Abstract
Heme compounds are an important source of iron for neisseriae. We have identified a neisserial gene, hemO, that is essential for heme, hemoglobin (Hb), and haptoglobin-Hb utilization. The hemO gene is located 178 bp upstream of the hmbR Hb receptor gene in Neisseria meningitidis isolates. The product of the hemO gene is homologous to enzymes that degrade heme; 21% of its amino acid residues are identical, and 44% are similar, to those of the human heme oxygenase-1. DNA sequences homologous to hemO were ubiquitous in commensal and pathogenic neisseriae. HemO genetic knockout strains of Neisseria gonorrhoeae and N. meningitidis were unable to use any heme source, while the assimilation of transferrin-iron and iron-citrate complexes was unaffected. A phenotypic characterization of a conditional hemO mutant, constructed by inserting an isopropyl-β-d-thiogalactopyranoside (IPTG)-regulated promoter upstream of the ribosomal binding site of hemO, confirmed the indispensability of the HemO protein in heme utilization. The expression of HemO also protected N. meningitidis cells against heme toxicity. hemO mutants were still able to transport heme into the cell, since both heme and Hb could complement an N. meningitidis hemA hemO double mutant for growth. The expression of the HmbR receptor was reduced significantly by the inactivation of the hemO gene, suggesting that hemO and hmbR are transcriptionally linked. The expression of the unlinked Hb receptor, HpuAB, was not altered. Comparison of the polypeptide patterns of the wild type and the hemO mutant led to detection of six protein spots with an altered expression pattern, suggesting a more general role of HemO in the regulation of gene expression in Neisseriae.
Heme is the most abundant source of iron in the body. Many pathogenic microorganisms possess specific heme uptake systems that harness heme iron for metabolic needs. Currently, the best studied heme assimilation systems are those of gram-negative bacteria (38). These bacteria express highly specific outer membrane receptors that bind different heme-containing compounds, extract heme from these compounds, and transport heme into the bacterial periplasm (17, 40, 43–45). Further processing of periplasmic heme depends on a HemTUV-like family of proteins that transports heme into the cytoplasm (41). The fate of heme after entering the cytoplasm is not well understood. Although heme iron accounts for less than 10% of total cell iron in Escherichia coli, heme is a very efficient source of iron for bacteria growing under conditions of iron restriction (15, 38). Therefore, externally supplied heme can not satisfy cells' requirements for iron without the involvement of heme degradation. Early studies that measured production of carbon monoxide, a byproduct of heme degradation, in the gram-positive organisms Bacillus cereus and Streptococcus mitis suggested the existence of a heme oxygenase-like activity in bacteria (9). In addition to their role in iron assimilation, heme oxygenases might protect cells against heme toxicity (3). Heme is toxic to bacteria: small concentrations of hemin inhibit growth of the gram-positive bacteria Staphylococcus aureus, Streptococcus faecalis, and B. cereus (16). Mucosal pathogens in general and especially those colonizing the female genital tract are exposed to large amounts of heme. However, oviduct infections by Neisseria gonorrhoeae usually occur within a week of an onset of menses, suggesting that this organism is able to withstand the toxicity of heme (10, 48). Since N. gonorrhoeae and Neisseria meningitidis possess multiple heme uptake pathways, protection against heme toxicity must be based on the destruction of heme and not on the inability of heme to reach the cell's interior (38).
A eukaryotic heme oxygenase enzyme was identified 30 years ago (22, 51, 56). Two mammalian heme oxygenases, HO-1 and HO-2, are particularly well studied. These enzymes play important roles in several different physiological processes, including iron reutilization, defense against oxidative damage, and neurotransmission (21, 29, 30, 52). In higher plants and algae, heme oxygenase is involved in the synthesis of tetrapyrrole chromophores, phycobilins, and phytochromobilins (5, 32, 34). All these different roles of heme oxygenases are based on the ability of the enzyme to cleave the heme molecule at the α-meso position, producing the open tetrapyrrole molecule, biliverdin, iron, and carbon monoxide (21). The first prokaryotic heme oxygenase-like enzyme, HmuO, has recently been identified and characterized in the gram-positive prokaryotes Corynebacterium diphtheriae and Corynebacterium ulcerans (36). hmuO gene mutants are unable to use heme and hemoglobin (Hb) as sources of iron, and the HmuO protein is 33% identical in its amino acid sequence to the human heme oxygenase HO-1 (36). A recent study confirmed the role of HmuO in heme cleavage, thus reaffirming the concept of the enzymatic destruction of heme in the assimilation of heme iron by bacteria (55).
In this communication we present the identification and characterization of a neisserial open reading frame (ORF), homologous to those encoding human heme oxygenases, that plays an essential role in the assimilation of heme iron. hemO mutants were unable to use heme, Hb, and haptoglobin-Hb as sources of iron but were fully capable of assimilating inorganic iron and iron-transferrin. HemO is the first reported product of gram-negative bacteria that resembles eukaryotic heme oxygenases both in its primary amino acid structure and in its function.
MATERIALS AND METHODS
Plasmids, bacteria, and media.
Strains and plasmids used and constructed in this study are listed in Table 1. The meningococci were grown on GCB (Difco) agar containing supplements as described elsewhere (14) and were incubated at 37°C in 5% CO2. E. coli was grown in Luria broth (LB). When necessary, the following antibiotics were used in work with E. coli: chloramphenicol at 30 mg/liter, kanamycin at 50 mg/liter, neomycin and ampicillin at 100 mg/liter, and erythromycin at 300 mg/liter. For neisseriae, neomycin at 100 mg/liter, erythromycin at 3 mg/liter, and spectinomycin at 100 mg/liter were used.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or description | Source or reference |
|---|---|---|
| Strains | ||
| E. coli K-12 | ||
| DH-5α | Stratagene | |
| TOPF10′ | Invitrogen | |
| BL21(DE3) | λ Lysogen; carries T7RNA polymerase gene | 47, 49 |
| N. gonorrhoeae | ||
| FA19 (IR1114) | Laboratory collection | |
| FA62 (IR2539) | Laboratory collection | |
| MS11(IR1113) | Laboratory collection | |
| FA6807 (IR1311) | Laboratory collection | |
| FA1090 (IR2865) | Laboratory collection | |
| GC340 (IR2864) | Laboratory collection | |
| IR3524 | MS11 hemO::Specr | This study |
| Commensal Neisseria strains IR2363 through IR2375 | 33 | |
| N. meningitidis | ||
| IR1072 | Serogroup C; wild type; hmbR+ | Laboratory collection |
| IR1073 | Serogroup A; wild type | Laboratory collection |
| IR1074 | Serogroup B; wild type | Laboratory collection |
| IR1075 | Serogroup C; wild type; hmbR+ hpuB+ | Laboratory collection |
| IR2855 | Serogroup A; wild type | Laboratory collection |
| IR3376 | IR1075 but hemO::Specr | This study |
| IR3377 | IR1075 but hemO::Specr | This study |
| IR3378 | IR1075 but hemO::Specr | This study |
| IR3380 | IR1072 but hemO::Specr | This study |
| IR3381 | IR1072 but hemO::Specr | This study |
| IR3509 | IR1072 but hemO::erm lacIOP; Ermr; IPTG inducible | This study |
| IR3510 | IR1072 but hemO::erm lacIOP; not IPTG inducible | This study |
| IR3499 | IR3381 but hemA::aphA3 | This study |
| IR3551 | IR1075 but hemO::erm lacIOP; Ermr; IPTG inducible | This study |
| IR3587 | IR1075 but hemO::Specr | This study |
| DMN502 | DMN2 but hemA::aphA3; Kanr Nalr | 18 |
| Plasmids | ||
| pCR2.1-TOPO | Invitrogen | |
| pHSX-ermC-LaIOP | Ermr cassette with inducible lac promoter | 25, 39 |
| pDJH1550 | 1.6-kb BglII DNA fragment carrying hemO and 5′ end of hmbR | This study |
| pDJH1560 | Like pDJH1550 but Ω cassette in BamHI site of hemO | This study |
| pWMZ1574 | 0.85 kb; 5′ ends of hmp and hemO from IR1072 | This study |
| pWMZ1575 | 0.85 kb; 5′ ends of hmp and hemO from IR2855 | This study |
| pWMZ1585 | 0.53 kb; 5′ end of hemO from IR1072 | This study |
| pWMZ1586 | 1 kb; 5′ end of hmp from IR1072 | This study |
| pWMZ1588 | 1-kb EcoRI fragment from pWMZ1586 cloned into pUC19 | This study |
| pWMZ1590 | 1-kb BglII/NotI fragment from pWMZ1588 and 0.53-kb XhoI/NotI fragment from pWMZ1585 cloned into pUC19 | This study |
| pWMZ1591a | 3-kb erm-LacIOP cassette from pHSX-ermC-LacIOP cloned into NotI fragment of pWMZ1590; lacOP close to hemO | This study |
| pWMZ1591b | Same as pWMZ1591a but with lacOP in opposite orientation | This study |
Recombinant DNA and other techniques.
Plasmid DNA preparation, restriction endonuclease analyses, and ligations were carried out by standard methods described in the work of Sambrook et al. (35). Southern blot analysis was done by using a DIG nonradioactive DNA labelling and detection kit (Genius system; Boehringer GmbH, Mannheim, Germany) under high-stringency conditions (65°C; two washes with 2× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate]–0.1% sodium dodecyl sulfate [SDS] at room temperature followed by two washes in 0.1× SSC–0.1% SDS at 65°C). PCRs were performed by using Taq DNA polymerase (Promega) according to the instructions of the supplier. Oligonucleotides were custom made by Gibco BRL. The hemO gene was amplified from the N. meningitidis serogroup A strain IR2855 (33) by using the primer PIG-C starting 6 bp upstream of the second ATG codon of hemO, 5′ CCAGATCTCTATATATGCGGGCAAGTGCGG 3′, and the HMBRΔ216-226B primer, 5′ CCAGATCTCCGTTGCGAATACAGCAAAGC 3′, whose sequence is located downstream of hemO in the hmbR gene (43). The DNA fragment was cloned into the pCR2.1-TOPO vector (Invitrogen), creating pDJH1550. The nucleotide sequence of the cloned insert was determined at the Emory DNA Sequencing Core Facility of the Veterans Administration Center—Atlanta on a model 377 DNA sequencer by using the Taq dideoxy terminator cycle sequencing kit (both from Applied Biosystems). The nucleotide sequence of the hemO gene from the N. meningitidis serogroup A IR2855 strain was 100% identical to the sequence of hemO from strain Z2491 (contig 355, as of March 5, 1999; Sanger Center N. meningitidis BLAST server). The hmbR poly(G) tract was sequenced as described in reference 33. The hpuA poly(G) tract was sequenced as described by Lewis et al. (19).
Iron assimilation assays.
The ability of neisseriae to use various iron-containing proteins as iron sources was tested by a plate assay. A suspension of bacteria was plated onto GCB agar containing 50 μM deferoxamine mesylate (Desferal; Ciba Geigy). Filter discs (1/4 in; Schleicher & Schuell, Inc., Keene, N.H.) impregnated with test compounds (10 μl of 5-mg/ml stock solutions of bovine hemin chloride and human Hb, 10 mg of iron ammonium citrate/ml, and 20 mg of human transferrin/ml) were placed on these plates. One milligram of a lyophilized pooled human haptoglobin (Sigma, St. Louis, Mo.) that binds 0.5 to 0.9 mg of human Hb was dissolved in 100 μl of 6.5 mg of human Hb/ml. Ten microliters of the haptoglobin-Hb complex was used in the growth promotion assay. Zones of growth around the discs were recorded after overnight incubation at 37°C in the presence of 5% CO2 (45). Bovine hemin, human Hb, and iron ammonium citrate were purchased from Sigma. Human plasma holotransferrin was purchased from Biodesign International (Kennebunk, Maine).
Outer membrane preparations and Western blotting.
Meningococcal outer membrane preparations and Western blotting were carried out essentially as described by Richardson and Stojiljkovic (33). Anti-HpuB antibodies were the gift of L. Lewis and D. Dyer and were used at a 1:500 dilution.
Construction of N. meningitidis and N. gonorrhoeae hemO gene knockouts.
Inactivation of hemO was carried out by using a 2-kb DNA Ω fragment encoding a spectinomycin resistance determinant (31). Plasmid pDJH1550 was partially digested with BamHI and ligated with the BamHI-digested and purified Ω cassette. The plasmid pDJH1560 containing the spectinomycin resistance cassette in the internal BamHI restriction site of the hemO gene was used in gene knockout experiments with N. meningitidis IR1072 and IR1075 (see Fig. 2). Genetic transformation of neisserial strains and isolation of chromosomal DNA were carried out as described by Richardson and Stojiljkovic (33). Chromosomal DNA from meningococcal transformants was digested with SspI and probed with the hemO DNA probe. The 0.9-kb hemO DNA probe, made by PCR using primers PIG-C (see above) and PIG-B (5′ CCAGATCTCAGCGCGGCGATAGGGAGCAT 3′), was labelled by using a DIG DNA labelling and detection kit (Boehringer GmbH). Southern blot analysis confirmed the insertion of the antibiotic cassette in the hemO genes of IR1072 and IR1075 (data not shown). The inactivation of hemO in both genetic backgrounds was also confirmed by PCR (data not shown). Chromosomal DNA of the meningococcal hemO mutant IR3381 was used to construct the hemO mutant in the N. gonorrhoeae MS11 (IR1113) genetic background.
FIG. 2.
(A) Schematic representation of the N. meningitidis hemO locus and some plasmids used in cloning and gene replacement experiments. The gene encoding the neisserial Hb receptor, hmbR, was found to lie 178 bp downstream of the hemO stop codon. (B) Schematic representation of the 5′ regions of neisserial hemO genes. The Fur boxes, inverted repeats of the Correia element, and three putative ATG codons are indicated. The inverted repeats are 26 bp long, but only the 100% complementary parts are shown.
Construction of a conditional, IPTG-inducible, hemO gene knockout.
The involvement of the hemO gene product in heme and Hb utilization was confirmed by constructing and phenotypically characterizing a conditional, isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible hemO gene knockout. An ermC-lacIOP cassette (25, 39) containing ermC and lacIq genes and lacOP promoter sequences was inserted upstream of the hemO putative ribosome binding site (see Fig. 2B). This was accomplished by PCR amplification of two DNA fragments flanking the insertion site. The DNA fragment upstream of the insertion site was amplified with primers HPMA.A-XhoI (5′ GGCTCGAGTCAGGTTCAAAGCGTTC 3′) and HEMO.A2-NotI (5′ CGCGGCCGCGACAAAGGAAAACTTATG 3′). The DNA fragment downstream of the insertion site was amplified by PCR with primers HPMA.B2-BglII (5′ GAGATCTATACGCCACCAAAGTGGA 3′) and HEMO.B2-NotI (5′ CGCGGCCGCGGTAAGGGATAAGGATGCG 3′). Two fragments were ligated together into a SalI/BamHI-digested pUC19 plasmid, creating pWMZ1590, which contains a unique NotI site upstream of the hemO ribosomal binding site. A 3-kb NotI fragment carrying the ermC-lacIOP cassette was inserted into the NotI site, creating plasmids pWMZ1591a and pWMZ1591b. These two plasmids differ in the orientation of the lacOP promoter; in pWMZ1591a, the promoter is facing the hemO gene, while in pWMZ1591b it is oriented away from the hemO gene. Both plasmids were linearized and transformed into N. meningitidis IR1072, and the mutants were selected on GCB-erythromycin plates. The conditionality of the hemO mutation was determined by assaying heme and Hb utilization in the presence or absence of paper discs soaked in 10 μl of 1 mM IPTG. The same mutation was also introduced into N. meningitidis IR1075, which expresses both HpuB and HmbR receptors (44). The genotypes of the constructs were confirmed by PCR using primers HPMA.A-XhoI and HPMA.B2-BglII (5′ GGAGATCTCCCATTTGATAAAATCCCG 3′) and by a restriction digest of the amplified DNA with the NotI restriction enzyme (data not shown).
Heme toxicity assay.
Bacterial suspensions of the heme oxygenase mutant (IR3381) and the wild-type strain (IR1072) were prepared from overnight cultures grown on GCB plates. Bacteria were diluted in fresh GCB broth containing Kellog's supplements I and II and incubated for 3 h at 37°C under 5% CO2 with aeration. Approximately 1 × 107 to 5 × 107 logarithmically grown bacteria were inoculated into GCB broth (final volume, 490 μl) containing Kellog's supplement I and 50 μM Desferal by using a 48-well tissue culture plate (14). Portions (10 μl) of the hemin chloride solutions of different concentrations (final concentrations, 130, 65, 32, 16, 8, 4, and 0 μg/ml) were added to the cultures, which were then incubated at 37°C under 5% CO2 with aeration. After 6 h of incubation, viable counts of bacteria were determined by diluting and plating onto GCB plates. Fresh hemin chloride solution was prepared by dissolving 65 mg of hemin chloride (Porphyrin Products, Inc., Logan, Utah) in 10 ml of 50 mM NaOH.
Two-dimensional electrophoresis.
The procedure of O'Farrell (28) for two-dimensional electrophoresis was followed. Isoelectric focusing was carried out in glass tubes using 2% Resolyte (pH 4 to 8) ampholines (BDH from Hoefer Scientific Instruments). Sodium dodecyl sulfate (SDS) slab gel electrophoresis was carried out in 10% acrylamide gels. Gels were stained with either a silver stain or Coomassie brilliant blue R-250 and dried between sheets of cellophane. Thirty-five (silver-stained) to 175 (Coomassie-stained) μg of total cell protein was loaded on the gel. The pattern of polypeptide spots was analyzed by eye. The analysis was performed at Kendrick Laboratories (Madison, Wis.). Protein samples were run three times, giving essentially identical results. Identification of protein spots was attempted by digestion of the spots with endoproteinase Lys-C followed by matrix-assisted laser desorption ionization-mass spectrometry (MALDI-MS) analysis (performed by the Protein Chemistry Core Facility at Columbia University, New York, N.Y.).
Determination of the N-terminal protein sequence of HemO.
The hemO product was overexpressed in E. coli BL21(DE3) cells carrying plasmids pDJH1550 and pDJH1560. The hemO gene was expressed from the T7 promoter by using 1 mM IPTG as an inducer (47, 49). Cell pellets were washed several times with phosphate-buffered saline and then lysed in a French press. Cell lysates from pDJH1550- and pDJH1560-expressing bacteria were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) (see Fig. 1B). SDS-PAGE-separated proteins were transferred onto a polyvinylidene difluoride membrane, and bands corresponding to the HemO gene product were cut and sent for determination of the N-terminal amino acid sequence at the Emory Microchemical Facility. The N-terminal sequence of the hemO gene product was determined by Edman degradation.
FIG. 1.
(A) Phylogenetic tree analysis of confirmed heme oxygenase enzymes and their neisserial and P. aeruginosa homologues performed by using the MegAlign program from the LASERGENE software package (DNASTAR, Inc.). HO-RV, heme oxygenase from Rhodatella violacea (34); HO-PP, heme oxygenase from Porphyra purpurea (32). (B) Expression of the hemO and hemO::Specr genetic constructs in E. coli BL21 cells. Lane A, cell lysate of E. coli carrying pDJH1550 (hemO); lane B, cell lysate of E. coli carrying pDJH1560 (hemO::Specr).
Amino acid comparison and protein searches.
Different amino acid sequences were compared with the program ALIGN, provided by the BCM Search Launcher (Human Genome Center, Baylor College of Medicine, Houston, Tex.) and the LASERGENE software package (DNASTAR, Inc.). The search for neisserial homologues of human heme oxygenases was conducted by using TBLASTN search engines provided by the Oklahoma N. gonorrhoeae genome sequencing project and the Sanger Center N. meningitidis genome sequencing project (The Wellcome Trust). The PSORT search engine was used to identify signal sequences and transmembrane helices in the HemO amino acid sequence.
Nucleotide sequence accession number.
The sequence of the IR2855 hemO gene has been submitted to GenBank under accession no. AF133695.
RESULTS
Identification of neisserial ORFs that are homologous to human heme oxygenases.
In order to identify candidates for a neisserial heme oxygenase-like gene, the nucleotide sequence of the N. gonorrhoeae FA1090 genome (>94% complete) was searched for ORFs homologous to the human heme oxygenases HO-1 and HO-2. The search identified an ORF encoding a 237-amino-acid polypeptide as a candidate for a neisserial heme oxygenase. The N. meningitidis Z2491 genome sequence (Sanger Center) also contained the same homologue, 91% identical to the N. gonorrhoeae ORF. Neisserial ORF products shared 19 to 22% identical amino acid residues with human HO-1 and HO-2 (Table 2). The C-terminal halves of the neisserial ORF products were most homologous to human heme oxygenases, with 24 of 90 residues identical to those of HO-1 (data not shown). The similarities of the neisserial ORF products to other heme oxygenases, including the only prokaryotic enzyme, HmuO from C. diphtheriae, were lower (Table 2; Fig. 1A).
TABLE 2.
Percent sequence identity and similarity determined by pairwise alignment of amino acid sequences of different heme oxygenase proteinsa
| Heme oxygenaseb | % Identity or similarityc
|
|||||||
|---|---|---|---|---|---|---|---|---|
| HemO-NG | HemO-NM | HO-1-HU | HO-2-HU | PigA-PA | HmuO-CD | HO-SYN | HO-3-RT | |
| HemO-NG | X | 91 | 22 | 20 | 30 | 15 | 17 | 19 |
| HemO-NM | 93 | X | 21 | 19 | 33 | 18 | 19 | 18 |
| HO-1-HU | 43 | 44 | X | 43 | 19 | 27 | 38 | 31 |
| HO-2-HU | 52 | 44 | 69 | X | 19 | 25 | 37 | 68 |
| PigA-PA | 54 | 57 | 40 | 40 | X | 23 | 21 | 17 |
| HmuO-CD | 48 | 50 | 51 | 46 | 54 | X | 31 | 23 |
| HO-SYN | 47 | 42 | 67 | 58 | 44 | 59 | X | 28 |
| HO-3-RT | 46 | 46 | 59 | 82 | 37 | 44 | 53 | X |
Determined with the ALIGN program.
NG, N. gonorrhoeae; NM, N. meningitidis; HU, human; PigA-PA, product of P. aeruginosa pigA (accession no. AF060193); HmuO-CD, product of C. diphtheriae hmuO (36); HO-SYN, heme oxygenase from Synechocystis sp. strain PCC6803 (13); HO-3-RT, rat heme oxygenase-3 (24).
Boldface values, identity; lightface values, similarity; X, no comparison (same protein).
The His-25 residue (corresponds to His-45 of HO-2) and a water molecule have been identified as heme ligands of human heme oxygenases (11). This conserved histidine residue was also identified in neisserial HemO proteins (data not shown). The HemO product does not have any hydrophobic amino acid clusters that would be characteristic for a signal sequence or transmembrane helices, suggesting its cytoplasmic localization. PigA, a Pseudomonas aeruginosa ORF of unknown function, showed the highest degree of similarity to neisserial ORFs. The putative neisserial heme oxygenase was named hemO.
Cloning of the hemO gene from an N. meningitidis serogroup A isolate.
Analysis of the nucleotide sequence around the hemO gene of strain Z2491 revealed that the gene encoding the Hb receptor, HmbR, is positioned 178 bp downstream of the hemO stop codon. Indeed, the nucleotide sequence of hemO downstream of its BamHI restriction site (see Fig. 2A) is 98% identical to the published nucleotide sequence upstream of the hmbR start codon (43). To test the function of the neisserial heme oxygenase homologue in iron assimilation, a 1.6-kb DNA fragment carrying hemO and the 5′ end of the hmbR gene was PCR amplified (see Materials and Methods) from the N. meningitidis serogroup A strain IR2855 and was cloned into the PCR2.1-TOPO vector to generate pDJH1550 (Fig. 2A). Overexpression of the hemO gene in E. coli from pDJH1550 produced cultures with a distinct green color, suggesting that these cells produce the heme degradation product biliverdin (Fig. 1B) (4, 55). Determination of the nucleotide sequence confirmed the presence of hemO and hmbR genes on the amplified fragment. The nucleotide sequence of the hemO gene was 100% identical to the sequence of the hemO of N. meningitidis Z2491 and 91% identical to the sequence of the N. gonorrhoeae FA1090 hemO gene. An ORF that is highly homologous to the product of the E. coli hmp gene encoding bacterial flavohemoglobin was identified upstream of the hemO gene (Fig. 2A) (26).
The gonococcal hemO gene has two potential ATG start codons separated by 18 bp and encodes an ORF that is 6 amino acids longer than its meningococcal homologue. Comparison of the promoter regions of the N. gonorrhoeae FA1090 and N. meningitidis IR2855 hemO genes revealed the existence of the putative Fur box. The sequence 5′ GATAATAAATTTCGTTTAT 3′ starts 17 bp upstream of the first ATG codon of the gonococcal hemO gene and has 11 of 19 optimal matches with the consensus E. coli Fur box (7, 42). The same putative Fur box is found 185 bp upstream of the ATG codon of the meningococcal hemO gene (i.e., the 2nd ATG codon of the gonococcal ORF). The difference in the location of Fur boxes is due to the insertion of a small repetitive sequence (Correia repeat) in front of the 2nd start codon of the hemO gene of N. meningitidis 2855 (Fig. 2B) (6). The hemO-hmp intergenic region of strain IR2855 contained a partial duplication of the right inverted repeat of the Correia element that is missing in the sequence in Z2491 (Sanger Center) (Fig. 2B). Potential promoter sequences were found in the Correia element and upstream of the Fur boxes (data not shown). A 3rd potential start codon is found 61 bp downstream of the 2nd start codon in both meningococcal and gonococcal sequences (Fig. 2B). Localization of a strong ribosomal binding site upstream of the 3rd ATG codon suggests that the HemO protein starts from this methionine. Indeed, insertion of the Erm-LacIqlacOP cassette upstream of this ribosomal binding site did not inactivate the hemO gene (see below). Conversely, insertion of the Erm-LacIqlacOP cassette upstream of the 2nd potential start codon did not produce a conditional mutant, suggesting that promoter elements are located between the 2nd and 3rd ATG codons.
Expression studies with E. coli BL21 carrying the hemO and hemO::Specr plasmids pDJH1550 and pDJH1560, respectively, showed that a protein product of approximately 25 kDa is expressed from pDJH1550 but not from pDJH1560 (Fig. 1B). The N-terminal sequence of the 25-kDa product was determined to be (S/A/M)ETENQALTFA, thus confirming that the hemO gene is translated from the 3rd start codon. It remains to be seen whether the same start codon is used in neisseriae.
Phenotypic characterization of N. gonorrhoeae and N. meningitidis hemO mutants.
A GCB-Desferal plate assay was used to test the ability of the N. meningitidis IR3381 and IR3376 hemO mutants to use heme compounds as sources of iron. hemO mutant cells were efficient in the use of iron-citrate complexes and human transferrin-iron but could not use heme, Hb, and haptoglobin-Hb complexes (Table 3; Fig. 3). These data indicated that the utilization defect of the hemO mutant was specific for heme compounds and did not affect the assimilation of iron from nonheme sources. A few heme-utilizing colonies appeared around heme but not Hb discs after prolonged incubation (data not shown). These colonies were not true revertants because, although they were resistant to spectinomycin, they did not show the wild-type phenotype of growth around Hb and heme discs when retested. The same heme- and Hb-negative phenotype was observed with the hemO mutant of N. gonorrhoeae MS11 IR3524 (Table 3).
TABLE 3.
Phenotypic characterization of neisserial hemO mutants
| Strain | Utilizationa of:
|
|||
|---|---|---|---|---|
| Heme | Hb | TF | Fe-Cit | |
| IR1072wt | +++ | +++ | +++ | +++ |
| IR3381 (hemO::Specr) | − | − | +++ | +++ |
| IR3509 (hemO::Ermrlac) | +++* | +++* | ND | ND |
| IR1075wt | +++ | +++ | +++ | +++ |
| IR3376 (hemO::Specr) | − | − | +++ | +++ |
| IR3551 (hemO::ermrlac) | +++* | +++* | ND | ND |
| MS11wt | +++ | − | +++ | +++ |
| IR3524 (hemO::Specr) | − | − | +++ | +++ |
TF, human transferrin; Fe-Cit, iron-citrate complex; +++, utilization; −, no utilization; ∗, growth only in the presence of IPTG; ND, not done.
FIG. 3.
Growth phenotypes of N. meningitidis hemO mutants on iron-restricted plates. Approximately 107 to 108 meningococci were streaked onto GCB-Desferal (50 μM) plates, and discs with heme and human Hb were placed on the plates. In the case of the IPTG-inducible hemO gene (3551), paper discs containing 10 μl of 1 mM IPTG were placed on top of Hb, heme (Hm), and haptoglobin-Hb (HH) discs. Phenotypes were recorded after 24 h of incubation at 37°C under 5% CO2 (see also Table 3).
The IR1072 hemO conditional mutant (IR3509) was able to use heme or Hb only in the presence of IPTG, indicating that the expression of hemO is essential for bacteria to use heme as a source of iron (Table 3). In addition to the defect in Hb and heme utilization, the N. meningitidis IR1075 hemO conditional mutant (IR3551) was also unable to use haptoglobin-Hb complexes as sources of iron (Table 3; Fig. 3). The wild-type IR1075 strain expresses the HpuAB receptor that is responsible for the use of haptoglobin-Hb complexes (17) (see also Fig. 5). The hpuB receptor gene is not present in IR1072 (43–45).
FIG. 5.
Western blot analysis of expression of Hb receptor proteins in wild-type meningococci and their hemO mutants. (A) Anti-HmbR at a 1:1,000 dilution; (B) anti-HpuB antibody at a 1:500 dilution. Lanes: 1072 and 1075, wild-type meningococci (1072 does not possess the hpuB gene); 3381 and 3376, hemO mutants in 1072 and 1075 genetic backgrounds, respectively; 3509, N. meningitidis 1072 but hemO::ermC-lacIOP; 3551, N. meningitidis 1075 but hemO::ermC-lacIOP; + IPTG or − IPTG, bacteria were grown in the presence or absence of 1 mM IPTG.
When tested on GCB plates, hemO mutants were not hypersensitive to heme. However, incubation of the hemO mutant under iron-restricted growth conditions and in the presence of different concentrations of hemin chloride reduced the survival of the mutant cells approximately 10- to 100-fold compared to that of the wild-type cells (Fig. 4). Only heme concentrations higher than 8 μg/ml were toxic for both wild-type and hemO mutant cells. At lower concentrations, heme promoted growth of the wild type but did not significantly affect the survival of the hemO mutant (i.e., a twofold reduction at 4 μg/ml) (Fig. 4). N. meningitidis hemO mutants were as sensitive to the toxic heme analogue gallium protoporphyrin IX (Ga-PPIX) as the wild type, indicating that the HemO product does not degrade Ga-PPIX (data not shown). Alternatively, since Ga-PPIX targets reside in the bacterial periplasm, cytoplasmatic HemO cannot protect cells against Ga-PPIX toxicity (46).
FIG. 4.
Toxicity of heme for wild-type and hemO mutant meningococci. Bacteria were incubated in the presence of different concentrations of hemin chloride for 6 h, and the viabilities of cultures were determined by plating. Three independent measurements were done. Bars, means ± standard errors of the means.
Inactivation of the hemO gene in N. meningitidis affects the expression of HmbR.
Western blot analysis was conducted by using anti-HmbR and anti-HpuB antibodies to determine whether inactivation of the hemO gene alters the expression of Hb receptors. As can be seen from Fig. 5, expression of the HmbR receptor in the outer membranes of iron-starved hemO mutants (IR3381 and IR3376) is severely affected; only a very weak band corresponding to HmbR is seen on Western blots. Altered expression of HmbR was not the result of a strand slippage mispairing event within the poly(G) sequence of hmbR, since the sequence analysis of hmbR genes in two hemO mutants, 3381 and 3376, revealed 9 and 12 G's, respectively, which would keep the gene in frame (data not shown) (33). The hmbR gene, located 178 bp downstream of the hemO stop codon, possesses several potential transcription start sites and a Fur box (data not shown and reference 43). However, lowered expression of hmbR in the hemO genetic background suggests that the inactivation of hemO has a polar effect on hmbR expression. Indeed, the induction of hemO expression leads to higher levels of HmbR in the outer membranes of two hemO conditional mutants, IR3509 and IR3551, suggesting transcriptional linkage between hemO and hmbR (Fig. 5). The level of HpuB in the outer membranes of the conditional hemO mutant (IR3551) was independent of IPTG addition (IPTG is an inducer of hemO transcription in IR3551 and IR3509). Two other hemO mutants, IR3376 and IR3381, did not express HpuB (Fig. 5). The IR3381 strain is a derivative of IR1072, a meningococcal isolate that does not possess the hpuB gene (33, 43–45). The IR3376 strain is a derivative of IR1075 which expresses HpuB (33, 44). The lack of HpuB expression in IR3376 is most probably caused by a phase variation event within the poly(G) sequence of the hpuA gene (19). The number of G's in the poly(G) region of the hpuA gene nucleotide sequence from IR3376 could not be unequivocally determined by PCR amplification and sequencing. Construction and phenotypic characterization of another 1075 hemO::Specr derivative (IR3587) confirmed that an inactivation of the hemO gene does not affect the expression of HpuB protein (data not shown).
N. meningitidis hemA hemO double mutants are able to use heme and Hb as sources of porphyrin.
It can be argued that the defect in heme transport and not the defect in heme degradation causes the inability of hemO mutants to use heme as a source of iron. Indeed, the expression of HmbR is drastically lowered in hemO conditional mutants although the expression of HpuB seems normal. However, our earlier studies has shown that neisseriae possess a yet unidentified heme transport mechanism that is independent of HmbR, HpuB, and TonB (43–45). Further, HemO mutants were still sensitive to Ga-PPIX (see above), indicating that the uptake of heme-like molecules is not drastically impaired. Another genetic test for the ability of hemO mutants to transport heme was devised by introducing a hemA mutation into an N. meningitidis hemO genetic background. Meningococcal hemA mutant cells are unable to grow on GCB plates due to the defect in heme biosynthesis, but the defect can be complemented by providing 5-aminolevulinic acid, heme, or Hb supplementation (18). If the hemO mutation blocks the uptake of heme, the hemA hemO double mutant will not be able to grow on heme as a source of porphyrin or iron. This genetic test confirmed the ability of meningococcal cells to transport heme, since the hemO hemA double mutant grew on GCB plates in the presence of heme and Hb. The same strain could not use heme or Hb as a source of iron. These data corroborated the hypothesis that hemO mutants have a defect in the intracellular processing of heme iron.
Patterns of protein expression of the wild-type strain and the N. meningitidis hemO mutant.
To learn whether there are any differences in gene expression between wild-type and hemO mutant meningococci, patterns of gene expression were assayed by two-dimensional polyacrylamide electrophoresis. Bacteria were grown under iron restriction conditions in GCB, lysed, and subjected to two-dimensional electrophoresis performed according to the method of O'Farrell (28). Comparison of two-dimensional gel patterns of the wild-type strain (1075) and its hemO mutant derivative (3376) revealed five polypeptide spots to be unique or darker in the wild-type strain and one spot to be significantly stronger in the mutant strain (data not shown). Polypeptides unique to the wild-type strain had the following molecular masses (in kilodaltons) and pI values (in parentheses): 13 (4.8), 16 (5.2), 25 (6.8), 31 (7.0), and 41 (7.2). The only polypeptide spot unique to the hemO mutant strain had a molecular mass of 41 kDa and pI of 7.4 (data not shown). A polypeptide spot corresponding to the hemO gene product was not identified by this analysis. In addition, spots corresponding to the HmbR, HpuB, and HpuA proteins were not resolved by the above method because these outer membrane proteins have pI values between 8.8 (HpuA) and 9.4 (HmbR).
Distribution of the hemO gene in pathogenic and commensal neisserial isolates.
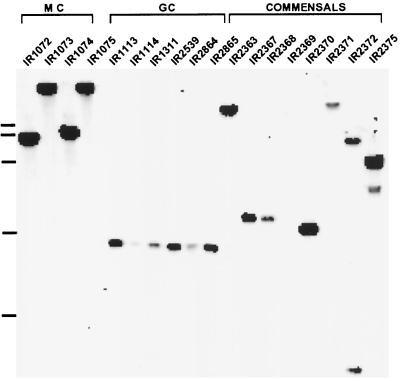
Southern blot analysis was performed on a limited number of pathogenic and commensal isolates in order to determine whether the hemO gene is present in other neisseriae (33). As can be seen from Fig. 6, all pathogenic and commensal strains except Neisseria ovis 72B (IR2369) contained nucleotide sequences that hybridized with the hemO DNA probe. This hybridization study has not been extended to other heme-acquiring bacteria thought to possess heme oxygenase-like enzymes. However, analysis of nucleotide data banks and unfinished genome nucleotide sequences identified hemO homologues in Pseudomonas aeruginosa (pigA; see above) and perhaps in Bordetella pertussis (data not shown).
FIG. 6.
Distribution of hemO-related sequences in different neisserial isolates detected by Southern blot hybridization. A 0.9-kb PCR-generated DNA probe was used. Chromosomal DNA was cleaved with ClaI. MC, strains 1072 to 1075 (N. meningitidis serogroup A, B, and C isolates); GC, different strains of N. gonorrhoeae (isolates 1113, 1114, 1311, 2359, 2864, and 2865); CM, commensal neisseriae (strains 2363, 2367, 2368, 2369, 2370, 2371, 2372, and 2375). Only N. ovis 72B (IR2369) did not hybridize with the hemO DNA probe. The molecular weight marker on the left indicates 3, 6, 9, and 12 kb).
DISCUSSION
The early work of Archibald and De Voe (1978) and Mickelsen and Sparling (1981) showed that pathogenic neisseriae use heme compounds as sources of iron (1, 27). Recently, the mechanism of heme utilization has been elucidated in gram-negative bacteria including neisseriae. Briefly, these bacteria express outer membrane proteins that bind heme compounds, extract heme from them, and transport heme into the periplasm (17, 40, 43). In yersiniae, a periplasmic binding protein-dependent system (HemTVU) transports heme further into the cytoplasm (41). Currently, a heme-specific cytoplasmic membrane transporter still awaits identification in neisseriae (38). The fate of the heme molecule after entering the cytoplasm is still not resolved in any gram-negative microorganism. It is clear that some sort of heme degradation must occur in order for bacteria to use heme as an iron source, since the vast majority of iron in meningococci is found in a nonheme iron pool (1).
A direct proof for the heme oxygenase hypothesis has recently been established for the gram-positive organism C. diphtheriae. In this organism, a cytoplasmic enzyme homologous to eukaryotic heme oxygenases degrades heme, liberating iron from it (36). A heme oxygenase-like enzyme has not been irrefutably identified in gram-negative bacteria; Stojiljkovic and Hantke (1994) have proposed that the Yersinia enterocolitica cytoplasmic protein HemS degrades heme (41). Alternatively, the last enzyme in the heme biosynthetic pathway, iron ferrochelatase, was implicated in the removal of iron from heme in Haemophilus influenzae (20).
Three lines of evidence strongly suggest that the HemO product is involved in the degradation of heme in neisseriae. (i) The presence of an intact hemO gene was essential for the use of heme, Hb, and haptoglobin-Hb complexes as sources of iron. Conversely, complementation of a hemA hemO double mutant with heme and Hb as sources of porphyrin was not affected, indicating that the ability to transport heme into hemO cells is intact. Furthermore, hemO mutants were sensitive to heme and the toxic heme analogue Ga-PPIX, thus confirming the ability of porphyrins to enter hemO cells. (ii) The overexpression of the hemO gene product in E. coli produces green bacterial cultures as a result of biliverdin formation from the degradation of heme. This phenomenon was observed with other, eukaryotic heme oxygenases and C. diphtheriae HmuO when they were overexpressed in E. coli (4, 55). (iii) Finally, HemO has significant amino acid homology with other heme oxygenases. Recent studies of the eukaryotic HO-1 have begun to analyze amino acid residues that are essential for the enzyme function. The His-25 residue and a water molecule have been identified as the heme ligands of the enzyme (11). This conserved histidine residue is also found in neisserial HemO proteins. Two glutamic acid-rich regions of the protein, 49…EEEIE…67 and 205…EELQEXXXD…226 (where X is any residue), were localized within the active site of the human heme oxygenase by cross-linking studies (54). HemO is rich in glutamic and aspartic acid residues, but the glutamic acid-rich motifs similar to those found in HO-1 are not well conserved. A 1996 study by Wilks et al. (53) implicated His-132 as the second heme ligand of HO-1. However, Matera et al. (1997) did not confirm the role of His-132 in heme degradation (23). The corresponding part of neisserial proteins is not well conserved and contains a tryptophan residue instead of a histidine at position 132. Similarly, the C-terminal part of the C. diphtheriae HmuO protein is also divergent from eukaryotic enzymes. The divergence in the primary structure is thought to be responsible for the difference in rates of heme catabolism between eukaryotic and prokaryotic heme oxygenases: the kinetics of heme degradation catalyzed by the C. diphtheriae HmuO is severalfold slower than the kinetics observed with eukaryotic enzymes (4, 55).
Meningococcal hemO mutants have drastically reduced levels of the HmbR receptor in their outer membranes. Phenotypic analysis of hemO conditional mutants showed that this is a local phenomenon, i.e., the expression of the genetically unlinked Hb receptor HpuB was not affected in the hemO conditional mutant. Genetic and in silico analysis identified several potential transcriptional starts and a Fur box in the hemO-hmbR intergenic region and within the hemO gene (43, 44). Analysis of the HmbR-mediated Hb binding confirmed the Fur-dependent nature of HmbR expression, suggesting that the intergenic promoter drives hmbR transcription (44). On the other hand, downregulation of HmbR expression in hemO knockouts and the induction of HmbR expression in hemO conditional mutants suggests transcriptional linkage between hemO and hmbR. Alternatively, the presence or absence of the HemO product might be regulating the levels of HmbR expression. It can be speculated that the lack of heme catabolism in hemO mutants and the concomitant accumulation of heme downregulates the expression of HmbR. Indeed, large amounts of externally supplied heme decrease the expression of heme/Hb receptors and outer membrane proteins in Porphyromonas gingivalis, H. influenzae, and Haemophilus ducreyi (2, 8, 12). Since heme is potentially a very toxic molecule, downregulation of heme uptake may be one of the mechanisms by which mucosal microorganisms defend themselves against heme toxicity. Conversely, the regulation of expression of the only prokaryotic heme oxygenase identified so far is more complex. The hmuO gene product of C. diphtheriae is positively regulated by the presence of heme but is fully repressed by an excess of inorganic iron in the medium (37).
In conclusion, preliminary characterization of the hemO gene revealed its indispensability for heme utilization by pathogenic neisseriae. The amino acid sequence and the phenotype of hemO mutants strongly suggest that HemO is the first heme oxygenase enzyme identified in gram-negative bacteria. HemO has a more complex role in the metabolism of neisseriae, since its inactivation led to a downregulation of HmbR expression and altered expression of at least six polypeptides.
ACKNOWLEDGMENTS
We thank the Gonococcal Genome Sequencing Project, supported by USPHS/NIH grant AI38399, and B. A. Roe, S. P. Lin, L. Song, X. Yuan, S. Clifton, T. Ducey, L. Lewis, and D. W. Dyer for gonococcal hemO nucleotide sequence data. The sequence data of the N. meningitidis Z2491 hemO gene were produced by the N. meningitidis Sequencing Group at the Sanger Center and were obtained from The Sanger Centre website (ftp://ftp.sanger.ac.uk/pub/pathogens/nm/). We thank L. Lewis and D. Dyer for the gift of anti-HpuB antibodies and for sending bacterial strains. We thank S. H. Seifert for sending us the Erm-Lac cassette. We thank C. Bracken for comments on the manuscript.
This work is supported by Public Service grant AI472870-01A1.
W. Zhu and D. J. Hunt contributed equally to this work.
REFERENCES
- 1.Archibald F S, De Voe I W. Iron in Neisseria meningitidis: minimum requirements, effects of limitation, and characteristics of uptake. J Bacteriol. 1978;136:35–48. doi: 10.1128/jb.136.1.35-48.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bramanti T E, Holt S C. Effect of porphyrins and host iron transport proteins on outer membrane protein expression in Porphyromonas (Bacteroides) gingivalis: identification of a novel 26 kDa hemin-repressible surface protein. Microb Pathog. 1992;13:61–73. doi: 10.1016/0882-4010(92)90032-j. [DOI] [PubMed] [Google Scholar]
- 3.Cannon J B. Pharmaceutics and drug delivery aspects of heme and porphyrin therapy. J Pharm Sci. 1993;82:435–446. doi: 10.1002/jps.2600820502. [DOI] [PubMed] [Google Scholar]
- 4.Chu G C, Katakuya K, Zhang X, Yoshida T, Keda-Saito M I. Heme degradation as catalyzed by a recombinant bacterial heme oxygenase (HmuO) from Corynebacterium diphtheriae. J Biol Chem. 1999;274:21319–21325. doi: 10.1074/jbc.274.30.21319. [DOI] [PubMed] [Google Scholar]
- 5.Cornejo J, Beale S I. Algal heme oxygenase from Cyanidium caldarium. J Biol Chem. 1988;263:11915–11921. [PubMed] [Google Scholar]
- 6.Correia F F, Inouye S, Inouye M. A family of small repeated elements with some transposon-like properties in the genome of Neisseria gonorrhoeae. J Mol Biol. 1988;263:12194–12198. [PubMed] [Google Scholar]
- 7.de Lorenzo V, Giovannini F, Herrero M, Neilands J B. Metal ion regulation of gene expression. Fur repressor-operator interaction at the promoter region of the aerobactin system of pColV-K30. J Mol Biol. 1988;203:875–884. doi: 10.1016/0022-2836(88)90113-1. [DOI] [PubMed] [Google Scholar]
- 8.Elkins C. Identification and purification of a conserved heme-regulated hemoglobin-binding outer membrane protein from Haemophilus ducreyi. Infect Immun. 1995;63:1241–1245. doi: 10.1128/iai.63.4.1241-1245.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engel R R, Matsen J M, Chapman S S, Swartz S. Carbon monoxide production from heme compounds by bacteria. J Bacteriol. 1972;112:1310–1315. doi: 10.1128/jb.112.3.1310-1315.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eschenbach D A, Harnisch J P, Holmes K K. Pathogenesis of acute pelvic inflammatory diseases: role of contraception and other risk factors. Am J Obstet Gynecol. 1977;128:838–850. doi: 10.1016/0002-9378(77)90051-5. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa K, Matera K M, Zhou H, Fujii H, Sato M, Yoshimura T, Ikeda-Saito M, Yoshida T. Identification of histidine 45 as the axial heme iron ligand of heme oxygenase-2. J Biol Chem. 1998;273:4317–4322. doi: 10.1074/jbc.273.8.4317. [DOI] [PubMed] [Google Scholar]
- 12.Jin H, Ren Z, Pozsgay J M, Elkins C, Whitby P W, Morton D J, Stull T. Cloning of a DNA fragment encoding a heme-repressible hemoglobin-binding outer membrane protein from Haemophilus influenzae. Infect Immun. 1996;64:3134–3141. doi: 10.1128/iai.64.8.3134-3141.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, Kimura T, Hosouchi T, Matsuno A, Muraki A, Nakazaki N, Naruo K, Okumura S, Shimpo S, Takeuchi C, Wada T, Watanabe A, Yamada M, Yasuda M, Tabata S. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 14.Kellogg D S, Jr, Peacock W L, Jr, Deacon W E, Brown L, Pirkle C I. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J Bacteriol. 1963;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim I C, Bragg P D. Properties of nonheme iron in a cell envelope fraction from Escherichia coli. J Bacteriol. 1971;107:664–670. doi: 10.1128/jb.107.3.664-670.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ladan H, Nitzan Y, Malik Z. The antibacterial activity of haemin compared with cobalt, zinc and magnesium protoporphyrin and its effect on potassium loss and ultrastructure of Staphylococcus aureus. FEMS Microbiol Lett. 1993;112:173–177. doi: 10.1111/j.1574-6968.1993.tb06444.x. [DOI] [PubMed] [Google Scholar]
- 17.Lewis L A, Gray E, Wang Y, Roe B A, Dyer D W. Molecular characterization of hpuAB, the haemoglobin-haptoglobin-utilization operon of Neisseria meningitidis. Mol Microbiol. 1997;23:737–749. doi: 10.1046/j.1365-2958.1997.2501619.x. [DOI] [PubMed] [Google Scholar]
- 18.Lewis L A, Sung M H, Gipson M, Hartman K, Dyer D W. Transport of intact porphyrin by HpuAB, the hemoglobin-haptoglobin utilization system of Neisseria meningitidis. J Bacteriol. 1998;180:6043–6047. doi: 10.1128/jb.180.22.6043-6047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis L A, Gipson M, Hartman K, Ownbey T, Vaughn J, Dyer D W. Phase variation of HpuAB and HmbR, two distinct haemoglobin receptors of neisseria. Mol Microbiol. 1999;32:9877–9889. doi: 10.1046/j.1365-2958.1999.01409.x. [DOI] [PubMed] [Google Scholar]
- 20.Loeb M. Ferrochelatase activity and protoporphyrin IX utilization in Haemophilus influenzae. J Bacteriol. 1995;177:3613–3615. doi: 10.1128/jb.177.12.3613-3615.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maines M D. Heme oxygenase: function, multiplicity, regulatory mechanism, and clinical applications. FASEB J. 1988;2:2557–2568. [PubMed] [Google Scholar]
- 22.Maines M D, Kappas A. Cobalt induction of hepatic heme oxygenase, with evidence that cytochrome P-450 is not essential for this enzyme activity. Proc Natl Acad Sci USA. 1974;71:4293–4297. doi: 10.1073/pnas.71.11.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matera M K, Zhou H, Migita C T, Hobert S E, Ishikawa K, Katakura K, Maeshima H, Yoshida T, Ikeda-Saito M. Histidine-132 does not stabilize a distal water ligand and is not an important residue for the enzyme activity in heme oxygenase-1. Biochemistry. 1997;36:4900–4915. doi: 10.1021/bi962321m. [DOI] [PubMed] [Google Scholar]
- 24.McCoubrey W K, Jr, Huang T J, Maines M D. Isolation and characterization of cDNA from rat brain that encodes hemoproteins heme oxygenase-3. Eur J Biochem. 1997;247:725–732. doi: 10.1111/j.1432-1033.1997.00725.x. [DOI] [PubMed] [Google Scholar]
- 25.Mehr I J, Seifert H S. Differential roles of homologous recombination pathways in Neisseria gonorrhoeae pilin antigenic variation, DNA transformation and DNA repair. Mol Microbiol. 1998;30:697–710. doi: 10.1046/j.1365-2958.1998.01089.x. [DOI] [PubMed] [Google Scholar]
- 26.Membrillo-Hernandez J, Coopamah M D, Chamma A, Hughes M N, Poole R K. A novel mechanism for upregulation of the Escherichia coli K-12 hmp (flavohaemoglobin) gene by the ‘NO releaser’, S-nitrosoglutathione: nitrosation of homocysteine and modulation of MetR binding to glyA-hmp intergenic region. Mol Microbiol. 1998;29:1101–1112. doi: 10.1046/j.1365-2958.1998.01000.x. [DOI] [PubMed] [Google Scholar]
- 27.Mickelsen P A, Sparling P F. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from transferrin and iron compounds. Infect Immun. 1981;33:555–564. doi: 10.1128/iai.33.2.555-564.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Farrell P H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 29.Poss K D, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci USA. 1997;94:10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poss K D, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci USA. 1997;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prentki P, Krisch H M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 32.Reith M E, Munholland J. Complete nucleotide sequence of the Porphyra purpurea chloroplast genome. Plant Mol Biol Rep. 1995;13:333–335. [Google Scholar]
- 33.Richardson A R, Stojiljkovic I. HmbR, a hemoglobin-binding outer membrane protein of Neisseria meningitidis, undergoes phase variation. J Bacteriol. 1999;181:2067–2074. doi: 10.1128/jb.181.7.2067-2074.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richaud C, Zabulon G. The heme oxygenase (pbsA) in the red alga Rhodella violacea is discontinuous and transcriptionally activated during iron limitation. Proc Natl Acad Sci USA. 1997;94:11736–11741. doi: 10.1073/pnas.94.21.11736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 36.Schmitt M P. Utilization of host iron sources by Corynebacterium diphtheriae: identification of a gene whose product is homologous to eukaryotic heme oxygenases and is required for acquisition of iron from heme and hemoglobin. J Bacteriol. 1997;179:838–845. doi: 10.1128/jb.179.3.838-845.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmitt M P. Transcription of the Corynebacterium diphtheriae hmuO gene is regulated by iron and heme. Infect Immun. 1997;65:4634–4641. doi: 10.1128/iai.65.11.4634-4641.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schryvers A B, Stojiljkovic I. Iron acquisition systems in the pathogenic neisseria. Mol Microbiol. 1999;32:1117–1123. doi: 10.1046/j.1365-2958.1999.01411.x. [DOI] [PubMed] [Google Scholar]
- 39.Seifert H S. Insertionally inactivated and inducible recA alleles for use in neisseria. Gene. 1997;188:215–220. doi: 10.1016/s0378-1119(96)00810-4. [DOI] [PubMed] [Google Scholar]
- 40.Stojiljkovic I, Hantke K. Haemin uptake system of Yersinia enterocolitica: similarities with other TonB-dependent systems in Gram-negative bacteria. EMBO J. 1992;11:4359–4367. doi: 10.1002/j.1460-2075.1992.tb05535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stojiljkovic I, Hantke K. Transport of haemin across the cytoplasmic membrane through a haemin-specific periplasmic binding-protein-dependent transport system in Yersinia enterocolitica. Mol Microbiol. 1994;13:719–732. doi: 10.1111/j.1365-2958.1994.tb00465.x. [DOI] [PubMed] [Google Scholar]
- 42.Stojiljkovic I, Baumler A J, Hantke K. Fur regulon in gram-negative bacteria. Identification and characterization of new iron-regulated Escherichia coli genes by a Fur titration assay. J Mol Biol. 1994;236:531545. doi: 10.1006/jmbi.1994.1163. [DOI] [PubMed] [Google Scholar]
- 43.Stojiljkovic I, Hwa B, de Saint Martin L, O'Gaora P, Nassif X, Hefferon F, So M. The Neisseria meningitidis haemoglobin receptor: its role in iron utilization and virulence. Mol Microbiol. 1995;15:531–541. doi: 10.1111/j.1365-2958.1995.tb02266.x. [DOI] [PubMed] [Google Scholar]
- 44.Stojiljkovic I, Hwa B, Larson J, Anic S, So M. HmbR outer membrane proteins of pathogenic Neisseria spp.: iron-regulated, hemoglobin-binding proteins with a high level of primary structure conservation. J Bacteriol. 1996;178:4670–4678. doi: 10.1128/jb.178.15.4670-4678.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stojiljkovic I, Srinivasan N. Neisseria meningitidis tonB, exbB, and exbD genes: Ton-dependent utilization of protein-bound iron in neisseriae. J Bacteriol. 1997;179:805–812. doi: 10.1128/jb.179.3.805-812.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stojiljkovic I, Kumar V, Srinivasan N. Non-iron metalloporphyrins: potent antibacterial compounds that exploit haem/Hb uptake systems of pathogenic bacteria. Mol Microbiol. 1999;31:429–442. doi: 10.1046/j.1365-2958.1999.01175.x. [DOI] [PubMed] [Google Scholar]
- 47.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 48.Sweet R L, Blankfort-Doyle M, Robbie M O, Schacter J. The occurrence of chlamydial and gonococcal salpingitis during the menstrual cycle. JAMA. 1986;255:2062–2064. [PubMed] [Google Scholar]
- 49.Tabor S, Richardson C C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci USA. 1985;82:1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takahashi S, Wang J, Rousseau D, Ishikawa K, Yoshida T, Takeuchi N, Ikeda-Saito M. Heme-heme oxygenase complex: structure and properties of the catalytic site from resonance Raman scattering. Biochemistry. 1994;33:5531–5538. doi: 10.1021/bi00184a023. [DOI] [PubMed] [Google Scholar]
- 51.Tenhunen R, Marver H S, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA. 1968;61:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verma A, Hirsch D J, Glatt C E, Ronnett G V, Snyder S H. Carbon monoxide: a putative neural messenger. Science. 1993;259:381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- 53.Wilks A, Ortiz de Montellano P R, Sun J, Loehr T M. Heme oxygenase (HO-1): His-132 stabilizes a distal water ligand and assists catalysis. Biochemistry. 1996;35:930–936. doi: 10.1021/bi952405f. [DOI] [PubMed] [Google Scholar]
- 54.Wilks A, Medzihradszky K F, Ortiz de Montellano P R. Heme oxygenase active-site residues identified by heme-protein cross-linking during reduction of CbrCl3 catalysis. Biochemistry. 1996;37:2889–2896. doi: 10.1021/bi972720x. [DOI] [PubMed] [Google Scholar]
- 55.Wilks A, Schmitt M P. Expression and characterization of a heme oxygenase (HmuO) from Corynebacterium diphtheriae. J Biol Chem. 1998;273:837–841. doi: 10.1074/jbc.273.2.837. [DOI] [PubMed] [Google Scholar]
- 56.Yoshida T, Kikuchi G. Partial purification and reconstitution of the heme oxygenase system from pig spleen microsomes. Biochem J. 1974;75:1187–1191. doi: 10.1093/oxfordjournals.jbchem.a130494. [DOI] [PubMed] [Google Scholar]