Abstract
Background
Plasma ceramides and sphingomyelins have been independently linked to diabetes risk, glucose and insulin levels, and the risk of several cardiovascular (CVD) outcomes. However, whether individual ceramide and sphingomyelin species contribute to CVD risk among people with type 2 diabetes is uncertain. Our goal was to evaluate associations of 4 ceramide and 4 sphingomyelin species with incident CVD in a longitudinal population-based study among American Indians with diabetes.
Methods
This analysis included participants with prevalent type 2 diabetes from two cohorts: a prospective cohort of 597 participants in the Strong Heart Family Study (116 incident CVD cases; mean age: 49 years; average length of follow-up: 14 years), and a nested case–control sample of 267 participants in the Strong Heart Study (78 cases of CVD and 189 controls; mean age: 61 years; average time until incident CVD in cases: 3.8 years). The average onset of diabetes was 7 years prior to sphingolipid measurement. Sphingolipid species were measured using liquid chromatography and mass spectrometry. Cox regression and logistic regression were used to assess associations of sphingolipid species with incident CVD; results were combined across cohorts using inverse-variance weighted meta-analysis.
Results
There were 194 cases of incident CVD in the two cohorts. In meta-analysis of the 2 cohort results, higher plasma levels of Cer-16 (ceramide with acylated palmitic acid) were associated with higher CVD risk (HR per two-fold higher Cer-16: 1.85; 95% CI 1.05–3.25), and higher plasma levels of sphingomyelin species with a very long chain saturated fatty acid were associated with lower CVD risk (HR per two-fold higher SM-22: 0.48; 95% CI 0.26–0.87), although none of the associations met our pre-specified threshold for statistical significance of p = 0.006.
Conclusions
While replication of the findings from the SHS in other populations is warranted, our findings add to a growing body of research suggesting that ceramides, in particular Cer-16, not only are associated with higher diabetes risk, but may also be associated with higher CVD risk after diabetes onset. We also find support for the hypothesis that sphingomyelins with a very long chain saturated fatty acid are associated with lower CVD risk among adults with type 2 diabetes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-022-01596-4.
Keywords: Sphingolipids, Ceramides, Sphingomyelins, Cardiovascular disease, Epidemiology
Background
Diabetes is a major risk factor for cardiovascular disease (CVD) [1], contributing to up to one fifth of vascular disease incidence worldwide [2]. Randomized clinical trials have shown that normalization of glucose does not effectively reduce CVD risk among patients with diabetes [3–6]; novel, effective therapies to reduce the burden of CVD among people living with diabetes are urgently needed.
There is abundant evidence that ceramides are involved in diabetes in experimental studies [7, 8]. In addition, we have previously reported that plasma ceramides are associated with risk of incident type 2 diabetes in two large population studies, suggesting an involvement in diabetes in humans as well [9, 10].
Ceramide and its precursor sphingomyelin are part of the large sphingolipid family. At their core is a “sphingoid” backbone to which one fatty acid is acylated. There is increasing evidence that the length of the fatty acid acylated to backbone impacts the biological activities of ceramides and sphingomyelins. Cell and animal studies have suggested that Cer-16 promotes apoptosis, while longer chain ceramides (Cer-20, -22, and -24) appear to prevent apoptosis [11–15]. In addition, we have shown in the Cardiovascular Health Study that plasma levels of Cer-16 and SM-16 are associated with a higher risk of incident heart failure, atrial fibrillation, and mortality, while longer-chain ceramides and sphingomyelins are associated with a lower risk of each of these outcomes [16, 17]. In contrast, we have recently reported that both Cer-16 and its longer chain counterparts are associated with higher risk of incident diabetes, as well as with elevated fasting insulin and glucose levels [10, 18, 19]. Whether ceramide and sphingomyelin species influence cardiovascular disease risk among patients with prevalent type 2 diabetes has received limited attention.
Diabetes is common among American Indians (AIs), and managing its potential complications is a public health priority [20]. The purpose of this study was to assess whether ceramide and sphingomyelin with 16:0 are associated with higher risk of incident CVD, while ceramide and sphingomyelin species with 20:0, 22:0 and 24:0 are associated with lower risk of incident CVD among participants in the Strong Heart Study with prevalent type-2 diabetes.
Methods
Study population
The Strong Heart Study (SHS) and Strong Heart Family Study (SHFS) are population-based longitudinal studies of CVD in several American Indian communities in Arizona, North and South Dakota, and Oklahoma. Details of the study designs have been described previously [21]. Briefly, in 1989, SHS recruited a cohort of 4549 individuals who participated in clinical exams in 1989–91, 1993–95, and 1998–99. At the 1998–99 exam, 1st, 2nd, and 3rd degree relatives of SHS participants were invited to participate in a family study, which became the Strong Heart Family Study (SHFS). The SHFS included two examinations: 2001–03 and 2006–09. The institutional review boards from each Indian Health Service region and all communities approved the studies, and written informed consent was obtained from all participants at each exam.
Analyses were restricted to participants with prevalent type-2 diabetes at the time of their sphingolipid measurement. Prevalent diabetes was defined as a fasting plasma glucose ≥ 126 mg/dL or use of insulin or oral anti-diabetic medications at the relevant study exam; in SHS, where 2-h oral glucose tolerance tests were performed, we also identified prevalent diabetes as an oral glucose tolerance test ≥ 200 mg/dL [22].
Sphingolipids were measured prospectively at two timepoints in SHFS, allowing for the inclusion of 508 participants with prevalent diabetes at the 2001–03 exam and an additional 202 participants who were subsequently diagnosed with diabetes before or at the 2006–09 exam. Of these 710 participants, we excluded 96 who had a history of CVD at the time of their sphingolipid measurement and 17 who had no available follow-up CVD data, leaving 597 SHFS participants eligible for this analysis.
In SHS, we used a nested case–control design and samples from the 1993–95 exam. Selected cases and controls had prevalent diabetes and no history of CVD at the 1993–1995 exam. For cases, we randomly selected 100 participants out of 444 who subsequently developed CVD during follow-up in order to preserve scarce samples. We used risk set matching; for each case, we selected two age (within 5 years) and sex matched controls who had not developed incident CVD. Of the 300 SHS participants we sampled, 281 had sufficient blood samples and were able to have their sphingolipids measured; 14 participants from the Arizona site were excluded due to a lack of appropriate controls (13 cases and 1 control), leaving 267 participants (78 cases and 189 controls) in the analysis.
Data collection
Each SHFS and SHS examination included a standardized personal interview, physical examination, and laboratory work-up. The data collection procedures were identical at all SHFS and SHS exams except for physical activity and diabetes ascertainment; these procedures have been described in detail previously [21, 23].
Sphingolipid measurement
Sphingolipids were measured from blood samples collected at the 2001–03 and 2006–09 SHFS study examinations and the 1993–95 SHS study examination. The same collection protocols were used at each study exam; blood samples were collected after a 12-h fast and were stored at − 70 °C until analyzed. A detailed description of the laboratory procedures can be found elsewhere; briefly, plasma sphingolipids were quantified from baseline EDTA-blood samples using liquid chromatography–tandem mass spectrometry [24].
This analysis includes eight sphingolipids that carry a saturated fatty acid acylated to the sphingoïd backbone: ceramide and sphingomyelin with palmitic acid (16:0 [16 carbons, 0 double bonds]; Cer-16 and SM-16), with arachidic acid (20:0; Cer-20 and SM-20), with behenic acid (22:0; Cer-22 and SM-22), and with lignoceric acid (24:0; Cer-24 and SM-24).
Incident cardiovascular disease
Incident CVD was defined as the first occurrence of myocardial infarction (MI; definite, probable, or fatal), ischemic stroke (definite or fatal), atherosclerotic cardiovascular disease (ASCVD; definite), or heart failure (HF; including fatal). Incident events in both SHFS and SHS were identified at each study examination and through subsequent surveillance of medical records and death certificates; after the 2006–09 examination and through December 2017, cases were ascertained through phone interviews with the participants and confirmed by medical record documentation. Stroke and HF were identified via chart review, MI was identified through chart reviews and/or evidence of MI by study visit ECG, and ASCVD was defined as incident MI, coronary heart disease (CHD), ischemic stroke, or abnormal study ECG with positive Rose Angina Questionnaire.
Statistical analysis
In the SHFS cohort, Cox regression models were used to examine the associations of each sphingolipid with the risk of incident CVD. SHFS participants began accruing time-at-risk at the earliest study exam at which they had prevalent diabetes and measured sphingolipids (n = 434 from the 2001–03 exam, and n = 163 from the 2006–09 exam), and were followed to the earliest of: date of incident CVD, death, or loss to follow up. Because the SHFS is comprised of extended families, robust standard error estimates that account for clustering of risk factors among family members were used. In the SHS cohort, logistic regression with robust standard errors was used to evaluate the associations of plasma sphingolipids and incident CVD.
We used 3 sets of models to examine associations of sphingolipids with incident CVD. Model 1 (minimally-adjusted model) included terms for age, sex, and study site (SHFS only). Model 2 (multivariable model) additionally adjusted for education (years), smoking (never, former, current), alcohol consumption (never, former, current), physical activity (in SHFS: average number of pedometer measured steps per day; in SHS: metabolic equivalent task hours per week), body mass index (BMI; log-transformed), waist circumference, low density lipoprotein-cholesterol (LDL), systolic blood pressure, treated hypertension, duration of diabetes (log-transformed), type of diabetes medication used (insulin + oral hypoglycemic, insulin only, oral hypoglycemic only, or neither), and treated hyperlipidemia. Model 3 (sphingolipid adjusted model) included Model 2 with additional adjustment for one of the other species: Cer-16 and SM-16 models include adjustment for Cer-22 and SM-22, respectively; Cer-20, -22, -24, and SM-20, -22, and -24 models include adjustment for Cer-16 and SM-16, respectively. In both cohorts, adjustment covariate values were drawn from the same study exam at which the sphingolipids were measured. Sphingolipid exposures were log base-2 transformed, so the relative risk represents the associations with incident CVD per doubling of each of the sphingolipid species concentrations (µM), which is roughly equivalent to the difference between the 10th and 90th percentiles (Table 2). With the exception of study site in SHS, the same set of adjustment variables were used in each of the models for both SHFS and SHS cohorts.
Table 2.
SHFS and SHS participant sphingolipid concentrations (µM)
| SHFS (n = 597) | SHS controls (n = 189) | SHS cases (n = 78) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | 90th/10th pct | Mean | SD | Range | 90th/10th pct | Mean | SD | Range | 90th/10th pct | |
| Cer-16 | 0.20 | 0.07 | 0.06–0.58 | 2.2 | 0.32 | 0.08 | 0.13–0.64 | 1.9 | 0.33 | 0.09 | 0.16–0.6 | 2.2 |
| Cer-20 | 0.07 | 0.03 | 0.01–0.29 | 2.8 | 0.11 | 0.04 | 0.04–0.38 | 2.5 | 0.11 | 0.04 | 0.06–0.27 | 2.2 |
| Cer-22 | 0.81 | 0.33 | 0.23–3.16 | 2.5 | 0.76 | 0.22 | 0.34–1.84 | 1.9 | 0.74 | 0.22 | 0.29–1.43 | 2.1 |
| Cer-24 | 5.62 | 2.06 | 1.75–18.96 | 2.4 | 5.15 | 1.36 | 2.33–9.94 | 1.9 | 5.06 | 1.41 | 2.23–9.93 | 1.9 |
| SM-16 | 135.21 | 28.73 | 59.79–275.51 | 1.6 | 131.37 | 18.38 | 84.36–186.86 | 1.4 | 134.74 | 25.97 | 76.13–226.01 | 1.6 |
| SM-20 | 20.33 | 5.00 | 8.76–44.53 | 1.9 | 15.70 | 3.17 | 9.17–23.97 | 1.8 | 15.15 | 3.49 | 6.65–28.98 | 1.7 |
| SM-22 | 35.82 | 9.40 | 13.09–76.09 | 1.9 | 26.21 | 5.52 | 14.03–39.15 | 1.8 | 25.23 | 6.06 | 12.41–48.77 | 1.8 |
| SM-24 | 19.36 | 5.45 | 7.35–44.67 | 2.0 | 14.06 | 3.36 | 7.3–23.76 | 1.8 | 13.42 | 3.42 | 6.46–24.12 | 1.9 |
As a secondary analysis, we evaluated associations of sphingolipids with incident MI, stroke, heart failure, and ASCVD within the SHFS cohort. These models followed the same analytic approach as the primary analysis.
We conducted a number of sensitivity analyses. To assess whether associations were robust to additional adjustment of high density lipoprotein-cholesterol (HDL), fibrinogen, or triglyceride levels, or of estimated glomerular filtration rate (eGFR), we repeated the multivariable analyses with additional terms for HDL-cholesterol, triglycerides, and fibrinogen in one model and chronic kidney disease (CKD; eGFR < 60) in another. We also examined whether associations of sphingolipids with incident CVD differed by age, sex, BMI, CKD, or duration of diabetes by adding product interaction terms to the multivariable models. To account for multiple comparisons in these sensitivity analyses, we used a significance threshold of p < 0.001 (8 sphingolipid species and 6 additional models; p < 0.05/48).
Inverse-variance-weighted fixed effects meta-analyses were conducted to combine results from the two cohorts using the log hazard ratios (SHFS), log odds ratios (SHS), and their corresponding standard errors in STATA 16.0 (Stata Corporation, College Station, TX); for the purposes of this manuscript, we refer to resulting meta-analyzed parameter as “relative risk.” Inverse-variance-weighted fixed-effects meta-analyses approximate results that would be obtained if the data from all studies could be analyzed together with adjustment for study [25]. Heterogeneity was assessed via I2 variance [26].
Multiple imputation with chained equations was used to impute missing values for smoking (SHFS: n = 2; SHS: n = 9), alcohol consumption (SHFS: n = 30; SHS: n = 5), education (SHFS: n = 9), BMI (SHFS: n = 7), waist circumference (SHFS: n = 4), LDL-cholesterol (SHFS: n = 10; SHS: n = 18), duration of diabetes (SHFS: n = 32; SHS: n = 1), physical activity (SHFS: n = 206; SHS: n = 12), systolic blood pressure (n = 1), fibrinogen (SHFS: n = 3; SHS: n = 3), triglycerides (SHFS: n = 3; SHS: n = 1), HDL-cholesterol (SHFS: n = 7; SHS: n = 2), and CKD (SHFS: n = 2; SHS: n = 4) using information on age, sex, site, treated hypertension, treated hyperlipidemia, and type of diabetes treatment [18, 27, 28]. Schoenfeld residuals were evaluated to test the proportional hazards assumption in SHFS Cox models; Martingale residuals were used to assess the linearity of associations; and delta-betas were reviewed to assess the impact of potential influential outliers.
A Bonferroni correction was used to adjust for multiple comparisons; a significance threshold of 0.0063 (0.05/8 sphingolipid species) was used.
Results
Participant characteristics at the time of their sphingolipid measurement are listed in Table 1. The average age of participants was 49 years (range: 15–86 years) in SHFS, 61 years (range: 49–78 years) in SHS controls, and 62 years (range 49–79 years) in SHS cases; 62% and 64% of SHFS and SHS participants, respectively, were women. On average, participants were diagnosed with diabetes 7 years before their sphingolipid measurement. SHFS participants had larger BMIs and waist circumferences, lower LDL-cholesterol, a lower prevalence of CKD, and a higher prevalence of treated hypertension and treated hyperlipidemia than SHS participants, despite being 12 years younger, on average. Smoking (current or past) was more common in SHS cases and controls, while current alcohol use was more common among SHFS participants.
Table 1.
Baseline characteristics of SHFS and SHS participants
| SHFS (n = 597) | SHS controls (n = 189) | SHS cases (n = 78) | ||||
|---|---|---|---|---|---|---|
| Mean or % | SD | Mean or % | SD | Mean or % | SD | |
| Age, years | 49 | 14 | 61 | 8 | 62 | 8 |
| Education, years | 12 | 2 | 11 | 3 | 11 | 9 |
| Smoking, % | ||||||
| Current | 33% | 37% | 35% | |||
| Past | 30% | 39% | 37% | |||
| Alcohol consumption, % | ||||||
| Current | 46% | 31% | 22% | |||
| Past | 41% | 47% | 55% | |||
| Physical activitya | 4299 | 3260 | 75 | 76 | 77 | 16 |
| BMI, kg/m2 | 36 | 8 | 31 | 5 | 32 | 17 |
| Waist circumference, cm | 115 | 18 | 107 | 11 | 110 | 18 |
| LDL-cholesterol, mg/dL | 101 | 31 | 123 | 31 | 127 | 19 |
| HDL-cholesterol, mg/dL | 47 | 14 | 37 | 10 | 36 | 20 |
| Triglycerides, mg/dL | 242 | 354 | 190 | 149 | 209 | 21 |
| Systolic blood pressure, mmHg | 128 | 18 | 127 | 18 | 136 | 22 |
| Treated hypertension, % | 49% | 33% | 45% | |||
| Duration of diabetes, years | 6 | 8 | 7 | 8 | 11 | 24 |
| Diabetes medication, % | ||||||
| Insulin | 10% | 19% | 27% | |||
| Oral | 46% | 37% | 33% | |||
| Both | 9% | 2% | 10% | |||
| Neither | 35% | 43% | 29% | |||
| Prevalent CKD, % | 9% | 16% | 32% | |||
| Treated lipidemia, % | 12% | 1% | 1% | |||
aPhysical activity was defined as pedometer measured steps per day in SHFS and metabolic equivalent task hours per week in SHS
Concentrations of each of the measured sphingolipids for each cohort are presented in Table 2. Overall, the distributions of each sphingolipid were similar in the two cohorts, and the concentration at the 90th percentile was roughly double that of concentration at the 10th percentile for each sphingolipid. Correlations of the measured sphingolipids are presented in Additional file 1: Fig. S1.
Over a median 14 years of follow-up (range: 0–16 years), we identified 116 cases of incident CVD among the 597 SHFS participants with diabetes. These included 39 incident MI events, 16 strokes (12 ischemic; 3 hemorrhagic, 1 fatal/undetermined), 99 incident total cases of ASCVD, and 33 incident cases of HF (outcomes not mutually exclusive). Among the 78 SHS cases, the median time until incident CVD was 3.8 years; these included 17 MI events, 11 strokes (7 ischemic, 2 hemorrhagic, 2 fatal/undetermined), 50 total cases of incident ASCVD, and 17 cases of incident HF.
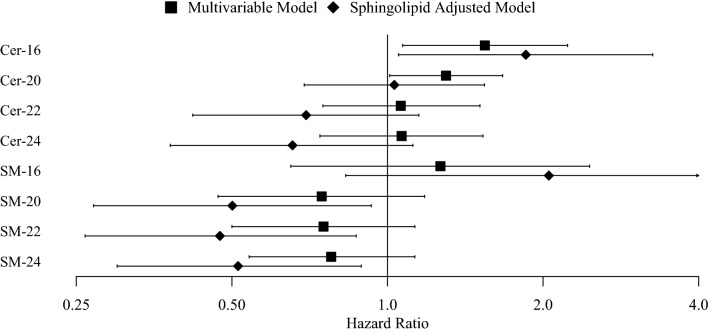
Meta-analyzed results from the SHFS cohort and from the case–control study nested in SHS that evaluate the associations between sphingolipids and risk of incident CVD among participants with prevalent diabetes are presented in Table 3; Fig. 1 (and Additional file 2: Tables S1 and S2). Although we could not demonstrate that any of the investigated sphingolipids were associated with incident CVD at our pre-specified Bonferroni threshold of 0.0063, there is evidence to suggest that Cer-16 is associated with elevated CVD risk (per two-fold increase in Cer16—hazard ratio (HR): 1.54; 95% confidence interval (CI) 1.07–2.23; p-value: 0.021). After adjustment for another sphingolipid species, Cer-16 was associated with increased risk, while SM-20, -22, -24, were associated with decreased risk of incident CVD at the 0.05 significance level.
Table 3.
Meta-analyzed adjusted risk of incident cardiovascular disease in SHFS and SHS
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | p-value | RR | 95% CI | p-value | RR | 95% CI | p-value | |
| Cer-16 | 1.71 | (1.18, 2.48) | 0.005 | 1.54 | (1.07, 2.23) | 0.021 | 1.85 | (1.05, 3.25) | 0.033 |
| Cer-20 | 1.27 | (0.98, 1.65) | 0.072 | 1.30 | (1.01, 1.68) | 0.044 | 1.03 | (0.69, 1.54) | 0.876 |
| Cer-22 | 1.09 | (0.76, 1.57) | 0.640 | 1.06 | (0.75, 1.5) | 0.741 | 0.70 | (0.42, 1.16) | 0.162 |
| Cer-24 | 1.13 | (0.77, 1.64) | 0.534 | 1.06 | (0.74, 1.52) | 0.738 | 0.65 | (0.38, 1.12) | 0.122 |
| SM-16 | 1.62 | (0.84, 3.14) | 0.154 | 1.26 | (0.65, 2.46) | 0.492 | 2.04 | (0.82, 5.05) | 0.125 |
| SM-20 | 0.80 | (0.52, 1.24) | 0.328 | 0.75 | (0.48, 1.18) | 0.209 | 0.50 | (0.27, 0.93) | 0.028 |
| SM-22 | 0.85 | (0.57, 1.27) | 0.414 | 0.75 | (0.51, 1.13) | 0.169 | 0.48 | (0.26, 0.87) | 0.016 |
| SM-24 | 0.88 | (0.61, 1.25) | 0.470 | 0.78 | (0.54, 1.14) | 0.198 | 0.52 | (0.3, 0.89) | 0.018 |
Model 1 includes terms for age, sex, and study site (SHFS only). Model 2 additionally includes terms for education, smoking, physical activity, BMI, waist circumference, LDL-cholesterol, systolic blood pressure, treated hypertension, duration of diabetes, type of diabetes medication used, and treated hyperlipidemia. Model 3 further adjusts for one of the other species: Cer-16 and SM-16 models include adjustment for Cer-22 and SM-22, respectively; Cer-20, -22, -24, and SM-20, -22, and -24 models include adjustment for Cer-16 and SM-16, respectively
Fig. 1.
Meta-analyzed adjusted risk of incident CVD in SHFS and SHS. Multivariable Model includes terms for age, sex, study site (in SHFS only), education, smoking, physical activity, BMI, waist circumference, LDL-cholesterol, systolic blood pressure, treated hypertension, duration of diabetes, type of diabetes medication used, and treated hyperlipidemia. Sphingolipid Adjusted Model further adjusts for one of the other species: Cer-16 and SM-16 models include adjustment for Cer-22 and SM-22, respectively; Cer-20, -22, -24, and SM-20, -22, and -24 models include adjustment for Cer-16 and SM-16, respectively
Results from models that adjusted for HDL-cholesterol, triglycerides, and fibrinogen and CKD were similar to our primary analysis (Additional file 2: Table S3). There was no evidence of interactions of any of the metabolites with age, sex, BMI, CKD, or duration of diabetes.
There was no evidence of significant heterogeneity in the multivariable model between the two cohorts, and differences between the cohorts had little effect on the associations of Cer-16, Cer-22, Cer-24, SM-16, and SM-18 (I2 < 25%) and a moderate effect on the associations of Cer-20, SM-22, and SM-24 (I2 < 50%). The proportional hazards assumption was not violated in any SHFS models, and there was no evidence of departure from linearity in any of the primary models.
Results from Cox regression models of sphingolipids with incident ASCVD and heart failure in SHFS are presented in Table 4. In our multivariable model, two of the investigated sphingolipids, Cer-16 and Cer-20, were associated with greater risk of incident ASCVD (Cer-16 HR: 2.01, 95% CI 1.35, 2.99, p-value: 0.0006; Cer-20 HR: 1.70, 95% CI 1.32, 2.19, p-value: < 0.0001). After adjustment for other sphingolipids, neither sphingolipid met the Bonferroni threshold for significance, but there was evidence to suggest that Cer-16 was associated with higher risk of ASCVD (HR: 2.33, 95% CI 1.14, 4.77, p-value: 0.03), while the association with Cer-20 was attenuated (HR: 1.29, 95% CI 0.74, 2.25, p-value: 0.22). We did not observe any significant associations of the investigated sphingolipids with incident heart failure.
Table 4.
Adjusted risk of incident atherosclerotic cardiovascular disease and heart failure in SHFS
| Atherosclerotic cardiovascular disease | Heart failure | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Multivariable model | Sphingolipid adjusted model | Multivariable model | Sphingolipid adjusted model | |||||||||
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| Cer-16 | 2.01 | (1.35, 2.99) | 0.0006 | 2.26 | (1.09, 4.66) | 0.0276 | 1.77 | (0.73, 4.28) | 0.21 | 2.57 | (0.76, 8.66) | 0.13 |
| Cer-20 | 1.7 | (1.32, 2.19) | 0.00004 | 1.42 | (0.81, 2.47) | 0.2180 | 1.24 | (0.64, 2.39) | 0.52 | 0.83 | (0.32, 2.18) | 0.71 |
| Cer-22 | 1.44 | (0.96, 2.17) | 0.08 | 0.88 | (0.44, 1.75) | 0.7133 | 1.22 | (0.59, 2.52) | 0.59 | 0.67 | (0.26, 1.72) | 0.41 |
| Cer-24 | 1.36 | (0.86, 2.14) | 0.19 | 0.72 | (0.35, 1.48) | 0.3705 | 1.07 | (0.45, 2.58) | 0.87 | 0.45 | (0.14, 1.44) | 0.18 |
| SM-16 | 2.07 | (0.92, 4.67) | 0.08 | 2.97 | (0.96, 9.18) | 0.0588 | 0.98 | (0.28, 3.50) | 0.98 | 0.69 | (0.13, 3.74) | 0.67 |
| SM-20 | 1.09 | (0.62, 1.91) | 0.76 | 0.61 | (0.27, 1.37) | 0.2343 | 1.14 | (0.46, 2.83) | 0.78 | 1.27 | (0.38, 4.22) | 0.69 |
| SM-22 | 1.08 | (0.64, 1.82) | 0.78 | 0.6 | (0.28, 1.31) | 0.2031 | 1.28 | (0.46, 3.57) | 0.63 | 1.59 | (0.41, 6.10) | 0.50 |
| SM-24 | 1.03 | (0.63, 1.70) | 0.91 | 0.61 | (0.30, 1.24) | 0.1709 | 1.28 | (0.49, 3.34) | 0.62 | 1.52 | (0.49, 4.77) | 0.47 |
Number of incident events: Atherosclerotic Heart Disease (n = 99); HF (n = 33). Models are adjusted for age, sex, study site, education, smoking, physical activity, BMI, waist circumference, LDL-cholesterol, systolic blood pressure, treated hypertension, duration of diabetes, type of diabetes medication used, and treated hyperlipidemia; for the sphingolipid adjusted models, Cer-16 and SM-16 models include additional adjustment for Cer-22 and SM-22, respectively; Cer-20, -22, -24, and SM-20, -22, and -24 models include additional adjustment for Cer-16 and SM-16, respectively
Discussion
Among 597 SHFS and 267 SH study participants with prevalent diabetes, we did not detect statistically significant associations of the investigated sphingolipids with our composite CVD outcome at our pre-specified significance threshold. However, in agreement with our stated hypothesis, we observed an association of Cer-16 with higher risk of CVD, and in analyses adjusted for SM-16, associations of SM-20, 22 and 24 with lower risk of CVD. Further, in secondary analyses, we report that Cer-16 and possibly Cer-20 are associated with increased risk of incident atherosclerotic CVD. Although previous studies have reported associations of specific ceramides with both CVD and diabetes independently, to our knowledge, this is the first prospective study to investigate associations of ceramide and sphingomyelin species with CVD among adults with type 2 diabetes.
An association of Cer-16 with higher risk of CVD has been previously reported. In particular, Cer-16 was associated with incident risk of major cardiovascular events in the FINRISK and EPIC-Potsdam studies [29, 30]. In addition, in a recent meta-analysis that pooled results of cohort studies of incident and recurrent CVD, Cer-16 was associated with CVD risk [15]. Our study attempts to extend these findings by exploring these associations in a different ethnicity and among high-risk adults with type 2 diabetes. Further, we previously reported that circulating ceramides are associated with higher risk of incident diabetes in the same population, as well as associate with early markers of diabetes [10, 18, 19]. The current study now suggests that ceramides, in particular Cer-16, not only are associated with higher risk of incident diabetes, but also higher risk of incident CVD after diabetes is diagnosed. In addition, the studies of circulating ceramides and CVD mentioned above did not examine sphingomyelins. A recent publication from the Da Qing Diabetes Study did not investigate sphingomyelins with a very long chain saturated fatty acid, but did report an association of SM-16 with higher CVD risk among people with diabetes, which is suggested (but not significant) in our findings [31]. Our study suggests for the first time that sphingomyelins with a very long chain saturated fatty acid are associated with lower risk of incident CVD among adults with type 2 diabetes.
As is common in longitudinal population-based studies, our CVD outcome was a composite of different cardiovascular events. Because 85% of the events of our study were either MI, CHD, or ischemic stroke, we examined the sphingolipid associations with the composite outcome of “atherosclerotic CVD” in secondary analyses. Circulating Cer-16 and Cer-20 were associated with higher risk of incident atherosclerotic CVD; however, these two ceramides are highly correlated (correlation = 0.76 in SHFS, 0.69 in SHS) and the association of Cer-20 was greatly diminished by adjustment for Cer-16, suggesting that Cer-16 is the primary species associated with risk. There is less support in the literature for an association of Cer-16 with atherosclerotic outcomes in cohort studies. An association of the ratio Cer-24/Cer-16 with lower risk of incident CHD has been reported in the Framingham Heart Study and the Study of Health in Pomerania; however, the CHD association was entirely due to Cer-24, and Cer-16 itself was not associated with CHD risk in both cohorts, with point estimates close to the null [8, 18, 24]. Further studies will be needed to replicate the association of Cer-16 with atherosclerotic outcomes.
We did not observe an association of ceramides and sphingomyelins with heart failure. This is in contrast with findings in the Cardiovascular Health Study where Cer-16 and SM-16 are associated with increased risk of HF while longer-chain Cer and SM are associated with decreased risk [16]. Given the small number of heart failure events and limited power to find HF associations in this current study, the apparent lack of association should be interpreted with caution.
Ceramides and sphingomyelins may play a direct role in cardiovascular disease through an effect on atherosclerosis and inflammation, which would be especially influential among people with diabetes [32–34]. Conversion of sphingomyelin in LDL-cholesterol particles to ceramide by sphingomyelinase promotes ceramide-ceramide aggregation, a possible early event in atherosclerosis, and lowered plasma sphingomyelin levels in mice resulted in reduced atherosclerosis [35–37]. In addition to their possible role in atherosclerosis, ceramides are implicated in reperfusion injury, lipotoxicity and apoptosis, biological activities that may influence the risks of heart failure and arrhythmia [32, 34, 38].
Strengths of this study include participants with diabetes from multi-site prospective studies of AIs—a population that itself suffers a relatively high prevalence of diabetes complications; detailed and thorough assessment of participant characteristics and potential confounders; and objective laboratory measurements of ceramide and sphingomyelin levels. This study also has several limitations. We used a composite measure of CVD as the primary outcome in this analysis, and the limited number of events of MI and stroke did not allow separate analyses of these outcomes. In SHFS we were able to categorize events as either ASCVD or HF, but due to a limited number of events in SH and the case–control sampling we were not able to make the same classification in this cohort and hence could not meta-analyze the CVD subtypes. Further, in the SH study, cases and controls were not matched on site. As a result, we were not able to adjust the SH analysis for site; since diet and lifestyle patterns differ between the three SH sites, SH estimates may be biased due to unmeasured confounding.
In conclusion, our findings in this population-based longitudinal study of AIs with type 2 diabetes suggest that several ceramide and sphingomyelin species may be associated with incident CVD, particularly with ASCVD. Although these associations did not meet our pre-specified threshold for statistical significance, our findings for Cer-16 add credence to recent findings in other cohorts, and our findings for SM with a very long chain saturated fatty acid encourage further research in other populations.
Supplementary Information
Additional file 1: Figure S1. Correlation of Sphingolipid Species in SHFS and SHS.
Additional file 2: Table S1. Risk of incident CVD per two-fold higher sphingolipid level in SHFS. Table S2. Odds of incident CVD per two-fold higher sphingolipid level in SHS. Table S3. Sensitivity analysis—associations of sphingolipids with incident CVD risk after adjustment for HDL and Triglycerides, Fibrinogen, and Chronic Kidney Disease.
Acknowledgements
We thank the study participants and the Strong Heart Study staff.
Abbreviations
- CVD
Cardiovascular disease
- Cer
Ceramide
- SM
Sphingomyelin
- AI
American Indian
- SHS
Strong Heart Study
- SHFS
Strong Heart Family Study
- MI
Myocardial infarction
- ASCVD
Atherosclerotic cardiovascular disease
- HF
Heart failure
- CHD
Coronary heart disease
- BMI
Body mass index
- LDL
Low density lipoprotein-cholesterol
- HDL
High density lipoprotein-cholesterol
- eGFR
Estimated glomerular filtration rate
- CKD
Chronic kidney disease
- HR
Hazard ratio
- CI
Confidence interval
Author contributions
PNJ contributed to the conception and design of the study, conducted the statistical analysis, and drafted the manuscript. AMF, CMS, and BMK contributed to the data analysis and critically reviewed the manuscript. ANH performed biospecimen measurements and critically reviewed the manuscript. BVH and JGU contributed to the acquisition of data and critically reviewed the manuscript. DSS, IBK, and NS contributed to the interpretation of results and critically reviewed the manuscript. RNL contributed to the conception and design of the study, the acquisition of data, and critically reviewed the manuscript. All authors read and approved the final manuscript.
Funding
Research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases under Award Number R01-DK103657 and P30 DK035816 and by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number R01HL109284. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Availability of data and materials
Due to privacy agreements with the tribal communities involved in this study, access to study data is restricted. Further information can be found at https://strongheartstudy.org/.
Declarations
Ethics approval and consent to participate
The institutional review board from each Indian Health Service region and each of the included communities approved the study, and written informed consent was obtained from all participants at each exam.
Consent for publication
Not applicable.
Competing interests
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Center for Disease Control and Prevention. Diabetes fact sheet: national estimates and general information on diabetes and pre-diabetes in the United States. Atlanta: US Department of Health and Human Services, Center for Disease Control and Prevention; 2011.
- 2.Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Emerging Risk Factors Collaboration et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 4.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Group AC. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 6.Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, Lafont S, Bergeonneau C, Kassai B, et al. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. BMJ. 2011;343:d4169. doi: 10.1136/bmj.d4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Field BC, Gordillo R, Scherer PE. The role of ceramides in diabetes and cardiovascular disease regulation of ceramides by adipokines. Front Endocrinol (Lausanne) 2020;11:569250. doi: 10.3389/fendo.2020.569250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Summers SA, Nelson DH. A role for sphingolipids in producing the common features of type 2 diabetes, metabolic syndrome X, and Cushing’s syndrome. Diabetes. 2005;54(3):591–602. doi: 10.2337/diabetes.54.3.591. [DOI] [PubMed] [Google Scholar]
- 9.Fretts AM, Jensen PN, Hoofnagle AN, McKnight B, Howard BV, Umans J, et al. Plasma ceramides containing saturated fatty acids are associated with risk of type 2 diabetes. J Lipid Res. 2021;62:100119. doi: 10.1016/j.jlr.2021.100119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fretts AM, Jensen PN, Hoofnagle A, McKnight B, Howard BV, Umans J, et al. Plasma ceramide species are associated with diabetes risk in participants of the strong heart study. J Nutr. 2020;150(5):1214–1222. doi: 10.1093/jn/nxz259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grosch S, Schiffmann S, Geisslinger G. Chain length-specific properties of ceramides. Prog Lipid Res. 2012;51(1):50–62. doi: 10.1016/j.plipres.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Deng X, Yin X, Allan R, Lu DD, Maurer CW, Haimovitz-Friedman A, et al. Ceramide biogenesis is required for radiation-induced apoptosis in the germ line of C. elegans. Science. 2008;322(5898):110–115. doi: 10.1126/science.1158111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crowder CM. Cell biology. Ceramides—friend or foe in hypoxia? Science. 2009;324(5925):343–344. doi: 10.1126/science.1173278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SY, Kim JR, Hu Y, Khan R, Kim SJ, Bharadwaj KG, et al. Cardiomyocyte specific deficiency of serine palmitoyltransferase subunit 2 reduces ceramide but leads to cardiac dysfunction. J Biol Chem. 2012;287(22):18429–18439. doi: 10.1074/jbc.M111.296947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mantovani A, Dugo C. Ceramides and risk of major adverse cardiovascular events: a meta-analysis of longitudinal studies. J Clin Lipidol. 2020;14(2):176–185. doi: 10.1016/j.jacl.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Lemaitre RN, Jensen PN, Hoofnagle A, McKnight B, Fretts AM, King IB, et al. Plasma ceramides and sphingomyelins in relation to heart failure risk. Circ Heart Fail. 2019;12(7):e005708. doi: 10.1161/CIRCHEARTFAILURE.118.005708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen PN, Fretts AM, Hoofnagle AN, Sitlani CM, McKnight B, King IB, et al. Plasma ceramides and sphingomyelins in relation to atrial fibrillation risk: the cardiovascular health study. J Am Heart Assoc. 2020;9(4):e012853. doi: 10.1161/JAHA.119.012853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemaitre RN, Yu C, Hoofnagle A, Hari N, Jensen PN, Fretts AM, et al. Circulating sphingolipids, insulin, HOMA-IR, and HOMA-B: the strong heart family study. Diabetes. 2018;67(8):1663–1672. doi: 10.2337/db17-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen PN, Fretts AM, Yu C, Hoofnagle AN, Umans JG, Howard BV, et al. Circulating sphingolipids, fasting glucose, and impaired fasting glucose: the strong heart family study. EBioMedicine. 2019;41:44–49. doi: 10.1016/j.ebiom.2018.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee ET, Welty TK, Fabsitz R, Cowan LD, Le NA, Oopik AJ, et al. The strong heart study. A study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol. 1990;132(6):1141–1155. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- 21.North KE, Howard BV, Welty TK, Best LG, Lee ET, Yeh JL, et al. Genetic and environmental contributions to cardiovascular disease risk in American Indians: the strong heart family study. Am J Epidemiol. 2003;157(4):303–314. doi: 10.1093/aje/kwf208. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization . Definition, diagnosis, and classification of diabetes mellitus and its complications. Geneva: World Health Organization; 1999. pp. 1–59. [Google Scholar]
- 23.Lee E, Welty T, Fabsitz R, Cowan L, Ngoc A, Oopik A, et al. The strong heart study: a study of cardiovascular disease in american indians: design and methods. Am J Epidemiol. 1990;132:1141–1155. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- 24.Lemaitre RN, Yu C, Hoofnagle A, Hari N, Jensen P, Fretts AM, et al. Circulating sphingolipids, insulin, HOMA-IR and HOMA-B: the strong heart family study. Diabetes. 2018;67(8):1663–1672. doi: 10.2337/db17-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin DY, Zeng D. Meta-analysis of genome-wide association studies: no efficiency gain in using individual participant data. Genet Epidemiol. 2010;34(1):60–66. doi: 10.1002/gepi.20435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8(1):3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- 28.Van Buuren S, Groothuis-Oudshoorn K. MICE: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. doi: 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- 29.Havulinna AS, Sysi-Aho M, Hilvo M, Kauhanen D, Hurme R, Ekroos K, et al. Circulating ceramides predict cardiovascular outcomes in the population-based FINRISK 2002 cohort. Arterioscler Thromb Vasc Biol. 2016;36(12):2424–2430. doi: 10.1161/ATVBAHA.116.307497. [DOI] [PubMed] [Google Scholar]
- 30.Wittenbecher C, Cuadrat R, Johnston L, Eichelmann F, Jager S, Kuxhaus O, et al. Dihydroceramide- and ceramide-profiling provides insights into human cardiometabolic disease etiology. Nat Commun. 2022;13(1):936. doi: 10.1038/s41467-022-28496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Jia H, Qian X, Wang J, Yu M, Gong Q, et al. Circulating palmitoyl sphingomyelin is associated with cardiovascular disease in individuals with type 2 diabetes: findings from the China Da Qing diabetes study. Diabetes Care. 2022;45(3):666–673. doi: 10.2337/dc21-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang XC, Goldberg IJ, Park TS. Sphingolipids and cardiovascular diseases: lipoprotein metabolism, atherosclerosis and cardiomyopathy. Adv Exp Med Biol. 2011;721:19–39. doi: 10.1007/978-1-4614-0650-1_2. [DOI] [PubMed] [Google Scholar]
- 33.Hornemann T, Worgall TS. Sphingolipids and atherosclerosis. Atherosclerosis. 2013;226(1):16–28. doi: 10.1016/j.atherosclerosis.2012.08.041. [DOI] [PubMed] [Google Scholar]
- 34.Baranowski M, Gorski J. Heart sphingolipids in health and disease. Adv Exp Med Biol. 2011;721:41–56. doi: 10.1007/978-1-4614-0650-1_3. [DOI] [PubMed] [Google Scholar]
- 35.Devlin CM, Leventhal AR, Kuriakose G, Schuchman EH, Williams KJ, Tabas I. Acid sphingomyelinase promotes lipoprotein retention within early atheromata and accelerates lesion progression. Arterioscler Thromb Vasc Biol. 2008;28(10):1723–1730. doi: 10.1161/ATVBAHA.108.173344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marathe S, Choi Y, Leventhal AR, Tabas I. Sphingomyelinase converts lipoproteins from apolipoprotein E knockout mice into potent inducers of macrophage foam cell formation. Arterioscler Thromb Vasc Biol. 2000;20(12):2607–2613. doi: 10.1161/01.ATV.20.12.2607. [DOI] [PubMed] [Google Scholar]
- 37.Hojjati MR, Li Z, Zhou H, Tang S, Huan C, Ooi E, et al. Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. J Biol Chem. 2005;280(11):10284–10289. doi: 10.1074/jbc.M412348200. [DOI] [PubMed] [Google Scholar]
- 38.Kolter T. A view on sphingolipids and disease. Chem Phys Lipids. 2011;164(6):590–606. doi: 10.1016/j.chemphyslip.2011.04.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Correlation of Sphingolipid Species in SHFS and SHS.
Additional file 2: Table S1. Risk of incident CVD per two-fold higher sphingolipid level in SHFS. Table S2. Odds of incident CVD per two-fold higher sphingolipid level in SHS. Table S3. Sensitivity analysis—associations of sphingolipids with incident CVD risk after adjustment for HDL and Triglycerides, Fibrinogen, and Chronic Kidney Disease.
Data Availability Statement
Due to privacy agreements with the tribal communities involved in this study, access to study data is restricted. Further information can be found at https://strongheartstudy.org/.