRAND study (Genetics and clinical characteristics of coRonary Artery disease in the ChiNese young aDults, GRAND) [6] to explore the genetic predisposition to EOCAD among Han Chinese (Supplementary Fig. S1A). We performed a two-stage analysis using whole-exome sequencing data from 7671 Han Chinese individuals to identify causal mutations in protein-coding regions and susceptible genes for the EOCAD risk (Supplementary Fig. S1). The clinical information of the GRAND study population, including 1950 EOCAD patients with a young age at onset (≤45 years) and 1006 non-CAD older controls (≥65 years, angiographically confirmed), is listed in Supplementary Table S1. In the discovery stage of 1000 randomly selected EOCAD patients and the 1006 non-CAD older controls from GRAND (≥65 years, angiographically confirmed), 1420 common mutations (minor allele frequency (MAF) ≥ 1%, P < 0.005), 107 rare mutations (MAF < 1%, odds ratio (OR) > 3.5 and P < 0.01) and 85 genes enriched with rare mutations (OR > 3.5 and P < 0.01) were selected for replication (Supplementary Fig. S1B). We selected and matched the general controls (20–60 years) from two independent populations [7,8] (Supplementary Methods) to the rest of the EOCAD patients from GRAND based on Euclidean distance in the space of the top 20 principal components to control population stratification (Supplementary Fig. S2 and Supplementary Data). The inflation factor analyses confirmed that the patients and the matched controls had similar genetic backgrounds (Supplementary Fig. S3). Eventually, from the analysis among the 950 EOCAD patients and 4715 genetically matched controls (Supplementary Fig. S2), 27 independent known common variants (P < 0.005), three rare mutations and two genes (OR > 3.5 and P < 0.05) reached suggestive significance (Supplementary Fig. S1B). When we further combined the populations from the discovery and replication stages with 10 588 Chinese general controls from the China-Map project [9], all of these three rare mutations (two novel mutations in LDLR, rs879255066 encoding p.A627T, OR = 117.49; rs875989921 encoding p.W483X, OR = 117.49; both P < 6.5 × 10–7; one mutation in the novel gene QTRT1, rs1195458367 encoding p.R220X, OR = 41.91, P < 6.5 × 10–7) and two genes (QTRT1 and LDLR, P < 2.97 × 10–6) achieved exome-wide significance (Fig. 1B and C, Supplementary Fig. S1B and Supplementary Table S2). Sanger sequencing confirmed these three rare mutations at a 100% verification rate.
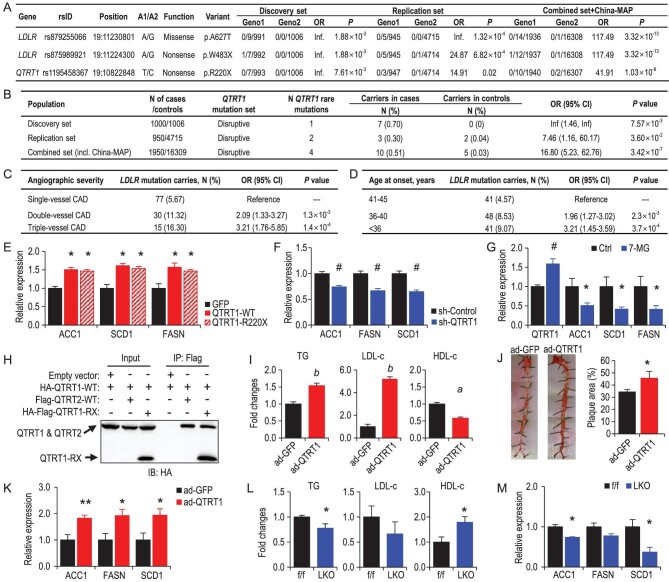
Figure 1.
Exome sequencing identifies rare mutations of LDLR and QTRT1 conferring risk for early-onset coronary artery disease. (A) Rare mutations in LDLR and QTRT1 were associated with EOCAD with a significance of P < 6.50 × 10–7 from two-sided Fisher's exact test. A1, minor allele; A2, major allele; Geno1, the genotype count for the homozygous minor/heterozygous/homozygous major alleles in EOCAD patients; Geno2, the genotype count for the homozygous minor/heterozygous/homozygous major alleles in controls. (B) QTRT1 gene enriched with rare mutations was associated with EOCAD from gene-wide association analysis with a significance of P < 2.97 × 10–6 from two-sided Fisher's exact test. (C) Associations between LDLR mutations and angiographic severity in EOCAD patients. OR, odds ratio; 95% CI, 95% confidence intervals. ORs were calculated from logistic regression models with adjustment of the top five principal components of ancestry. (D) Associations between LDLR mutations and age at onset in EOCAD patients. ORs were calculated from logistic regression models with adjustment of the top five principal components of ancestry. (E) Relative expression of genes related to de novo lipogenesis (DNL) in QTRT1-overexpressing HepG2 cells with overexpression of wild-type QTRT1 (QTRT1-WT) or mutated QTRT1 (QTRT1-R220X) or GFP control (GFP). (F) Relative expression of genes related to DNL in QTRT1-knock-down HepG2 cells (sh-QTRT1) and control HepG2 cells (sh-Control). (G) Relative expression of QTRT1 and genes related to DNL in QTRT1 inhibitor (7-methylguanine, 7-MG) treated HepG2. (H) The physical interaction between QTRT1 with QTRT2 and mutated QTRT1. (I) Levels of serum triglycerides (TG), LDL-c and HDL-c in ad-QTRT1 and ad-GFP mice; n = 6–9. (J) Representative images of Oil Red O-stained arteries in ad-QTRT1 and ad-GFP mice, and quantification of the percentage of plaque area in the Oil Red O-stained arteries; n = 6–9. (K) Relative hepatic expression of genes related to DNL in ad-QTRT1 and ad-GFP control mice; n = 6–9. (L) Levels of hepatic triglycerides (TG), LDL-c and HDL-c in liver-specific QTRT1 knockout mice (LKO) and control flox/flox littermates (f/f); n = 5. (M) Relative hepatic expression of genes related to DNL in liver-specific QTRT1 knockout mice (LKO) and control flox/flox littermates (f/f); n = 5. *P < 0.05; **P < 0.01; #P < 0.005; aP < 0.001; bP < 0.0005. Mann-Whitney test was used for the statistical analyses in Fig. 1E–M.
The two novel rare mutations in LDLR from the Chinese population were found to be monomorphic (p.A627T) or absent (p.W483X) in European participants in the Genome Aggregation Database (gnomAD) [10]. Notably, rare LDLR copy number variations (Supplementary Fig. S4) were more likely to be found in this study compared with a previous report (nine carriers in 1950 Han Chinese patients with whole-exome sequencing data versus one carrier in 2081 European patients with whole-genome sequencing data) [4]. In our GRAND study population, LDLR mutation carriers showed geographical clustering characteristics, mainly from East and Central China (Supplementary Fig. S4). Among EOCAD patients, rare LDLR mutations were associated with more severe atherosclerosis (OR [95% confidence interval] of double-vessel disease 2.09 [1.33–3.27], P = 0.001; and of triple-vessel disease 3.21 [1.61–3.52], P = 1.4 × 10–4; Fig. 1C), an earlier onset age (for those with an onset age of 36–40 years 1.96 [1.27–3.02], P = 0.002; for those with an onset age of <36 years 3.21 [1.45–3.59], P = 3.7 × 10–4; Fig. 1D) and a higher risk of major adverse cardiac events in 20 months (adjusted hazard ratio [95% confidence interval] 6.18 [3.86–9.89] in carriers vs. non-carriers, P = 3.1 × 10–14) (Supplementary Fig. S4 and Supplementary Table S3).
Our study is the first to report that QTRT1 was associated with CAD risk to the best of our knowledge. We found three extremely rare loss-of-function mutations of QTRT1 in the China-MAP database (Supplementary Table S2) but not in another large database (PGG.SNV) [11], implying that this gene might be relatively conserved across populations. In the association of QTRT1 with EOCAD, an extremely rare nonsense mutation (rs1195458367 encoding p.R220X) was the leading mutation. This rare mutation in QTRT1 had a MAF of 0.256% in patients, 0.0087% in our controls, 0.005% in 10 588 Han Chinese [9] (Supplementary Table S2) and 0.003% in Europeans in gnomAD. EOCAD patients carrying the QTRT1 p.R220X mutation had higher plasma low-density lipoprotein cholesterol (LDL-c) levels (mean ± SD: 198.26 ± 76.29 mg/dL in carriers and 120.63 ± 65.56 mg/dL in non-carriers, P = 0.003) and lower high-density lipoprotein cholesterol (HDL-c) levels (28.67 ± 15.36 mg/dL in carriers and 37.36 ± 12.10 mg/dL in non-carriers, P = 0.04). In additional data sets (https://t2d.hugeamp.org/), another intronic QTRT1 mutation (rs4425006, an extremely rare mutation in East Asians but common in Europeans) was also associated with multiple CAD-related phenotypes (Supplementary Fig. S5).
Because LDLR is known to cause hypercholesterolemia and CAD risk [3] and QTRT1 is the primary novel finding from the above analyses, we employed in vitro and in vivo experiments to explore the function of QTRT1. First, we overexpressed QTRT1-p.R220X or wild-type QTRT1 in human hepatocytes and tested whether it would impact the hepatic lipid metabolism (Supplementary Fig. S6A). Our results showed that both QTRT1-p.R220X and wild-type QTRT1 increased the expression of the essential genes of de novo lipogenesis (DNL) (ACC1, FASN and SCD1) (Fig. 1E), suggesting that QTRT1-p.R220X might be a gain-of-function mutation and upregulate lipid synthesis. Consistently, the knock-down of QTRT1 significantly downregulated the expression of the genes related to DNL (Fig. 1F and Supplementary Fig. S6B). In addition, treatment with 7-methylguanine, a known QTRT1 inhibitor, significantly downregulated the expression of ACC1, SCD1 and FASN in hepatocytes (Fig. 1G). We noticed a simultaneous increase in the expression of QTRT1 in the inhibitor-treated cells, suggesting a feedback regulation on QTRT1 expression (Fig. 1G). Previously, it has been reported that QTRT2 shared a high degree of homology with QTRT1, but the different amino acids in the catalytic area caused the inability of QTRT2 to catalyse the reaction. However, forming a heterodimer with QTRT2 is critical for QTRT1 activity [12]. Thus, we hypothesized that the mutated QTRT1 might also form a protein complex with wild-type QTRT1. To test this hypothesis, we performed a Co-immunoprecipitation experiment between mutated and wild-type QTRT1. Our data confirmed that, similarly to QTRT2, the mutated QTRT1 could physically interact with wild-type QTRT1 (Fig. 1H), which may explain the gain-of-function of the mutated QTRT1. Combined with the above-mentioned in vitro data, our observations suggested that QTRT1 regulates DNL in hepatocytes in a cell-autonomous manner.
Next, we tested whether the QTRT1 would play a role in lipid metabolism in vivo. We overexpressed QTRT1 in the liver of ApoE null mice through tail vein injection of adenovirus. qPCR data confirmed the expression of QTRT1 in the livers of overexpressed mice (ad-QTRT1) was 2.77-fold higher than that in the control mice (ad-GFP) 3 months after the virus injection (Supplementary Fig. S6C). Furthermore, ad-QTRT1-treated mice had significantly higher circulating levels of LDL-c (5.19-fold) and triglycerides (1.54-fold) but lower levels of HDL-c (0.58-fold) compared with the control group (Fig. 1I). A similar but milder trend appeared 2 months after the virus injection (Supplementary Figs. S6D and E), but no difference was observed at 1 month since injection (Supplementary Fig. S6E). QTRT1 overexpression seemed to result in more arterial plaques stained by Oil Red O (Fig. 1J), which was in line with the higher serum levels of LDL-c and triglycerides in ad-QTRT1 mice. Consistently with our in vitro data, the expression level of essential genes (ACC1, FASN and SCD1) for DNL was upregulated in QTRT1-overexpressing mice (Fig. 1K). QTRT1 overexpression did not affect the expression of other genes related to lipoprotein transportation in the liver (i.e. LDLR, ApoA1 and ApoB; Supplementary Fig. S6F). Further, we generated a conditional liver-specific QTRT1 knockout mouse model to validate the function of QTRT1 in lipid metabolism (Supplementary Fig. S6G). In these QTRT1 knockout mice, we observed lower total triglyceride (0.77-fold) and trending lower LDL-c (0.66-fold) but significantly higher HDL-c content (1.79-fold) in the livers (Fig. 1L), which is in line with the observation found in QTRT1-overexpressing mice. Consistently, a reduced expression of ACC1 and SCD1, and a trending lower expression of FASN were also found in liver-specific QTRT1 knockout mice compared to wild-type control mice (Fig. 1M). These results confirmed that QTRT1 was essential in lipid metabolism and atherosclerosis development.
In this most extensive whole-exome sequencing study of CAD among the non-European population, we identified that novel gene QTRT1 and rare novel mutations in LDLR contribute to EOCAD risk. QTRT1 mutations influenced EOCAD risk through DNL dysregulation and accelerated atherosclerosis. These findings might provide new insights into genetic screening, early diagnosis and future drug discovery for CAD in young individuals.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank all participants and the research staff of the 38 recruiting centers of the GRAND Collaboration/Consortium for their valuable contributions.
Contributor Information
Kang Yao, Department of Cardiology, Zhongshan Hospital, Fudan University, China; Shanghai Institute of Cardiovascular Disease, China.
Yuxiang Dai, Department of Cardiology, Zhongshan Hospital, Fudan University, China; Shanghai Institute of Cardiovascular Disease, China.
Juan Shen, BGI-Shenzhen, China.
Yi Wang, State Key Laboratory of Genetic Engineering, School of Life Sciences and Human Phenome Institute, Fudan University, China.
Huanjie Yang, BGI Genomics, BGI-Shenzhen, China.
Runda Wu, Department of Cardiology, Zhongshan Hospital, Fudan University, China; Shanghai Institute of Cardiovascular Disease, China.
Qijun Liao, BGI-Shenzhen, China.
Hongyi Wu, Department of Cardiology, Zhongshan Hospital, Fudan University, China; Shanghai Institute of Cardiovascular Disease, China.
Xiaodong Fang, BGI-Shenzhen, China.
Shalaimaiti Shali, Department of Cardiology, Zhongshan Hospital, Fudan University, China; Shanghai Institute of Cardiovascular Disease, China.
Lili Xu, Department of Cardiology, Zhongshan Hospital, Fudan University, China; Shanghai Institute of Cardiovascular Disease, China.
Meng Hao, State Key Laboratory of Genetic Engineering, School of Life Sciences and Human Phenome Institute, Fudan University, China.
Chenhao Lin, State Key Laboratory of Genetic Engineering, School of Life Sciences and Human Phenome Institute, Fudan University, China.
Zhonghan Sun, State Key Laboratory of Genetic Engineering, School of Life Sciences and Human Phenome Institute, Fudan University, China.
Yilian Liu, State Key Laboratory of Genetic Engineering, School of Life Sciences and Human Phenome Institute, Fudan University, China.
Mengxin Li, State Key Laboratory of Genetic Engineering, School of Life Sciences and Human Phenome Institute, Fudan University, China.
Zhen Wang, Department of Cardiology, Zhongshan Hospital, Fudan University, China; Shanghai Institute of Cardiovascular Disease, China.
Qiang Gao, BGI Genomics, BGI-Shenzhen, China.
Shuning Zhang, Department of Cardiology, Zhongshan Hospital, Fudan University, China; Shanghai Institute of Cardiovascular Disease, China.
Chenguang Li, Department of Cardiology, Zhongshan Hospital, Fudan University, China; Shanghai Institute of Cardiovascular Disease, China.
Wei Gao, Department of Cardiology, Zhongshan Hospital, Fudan University, China; Shanghai Institute of Cardiovascular Disease, China.
Lei Ge, Department of Cardiology, Zhongshan Hospital, Fudan University, China; Shanghai Institute of Cardiovascular Disease, China.
Yunzeng Zou, Department of Cardiology, Zhongshan Hospital, Fudan University, China; Shanghai Institute of Cardiovascular Disease, China.
Aijun Sun, Department of Cardiology, Zhongshan Hospital, Fudan University, China; Shanghai Institute of Cardiovascular Disease, China.
Juying Qian, Department of Cardiology, Zhongshan Hospital, Fudan University, China; Shanghai Institute of Cardiovascular Disease, China.
Li Jin, State Key Laboratory of Genetic Engineering, School of Life Sciences and Human Phenome Institute, Fudan University, China.
Shangyu Hong, State Key Laboratory of Genetic Engineering, School of Life Sciences and Human Phenome Institute, Fudan University, China.
Yan Zheng, Department of Cardiology, Zhongshan Hospital, Fudan University, China; State Key Laboratory of Genetic Engineering, School of Life Sciences and Human Phenome Institute, Fudan University, China.
Junbo Ge, Department of Cardiology, Zhongshan Hospital, Fudan University, China; Shanghai Institute of Cardiovascular Disease, China.
FUNDING
This work was supported by the National Key Research and Development Program of China (2021YFA1301004 and 2020YFC2005000), the Clinical Research Plan of Shanghai Hospital Development Center (SHDC2020CR1007A), the Shanghai Municipal Science and Technology Major Project (2017SHZDZX01), the National Natural Science Foundation of China (82170460 and 81973032), the Clinical Research Plan of Shanghai Hospital Development Center (SHDC2020CR1007A). Dr. Zheng was supported by the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning. Dr. Hong was supported by the National Key Research and Development Program of China (2019YFA0802300 and 2021YFA0804801), the Training Program of the Major Research Plan of the National Natural Science Foundation of China (91957117) and the National Natural Science Foundation of China (31971082).
Conflict of interest statement . None declared.
REFERENCES
- 1. Hartiala J, Schwartzman WS, Gabbay Jet al. Curr Atheroscler Rep 2017; 19: 6. 10.1007/s11883-017-0641-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nikpay M, Goel A, Won HHet al. Nat Genet 2015; 47: 1121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Do R, Stitziel NO, Won HHet al. Nature 2015; 518: 102–6. 10.1038/nature13917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khera AV, Chaffin M, Zekavat SMet al. Circulation 2019; 139: 1593–602. 10.1161/CIRCULATIONAHA.118.035658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pasupathy S, Tavella R, Beltrame JF. Circulation 2017; 135: 1490–3. 10.1161/CIRCULATIONAHA.117.027666 [DOI] [PubMed] [Google Scholar]
- 6. Shalaimaiti S, Dai Y-X, Wu H-Yet al. Cardiology Plus 2021; 6: 65–73. 10.4103/2470-7511.312594 [DOI] [Google Scholar]
- 7. Liu X, Tong X, Zou Yet al. Nat Genet 2022; 54: 52–61. 10.1038/s41588-021-00968-y [DOI] [PubMed] [Google Scholar]
- 8. Hao M, Pu W, Li Yet al. J Genet Genomics 2021; 48: 1032–5. 10.1016/j.jgg.2021.07.013 [DOI] [PubMed] [Google Scholar]
- 9. Cao Y, Li L, Xu Met al. Cell Res 2020; 30: 717–31. 10.1038/s41422-020-0322-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karczewski KJ, Francioli LC, Tiao Get al. Nature 2020; 581: 434–43. 10.1038/s41586-020-2308-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang C, Gao Y, Ning Zet al. Genome Biol 2019; 20: 215. 10.1186/s13059-019-1838-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen YC, Kelly VP, Stachura SVet al. RNA 2010; 16: 958–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.