Abstract
Background
Observational studies have revealed that type 2 diabetes (T2D) is associated with an increased risk of peripheral artery disease (PAD). However, whether the two diseases share a genetic basis and whether the relationship is causal remain unclear. It is also unclear as to whether these relationships differ between ethnic groups.
Methods
By leveraging large-scale genome-wide association study (GWAS) summary statistics of T2D (European-based: Ncase = 21,926, Ncontrol = 342,747; East Asian-based: Ncase = 36,614, Ncontrol = 155,150) and PAD (European-based: Ncase = 5673, Ncontrol = 359,551; East Asian-based: Ncase = 3593, Ncontrol = 208,860), we explored the genetic correlation and putative causal relationship between T2D and PAD in both Europeans and East Asians using linkage disequilibrium score regression and seven Mendelian randomization (MR) models. We also performed multi-trait analysis of GWAS and two gene-based analyses to reveal candidate variants and risk genes involved in the shared genetic basis between T2D and PAD.
Results
We observed a strong genetic correlation (rg) between T2D and PAD in both Europeans (rg = 0.51; p-value = 9.34 × 10−15) and East Asians (rg = 0.46; p-value = 1.67 × 10−12). The MR analyses provided consistent evidence for a causal effect of T2D on PAD in both ethnicities (odds ratio [OR] = 1.05 to 1.28 for Europeans and 1.15 to 1.27 for East Asians) but not PAD on T2D. This putative causal effect was not influenced by total cholesterol, body mass index, systolic blood pressure, or smoking initiation according to multivariable MR analysis, and the genetic overlap between T2D and PAD was further explored employing an independent European sample through polygenic risk score regression. Multi-trait analysis of GWAS revealed two novel European-specific single nucleotide polymorphisms (rs927742 and rs1734409) associated with the shared genetic basis of T2D and PAD. Gene-based analyses consistently identified one gene ANKFY1 and gene-gene interactions (e.g., STARD10 [European-specific] to AP3S2 [East Asian-specific]; KCNJ11 [European-specific] to KCNQ1 [East Asian-specific]) associated with the trans-ethnic genetic overlap between T2D and PAD, reflecting a common genetic basis for the co-occurrence of T2D and PAD in both Europeans and East Asians.
Conclusions
Our study provides the first evidence for a genetically causal effect of T2D on PAD in both Europeans and East Asians. Several candidate variants and risk genes were identified as being associated with this genetic overlap. Our findings emphasize the importance of monitoring PAD status in T2D patients and suggest new genetic biomarkers for screening PAD risk among patients with T2D.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-022-02476-0.
Keywords: Type 2 diabetes, Peripheral artery disease, Shared genetics, Causality
Introduction
Type 2 diabetes (T2D) is a severe metabolic disease caused primarily by the inadequate production or secretion of insulin [1]. Previous studies have revealed that T2D patients have a significantly increased risk of suffering from peripheral artery disease (PAD) [2, 3], a chronic circulatory condition characterized by the constriction of arteries that serves to slow the blood flow in the limbs [4, 5]. Evidence has accumulated for a higher mortality rate among patients with co-morbid T2D and PAD than among patients with PAD alone [6], illustrating the importance of understanding the pathophysiological link between T2D and PAD.
The observed association between T2D and PAD can be explained in part by a shared genetic basis [7–9]. For example, Strawbridge and van Zuydam identified CDKN2A/B as candidate genes likely to be involved in regulating both T2D and PAD [8]. A recent study also replicated CDKN2A as being associated with PAD in individuals with diabetes [10]. Vujkovic et al. analyzed ~ 1.4 million individuals of multiple ethnicities (including Europeans, African Americans, Hispanics, South Asians, and East Asians), thereby disclosing significant associations between 318 genetic variants and the risk of both T2D and PAD [9]. Nevertheless, our knowledge of the nature and extent of the genetic associations between T2D and PAD is still far from clear. Furthermore, whether the associations between T2D and PAD differ among ethnic groups remains uncertain, and whether the genetic associations between the two diseases reflect a causal relationship or pleiotropy is unknown.
The growth of genome-wide association studies (GWAS) over the past two decades has stimulated the development of approaches to analyze cross-trait shared genetic architecture based on GWAS summary statistics. For example, Bulik-Sullivan et al. proposed the GWAS-based linkage disequilibrium score regression (LDSC) statistic as a means to estimate the single nucleotide polymorphism (SNP)-based genetic correlation between traits [11]. Mendelian randomization (MR) has been proposed as a way to measure the putative causal relationship between traits. MR uses genetic variants as instruments to mimic a random allocation procedure in randomized controlled trials [12], thereby avoiding issues of confounding and reverse causation [13]. With the advent of large-scale biobanks involving multiple ethnicities (e.g., UK Biobank [UKB], BioBank Japan [BBJ]), it became possible to compare the cross-trait shared genetic architecture between different ethnicities in order to explore the possibility of trans-ethnic genetic heterogeneity. To date, the applications of these approaches and datasets have achieved considerable success in increasing our understanding of the shared genetic architecture between complex diseases [14–16], thereby superseding traditional experimental models which are much more time-consuming and costlier to implement. Thus, using GWAS-based approaches to analyze GWAS summary data for T2D and PAD derived from different ethnic groups provides an opportunity for us to study the shared genetic basis between the two diseases.
In this study, we applied LDSC and seven MR or MR-equivalent approaches to estimate the genetic correlation and potential causal relationship between T2D and PAD in Europeans and East Asians. Further multivariable Mendelian randomization analysis (MVMR) was used to examine whether the putative causal relationship between T2D and PAD can be affected by traits associated with the increased risk of PAD or T2D. A validation of genetic overlap between T2D and PAD was then performed using independent samples to explore whether the polygenic risk scores (PRS) for T2D could predict the status of PAD, and vice versa. Finally, we performed a multi-trait analysis of GWAS (MTAG) and two gene-based analyses (i.e., multi-marker analysis of genomic annotation [MAGMA] and summary data-based Mendelian randomization [SMR]) to identify the risk SNPs and functional genes that are likely to be responsible for the shared genetic etiology underlying T2D and PAD. We applied the analytical pipeline to Europeans, and also to East Asians independently as a replicated analysis, to investigate the common and distinct mechanisms of co-occurring T2D-PAD between Europeans and East Asians.
Methods
Data sources for Europeans
Analyses were performed by utilizing more than 400,000 individuals of European ancestry from the UKB cohort. Individuals of European ancestry were selected using a two-stage approach: first, we calculated the top two principal components (PCs) for each individual from HapMap 3 (HM3) SNPs of 1000 Genomes Project [17]; second, we assigned individuals to European if their PC information was optimally matched to the 1000 European Genomes reference (compared to other population reference, e.g., African, East Asian, South Asian and Admixed) [18]. Individuals were classified as T2D or PAD patients if they were coded with the International Classification of Diseases 10th (ICD-10) based on non-insulin-dependent (type 2) diabetes mellitus (UKB Field ID: 41270 [E11]; Additional file 1: Table S1) or ICD-10 based diseases of arteries, arterioles, and capillaries (UKB Field ID: 41270 [I70-I79]; Additional file 1: Table S1), respectively [19]. They were classified as controls in relation to T2D or PAD if they had not been ascribed the corresponding T2D or PAD code, respectively. In total, we obtained a T2D sample of 364,673 individuals (Ncase = 21,926; Ncontrol = 342,747) and a PAD sample of 365,224 individuals (Ncase = 5673; Ncontrol = 359,551).
Next, we randomly divided these individuals (including cases and controls) into two subsets, comprising 80% and 20% of the sample, respectively. The former (i.e., 80% of the sample) was used as the GWAS discovery sample whereas the latter (i.e., 20% of the sample) was used in the subsequent PRS analysis (see below). GWAS was carried out using BOLT-LMM [20], which is a Bayesian-based linear mixed model (LMM) that allows for the relatedness of individuals by adjusting the population structure and cryptic relatedness. BOLT-LMM was corrected for age, sex, genotype batch, and assessment center and was fitted by random effects of the restricted SNPs (with LD r2 < 0.9). The SNPs used in BOLT-LMM were calibrated by the LD scores calculated from the 1000 Genomes Project Europeans reference [21]. Genotyped SNPs were imputed according to the Haplotype Reference Consortium reference panel [22]. We further excluded SNPs with minor allele frequency (MAF) < 0.01, imputation INFO score < 0.3, p-value of Hardy-Weinberg test < 1 × 10−6, minor allele count < 5, or the call rate < 0.05, yielding a final set of 8.54M SNPs. SNP effects (i.e., βBOLT − LMM) and their associated standard errors (i.e., SEBOLT − LMM) from BOLT-LMM were converted to a quantitative scale using the approximate approach , where μ is the proportion of cases. The remaining individuals (i.e., 20% of cases and 20% of controls) were employed for the PRS analysis (NT2D case = 3655, NT2D control = 59,801; NPAD case = 863, NPAD control = 62,593), after excluding the individuals who were genetically related (with a cryptic relatedness r2 > 0.05) to the individuals of the discovery sample for GWAS.
Data sources for East Asians
The GWAS summary statistics of T2D [23] and PAD [24] for East Asians were accessed via the BBJ cohort [25]. The GWAS of T2D was a fixed-effect inverse variance-weighted meta-analysis (via METAL [26]) of four Japanese ancestry-based cohorts comprising 36,614 cases and 155,150 controls. The GWAS of PAD included 3593 cases and 208,860 controls and was generated using a LMM via SAIGE [27], adjusted by age, sex, and the top five genetic principal components. Additionally, unrelated East Asian individuals (NT2D case = 241, NT2D control = 2,483; NPAD case = 26, NPAD control = 2698) were extracted from UKB (independent from BBJ) for the downstream PRS analysis.
eQTL summary data
Expression quantitative trait loci (eQTL) are defined as loci which are associated with genetic variants that alter gene expression levels [28]. In our study, we obtained the publicly available blood-based cis-eQTL summary data from the eQTLGen Consortium for SMR analysis (see below) [29]. The cis-eQTL summary data were generated from 31,684 individuals of European ancestry using a weighted Z-score [30] based on a meta-analysis of 37 cohorts comprising 19,250 probes [31].
Linkage disequilibrium score regression
We performed single-trait and cross-trait LDSC to estimate the liability-scale heritability (h2) of T2D and PAD as well as their genetic correlation (rg) [32, 33], respectively, according to the population and sample prevalence values of T2D (or PAD) to be 10.00% (or 5.30%) and 6.01% (or 1.55%) in Europeans [34, 35]. The corresponding population and sample prevalence values of T2D (or PAD) were 7.50% (or 4.30%) and 19.09% (or 1.69%) in East Asians (as a test of replicability of the analysis in Europeans) [23, 36]. SNPs were excluded from LDSC if they resided within the major histocompatibility complex (MHC) region (chromosome 6: 28,477,797–33,448,354), were strand-ambiguous (i.e., the A/T and G/C SNPs), or had an MAF less than 0.01. The default 1000 Genomes Project European- and East Asian-based LD score reference panels were employed throughout the analyses. LDSC were performed with and without intercept constraint to explore the impact of potential inflation of the GWAS summary statistics due to the presence of population stratification. The significant genetic correlation was assumed on the basis of a p-value < 0.05.
Mendelian randomization analysis
We applied multiple MR models to investigate the putative causal relationship between T2D and PAD. MR evaluates the causal effect of a risk factor (i.e., exposure) on a target trait (i.e., outcome) using genetic variants as instruments, assuming that the genetic variants concerned are significantly associated with the exposure and have causal effects on the outcome only through the exposure. This latter assumption may be violated due to the presence of horizontal pleiotropy, which occurs if the genetic variants affect the outcome through non-causal pathways [32]. Horizontal pleiotropy can itself be subclassified into correlated pleiotropy which is defined as the genetic variants acting on exposure and outcome via shared factors and uncorrelated pleiotropy that occurs if the genetic variants act on exposure and outcome via other independent pathways. To distinguish true causality from horizontal pleiotropy, we applied multiple MR and MR-equivalent models employing different assumptions on horizontal pleiotropy, namely inverse variance-weighted (IVW), MR-Egger, weighted mode, weighed median, generalized summary data-based Mendelian randomization (GSMR), the causal analysis using summary effect estimates (CAUSE), and MR-equivalent latent causal variable (LCV) analysis. IVW estimates the Wald ratio for each SNP and calculates the causal estimate using a weighted linear regression which does not correct for horizontal pleiotropy [37]. MR-Egger adds an extra intercept to IVW to weigh the possible deviations attributable to uncorrelated pleiotropy [38]. Weighted mode greatly loosens the assumptions made on correlated and uncorrelated pleiotropy and measures the causal effect only from the most frequent SNP set with consistent effect [39]. The weighted median calculates the causal effect using the weighted median of the SNP ratio under the assumption that most instrumental variants are valid (i.e., more than half the instrumental variants are valid instrumental SNPs) [40]. GSMR is an extension of IVW which applies the heterogeneity in dependent instruments (HEIDI) test to exclude instrumental SNPs with potentially uncorrelated pleiotropic effects [29, 41]. Bayesian-based CAUSE corrects both correlated and uncorrelated pleiotropy via a multivariate linear model adjusted by a joint distribution of instrumental SNPs, assuming that true causality can be attributed to all instrumental SNPs whereas correlated and uncorrelated pleiotropy only affects partial instrumental SNPs [42]. CAUSE further examines the model fitness using the expected log pointwise posterior density (ELPD) by comparing the causal model (i.e., exposure affects outcome via both causal effect and pleiotropic effects), the shared model (i.e., exposure affects outcome only via pleiotropic effects), and the null model (i.e., no causal effect or pleiotropic effects between exposure and outcome). Finally, LCV supposes that the rg between the two disease entities is regulated by a latent variable with causal effects on both exposure and outcome and evaluates this latent causal variable using the genetic causality proportion (GCP) [43].
MR analyses were carried out using R packages cause (version 1.0.0), LCV, gsmr (version 1.0.9), and TwoSampleMR (version 0.5.5). LCV recruits all HapMap3 variants as instrumental SNPs [44]; CAUSE selects independent instrumental SNPs with an arbitrary p-value of exposure GWAS < 1 × 10−5 by LD pruning; other MR approaches determine independent instrumental SNPs (with GWAS p-value < 5 × 10−8 or < 1 × 10−5 when employing T2D or PAD as exposure) by LD clumping (LD r2 < 0.05 within 1000-kb windows) using PLINK (v1.90) [45]. Instrumental SNPs were further filtered out if they were located within the MHC region [33], had a MAF less than 0.01, or were nominally significantly associated with the outcome (as potential pleiotropic SNPs).
The putative causal relationship was determined if consistent results were identified by multiple MR models with Bonferroni-corrected p-value < 3.85 × 10−3 (= 0.05/13, six methods with bi-directional analyses and LCV model). Post hoc power calculations were performed using an online web tool (https://sb452.shinyapps.io/power/) [46]. The causal effects (i.e., β) were converted from logit scale to liability scale using the method proposed by Byrne et al. [47]: , where Kx and Ky are the population prevalence of exposure and outcome, and and are the values (heights) of the standard normal distribution at the two population prevalence, respectively. The liability-scale β was then transformed to odds ratios (OR), assuming the population prevalence for T2D and PAD to be 10.00% and 5.30% in Europeans [34, 35] and 7.50% and 4.30% in East Asians (as a replicated analysis) [23, 36], respectively.
Multivariable MR analysis
Multivariable MR (MVMR) is an extension of standard MR that re-estimates the causal effect of exposure on the outcome after adjusting the potential pleiotropic effects from the outcome-related risk factors (i.e., risk factors that may affect the outcome through exposure) [48]. Here, we performed MVMR by considering T2D (or PAD) as the initial exposure, together with additional exposures including total cholesterol (TC) [49, 50], body mass index (BMI) [51, 52], systolic blood pressure (SBP) [53, 54], and smoking initiation [55, 56], which had publicly available GWAS in both Europeans and East Asians. MVMR-based independent instrumental SNPs were selected if they were significantly (p-value < 5 × 10−8 or < 1 × 10−5 when employing T2D or PAD as exposure) associated with at least one exposure. We applied MVMR to ascertain the causality of T2D on PAD (or PAD on T2D) for Europeans and East Asians (for replication), adjusted for each outcome-related risk factor individually as well as for all four risk factors (TC, BMI, SBP, and smoking initiation) acting combinatorically. MVMR was performed using the R package TwoSampleMR (version 0.5.5).
Polygenic risk score analysis
Whenever a genetic correlation between T2D and PAD was identified, PRS analysis was performed to explore whether the aggregated T2D-related genetic effects could predict the status of PAD (and vice versa) based on the genetic profile, using independent European- or East Asian-based samples. SNP sets for PRS calculation were selected by employing 9 different p-value cutoffs (i.e., 5 × 10−8, 1 × 10−5, 1 × 10−3, 0.01, 0.05, 0.1, 0.3, 0.5, and 1), which were then filtered by LD clumping (LD r2 < 0.05 within 1000-kb windows) using PLINK (v1.90) [45]. These independent SNPs were used to calculate PRS through the PLINK “score” function. PRS analysis was performed via logistic regression for the binary T2D status on the scaled PRS for PAD, and the binary PAD status on the scaled PRS for T2D, respectively. The regression model was constructed based on independent samples to test whether PRS from combined effects of multiple T2D risk-associated SNPs can predict the status of PAD, and vice versa. All the regressions were adjusted by age, sex, genotype batch, and assessment center. The variance of the susceptibility to a target trait explained by PRS was quantified by Nagelkerke’s pseudo-coefficient of determination (R2) [57]. We converted the observed Nagelkerke’s pseudo-R2 (and their upper and lower bounds of 95% confidence intervals [CI]) to the liability scale, assuming the population and sample prevalence of T2D (or PAD) to be 10.00% (or 5.30%) and 5.76% (or 1.36%) in Europeans [34, 35] and T2D (or PAD) at 7.50% (or 4.30%) and 8.85% (or 0.95%) in East Asians [23, 36], respectively. The standard error of R2 was estimated using Olkin and Finn’s approximation (, where N is the sample size and K is the number of predictors used in the full model) [58]. The power of each PRS regression was calculated via the R package AVENGEME [59].
Multi-trait analysis of T2D and PAD
To detect risk SNPs shared between T2D and PAD, we performed an inverse variance-weighted cross-trait meta-analysis using MTAG [60], which has an advantage over other meta-analysis tools because MTAG can account for potential sample overlap between GWAS summary data via cross-trait LDSC results. We also calculated “maxFDR” (the approximate upper limit for the false discovery rate [FDR] of the MTAG results) to assess the validity of the assumption that more than 10% of SNPs were causal for each trait. The lower maxFDR value suggests that the MTAG results were unlikely to be false-positive. Novel risk SNPs were identified if they were (1) independent of each other (i.e., LD clumping r2 < 0.05 in a window of 1000 kb), (2) genome-wide significant (p-value < 5 × 10−8) associations with the cross-trait shared architecture of T2D and PAD but not single-trait T2D or PAD, and (3) independent from genome-wide significant SNPs of single-trait T2D or PAD (i.e., r2 < 0.05 in a window of 1000 kb).
Gene-based association analysis
Whenever a significant shared genetic architecture between T2D and PAD was determined, we then extended the association results from the SNP level to the gene level and applied powerful MAGMA to identify the genes associated with T2D and PAD [61], as well as the cross-trait shared architecture of T2D and PAD in Europeans and East Asians (for replication). A total of 19,259 protein-coding genes (based on NCBI 37.3 build; excluding the MHC genes) were analyzed. SNPs were mapped to a gene if they were located within 50 kb upstream or downstream of the gene length boundary. The 1000 Genomes Project European- and East Asian-based LD reference panels were utilized to correct LD structure. Candidate pleiotropic genes underlying T2D and PAD were identified as those exhibiting associations with the cross-trait shared architecture of T2D and PAD at the FDR 5% significance level.
Summary data-based Mendelian randomization analysis
When genes were indicated as being significantly associated with the cross-trait shared architecture of T2D and PAD by MAGMA analysis, we further evaluated whether the expression levels of these genes were significantly associated with the cross-trait shared architecture of T2D and PAD using SMR [29]. SMR recruits functional genes as exposure and a focal trait as an outcome, using the top SNPs of eQTLs (p-value < 5 × 10−8) as instrumental variables, which expects to yield sufficient power to identify significant gene-trait associations [62]. The blood-based cis-eQTL summary data were obtained from the eQTLGen Consortium [31]. The HEIDI test [29] was utilized to exclude significant SMR associations due to linkage (i.e., different causal SNPs in LD that influence gene expression and disease separately), rather than causality (i.e., instrumental SNP influences disease via gene expression) or pleiotropy (i.e., instrumental SNP influences both gene expression and disease via shared effects). Significant SMR associations were determined with a Bonferroni-corrected SMR p-value (= 0.05/number of tested genes) and a HEIDI p-value > 0.01 from a minimum of 10 SNPs. Novel functional genes were ascertained if they exhibited significant associations with the cross-trait shared architecture of T2D and PAD using both MAGMA and SMR.
Results
Shared genetics and putative causal relationship between T2D and PAD in Europeans
Genetic correlation between T2D and PAD
Using the single-trait LDSC (without constrained intercept, Table 1), we estimated the European-based liability-scale h2 for T2D and PAD to be 23.47% (p-value = 9.42 × 10−52) and 10.93% (p-value = 8.54 × 10−9), respectively. The h2 values estimated with constrained intercept (T2D: h2 = 28.08%, p-value = 2.29 × 10−85; PAD: h2 = 12.23%, p-value = 3.11 × 10−17) were compatible with those without constrained intercept, implying mild inflations in these GWAS summary data. Using the cross-trait LDSC with constrained intercept (Table 1), we estimated the rg between T2D and PAD to be 0.44 (p-value = 5.23 × 10−17) whereas when we used the cross-trait LDSC without constrained intercept, the rg between T2D and PAD was estimated to be 0.51 (p-value = 9.34 × 10−15). These results were held to indicate strong shared genetics between the two conditions in Europeans.
Table 1.
Genetic correlation between T2D and PAD in Europeans and East Asians
| Constrained intercept | Unconstrained intercept | |||||
|---|---|---|---|---|---|---|
| T2D | PAD | T2D | PAD | |||
| EUR | Single-trait LDSC | h2 ± SE | 28.08% ± 1.35% | 12.23% ± 1.40% | 23.47% ± 1.47% | 10.93% ± 1.86% |
| Ph2 | 2.29 × 10−85 | 3.11 × 10−17 | 9.42 × 10−52 | 8.54 × 10−9 | ||
| Cross-trait LDSC | rg ± SE | 0.44 ± 0.05 | 0.51 ± 0.07 | |||
| Prg | 5.23 × 10−17 | 9.34 × 10−15 | ||||
| EAS | Single-trait LDSC | h2 ± SE | 21.48% ± 1.17% | 13.58% ± 2.04% | 15.12% ± 1.37% | 18.83% ± 2.93% |
| Ph2 | 2.45 × 10−67 | 8.34 × 10−11 | 3.61 × 10−26 | 3.44 × 10−10 | ||
| Cross-trait LDSC | rg ± SE | 0.45 ± 0.07 | 0.46 ± 0.06 | |||
| Prg | 1.16 × 10−10 | 1.67 × 10−12 | ||||
PAD peripheral artery disease, T2D type 2 diabetes, h2 heritability, rg genetic correlation, SE standard error, Ph2p-value for estimated h2, Prgp-value for estimated rg, LDSC linkage disequilibrium score regression, EUR Europeans, EAS East Asians
Putative causal effect of T2D on PAD
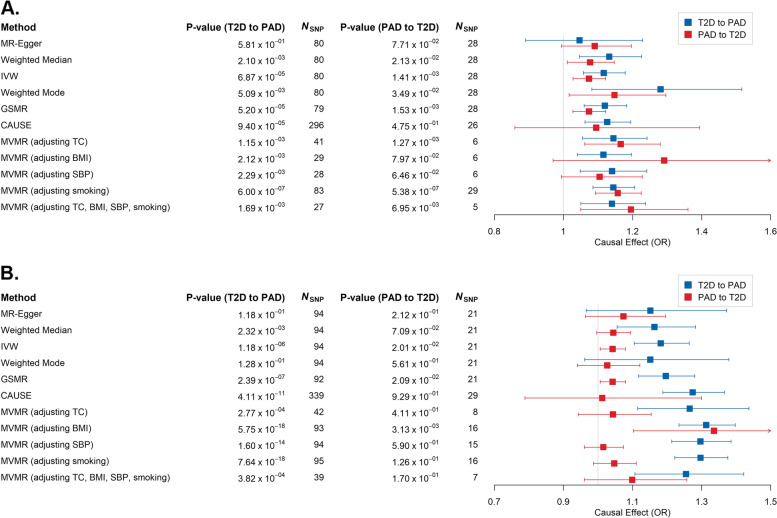
The application of multiple MR methods provided suggestive evidence for a causal effect of T2D on PAD (Fig. 1A, Additional file 1: Table S2 and S3) in Europeans. We observed that four out of seven MR models (with the exception of MR-Egger [p-value = 0.58], weighted mode [p-value = 5.09 × 10−3], and LCV [p-value = 0.28]) surpassed the Bonferroni-corrected level of significance (p-value < 3.85 × 10−3), with estimated liability-scale odds ratios (ORs) at ~ 1.14 on average, suggesting that T2D patients had approximately 1.14 times the risk (mean standard error = 5.83 × 10−2) of developing PAD compared to healthy individuals. The power of MR models was evaluated to be in the range of 78–100% (except for the MR-Egger model that failed to reach the 5% level of significance), corresponding to the causal effect estimated between 1.12 and 1.28 (Additional file 2: Fig. S1). These results suggested the sufficiency of study power of our GWAS summary statistics to identify causality between T2D and PAD, although the power of MR models for PAD on T2D is relatively limited compared to those for T2D on PAD. Horizontal pleiotropy would appear to have had a limited impact on this putative causal relationship, since MR-Egger suggested a close-to-zero intercept (intercept = 6.75 × 10−3, p-value = 0.41) while CAUSE indicated a better fit for the causal model compared to either the sharing model (ELPD p-value = 4.77 × 10−3) or the null model (ELPD p-value = 8.57 × 10−3). Furthermore, recruiting weaker instrumental SNPs (p-value < 1 × 10−5) for the CAUSE model is unlikely to have affected the accuracy of the MR estimates, as similar results (Additional file 1: Table S2 and S3) were obtained in a parallel CAUSE analysis utilizing a stricter p-value threshold of 5 × 10−8. Applications of MVMR analyses found that the putative causal effect of T2D on PAD was largely unchanged after adjusting for TC, BMI, SBP, and smoking individually, as well as adjusting for these risk factors as a whole (Fig. 1A and Additional file 1: Table S4). Furthermore, there was no consistent causal effect of PAD on T2D using MVMR after adjusting for the same risk factors (Fig. 1A and Additional file 1: Table S4).
Fig. 1.
The causal effect (in liability-scale odds ratio [OR]) of type 2 diabetes (T2D) on peripheral artery disease (PAD) as estimated by six Mendelian randomization (MR) models (MR-Egger, weighted median, IVW, weighted mode, GSMR, and CAUSE) in A Europeans and B East Asians. MVMR analyses were performed by adjusting TC [“MVMR (adjusting TC)”], BMI [“MVMR (adjusting BMI)”], SBP [“MVMR (adjusting SBP)”], and smoking [“MVMR (adjusting smoking)”] and by adjusting all these factors jointly [“MVMR (adjusting TC, BMI, SBP, smoking)”] in Europeans and East Asians. Error bars represent the 95% confidence intervals of the estimates. IVW, inverse variance weighted; GSMR, generalized summary data-based Mendelian randomization; CAUSE, causal analysis using summary effect estimates; MVMR, multivariable MR; TC, total cholesterol; BMI, body mass index; SBP, systolic blood pressure
Higher PRS for T2D in PAD patients than in non-PAD controls
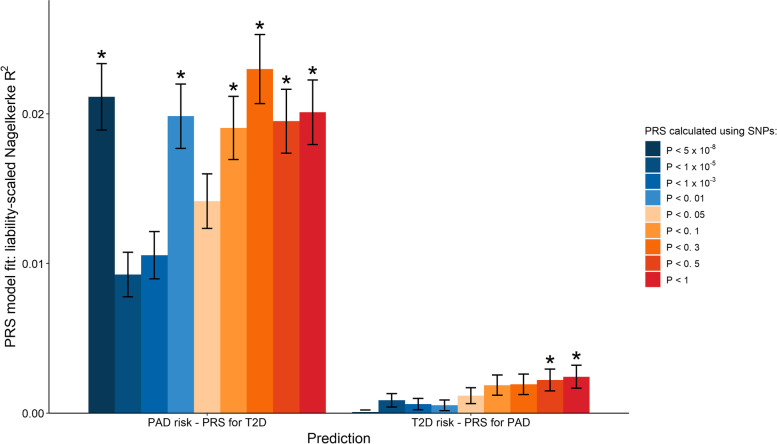
Irrespective of the p-value cutoffs in the PRS calculations, we observed consistently higher PRS for T2D in patients with PAD compared to those without PAD (Additional file 1: Table S5). The increased p-value cutoffs appeared to drive up the values of the liability-scaled Nagelkerke’s pseudo-R2 (except for the results below p-value < 5 × 10−8), suggesting that PRS for T2D calculated using additional SNPs could explain more of the variance of PAD, consistent with the expectation that PRS regression (Additional file 2: Fig. S2) had better predictive power when additional SNPs were included and when the sample size of GWAS summary statistics was increased. This notwithstanding, the reverse (PRS for PAD in the prediction of T2D status) was non-significant (Additional file 1: Table S6). Liability-scaled Nagelkerke’s pseudo-R2 of PRS for PAD on T2D status were also significantly lower (p-valuepaired t-test = 6.15 × 10−6) than those estimations of PRS for T2D on PAD status (Fig. 2). These results provided further evidence in support of the genetic overlap between T2D on PAD in Europeans.
Fig. 2.
European-based liability-scaled Nagelkerke’s R2 presents the goodness of fit of the logistic regression model to peripheral artery disease (PAD) status with polygenic risk score (PRS) for type 2 diabetes (T2D) (“PAD status – PRS for T2D”) and the goodness of fit of the logistic regression model to T2D status with PRS for PAD (“T2D status – PRS for PAD”). Error bars represent the 95% confidence intervals of the estimated Nagelkerke’s R2. Asterisk represents a significant coefficient (p-value < 2.78 × 10−3 = 0.05/18, Bonferroni correction) of the PRS terms in regression
Novel SNPs and candidate genes associated with the shared genetics of T2D and PAD
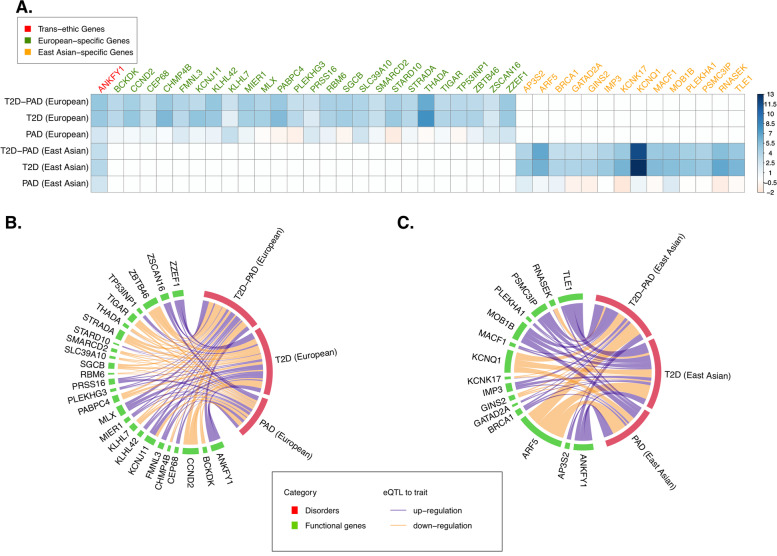
Performing a meta-analysis of the T2D and PAD GWAS data using MTAG revealed two novel SNPs, rs927742 and rs1734409 (Additional file 1: Table S7) that were significantly (p-value < 5 × 10−8) associated with the cross-trait shared architecture of T2D and PAD (MTAG MaxFDR = 4.54 × 10−7) and independent from the top SNPs of single-trait T2D or PAD. We also applied gene-based MAGMA analysis to identify a total of 898 FDR significant genes associated with the cross-trait shared architecture of T2D and PAD (Additional file 1: Table S8 and Fig. 3A). Among them, 136 genes were only associated with the cross-trait shared architecture of T2D and PAD (i.e., novel pleiotropic genes); the rest of the genes were additionally associated with T2D (N = 761; i.e., T2D-specific genes) or both T2D and PAD (N = 1; i.e., pleiotropic gene). Furthermore, SMR and HEIDI analysis implied 26 genes (out of 898 MAGMA genes; three as novel pleiotropic genes and 23 as T2D-specific genes) whose expression levels were significantly (p-value < 8.05 × 10−5 with Bonferroni correction) associated with the risk of the cross-trait shared architecture of T2D and PAD due to either pleiotropy or causality (Additional file 1: Table S9 and Fig. 3B).
Fig. 3.
Genes significantly associated with type 2 diabetes (T2D), peripheral artery disease (PAD), or cross-trait shared architecture of T2D and PAD identified by MAGMA or SMR analysis in Europeans and East Asians. A The genes significantly associated with T2D, PAD, or cross-trait shared architecture of T2D and PAD identified by multi-marker analysis of genomic annotation (MAGMA) in Europeans and East Asians, and the genes associated with T2D, PAD, or cross-trait shared architecture of T2D and PAD identified by summary data-based Mendelian randomization (SMR) in B Europeans and C East Asians. The color bar in A represents the z-score in MAGMA analysis. The width of the line in B and C denotes the strength of the SMR association. In B and C, lines in purple and orange represent respectively up- and downregulated genes associated with the increasing risk of the disease in question
Replicability of the putative causal relationship between T2D and PAD in East Asians
In East Asians, the h2 values for T2D and PAD were estimated to be 15.12% (p-value = 3.61 × 10−26) and 18.83% (p-value = 3.44 × 10−10) without constrained intercept and 21.48% (p-value = 2.45 × 10−67) and 13.58% (p-value = 8.34 × 10−11) with constrained intercept (Table 1). The rg values between T2D and PAD were estimated to be 0.45 (p-value = 1.16 × 10–10) and 0.46 (p-value = 1.67 × 10−12) with and without constrained intercept, respectively. These results were largely similar to those derived from Europeans, indicating strong shared genetics between T2D and PAD in different ethnicities.
We also identified a putative causal effect of T2D on PAD in East Asians (Fig. 1B, Additional file 1: Table S10 and S11), with five of seven MR models surpassing the Bonferroni-corrected threshold (except for MR-Egger [p-value = 0.12] and weighted mode [p-value = 0.13]). The estimated ORs for the causality of T2D on PAD in East Asians ranged from 1.15 to 1.27, which were slightly higher than those noted in Europeans. The power of MR models in East Asians was estimated to be in excess of 99% (Additional file 2: Fig. S1) for T2D on PAD, slightly higher than those in Europeans, while the power of MR models in East Asians for PAD on T2D was still significantly lower than those for T2D on PAD. These results were deemed to be less likely to be affected by horizontal pleiotropy (MR-Egger intercept = 2.54 × 10−3, p-value = 0.76; CAUSE causal model vs. sharing model ELPD p-value = 2.63 × 10−4 and vs. null model ELPD p-value = 1.60 × 10−5) or the application of weaker instrumental SNPs (similar results from the parallel CAUSE model using instrumental SNPs with p-value < 5 × 10−8; Additional file 1: Table S10). Additionally, the causal effect of T2D on PAD was less likely to be influenced by TC, BMI, SBP, or smoking, according to the MVMR results (Fig. 1B and Additional file 1: Table S12).
We next attempted to investigate whether the aggregated T2D (or PAD)-related genetic effects could be used to predict the status of PAD (or T2D) using PRS regression (Additional file 1: Table S13 and S14), but failed to identify significant results, probably on account of the insufficient sample size limiting the statistical power of regression (Additional file 2: Fig. S2). Nevertheless, we still observed significantly higher (p-valuepaired t-test = 1.98 × 10−3) liability-scaled Nagelkerke’s pseudo-R2 values estimated from the regression of PRS for T2D on PAD status than those of PRS for PAD on T2D status, suggesting that PAD maybe genetically a consequence of T2D but not the other way around.
We performed MTAG analysis but did not identify any novel genetic loci significantly associated with the cross-trait shared architecture of T2D and PAD in East Asians (MTAG MaxFDR = 4.54 × 10−7). Whereas gene-based MAGMA analysis found 372 genes exhibiting significant associations (FDR < 0.05) with cross-trait shared architecture of T2D and PAD (all T2D-specific genes; see Additional file 1: Table S15 and Fig. 3A), 15 of which were further observed as significant associations by SMR and HEIDI analysis (Bonferroni-corrected SMR p-value < 1.92 × 10−4; Additional file 1: Table S16 and Fig. 3C).
Common and distinct mechanisms underlying the co-occurrence of T2D and PAD between Europeans and East Asians
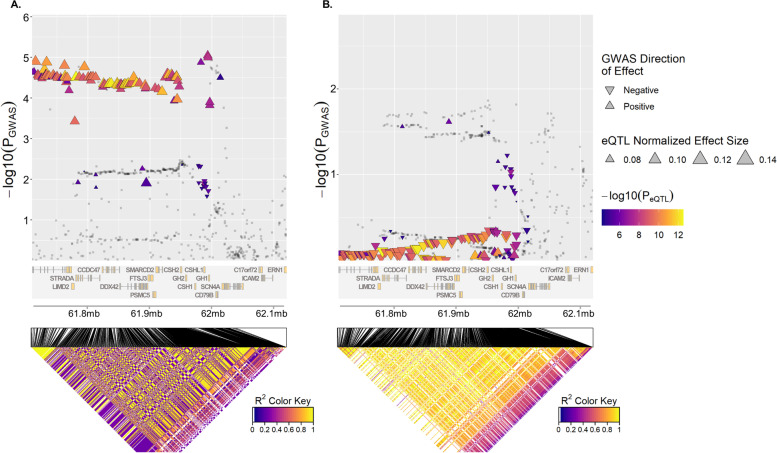
Gene-based analyses (i.e., MAGAMA and SMR) in Europeans and East Asians consistently identified the ANKFY1 gene as being significantly associated with the cross-trait shared architecture of T2D and PAD in both Europeans (p-valueMAGAMA = 3.28 × 10−7; βSMR = 0.44, p-valueSMR = 1.18 × 10−5) and East Asians (p-valueMAGAMA = 6.90 × 10−4; βSMR = 0.42, p-valueSMR = 1.83 × 10−4). MAGAMA and SMR additionally identified 25 and 14 genes which were significantly associated with cross-trait shared architecture of T2D and PAD in Europeans (FDRMAGAMA < 2.49 × 10−3; p-valueSMR < 8.05 × 10−5) and East Asians (FDRMAGAMA < 1.06 × 10−3; p-valueSMR < 1.94 × 10−4), respectively. These genes showed a significant (p-value = 1.53 × 10−2) enrichment in their protein-protein interactions (PPI) with each other according to STRING (Additional file 2: Fig. S3) [63], including seven interactions between genes significantly associated with the cross-trait shared architecture of T2D and PAD in Europeans and genes associated with the cross-trait shared architecture of T2D and PAD in East Asians (e.g., interactions of STARD10 [European-specific] with AP3S2 [East Asian-specific], and KCNJ11 [European-specific] with KCNQ1 [East Asian-specific]). These findings point to the existence of common trans-ethnic mechanisms underlying T2D and PAD. Nevertheless, we also noted distinct mechanisms underlying the co-occurrence of T2D and PAD between Europeans and East Asians, which might be attributed to the different LD structures in the two ethnicities (Fig. 4 taking gene SMARCD2 as an example).
Fig. 4.
The genomic region near to SMARCD2 gene was used as an example to illustrate the different genetic structures between Europeans (A) and East Asians (B). SMARCD2 was found to be significantly associated with the cross-trait shared architecture of type 2 diabetes (T2D) and peripheral artery disease (PAD) in Europeans but not in East Asians by MGAMA and SMR analysis. The dot and the triangle represent respectively p-values and effect sizes of SNPs in GWAS and eQTL. The directions of the triangles indicate the direction of SNPs in GWAS. Most of the SNPs plotted in A and B show opposite GWAS directions. The heatmap represents the linkage disequilibrium (LD) of the SNPs, which are obviously stronger in East Asians than in Europeans. GWAS, genome-wide association studies; eQTL, expression quantitative trait loci
Discussion
In this study, we systematically investigated the shared genetic architecture between T2D and PAD in Europeans and East Asians by leveraging their large-scale GWAS summary statistics. Our study disclosed a putative causal effect of T2D upon PAD in both Europeans and East Asians and identified several novel loci/functional genes that might be relevant to the shared genetics underlying T2D and PAD. We additionally used MVMR analysis to exclude an underlying heritable trait as the basis of the causal relationship between T2D and PAD and indicated that the causal effect of T2D on PAD is not influenced by TC, BMI, SBP, or smoking initiation. All these traits are generally considered to be factors that are associated with a high risk of PAD and T2D, and they are known to be related with dysfunction of lipoproteins or hemostasis [64, 65], etc. Indeed, recent studies have revealed lipoproteins and hemostatic factors to be risk factors for PAD [66–68]. Our study may indirectly indicate that neither the lipoproteins nor hemostatic factors have much influence on the causal relationship between T2D and PAD. Taken together, this study not only improves our understanding of the genetic etiology shared by T2D and PAD but also provides evidence to support the concept of genetically screening T2D patients in order to pre-symptomatically detect/prevent PAD.
To our knowledge, this is the first study to investigate the shared genetic architecture between T2D and PAD, although a significant clinical/phenotypic correlation has been previously reported [69, 70]. One of the main aims of this study was to investigate the shared genetic relationship between T2D and PAD in Europeans and East Asians. On the basis of an online LDSC power calculation (https://nealelab.github.io/UKBB_ldsc/viz_sampsize.html), we believe our input GWAS summary statistics have sufficient power (> 95%) to uncover shared genetic associations between T2D and PAD in either Europeans or East Asians. Here, we observed a genetic correlation between the two disease entities in both Europeans and East Asians. The estimated h2 of T2D and PAD, as well as the rg between T2D and PAD in Europeans, were largely similar to those in East Asians, suggesting a strong genetic correlation between T2D and PAD in both ethnicities.
In both Europeans and East Asians, we applied multiple MR methods and consistently identified the putative causal effect of T2D in relation to PAD, suggesting a common causal genetic relationship between T2D and PAD in Europeans and East Asians. Among multiple MR methods, CAUSE and LCV were the only models capable of distinguishing both correlated and uncorrelated pleiotropy from causality. LCV can yield conservative results when the genetic correlation is mild and cannot measure the extent of the causal effect directly. Other MR models can only deal with uncorrelated pleiotropy or partially correlated pleiotropy. Therefore, we consider CAUSE to be a prior MR method compared to other models. In our study, CAUSE showed consistent evidence for a putative causal effect of T2D on PAD, indicating that our findings are credible. Furthermore, MVMR analysis indicated that the putative causal effect of T2D on PAD is less likely to be influenced by TC, BMI, SBP, and smoking which have generally been considered to be common factors associated with the increased risk of PAD [71–76]. We further identified a higher PRS for T2D among PAD patients compared to non-PAD individuals using PRS regression, in line with previous studies that have reported T2D as a risk factor for arterial disease (such as coronary artery disease and arterial stiffness [77, 78]). Taken together, we conclude that T2D is a causal risk factor for PAD, although further studies with larger sample sizes (particularly with reference to additional PAD patients) will be required to establish this causal relationship conclusively. Nevertheless, several MR models (MR-Egger, weighted mode, and LCV) failed to fully distinguish causality from horizontal pleiotropy, after allowing for Bonferroni correction. The underlying reason may be the limitations inherent to these models. For instance, MR-Egger may inflate type I errors when horizontal pleiotropy from instrumental SNPs occurs via the same confounder [79]. The weighted mode is thought to be less powerful in comparison with other MR methods as it uses a smaller set of instrumental SNPs [80]. The standard error of GCP statistics produced by LCV analysis may be enhanced when analyzing the trait pair with mild or moderate genetic correlation [43].
Previous studies have provided support for the conclusion that while East Asians are more prone to T2D than Europeans [1, 81, 82], the prevalence of PAD in T2D patients is lower in East Asians than in Europeans [83]. The underlying reason has remained unclear, although genetic factors are thought to make important contributions to the ethnic disparities in relation to T2D and PAD prevalence [83–86]. Herein, we provide the first evidence for the common and distinct mechanisms underlying the shared genetics of T2D and PAD between Europeans and East Asians. We used MAGMA and SMR analyses to identify the ANKFY1 gene as showing significant associations with the cross-trait shared architecture of T2D and PAD in both Europeans and East Asians. ANKFY1 is thought to play a role in angiogenesis on the basis of experiments in endothelial cells [87, 88] and has been reported as a gene conferring T2D risk in a GWAS study [34]. Another study has identified a homozygous missense mutation in ANKFY1 to be a potential cause of nephrotic syndrome [89], a disease commonly comorbid with diabetes and cardiovascular disease [90, 91]. Moreover, protein-protein interaction analysis provided further evidence for common mechanisms shared between Europeans and East Asians by revealing gene-gene interactions underlying the comorbidity of T2D and PAD, specifically the interactions between STARD10 (European-specific) and AP3S2 (East Asian-specific) and between KCNJ11 (European-specific) and KCNQ1 (East Asian-specific). In the context of the interaction of STARD10 (European-specific) and AP3S2 (East Asian-specific), STARD10 is a phospholipid transfer protein whose expression in isolated pancreatic islets has been found to influence the production and processing of insulin in the mouse [92]. Prior studies indicated that STARD10 harbors SNPs associated with T2D [93, 94]. AP3S2 encodes a protein involved in protein transport whose variants are associated with the risk of T2D in South Asians and Japanese [95, 96]. Other studies have implicated AP3S2 as being important for the pathogenesis of T2D [97–101]. Regarding the interaction between KCNJ11 and KCNQ1, both genes contribute to potassium channels whereas genetic variants located near both KCNJ11 and KCNQ1 are known to be significantly associated with T2D [9, 102]. Here, we revealed their potential roles in the occurrence of PAD in T2D patients in both Europeans and East Asians. In addition, numerous genes identified as being associated with the cross-trait shared architecture of T2D and PAD in Europeans have similar functions to those in East Asians. For example, TP53INP1 (European-specific) and MOB1B (East Asian-specific) both have functions related to cell migration and death [103, 104]; CHMP4B (European-specific) and AP3S2 (East Asian-specific) are involved in protein transport and intracellular trafficking [105, 106]. These genes were reported as harboring SNPs significantly associated with T2D [1, 107, 108]. Here, they are suggestive of common genetic mechanisms of comorbid T2D and PAD between the two populations.
The distinct genetic mechanisms of co-occurrence of T2D and PAD between Europeans and East Asians may be explicable in terms of the discrepant prevalence of T2D/PAD between Europeans and East Asians. The discrepant prevalence of T2D between ethnic groups may be attributed to insulin resistance and β-cell dysfunction [109]. T2D in Europeans tends to be caused by obesity and insulin resistance whereas T2D in East Asians, who are generally characterized by a leaner body mass, is caused by more severe β-cell dysfunction [82]. Our study potentially supports the view that the distinct genetic mechanisms of co-occurrence of T2D and PAD could be due, at least in part, to the differences in the prevalence of insulin resistance and β-cell dysfunction between the two populations. For instance, we identified the insulin resistance-related gene TIGAR in Europeans and the β-cell dysfunction-related gene TLE1 in East Asians as being associated with the cross-trait shared architecture of T2D and PAD (Additional file 1: Table S9) [110, 111]. TIGAR, activated by p53, promotes insulin resistance in the rat whereas TLE1 has been found to play a role in inducing pancreatic β-cells and converting α-cells to β-cells [110, 111]. Further studies are required to explore the different roles that insulin resistance and β-cell dysfunction play in causing T2D-PAD comorbidity in Europeans and East Asians.
Our findings are potentially important for pre-symptomatic screening in order to prevent the occurrence of PAD in those T2D patients with genetic defects associated with PAD. Identifying incipient PAD among T2D patients and carrying out clinical interventions prior to the onset of arterial disease has the potential to dramatically improve the life quality and prognosis of T2D patients [112]. Our results could lead to the early-stage genetic screening for PAD in T2D patients thereby offering more effective diagnostic and therapeutic approaches for both diseases. Additionally, our study revealed the putative shared/distinct mechanisms underlying the causal relationship between T2D and PAD in Europeans and East Asians, which highlights the need for personalized intervention in designing therapies for PAD in T2D patients with different ethnic backgrounds.
Study limitation
Our study has several limitations. First, the sample size of East Asians in the UKB cohort is insufficient, thereby limiting the power of the study to confirm the putative causal relationship between T2D and PAD in East Asians via PRS analysis. Clearly, the results of this study should be replicated on a much larger group of East Asians. Second, neither European- nor East Asian-based PAD GWAS have enough instrumental SNPs (p-value < 5 × 10−8) for testing with some MR models. Alternatively, we relaxed the p-value threshold and recruited the “proxy” instrumental SNPs (with p-value < 1 × 10−5), which may have led to a violation of MR assumptions. Nevertheless, this influence is likely to be negligible as we obtained very similar results when applying multiple MR methods for sensitivity analysis. Third, the SMR results for East Asian-based GWAS were probably underpowered, as the eQTL summary data from eQTLGen were of European descent, which hampered our ability to identify trans-ethnic functional genes underlying T2D and PAD. Fourth, we excluded SNPs in the MHC region from all our analyses because of the complicated LD pattern within the MHC region; this could have led to an underestimation of the shared genetic basis between T2D and PAD. Finally, the classification of ICD-10-based T2D cases in our study may be less precise because we cannot filter out some rare cases of maturity-onset diabetes of the young (MODY). Nevertheless, as the proportion of MODY comprises only 1-5% of total diabetes [113], we believe such influence is likely to be minimal.
Conclusions
In conclusion, our study has provided the first evidence for a trans-ethnic genetic association between T2D and PAD, a trans-ethnic putative causal effect of T2D on PAD, and the identification of multiple genes which represent candidates for involvement in the shared genetic etiology between T2D and PAD in different ethnic groups. Acquiring a better understanding of the shared genetic architecture underlying T2D and PAD should help to improve clinical treatment and disease prevention of PAD in patients with T2D.
Web resources: GWAS summary statistics from Biobank Japan, http://jenger.riken.jp/en/; BOLT-LMM (v2.3.4), https://alkesgroup.broadinstitute.org/BOLT-LMM/; eQTLGen Consortium, https://eqtlgen.org/cis-eqtls.html; 1000 Genomics LD score reference panels, https://alkesgroup.broadinstitute.org/LDSCORE/; STRING, https://string-db.org/; and Online tool for MR power calculation, https://sb452.shinyapps.io/power/.
Supplementary Information
Additional file 1: Table S1. ICD codes of PAD and T2D. Table S2. MR results between T2D and PAD in Europeans. Table S3. MR results between T2D and PAD in Europeans (without excluding potential pleiotropy SNPs). Table S4. MVMR results between T2D and PAD adjusted by potential risk factors in Europeans. Table S5. Summary of PRS regression of PRS for T2D on PAD status in Europeans. Table S6. Summary of PRS regression of PRS for PAD on T2D status in Europeans. Table S7. Novel SNPs underlying the shared genetics of T2D and PAD in Europeans detected by MTAG. Table S8. Candidate genes associated with comorbid T2D and PAD in Europeans detected by MAGMA. Table S9. Significant SMR associations of gene expression (from Table S8) with T2D, PAD, and their cross-trait. Table S10. MR results between T2D and PAD in East Asians. Table S11. MR results between T2D and PAD in East Asians (without excluding potential pleiotropy SNPs). Table S12. MVMR results between T2D and PAD adjusted by potential risk factors in East Asians. Table S13. Summary of PRS regression of PRS for T2D on PAD status in East Asians. Table S14. Summary of PRS regression of PRS for PAD on T2D status in East Asians. Table S15. Candidate genes associated with comorbid T2D and PAD in East Asians detected by MAGMA. Table S16. Significant SMR associations of gene expression (from Table S15) with T2D, PAD, and their cross-trait. (XLS 383 kb)
Additional file 2: Figure S1. Estimated powers of European- or East Asian-based MR models for the causality between T2D and PAD. Figure S2. The power of PRS regression regarding SNP p-value thresholds of different discovery GWAS. Figure S3. The protein-protein interaction (PPI) network for candidate genes.
Acknowledgements
The authors thank the BioBank Japan project for making the data available.
Data for this study were also obtained from the UK Biobank (application number #65805).
Abbreviations
- T2D
Type 2 diabetes
- PAD
Peripheral artery disease
- PRS
Polygenic risk score
- MAGMA
Multi-marker analysis of genomic annotation
- SMR
Summary data-based Mendelian randomization
- GWAS
Genome-wide association studies
- LDSC
Linkage disequilibrium score regression
- PCs
Principal components
- HM 3
HapMap 3SNP: single nucleotide polymorphism
- MR
Mendelian randomization
- UKB
UK Biobank
- BBJ
BioBank Japan
- MVMR
Multivariable Mendelian randomization analysis
- MTAG
Multi-trait analysis of GWAS
- LMM
Linear mixed model
- MAF
minor allele frequency
- eQTL
Expression quantitative trait loci
- h2
Heritability
- rg
Genetic correlation
- MHC
Major histocompatibility complex
- IVW
Inverse variance-weighted
- GSMR
Generalized summary data-based Mendelian randomization
- CAUSE
Causal analysis using summary effect estimates
- LCV
Latent causal variable
- HEIDI
Heterogeneity in dependent instruments
- ELPD
Expected log pointwise posterior density
- GCP
Genetic causality proportion
- TC
Total cholesterol
- BMI
Body mass index
- SBP
Systolic blood pressure
- CI
Confidence intervals
Authors’ contributions
YH.Y and H. Zhao designed the study. X.X and H. Zhang performed the analyses, with assistance from YH. Y and H. Zhao. X.X, YH.Y, D.N.C, and H. Zhao wrote the manuscript. YD.Y, YH.Y, and H. Zhao supervised the study. All authors discussed and approved the results and interpretation and contributed to the final version of the paper.
Funding
The work was funded by the National Key Research and Development Program of China (2020YFB0204803), Natural Science Foundation of China (81801132, 81971190, 61772566), Guangdong Key Field Research and Development Plan (2019B020228001, 2018B010109006, and 2021A1515010256), Introducing Innovative and Entrepreneurial Teams (2016ZT06D211), Guangzhou Science and Technology Research Plan (202007030010), and Mater Foundation.
Availability of data and materials
The datasets analyzed during the current study are available in the BBJ, http://jenger.riken.jp/en/.
Declarations
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xuehao Xiu, Haoyang Zhang, Yuedong Yang, Yuanhao Yang and Huiying Zhao contributed equally to this work.
Contributor Information
Yuedong Yang, Email: yangyd25@mail.sysu.edu.cn.
Yuanhao Yang, Email: yuanhao.yang@mater.uq.edu.au.
Huiying Zhao, Email: zhaohy8@mail.sysu.edu.cn.
References
- 1.Spracklen CN, Horikoshi M, Kim YJ, Lin K, Bragg F, Moon S, Suzuki K, Tam CHT, Tabara Y, Kwak SH, et al. Identification of type 2 diabetes loci in 433,540 East Asian individuals. Nature. 2020;582(7811):240–245. doi: 10.1038/s41586-020-2263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thiruvoipati T, Kielhorn CE, Armstrong EJ. Peripheral artery disease in patients with diabetes: epidemiology, mechanisms, and outcomes. World J Diabetes. 2015;6(7):961. doi: 10.4239/wjd.v6.i7.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tresierra-Ayala M, Rojas AG. Association between peripheral arterial disease and diabetic foot ulcers in patients with diabetes mellitus type 2. Medicina Universitaria. 2017;19(76):123–126. doi: 10.1016/j.rmu.2017.07.002. [DOI] [Google Scholar]
- 4.Schorr EN, Treat-Jacobson D, Lindquist R. The relationship between peripheral artery disease symptomatology and ischemia. Nurs Res. 2017;66(5):378–387. doi: 10.1097/NNR.0000000000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsukura M, Ozaki K, Takahashi A, Onouchi Y, Morizono T, Komai H, Shigematsu H, Kudo T, Inoue Y, Kimura H, et al. Genome-wide association study of peripheral arterial disease in a Japanese population. PLoS One. 2015;10(10):e0139262. doi: 10.1371/journal.pone.0139262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. Jama. 2002;287(19):2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 7.Qin J, Tian J, Liu G, Zhang Y, Tian L, Zhen Y, Zhang H, Xu J, Sun X, Fang H. Association between 1p13 polymorphisms and peripheral arterial disease in a Chinese population with diabetes. J Diabetes Investig. 2018;9(5):1189–1195. doi: 10.1111/jdi.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strawbridge RJ, van Zuydam NR. Shared genetic contribution of type 2 diabetes and cardiovascular disease: implications for prognosis and treatment. Curr Diab Rep. 2018;18(8):59. doi: 10.1007/s11892-018-1021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vujkovic M, Keaton JM, Lynch JA, Miller DR, Zhou J, Tcheandjieu C, Huffman JE, Assimes TL, Lorenz K, Zhu X, et al. Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat Genet. 2020;52(7):680–691. doi: 10.1038/s41588-020-0637-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Zuydam NR, Stiby A, Abdalla M, Austin E, Dahlstrom EH, McLachlan S, Vlachopoulou E, Ahlqvist E, Di Liao C, Sandholm N, et al. Genome-wide association study of peripheral artery disease. Circ Genom Precis Med. 2021;14(5):e002862. doi: 10.1161/CIRCGEN.119.002862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bulik-Sullivan BK, Loh PR, Finucane HK, Ripke S, Yang J, Schizophrenia Working Group of the Psychiatric Genomics C. Patterson N, Daly MJ, Price AL, Neale BM. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47(3):291–295. doi: 10.1038/ng.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hemani G, Bowden J, Davey Smith G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet. 2018;27(R2):R195–R208. doi: 10.1093/hmg/ddy163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wurtz P, Wang Q, Kangas AJ, Richmond RC, Skarp J, Tiainen M, Tynkkynen T, Soininen P, Havulinna AS, Kaakinen M, et al. Metabolic signatures of adiposity in young adults: Mendelian randomization analysis and effects of weight change. PLoS Med. 2014;11(12):e1001765. doi: 10.1371/journal.pmed.1001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Y, Rist PM, Daghlas I, Giulianini F, Kurth T, Chasman DI. A genome-wide cross-phenotype meta-analysis of the association of blood pressure with migraine. Nat Commun. 2020;11(1):3368. doi: 10.1038/s41467-020-17002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y, Musco H, Simpson-Yap S, Zhu Z, Wang Y, Lin X, Zhang J, Taylor B, Gratten J, Zhou Y. Investigating the shared genetic architecture between multiple sclerosis and inflammatory bowel diseases. Nat Commun. 2021;12(1):5641. doi: 10.1038/s41467-021-25768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Xiu X, Xue A, Yang Y, Yang Y, Zhao H. Mendelian randomization study reveals a population-specific putative causal effect of type 2 diabetes in risk of cataract. Int J Epidemiol. 2022;50(6):2024–37. [DOI] [PubMed]
- 17.Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, Abecasis GR. A global reference for human genetic variation. Nature. 2015;526(7571):68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yengo L, Sidorenko J, Kemper KE, Zheng Z, Wood AR, Weedon MN, Frayling TM, Hirschhorn J, Yang J, Visscher PM, et al. Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum Mol Genet. 2018;27(20):3641–3649. doi: 10.1093/hmg/ddy271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization . The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. Geneva: World Health Organization; 1992. [Google Scholar]
- 20.Loh PR, Tucker G, Bulik-Sullivan BK, Vilhjálmsson BJ, Finucane HK, Salem RM, Chasman DI, Ridker PM, Neale BM, Berger B, et al. Efficient Bayesian mixed-model analysis increases association power in large cohorts. Nat Genet. 2015;47(3):284–290. doi: 10.1038/ng.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abecasis GR, Altshuler D, Auton A, Brooks LD, Durbin RM, Gibbs RA, Hurles ME, McVean GA. A map of human genome variation from population-scale sequencing. Nature. 2010;467(7319):1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loh PR, Danecek P, Palamara PF, Fuchsberger C, YAR, HKF, Schoenherr S, Forer L, McCarthy S, Abecasis GR, et al. Reference-based phasing using the Haplotype Reference Consortium panel. Nat Genet. 2016;48(11):1443–1448. doi: 10.1038/ng.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki K, Akiyama M, Ishigaki K, Kanai M, Hosoe J, Shojima N, Hozawa A, Kadota A, Kuriki K, Naito M, et al. Identification of 28 new susceptibility loci for type 2 diabetes in the Japanese population. Nat Genet. 2019;51(3):379–386. doi: 10.1038/s41588-018-0332-4. [DOI] [PubMed] [Google Scholar]
- 24.Ishigaki K, Akiyama M, Kanai M, Takahashi A, Kawakami E, Sugishita H, Sakaue S, Matoba N, Low SK, Okada Y, et al. Large-scale genome-wide association study in a Japanese population identifies novel susceptibility loci across different diseases. Nat Genet. 2020;52(7):669–679. doi: 10.1038/s41588-020-0640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagai A, Hirata M, Kamatani Y, Muto K, Matsuda K, Kiyohara Y, Ninomiya T, Tamakoshi A, Yamagata Z, Mushiroda T, et al. Overview of the BioBank Japan Project: study design and profile. J Epidemiol. 2017;27(3s):S2–s8. doi: 10.1016/j.je.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26(17):2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou W, Nielsen JB, Fritsche LG, Dey R, Gabrielsen ME, Wolford BN, LeFaive J, VandeHaar P, Gagliano SA, Gifford A, et al. Efficiently controlling for case-control imbalance and sample relatedness in large-scale genetic association studies. Nat Genet. 2018;50(9):1335–1341. doi: 10.1038/s41588-018-0184-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Majewski J, Pastinen T. The study of eQTL variations by RNA-seq: from SNPs to phenotypes. Trends Genet. 2011;27(2):72–79. doi: 10.1016/j.tig.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Z, Zhang F, Hu H, Bakshi A, Robinson MR, Powell JE, Montgomery GW, Goddard ME, Wray NR, Visscher PM, et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat Genet. 2016;48(5):481–487. doi: 10.1038/ng.3538. [DOI] [PubMed] [Google Scholar]
- 30.Westra HJ, Peters MJ, Esko T, Yaghootkar H, Schurmann C, Kettunen J, Christiansen MW, Fairfax BP, Schramm K, Powell JE, et al. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat Genet. 2013;45(10):1238–1243. doi: 10.1038/ng.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Võsa U, Claringbould A, Westra H-J, Bonder MJ, Deelen P, Zeng B, et al. Unraveling the polygenic architecture of complex traits using blood eQTL metaanalysis. bioRxiv. 2018. Preprint at https://www.biorxiv.org/content/10.1101/447367v1.
- 32.Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601. doi: 10.1136/bmj.k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.da Silva JS, Wowk PF, Poerner F, Santos PS, Bicalho Mda G. Absence of strong linkage disequilibrium between odorant receptor alleles and the major histocompatibility complex. Hum Immunol. 2013;74(12):1619–1623. doi: 10.1016/j.humimm.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Xue A, Wu Y, Zhu Z, Zhang F, Kemper KE, Zheng Z, Yengo L, Lloyd-Jones LR, Sidorenko J, Wu Y, et al. Genome-wide association analyses identify 143 risk variants and putative regulatory mechanisms for type 2 diabetes. Nat Commun. 2018;9(1):2941. doi: 10.1038/s41467-018-04951-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olinic DM, Spinu M, Olinic M, Homorodean C, Tataru DA, Liew A, Schernthaner GH, Stanek A, Fowkes G, Catalano M. Epidemiology of peripheral artery disease in Europe: VAS Educational Paper. Int Angiol. 2018;37(4):327–334. doi: 10.23736/S0392-9590.18.03996-2. [DOI] [PubMed] [Google Scholar]
- 36.Subramaniam T, Nang EE, Lim SC, Wu Y, Khoo CM, Lee J, Heng D, Chew SK, Wong TY, Tai ES. Distribution of ankle–brachial index and the risk factors of peripheral artery disease in a multi-ethnic Asian population. Vasc Med. 2011;16(2):87–95. doi: 10.1177/1358863X11400781. [DOI] [PubMed] [Google Scholar]
- 37.Burgess S, Dudbridge F, Thompson SG. Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat Med. 2016;35(11):1880–1906. doi: 10.1002/sim.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46(6):1985–1998. doi: 10.1093/ije/dyx102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted Median estimator. Genet Epidemiol. 2016;40(4):304–314. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu Z, Zheng Z, Zhang F, Wu Y, Trzaskowski M, Maier R, Robinson MR, McGrath JJ, Visscher PM, Wray NR, et al. Causal associations between risk factors and common diseases inferred from GWAS summary data. Nat Commun. 2018;9(1):224. doi: 10.1038/s41467-017-02317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morrison J, Knoblauch N, Marcus JH, Stephens M, He X. Mendelian randomization accounting for correlated and uncorrelated pleiotropic effects using genome-wide summary statistics. Nat Genet. 2020;52(7):740–747. doi: 10.1038/s41588-020-0631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Connor LJ, Price AL. Distinguishing genetic correlation from causation across 52 diseases and complex traits. Nat Genet. 2018;50(12):1728–1734. doi: 10.1038/s41588-018-0255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Altshuler DM, Gibbs RA, Peltonen L, Altshuler DM, Gibbs RA, Peltonen L, Dermitzakis E, Schaffner SF, Yu F, Peltonen L, et al. Integrating common and rare genetic variation in diverse human populations. Nature. 2010;467(7311):52–58. doi: 10.1038/nature09298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burgess S. Sample size and power calculations in Mendelian randomization with a single instrumental variable and a binary outcome. Int J Epidemiol. 2014;43(3):922–929. doi: 10.1093/ije/dyu005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Byrne EM, Zhu Z, Qi T, Skene NG, Bryois J, Pardinas AF, et al. Conditional GWAS analysis to identify disorder-specific SNPs for psychiatric disorders. Mol Psychiatry. 2021;26(6):2070–81. [DOI] [PMC free article] [PubMed]
- 48.Burgess S, Thompson SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol. 2015;181(4):251–260. doi: 10.1093/aje/kwu283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spracklen CN, Sim X. Progress in defining the genetic contribution to type 2 diabetes in individuals of East Asian ancestry. Curr Diab Rep. 2021;21(6):17. doi: 10.1007/s11892-021-01388-2. [DOI] [PubMed] [Google Scholar]
- 50.Spracklen CN, Chen P, Kim YJ, Wang X, Cai H, Li S, Long J, Wu Y, Wang YX, Takeuchi F. Association analyses of East Asian individuals and trans-ancestry analyses with European individuals reveal new loci associated with cholesterol and triglyceride levels. Hum Mol Genet. 2018;27(6):1122. doi: 10.1093/hmg/ddx439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akiyama M, Okada Y, Kanai M, Takahashi A, Momozawa Y, Ikeda M, Iwata N, Ikegawa S, Hirata M, Matsuda K, et al. Genome-wide association study identifies 112 new loci for body mass index in the Japanese population. Nat Genet. 2017;49(10):1458–1467. doi: 10.1038/ng.3951. [DOI] [PubMed] [Google Scholar]
- 53.Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, Smith AV, Tobin MD, Verwoert GC, Hwang SJ, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478(7367):103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kanai M, Akiyama M, Takahashi A, Matoba N, Momozawa Y, Ikeda M, Iwata N, Ikegawa S, Hirata M, Matsuda K, et al. Genetic analysis of quantitative traits in the Japanese population links cell types to complex human diseases. Nat Genet. 2018;50(3):390–400. doi: 10.1038/s41588-018-0047-6. [DOI] [PubMed] [Google Scholar]
- 55.Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, Datta G, Davila-Velderrain J, McGuire D, Tian C, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. 2019;51(2):237–244. doi: 10.1038/s41588-018-0307-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matoba N, Akiyama M, Ishigaki K, Kanai M, Takahashi A, Momozawa Y, Ikegawa S, Ikeda M, Iwata N, Hirata M, et al. GWAS of smoking behaviour in 165,436 Japanese people reveals seven new loci and shared genetic architecture. Nat Hum Behav. 2019;3(5):471–477. doi: 10.1038/s41562-019-0557-y. [DOI] [PubMed] [Google Scholar]
- 57.Nagelkerke NJD. A note on a general definition of the coefficient of determination. Biometrika. 1991;78(3):691–692. doi: 10.1093/biomet/78.3.691. [DOI] [Google Scholar]
- 58.Olkin I, Finn JD. Correlations redux. Psychol Bull. 1995;118(1):115–164. doi: 10.1037/0033-2909.118.1.155. [DOI] [Google Scholar]
- 59.Palla L, Dudbridge F. A fast method that uses polygenic scores to estimate the variance explained by genome-wide marker panels and the proportion of variants affecting a trait. Am J Hum Genet. 2015;97(2):250–259. doi: 10.1016/j.ajhg.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turley P, Walters RK, Maghzian O, Okbay A, Lee JJ, Fontana MA, Nguyen-Viet TA, Wedow R, Zacher M, Furlotte NA, et al. Multi-trait analysis of genome-wide association summary statistics using MTAG. Nat Genet. 2018;50(2):229–237. doi: 10.1038/s41588-017-0009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Leeuw CA, Mooij JM, Heskes T, Posthuma D. MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput Biol. 2015;11(4):e1004219. doi: 10.1371/journal.pcbi.1004219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Veturi Y, Ritchie MD. How powerful are summary-based methods for identifying expression-trait associations under different genetic architectures? Pac Symp Biocomput. 2018;23:228–239. [PMC free article] [PubMed] [Google Scholar]
- 63.Jensen LJ, Kuhn M, Stark M, Chaffron S, Creevey C, Muller J, Doerks T, Julien P, Roth A, Simonovic M, et al. STRING 8—a global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res. 2009;37(Database issue):D412–D416. doi: 10.1093/nar/gkn760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Awodu OA, Famodu AA. Haemostatic variables and their relationship to body mass index and blood pressure in adult Nigerians with the sickle cell trait. Clin Hemorheol Microcirc. 2007;36(1):89–94. [PubMed] [Google Scholar]
- 65.Langer RD, Criqui MH, Reed DM. Lipoproteins and blood pressure as biological pathways for effect of moderate alcohol consumption on coronary heart disease. Circulation. 1992;85(3):910–915. doi: 10.1161/01.CIR.85.3.910. [DOI] [PubMed] [Google Scholar]
- 66.Levin MG, Zuber V, Walker VM, Klarin D, Lynch J, Malik R, Aday AW, Bottolo L, Pradhan AD, Dichgans M, et al. Prioritizing the role of major lipoproteins and subfractions as risk factors for peripheral artery disease. Circulation. 2021;144(5):353–364. doi: 10.1161/CIRCULATIONAHA.121.053797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Small AM, Huffman JE, Klarin D, Sabater-Lleal M, Lynch JA, Assimes TL, Sun YV, Miller D, Freiberg MS, Morrison AC, et al. Mendelian randomization analysis of hemostatic factors and their contribution to peripheral artery disease-brief report. Arterioscler Thromb Vasc Biol. 2021;41(1):380–386. doi: 10.1161/ATVBAHA.119.313847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klarin D, Lynch J, Aragam K, Chaffin M, Assimes TL, Huang J, Lee KM, Shao Q, Huffman JE, Natarajan P, et al. Genome-wide association study of peripheral artery disease in the Million Veteran Program. Nat Med. 2019;25(8):1274–1279. doi: 10.1038/s41591-019-0492-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hayfron-Benjamin C, van den Born BJ, Maitland-van der Zee AH, Amoah AGB, Meeks KAC, Klipstein-Grobusch K, Bahendeka S, Spranger J, Danquah I, Mockenhaupt F, et al. Microvascular and macrovascular complications in type 2 diabetes Ghanaian residents in Ghana and Europe: the RODAM study. J Diabetes Complicat. 2019;33(8):572–578. doi: 10.1016/j.jdiacomp.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 70.Ruscitti P, Cipriani P, Liakouli V, Iacono D, Pantano I, Margiotta DPE, Navarini L, Destro Castaniti GM, Maruotti N, Di Scala G, et al. Subclinical and clinical atherosclerosis in rheumatoid arthritis: results from the 3-year, multicentre, prospective, observational GIRRCS (Gruppo Italiano di Ricerca in Reumatologia Clinica e Sperimentale) study. Arthritis Res Ther. 2019;21(1):204. doi: 10.1186/s13075-019-1975-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aday AW, Everett BM. Dyslipidemia profiles in patients with peripheral artery disease. Curr Cardiol Rep. 2019;21(6):42. doi: 10.1007/s11886-019-1129-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Joshi PH, Martin SS. Unraveling the risk of peripheral artery disease. Circulation. 2018;138(21):2342–2344. doi: 10.1161/CIRCULATIONAHA.118.036347. [DOI] [PubMed] [Google Scholar]
- 73.Heffron SP, Dwivedi A, Rockman CB, Xia Y, Guo Y, Zhong J, Berger JS. Body mass index and peripheral artery disease. Atherosclerosis. 2020;292:31–36. doi: 10.1016/j.atherosclerosis.2019.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yeh CH, Yu HC, Huang TY, Huang PF, Wang YC, Chen TP, Yin SY. High systolic and diastolic blood pressure variability is correlated with the occurrence of peripheral arterial disease in the first decade following a diagnosis of type 2 diabetes mellitus: a new biomarker from old measurement. Biomed Res Int. 2016;2016:9872945. doi: 10.1155/2016/9872945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clement DL, De Buyzere ML, Duprez DA. Hypertension in peripheral arterial disease. Curr Pharm Des. 2004;10(29):3615–3620. doi: 10.2174/1381612043382819. [DOI] [PubMed] [Google Scholar]
- 76.Clark D, 3rd, Cain LR, Blaha MJ, DeFilippis AP, Mentz RJ, Kamimura D, White WB, Butler KR, Robertson RM, Bhatnagar A, et al. Cigarette smoking and subclinical peripheral arterial disease in Blacks of the Jackson Heart Study. J Am Heart Assoc. 2019;8(3):e010674. doi: 10.1161/JAHA.118.010674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jansen H, Loley C, Lieb W, Pencina MJ, Nelson CP, Kathiresan S, Peloso GM, Voight BF, Reilly MP, Assimes TL, et al. Genetic variants primarily associated with type 2 diabetes are related to coronary artery disease risk. Atherosclerosis. 2015;241(2):419–426. doi: 10.1016/j.atherosclerosis.2015.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu M, Huang Y, Xie L, Peng K, Ding L, Lin L, Wang P, Hao M, Chen Y, Sun Y, et al. Diabetes and risk of arterial stiffness: a Mendelian randomization analysis. Diabetes. 2016;65(6):1731–1740. doi: 10.2337/db15-1533. [DOI] [PubMed] [Google Scholar]
- 79.Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377–389. doi: 10.1007/s10654-017-0255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Burgess S, Foley CN, Allara E, Staley JR, Howson JMM. A robust and efficient method for Mendelian randomization with hundreds of genetic variants. Nat Commun. 2020;11(1):376. doi: 10.1038/s41467-019-14156-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li J, Dong Y, Wu T, Tong N. Differences between Western and Asian type 2 diabetes patients in the incidence of vascular complications and mortality: a systematic review of randomized controlled trials on lowering blood glucose. J Diabetes. 2016;8(6):824–833. doi: 10.1111/1753-0407.12361. [DOI] [PubMed] [Google Scholar]
- 82.Narayan KMV. Type 2 diabetes: why we are winning the battle but losing the war? 2015 Kelly West Award Lecture. Diabetes Care. 2016;39(5):653. doi: 10.2337/dc16-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vitalis A, Lip GY, Kay M, Vohra RK, Shantsila A. Ethnic differences in the prevalence of peripheral arterial disease: a systematic review and meta-analysis. Expert Rev Cardiovasc Ther. 2017;15(4):327–338. doi: 10.1080/14779072.2017.1305890. [DOI] [PubMed] [Google Scholar]
- 84.Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281:64–91. doi: 10.1111/nyas.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Musunuru K, Strong A, Frank-Kamenetsky M, Lee NE, Ahfeldt T, Sachs KV, Li X, Li H, Kuperwasser N, Ruda VM, et al. From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature. 2010;466(7307):714–719. doi: 10.1038/nature09266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee JY, Lee BS, Shin DJ, Woo Park K, Shin YA, Joong Kim K, Heo L, Young Lee J, Kyoung Kim Y, Jin Kim Y, et al. A genome-wide association study of a coronary artery disease risk variant. J Hum Genet. 2013;58(3):120–126. doi: 10.1038/jhg.2012.124. [DOI] [PubMed] [Google Scholar]
- 87.Maekawa M, Tanigawa K, Sakaue T, Hiyoshi H, Kubota E, Joh T, Watanabe Y, Taguchi T, Higashiyama S. Cullin-3 and its adaptor protein ANKFY1 determine the surface level of integrin beta1 in endothelial cells. Biol Open. 2017;6(11):1707–1719. doi: 10.1242/bio.029579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tanaka M, Nakamura S, Maekawa M, Higashiyama S, Hara H. ANKFY1 is essential for retinal endothelial cell proliferation and migration via VEGFR2/Akt/eNOS pathway. Biochem Biophys Res Commun. 2020;533(4):1406–1412. doi: 10.1016/j.bbrc.2020.10.032. [DOI] [PubMed] [Google Scholar]
- 89.Hermle T, Schneider R, Schapiro D, Braun DA, van der Ven AT, Warejko JK, Daga A, Widmeier E, Nakayama M, Jobst-Schwan T, et al. GAPVD1 and ANKFY1 mutations implicate RAB5 regulation in nephrotic syndrome. J Am Soc Nephrol. 2018;29(8):2123–2138. doi: 10.1681/ASN.2017121312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kodner C. Diagnosis and management of nephrotic syndrome in adults. Am Fam Physician. 2016;93(6):479–485. [PubMed] [Google Scholar]
- 91.Gigante A, Barbano B, Sardo L, Martina P, Gasperini ML, Labbadia R, Liberatori M, Amoroso A, Cianci R. Hypercoagulability and nephrotic syndrome. Curr Vasc Pharmacol. 2014;12(3):512–517. doi: 10.2174/157016111203140518172048. [DOI] [PubMed] [Google Scholar]
- 92.Carrat GR, Haythorne E, Tomas A, Haataja L, Müller A, Arvan P, Piunti A, Cheng K, Huang M, Pullen TJ, et al. The type 2 diabetes gene product STARD10 is a phosphoinositide-binding protein that controls insulin secretory granule biogenesis. Mol Metab. 2020;40:101015. doi: 10.1016/j.molmet.2020.101015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Imamura M, Takahashi A, Yamauchi T, Hara K, Yasuda K, Grarup N, Zhao W, Wang X, Huerta-Chagoya A, Hu C, et al. Genome-wide association studies in the Japanese population identify seven novel loci for type 2 diabetes. Nat Commun. 2016;7:10531. doi: 10.1038/ncomms10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sakaue S, Kanai M, Tanigawa Y, Karjalainen J, Kurki M, Koshiba S, Narita A, Konuma T, Yamamoto K, Akiyama M, et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat Genet. 2021;53(10):1415–1424. doi: 10.1038/s41588-021-00931-x. [DOI] [PubMed] [Google Scholar]
- 95.Kooner JS, Saleheen D, Sim X, Sehmi J, Zhang W, Frossard P, Been LF, Chia KS, Dimas AS, Hassanali N, et al. Genome-wide association study in individuals of South Asian ancestry identifies six new type 2 diabetes susceptibility loci. Nat Genet. 2011;43(10):984–989. doi: 10.1038/ng.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fukuda H, Imamura M, Tanaka Y, Iwata M, Hirose H, Kaku K, Maegawa H, Watada H, Tobe K, Kashiwagi A, et al. A single nucleotide polymorphism within DUSP9 is associated with susceptibility to type 2 diabetes in a Japanese population. PLoS One. 2012;7(9):e46263. doi: 10.1371/journal.pone.0046263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fadason T, Ekblad C, Ingram JR, Schierding WS, O’Sullivan JM. Physical interactions and expression quantitative traits loci identify regulatory connections for obesity and type 2 diabetes associated SNPs. Front Genet. 2017;8:150. doi: 10.3389/fgene.2017.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wood AR, Jonsson A, Jackson AU, Wang N, van Leewen N, Palmer ND, Kobes S, Deelen J, Boquete-Vilarino L, Paananen J, et al. A genome-wide association study of IVGTT-based measures of first-phase insulin secretion refines the underlying physiology of type 2 diabetes variants. Diabetes. 2017;66(8):2296–2309. doi: 10.2337/db16-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kanthimathi S, Chidambaram M, Bodhini D, Liju S, Bhavatharini A, Uma R, Anjana RM, Mohan V, Radha V. Association of recently identified type 2 diabetes gene variants with gestational diabetes in Asian Indian population. Mol Genet Genomics. 2017;292(3):585–591. doi: 10.1007/s00438-017-1292-6. [DOI] [PubMed] [Google Scholar]
- 100.Kazakova EV, Zghuang T, Li T, Fang Q, Han J, Qiao H. The Gas6 gene rs8191974 and Ap3s2 gene rs2028299 are associated with type 2 diabetes in the northern Chinese Han population. Acta Biochim Pol. 2017;64(2):227–231. doi: 10.18388/abp.2016_1299. [DOI] [PubMed] [Google Scholar]
- 101.Shabana, Ullah Shahid S, Wah Li K, Acharya J, Cooper JA, Hasnain S, Humphries SE. Effect of six type II diabetes susceptibility loci and an FTO variant on obesity in Pakistani subjects. Eur J Hum Genet. 2016;24(6):903–910. doi: 10.1038/ejhg.2015.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mahajan A, Taliun D, Thurner M, Robertson NR, Torres JM, Rayner NW, Payne AJ, Steinthorsdottir V, Scott RA, Grarup N, et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet. 2018;50(11):1505–1513. doi: 10.1038/s41588-018-0241-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Han L, Huang Z, Liu Y, Ye L, Li D, Yao Z, Wang C, Zhang Y, Yang H, Tan Z, et al. MicroRNA-106a regulates autophagy-related cell death and EMT by targeting TP53INP1 in lung cancer with bone metastasis. Cell Death Dis. 2021;12(11):1037. doi: 10.1038/s41419-021-04324-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fan S, Price T, Huang W, Plue M, Warren J, Sundaramoorthy P, Paul B, Feinberg D, MacIver N, Chao N, et al. PINK1-dependent mitophagy regulates the migration and homing of multiple myeloma cells via the MOB1B-mediated Hippo-YAP/TAZ pathway. Adv Sci (Weinh) 2020;7(5):1900860. doi: 10.1002/advs.201900860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mercier V, Larios J, Molinard G, Goujon A, Matile S, Gruenberg J, Roux A. Endosomal membrane tension regulates ESCRT-III-dependent intra-lumenal vesicle formation. Nat Cell Biol. 2020;22(8):947–959. doi: 10.1038/s41556-020-0546-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lefrançois S, Janvier K, Boehm M, Ooi CE, Bonifacino JS. An ear-core interaction regulates the recruitment of the AP-3 complex to membranes. Dev Cell. 2004;7(4):619–625. doi: 10.1016/j.devcel.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 107.Voight BF, Scott LJ, Steinthorsdottir V, Morris AP, Dina C, Welch RP, Zeggini E, Huth C, Aulchenko YS, Thorleifsson G, et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet. 2010;42(7):579–589. doi: 10.1038/ng.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kichaev G, Bhatia G, Loh PR, Gazal S, Burch K, Freund MK, Schoech A, Pasaniuc B, Price AL. Leveraging polygenic functional enrichment to improve GWAS power. Am J Hum Genet. 2019;104(1):65–75. doi: 10.1016/j.ajhg.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Unnikrishnan R, Pradeepa R, Joshi SR, Mohan V. Type 2 diabetes: demystifying the global epidemic. Diabetes. 2017;66(6):1432. doi: 10.2337/db16-0766. [DOI] [PubMed] [Google Scholar]
- 110.Derdak Z, Lang CH, Villegas KA, Tong M, Mark NM, de la Monte SM, Wands JR. Activation of p53 enhances apoptosis and insulin resistance in a rat model of alcoholic liver disease. J Hepatol. 2011;54(1):164–172. doi: 10.1016/j.jhep.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Metzger DE, Liu C, Ziaie AS, Naji A, Zaret KS. Grg3/TLE3 and Grg1/TLE1 induce monohormonal pancreatic β-cells while repressing α-cell functions. Diabetes. 2014;63(5):1804–1816. doi: 10.2337/db13-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kullo IJ, Leeper NJ. The genetic basis of peripheral arterial disease: current knowledge, challenges, and future directions. Circ Res. 2015;116(9):1551–1560. doi: 10.1161/CIRCRESAHA.116.303518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Firdous P, Nissar K, Ali S, Ganai BA, Shabir U, Hassan T, Masoodi SR. Genetic testing of maturity-onset diabetes of the young current status and future perspectives. Front Endocrinol (Lausanne) 2018;9:253. doi: 10.3389/fendo.2018.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. ICD codes of PAD and T2D. Table S2. MR results between T2D and PAD in Europeans. Table S3. MR results between T2D and PAD in Europeans (without excluding potential pleiotropy SNPs). Table S4. MVMR results between T2D and PAD adjusted by potential risk factors in Europeans. Table S5. Summary of PRS regression of PRS for T2D on PAD status in Europeans. Table S6. Summary of PRS regression of PRS for PAD on T2D status in Europeans. Table S7. Novel SNPs underlying the shared genetics of T2D and PAD in Europeans detected by MTAG. Table S8. Candidate genes associated with comorbid T2D and PAD in Europeans detected by MAGMA. Table S9. Significant SMR associations of gene expression (from Table S8) with T2D, PAD, and their cross-trait. Table S10. MR results between T2D and PAD in East Asians. Table S11. MR results between T2D and PAD in East Asians (without excluding potential pleiotropy SNPs). Table S12. MVMR results between T2D and PAD adjusted by potential risk factors in East Asians. Table S13. Summary of PRS regression of PRS for T2D on PAD status in East Asians. Table S14. Summary of PRS regression of PRS for PAD on T2D status in East Asians. Table S15. Candidate genes associated with comorbid T2D and PAD in East Asians detected by MAGMA. Table S16. Significant SMR associations of gene expression (from Table S15) with T2D, PAD, and their cross-trait. (XLS 383 kb)
Additional file 2: Figure S1. Estimated powers of European- or East Asian-based MR models for the causality between T2D and PAD. Figure S2. The power of PRS regression regarding SNP p-value thresholds of different discovery GWAS. Figure S3. The protein-protein interaction (PPI) network for candidate genes.
Data Availability Statement
The datasets analyzed during the current study are available in the BBJ, http://jenger.riken.jp/en/.