Abstract
Neurocutaneous melanocytosis (NCM) is characterized by melanocyte deposition in the leptomeninges and brain parenchyma, primarily occurring in children with large (or giant) congenital melanocytic nevi (LCMN) or multiple melanocytic nevi. Patients with NCM may develop hydrocephalus and increased intracranial pressure, which can be managed with ventriculoperitoneal (VP) shunting. We present the case of a 16-month-old girl who developed peritoneal carcinomatosis and malignant ascites following VP shunting for hydrocephalus secondary to NCM to increase awareness of this rare, but serious complication of cerebrospinal fluid diversion.
Keywords: Neoplasms-malignant, nevi-melanocytic, neurocutaneous disorders, peritoneal carcinomatosis, meningeal carcinomatosis
Introduction:
Neurocutaneous melanocytosis (NCM) is a rare disease characterized by excessive proliferation of melanocytes in the leptomeninges and brain parenchyma, primarily occurring in children with large (or giant) congenital melanocytic nevi (LCMN) or multiple melanocytic nevi.1–3 LCMN is diagnosed in an estimated 1/20,000 to 1/50,000 births with NCM identified in up to 30% of these individuals.1,3 On magnetic resonance imaging (MRI), NCM can be identified as multifocal T1 shortening involving the brain or spine or diffuse leptomeningeal thickening.4,5 Clinical manifestations of NCM are variable, ranging from asymptomatic melanocytosis to multiple cranial neuropathies, seizures, and symptomatic hydrocephalus.1–5
LCMN or multiple melanocytic nevi and NCM are associated with risk of cutaneous and central nervous system (CNS) melanoma.1,6,7 In patients with LCMN or multiple melanocytic nevi, an abnormal MRI represents the most significant predictor for development of melanoma.7 Despite advances in management of non-NCM associated malignant melanoma with use of immune checkpoint inhibitors and targeted therapy directed at BRAF mutations,8,9 the prognosis of NCM-associated melanoma remains guarded.10,11 NCM-associated melanoma most frequently harbors NRAS mutations, but MEK inhibitors such as trametinib have not been successful in treating these patients.12
Ventriculoperitoneal (VP) shunting is used for management of hydrocephalus secondary to NCM and can improve symptoms of increased intracranial pressure such as headache, nausea, vomiting, or lethargy.13,14 Peritoneal seeding is a rare complication of VP shunting that can occur in the setting of NCM-associated melanoma and other tumors.15–19 Here we present the case of a 16-month-old girl with multiple melanocytic nevi and NCM who developed peritoneal carcinomatosis and malignant ascites after VP shunting for symptomatic hydrocephalus secondary to NCM.
Case Report:
The patient presented at birth with multiple congenital melanocytic nevi. The largest nevus involved the scalp, measured at 4 cm (M1), did not have color heterogeneity, irregular surface rugosity, hypertrichosis, or associated dermal or subcutaneous nodules.20 A nevus on the left side of the neck measured 2 cm, and had moderate hypertrichosis. Additional small nevi were noted involving the right arm, right knee, and posterior left leg. Hypotonia was noted on her first physical exam at birth. She underwent MRI brain imaging during the second month of life, which demonstrated increased T1 pre-contrast weighted signal in cerebellar hemispheres, pons, and anteromedial temporal lobes, radiographically consistent with NCM (Figure 1A). No ventriculomegaly was noted on initial imaging studies. No spine imaging was obtained at this time.
Figure 1:
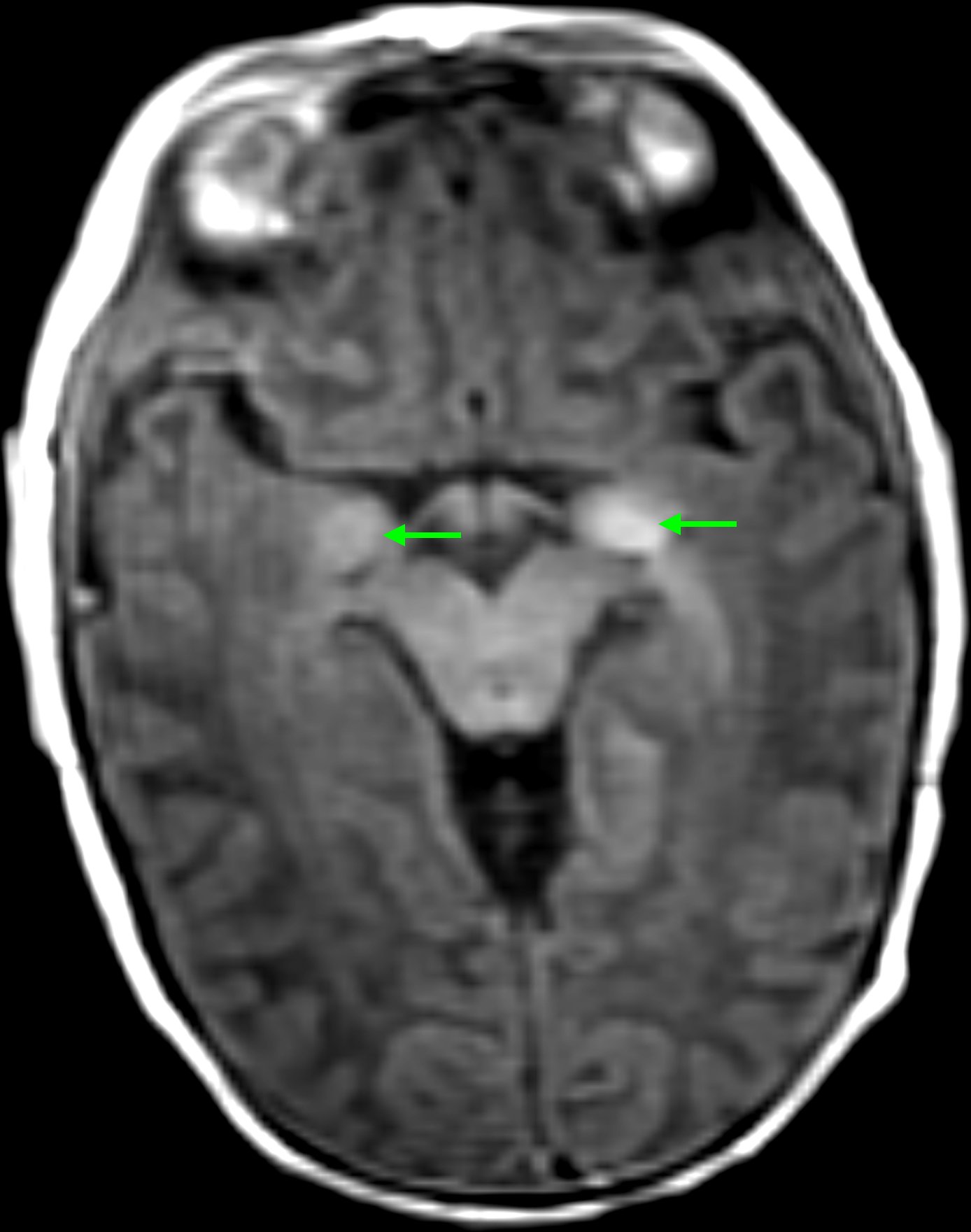
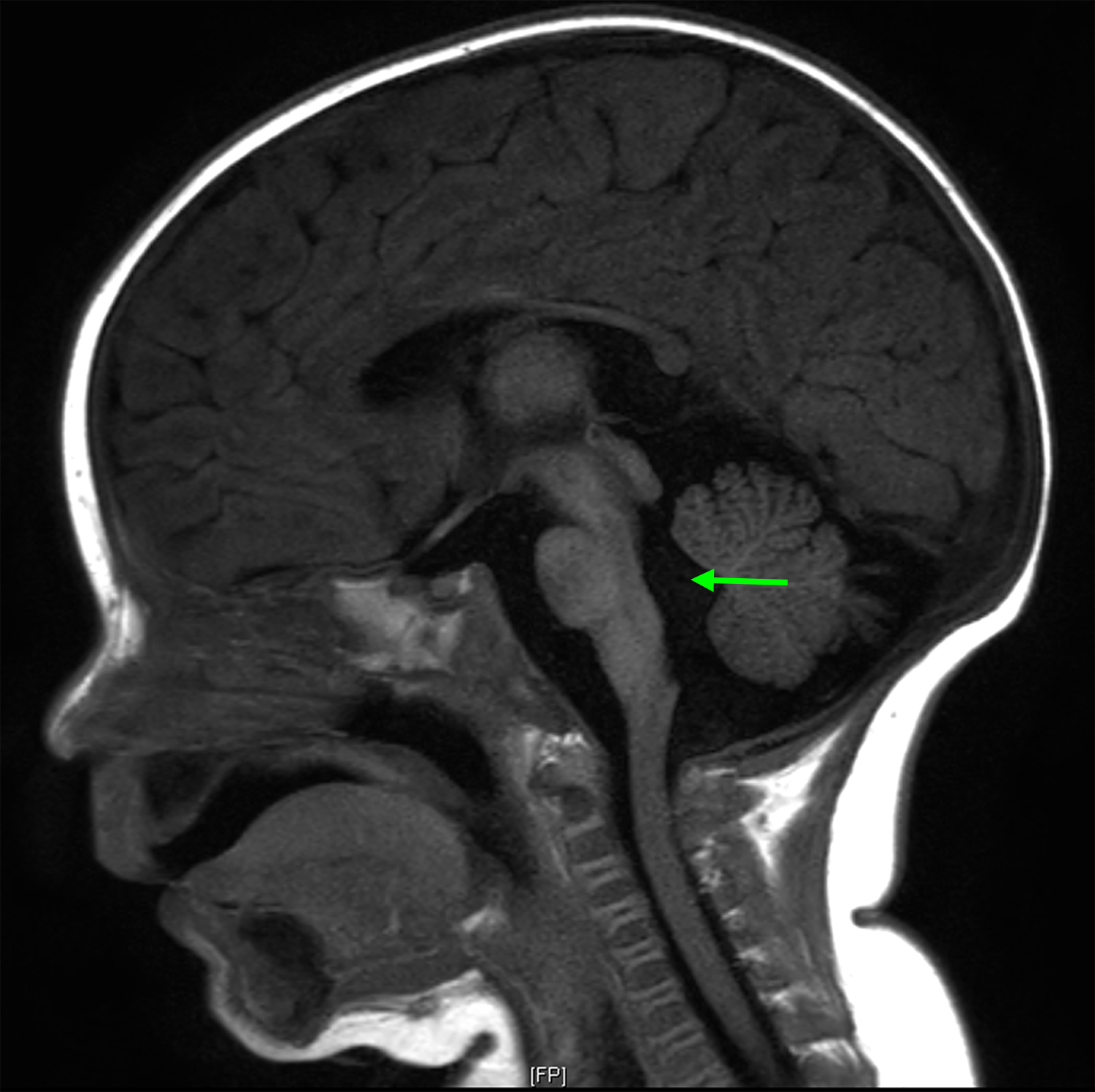
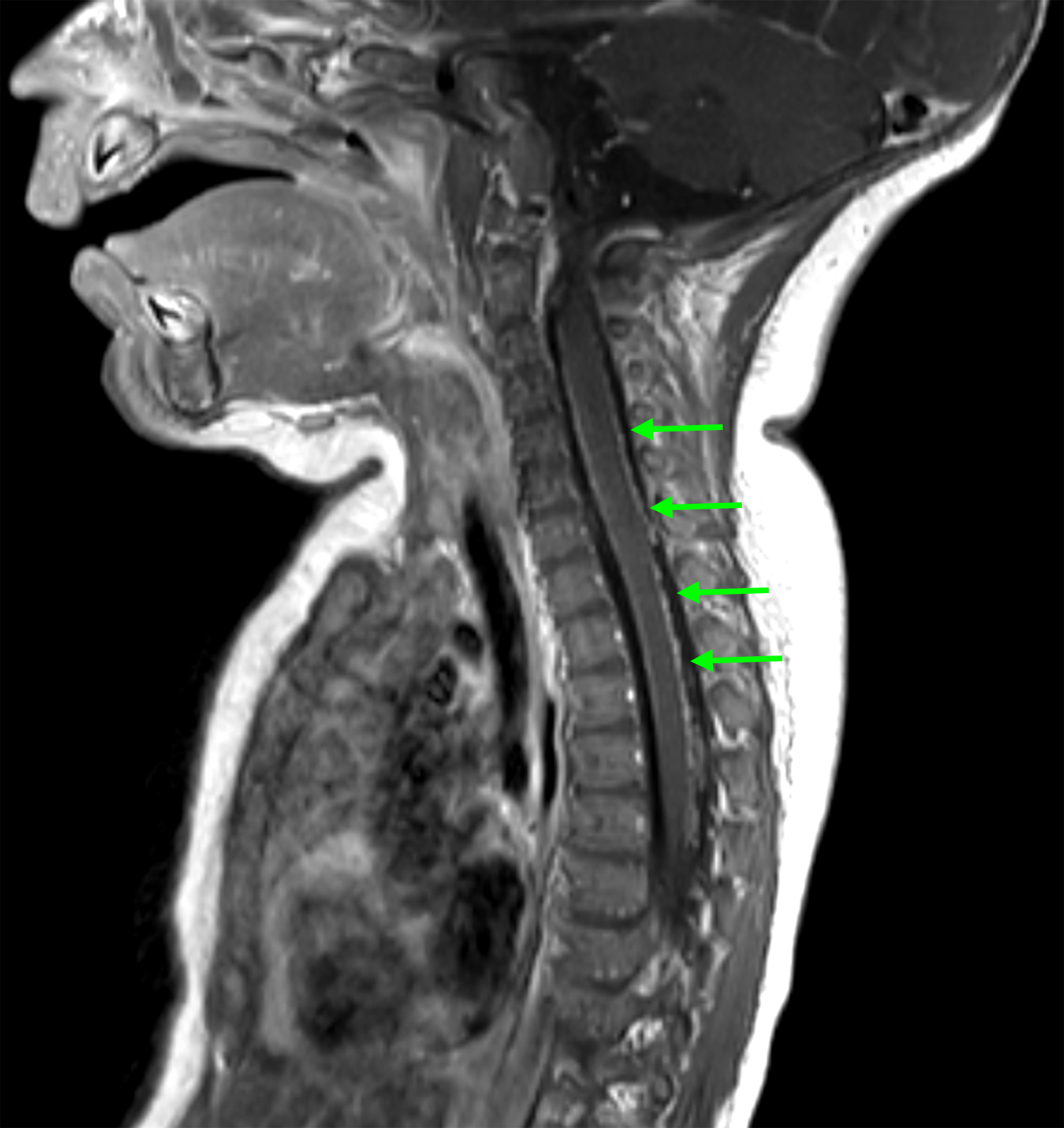
(A) Axial T1 MRI brain obtained shortly after birth, demonstrating T1 shortening in the cerebellum and brainstem (arrows), appearance radiographically consistent with NCM; (B) Sagittal T1 MRI brain at five months of age demonstrating hydrocephalus. Enlargement of the fourth ventricle is evident (arrow); (C) Sagittal T1 post-contrast MRI of the cervical and upper thoracic spine, obtained at the time patient presented with symptomatic hydrocephalus. Diffuse leptomeningeal enhancement is evident along the ventral and dorsal surfaces of the spinal cord (arrows).
At age 5 months, she presented with lethargy, ocular bobbing, and a bulging fontanelle. Repeat brain MRI was significant for interval development of hydrocephalus (Figure 1B). MRI spine demonstrated linear enhancement from the level of T1 to the conus medullaris, concerning for leptomeningeal disease (Figure 1C). She underwent placement of a programmable VP shunt, and the procedure did not involve incision through a cutaneous nevus. Cerebrospinal fluid (CSF) analysis with gram stain, culture, protein, glucose, cell count, and cytology was normal and revealed no evidence of malignant melanoma. Additional episodes of increased intracranial pressure characterized by projectile vomiting and ocular bobbing were managed with shunt adjustments with improvement in symptoms. Gross motor delay was evident with patient cruising at 15 months, but not taking independent steps. She was referred to physical therapy for early intervention, but she continued to have delay in milestone acquisition.
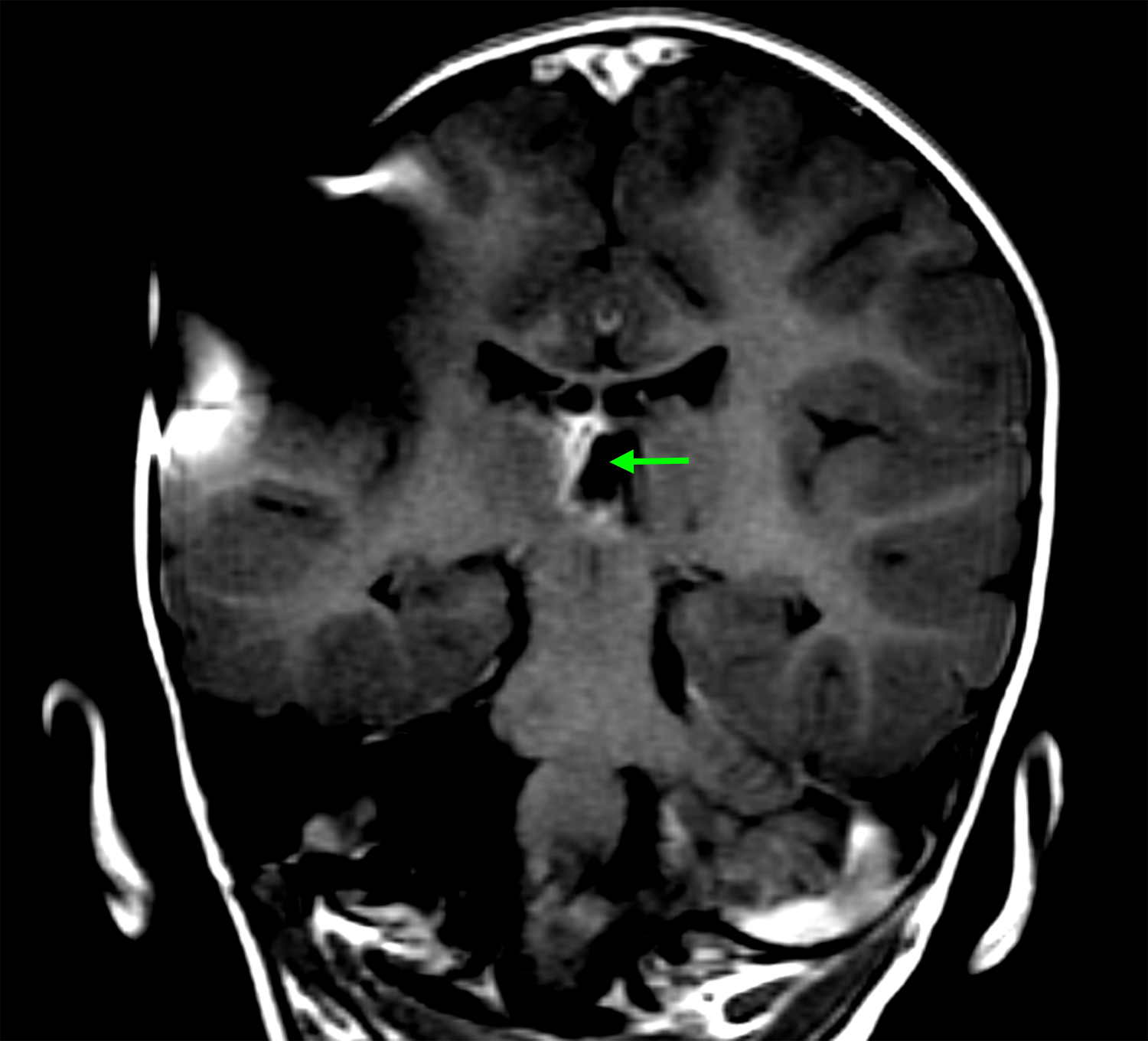
At 16 months of age, she developed a prominent inguinal hernia, which was repaired with herniorrhaphy. During the procedure, several peritoneal deposits were noted with biopsy confirming malignant melanocytic neoplasm with NRAS Q61K mutation (Figure 2A, 2B, 2C). Despite peritoneal involvement, her repeat MRI brain and spine were stable except for increased ependymal enhancement along the right medial thalamus and her VP shunt remained functional (Figure 1D).
Figure 2:
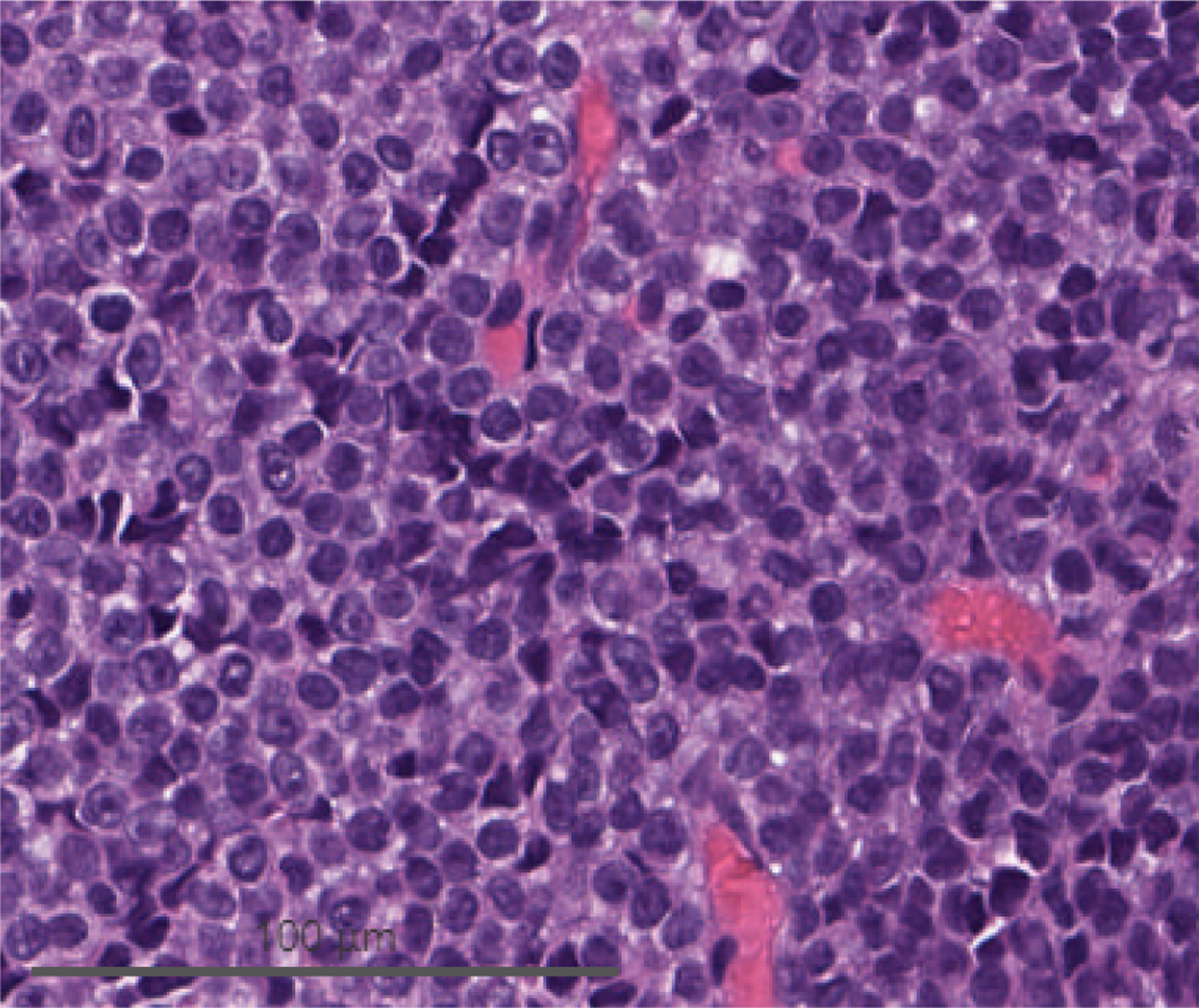
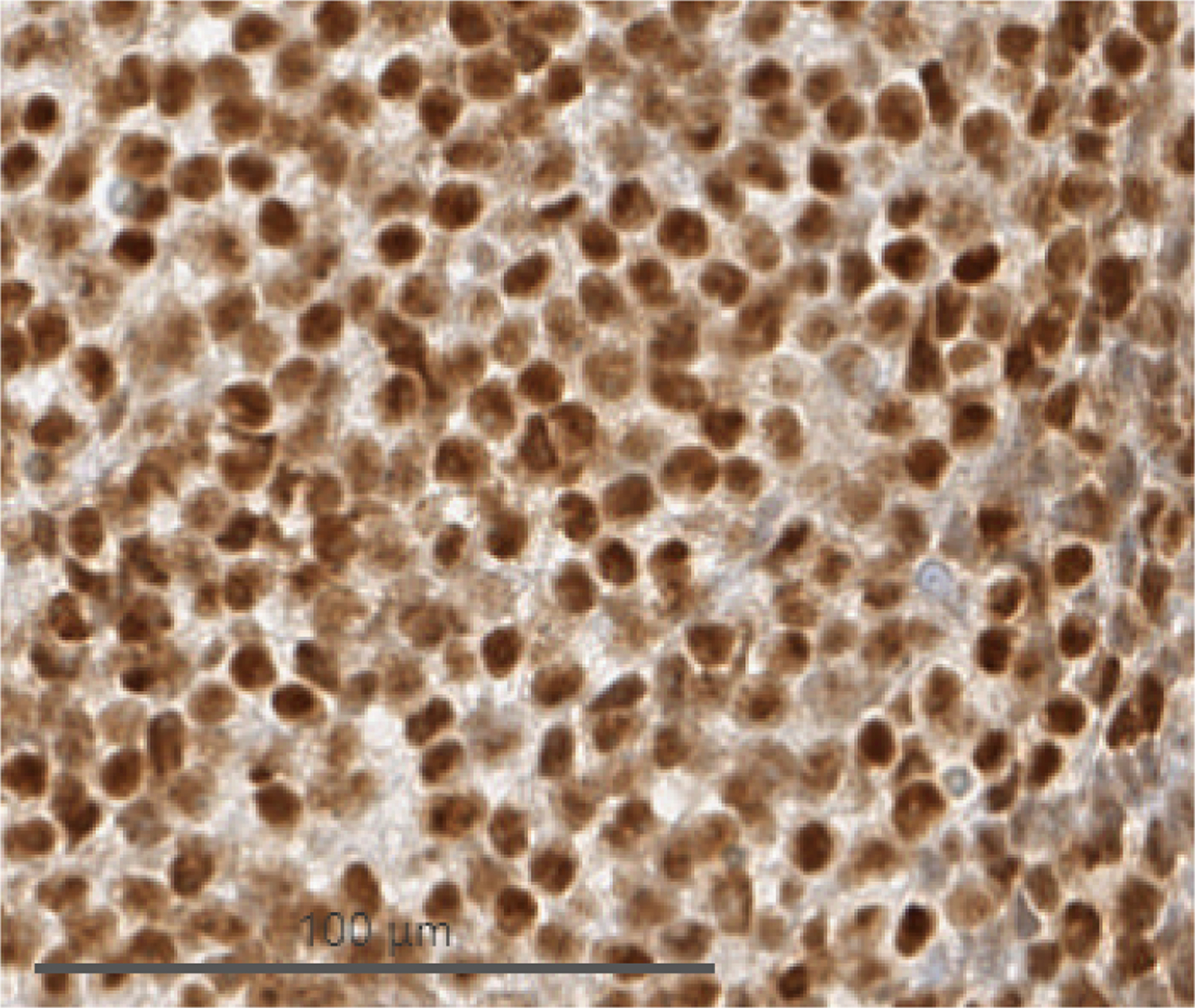
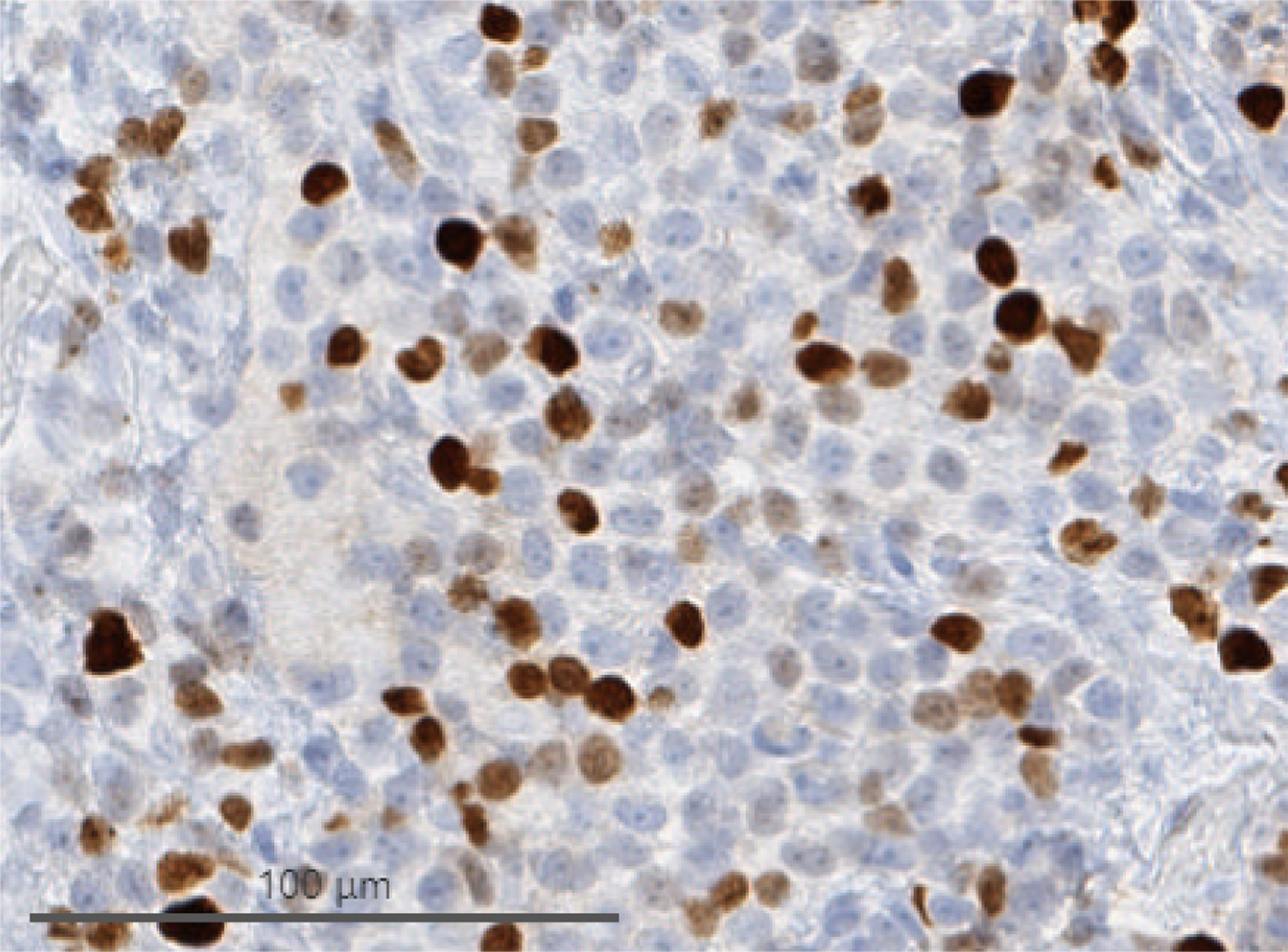
Cells from biopsy of peritoneal deposits identified during herniorrhaphy. (A) H&E demonstrating monomorphous, densely cellular neoplasm that is amelanotic. (B) Immunohistochemistry demonstrating tumor cell expression of SOX10, a melanocytic marker. (C) Immunohistochemistry demonstrating elevated Ki-67 activity.
Two weeks after hernia repair the patient developed abdominal distension; CT of the abdomen demonstrated ascites. Atypical cells were identified on paracentesis. A pigtail catheter was placed for symptomatic management and the fluid was sent to our lab to develop a patient-derived xenograft to study tumor behavior and predict the efficacy of chemotherapeutics, which was unsuccessful. She started systemic treatment with nivolumab, which was well-tolerated except for CTCAE grade I diarrhea.21 The peritoneal fluid developed increased cellularity several days after treatment with nivolumab and subsequently cleared.
Output from the abdominal catheter decreased over time despite worsening abdominal distension, consistent with obstruction of the peritoneal catheter due to malignant cells and proteinaceous discharge. She developed increasing lethargy, decreased appetite, and worsening abdominal distension with tachypnea and tachycardia. Her family opted for palliative care without further tumor-directed therapy. Ultimately, she died from complications of disease progression and ascites at age 21 months.
Discussion:
Leptomeningeal disease from melanoma or other tumors can result in increased intracranial pressure and symptomatic hydrocephalus.22 Some patients with leptomeningeal metastases experience transient neurologic symptoms such as sudden unresponsiveness or lethargy due to paroxysmal increases in intracranial pressure known as plateau waves.23,24 CSF diversion via VP shunting can reduce intracranial pressure and has been used for symptomatic management of leptomeningeal disease.13–15
In this case, our patient experienced hydrocephalus with plateau waves manifested as episodes of vomiting, ocular bobbing, and lethargy. The patient’s hydrocephalus was symptomatic and would have resulted in further neurologic decline and mortality if untreated. Shunting led to symptomatic improvement. However, during herniorrhaphy performed 11 months after shunt placement, peritoneal malignant melanoma deposits were identified. Neuroaxis imaging at the time of peritoneal melanoma diagnosis was stable with no radiographic progression of leptomeningeal melanocytosis and no evidence of solid melanoma within CNS parenchyma. Additionally, no changes were identified on examination of cutaneous nevi. While the precise location of malignant transformation is unclear and spread to the peritoneum from other sources could have occurred, we speculate melanocytes were deposited into the peritoneal cavity through the VP shunt and subsequently underwent malignant transformation. Peritoneal tumor led to development of malignant ascites. The proteinaceous malignant ascites proved difficult to manage, resulting in progressive lethargy, abdominal distention, and death. Despite the uniformly poor prognosis of NCM-associated melanoma in the CNS, she experienced progressive peritoneal disease in the setting of relatively stable radiographic appearance of CNS leptomeningeal melanocytosis.
VP shunting is generally safe with complications including shunt malfunction or infection.15 Peritoneal metastasis of malignant cells from CSF to the peritoneum in patient with primary or metastatic brain tumors has been described in several case reports including melanoma associated with NCM and other tumors.16–19,25 However, this appears to be a rare event as multiple retrospective reviews in patients with leptomeningeal carcinomatosis, primary brain tumors, and pediatric brain tumors have failed to show an increased risk of peritoneal metastasis after VP shunting.15,16,25 Optimal management of peritoneal metastasis related to VP shunting is yet to be established though intraperitoneal therapy may be considered.
Leptomeningeal melanocytic deposition and melanoma associated with NCM can develop within the CNS in absence of cutaneous melanoma and changes in nevus characteristics.1,8 While VP shunting remains an important tool for symptomatic management of increased intracranial pressure in patients with leptomeningeal disease from NCM, patients and family members should be counseled about potential risk of peritoneal seeding and associated tumor spread.
Acknowledgments:
The authors acknowledge the support of the NIH Cancer Center Support Grant P30 CA008748. Nevus Outreach Inc. provided funding to YK for travel and data collection.
References:
- 1.Mitre V, Heym K, Clark GD, Venkatramani R. Neurocutaneous Melanocytosis and Leptomeningeal Melanoma. J Pediatr Hematol Oncol. 2019. [DOI] [PubMed] [Google Scholar]
- 2.Kadonaga JN, Frieden IJ. Neurocutaneous melanosis: definition and review of the literature. J Am Acad Dermatol. 1991;24(5 Pt 1):747–755. [DOI] [PubMed] [Google Scholar]
- 3.Schaff LR, Marghoob A, Rosenblum MK, Meyer R, Khakoo Y. Malignant transformation of neurocutaneous melanosis (NCM) following immunosuppression. Pediatr Dermatol. 2019;36(4):497–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jakchairoongruang K, Khakoo Y, Beckwith M, Barkovich AJ. New insights into neurocutaneous melanosis. Pediatr Radiol. 2018;48(12):1786–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramaswamy V, Delaney H, Haque S, Marghoob A, Khakoo Y. Spectrum of central nervous system abnormalities in neurocutaneous melanocytosis. Dev Med Child Neurol. 2012;54(6):563–568. [DOI] [PubMed] [Google Scholar]
- 6.Hale EK, Stein J, Ben-Porat L, et al. Association of melanoma and neurocutaneous melanocytosis with large congenital melanocytic naevi--results from the NYU-LCMN registry. Br J Dermatol. 2005;152(3):512–517. [DOI] [PubMed] [Google Scholar]
- 7.Waelchli R, Aylett SE, Atherton D, Thompson DJ, Chong WK, Kinsler VA. Classification of neurological abnormalities in children with congenital melanocytic naevus syndrome identifies magnetic resonance imaging as the best predictor of clinical outcome. Br J Dermatol. 2015;173(3):739–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2019;381(16):1535–1546. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan RJ, Hamid O, Gonzalez R, et al. Atezolizumab plus cobimetinib and vemurafenib in BRAF-mutated melanoma patients. Nat Med. 2019;25(6):929–935. [DOI] [PubMed] [Google Scholar]
- 10.Kinsler VA, Aylett SE, Coley SC, Chong WK, Atherton DJ. Central nervous system imaging and congenital melanocytic naevi. Arch Dis Child. 2001;84(2):152–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas S, Patel B, Varghese SS, Backianathan S. Neurocutaneous Melanosis with Leptomeningeal Melanoma Involving Supratentorium and Infratentorium. Cureus. 2018;10(9):e3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polubothu S, McGuire N, Al-Olabi L, et al. Does the gene matter? Genotype-phenotype and genotype-outcome associations in congenital melanocytic naevi. Br J Dermatol. 2020;182(2):434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharouf F, Zaben M, Lammie A, Leach P, Bhatti MI. Neurocutaneous melanosis presenting with hydrocephalus and malignant transformation: case-based update. Childs Nerv Syst. 2018;34(8):1471–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsueh CW, Ho CS, Chiu NC, Shen EY. Neurocutaneous melanosis with hydrocephalus: report of one case. Acta Neurol Taiwan. 2004;13(1):29–33. [PubMed] [Google Scholar]
- 15.Kim HS, Park JB, Gwak HS, Kwon JW, Shin SH, Yoo H. Clinical outcome of cerebrospinal fluid shunts in patients with leptomeningeal carcinomatosis. World J Surg Oncol. 2019;17(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berger MS, Baumeister B, Geyer JR, Milstein J, Kanev PM, LeRoux PD. The risks of metastases from shunting in children with primary central nervous system tumors. J Neurosurg. 1991;74(6):872–877. [DOI] [PubMed] [Google Scholar]
- 17.Narayan A, Jallo G, Huisman TA. Extracranial, peritoneal seeding of primary malignant brain tumors through ventriculo-peritoneal shunts in children: Case report and review of the literature. Neuroradiol J. 2015;28(5):536–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cajaiba MM, Benjamin D, Halaban R, Reyes-Mugica M. Metastatic peritoneal neurocutaneous melanocytosis. Am J Surg Pathol. 2008;32(1):156–161. [DOI] [PubMed] [Google Scholar]
- 19.Humes RA, Roskamp J, Eisenbrey AB. Melanosis and hydrocephalus. Report of four cases. J Neurosurg. 1984;61(2):365–368. [DOI] [PubMed] [Google Scholar]
- 20.Krengel S, Scope A, Dusza SW, Vonthein R, Marghoob AA. New recommendations for the categorization of cutaneous features of congenital melanocytic nevi. J Am Acad Dermatol. 2013;68(3):441–451. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. published November 27, 2017. Accessed August 4, 2020. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf.
- 22.Clarke JL, Perez HR, Jacks LM, Panageas KS, Deangelis LM. Leptomeningeal metastases in the MRI era. Neurology. 2010;74(18):1449–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gold CA, Odom N, Srinivasan S, Schaff L, Haggiagi A, Odia Y. Electrographic Correlates of Plateau Waves in Patients With Leptomeningeal Metastases. Neurohospitalist. 2016;6(4):161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang EW, Kasprowicz M, Smielewski P, Pickard J, Czosnyka M. Changes in Cerebral Partial Oxygen Pressure and Cerebrovascular Reactivity During Intracranial Pressure Plateau Waves. Neurocrit Care. 2015;23(1):85–91. [DOI] [PubMed] [Google Scholar]
- 25.Nigim F, Critchlow JF, Kasper EM. Role of ventriculoperitoneal shunting in patients with neoplasms of the central nervous system: An analysis of 59 cases. Mol Clin Oncol. 2015;3(6):1381–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]


