ABSTRACT
Engineered conditional gene expression is used in appraisal of gene function and pathway relationships. For pathogens like the fungus Candida albicans, conditional expression systems are most useful if they are active in the infection environment and if they can be utilized in multiple clinical isolates. Here, we describe such a system. It employs the RBT5 promoter and can be implemented with a few PCRs. We validated the system with RBT5 promoter fusions to two genes that promote filamentation and polarized growth, UME6 and HGC1, and with efg1Δ/Δ mutants, which are defective in an activator of filamentous growth. An RBT5 promoter fusion to either gene enabled filamentous growth of an efg1Δ/Δ mutant of strain SC5314 in iron-limited media, including RPMI with serum and yeast extract-peptone-dextrose with bathophenanthrolinedisulfonic acid. The RBT5-UME6 fusion promoted filamentation of efg1Δ/Δ mutants in RPMI with serum of four other clinical C. albicans isolates as well. In a mouse model of disseminated candidiasis, the RBT5-UME6 fusion promoted filamentation of the SC5314 efg1Δ/Δ mutant in kidney tissue, an indication that the RBT5 promoter is active in the iron-limited host environment. The RBT5 promoter expands the conditional expression toolkit for C. albicans genetics.
IMPORTANCE Genetic strategies have been vital for mechanistic analysis of biological processes. Here, we describe a genetic tool for the fungal pathogen Candida albicans.
KEYWORDS: Candida albicans, gene expression, genetics, hyphal development
INTRODUCTION
Analysis of gene function often tests the impact of a gene alteration on phenotype. Such alterations can include reductions or increases in gene function or expression (1–3), as well as sequence variations that can result in quantitative or qualitative functional changes (4, 5). For pathogens like Candida albicans, the focus of our study, it is important that the impact of genetic alteration is manifested during proliferation in infection models. This feature allows assessment of the potential role of a gene in virulence-associated processes.
C. albicans is commensal on mucosal surfaces of the gastrointestinal and urogenital tracts in healthy individuals and can cause severe systemic infections in at-risk patients (6). Our understanding of virulence determinants is based mainly on deletion mutations, which cause a loss of gene function (7). However, as discussed in depth by Rai et al. (3), gene overexpression approaches have exceptional value for analysis of genetic redundancy, functional sufficiency, and epistasis or pathway relationships. Gene overexpression studies have been vital for study of virulence-associated processes that include the yeast-hypha transition and biofilm development (3).
Several constitutive promoters, such as PACT1 and PTDH3, are employed in C. albicans to induce stable high-level expression of the downstream gene (8, 9). Several conditional promoters regulate the expression of genes in a specific condition. For instance, the expression of genes under the control of PPCK1, PMAL2, or PMET3 can be shut off by medium with glucose or methionine and cysteine (10–12). For conditional expression that can be controlled in vitro or in vivo, the PTET-OFF and PTET-ON systems respond to tetracycline analogs that do not affect metabolism (13). Thus, many platforms are already available for C. albicans gene overexpression studies.
Does the C. albicans research community need yet another gene overexpression platform? The answer is an unequivocal “maybe.” We sought features in our system that may be relevant to other investigators’ interests. We wanted the ability to overexpress a gene in a murine infection model; we wanted a system that worked with multiple clinical C. albicans isolates; we wanted a system that used a native C. albicans promoter to avoid the spiral of materials requests, material transfer agreement negotiations, and shipping and handling costs that can try an investigator’s patience and pocketbook. We expect that the RBT5 promoter will be a useful arrow in the Candida geneticist’s quiver.
RESULTS AND DISCUSSION
Design and construction of an RBT5-based gene expression system.
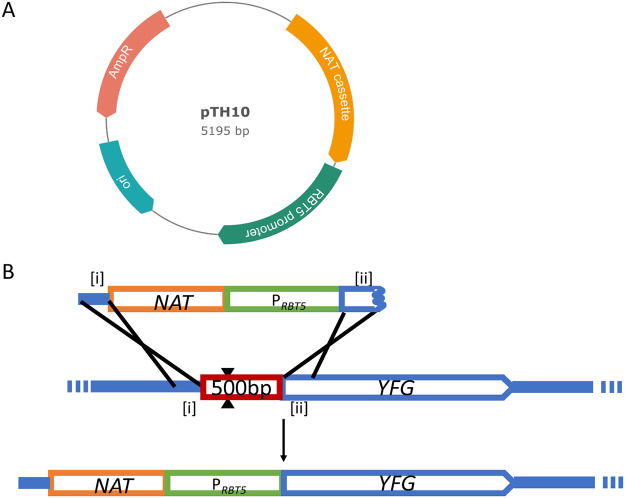
To achieve regulated overexpression of target genes in C. albicans, we designed a cassette containing the ~1-kbp RBT5 5′ region and the nourseothricin resistance (NAT) marker for selection of transformants (Fig. 1A). RBT5 was chosen because its RNA displays a large expression difference between iron-replete and iron-limited growth conditions (14) and it is highly expressed in vivo (15). In addition, its modest size facilitates DNA manipulations. The NAT expression cassette, from plasmid pCJN542 (16), has been used for transformation of several different clinical isolates (17). These two components were assembled in plasmid pTH10 (Fig. 1B), and the RBT5 segment was sequence verified.
FIG 1.
PRBT5 expression system. (A) DNA segments containing the NAT1 marker and RBT5 promoter, assembled as PCR products in plasmid pTH10. (B) Sequence [i], with homology to the upstream region of YFG, is appended to the cassette with a long primer containing an 80-bp region of homology. Sequence [ii], with homology to the start of the YFG open reading frame, is also appended to the cassette with long primer containing an 80-bp region of homology. The cassette is transformed into a recipient strain along with DNA cassettes expressing CAS9 and a single guide RNA targeting upstream of the YFG locus. The double-strand break introduced by Cas9 complexed with an sgRNA targeting the YFG upstream region is indicated by two black triangles. Expected homologous recombination events are depicted as single crosses, and together they should yield a locus containing NatR and PRBT5 fused to the YFG open reading frame.
RBT5 promoter activity in vitro.
We first tested the system with the UME6 gene in the SC5314 strain background. Ume6 activates filamentation, so expression of UME6 can be inferred from cell morphology (18). We used PCR to add 80-bp arms to the NAT-PRBT5 cassette. The 80-bp arms were homologous to the UME6 5′ region (Fig. 1A) and were designed to direct homologous integration into the native alleles in the C. albicans genome. The NAT-PRBT5 construct was transformed into wild-type and filamentation-defective efg1Δ/Δ strains, using a transient CRISPR approach (19), and NatR transformants were selected and genotyped. Previous studies have shown that the efg1Δ/Δ filamentation defect is overcome when UME6 is overexpressed (20). Therefore, the activity of PRBT5-UME6 can be measured by the resulting filamentation level.
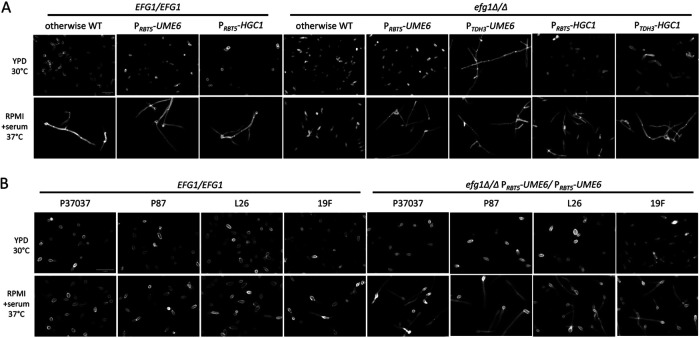
We first examined filamentation of the otherwise-wild-type strain homozygous for either UME6 or PRBT5-UME6 (Fig. 2A). We examined a yeast extract-peptone-dextrose (YPD), 30°C overnight culture, which was noninducing for filamentation and iron replete, and an RPMI with serum, 37°C 4-h culture, which was inducing for filamentation and was iron limited. Both strains grew as yeast cells in YPD and formed filaments in RPMI with serum (Fig. 2A). These results matched expectations and indicated that PRBT5-UME6 has low activity in YPD at 30°C. A more discerning test of PRBT5-UME6 function was provided by responses of efg1Δ/Δ mutants homozygous for either UME6 or PRBT5-UME6. Both strains grew as yeast cells in YPD, but only the PRBT5-UME6 strain formed filaments in RPMI with serum (Fig. 2A). Results with the efg1Δ/Δ UME6/UME6 strain matched expectations, given that Efg1 is required for filamentation. Results with the efg1Δ/Δ PRBT5-UME6/PRBT5-UME6 strain indicated that PRBT5-UME6 is expressed in RPMI with serum at 37°C. A control efg1Δ/Δ PTDH3-UME6/PTDH3-UME6 strain, in which UME6 expression is driven by the constitutive TDH3 promoter (16), produced hyphae in both YPD and RPMI with serum cultures (Fig. 2A), matching expectations for constitutive UME6 expression. These results indicate that the transplanted RBT5 promoter directs regulated expression of UME6.
FIG 2.
Filamentation assays in YPD and RPMI with serum media. (A) Cells of genotype EFG1/EFG1 or efg1Δ/Δ and otherwise wild type or otherwise homozygous for PRBT5-UME6, PTDH3-UME6, PRBT5-HGC1, or PTDH3-HGC1 as indicated were assayed for filamentation under planktonic growth conditions. Strains were grown in YPD medium overnight at 30°C with shaking or transferred to RPMI with 10% serum at 37°C for 4 h after YPD overnight growth with shaking. Fixed cells were stained with calcofluor white for confocal microscopy. White scale bar, 20 μm. (B) EFG1/EFG1 and efg1Δ/Δ PRBT5-UME6/PRBT5-UME6 strains from the indicated clinical isolate backgrounds were assayed for filamentation as described for panel A. White scale bar, 20 μm.
Most studies of gene function have been conducted with the SC5314 type strain of C. albicans and its derivatives. However, the filamentation gene regulatory network is variable among clinical isolates (17). This consideration prompted us to test the RBT5 promoter in multiple genetic backgrounds. We constructed efg1Δ/Δ PRBT5-UME6/PRBT5-UME6 strains in the P37037, P87, L26, and 19F backgrounds (21, 22). All strains grew as yeast cells in YPD and formed filaments in RPMI with serum (Fig. 2B). Filamentation levels in RPMI with serum for the efg1Δ/Δ PRBT5-UME6/PRBT5-UME6 strains were greater than those for the wild-type clinical isolates (Fig. 2B). These results indicate that the transplanted RBT5 promoter directs expression of UME6 in multiple strain backgrounds in RPMI with serum medium.
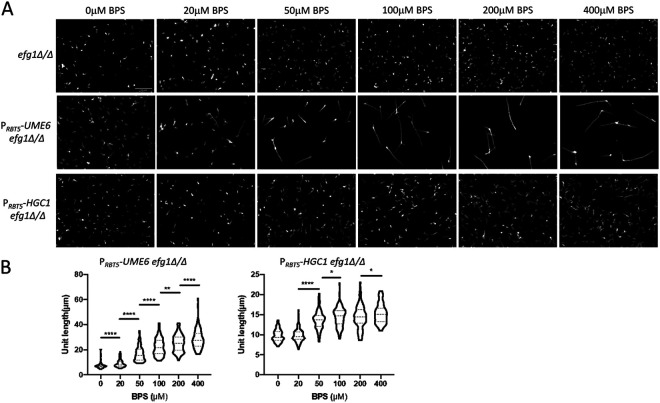
To establish that iron levels regulate RBT5 promoter activity in the PRBT5-UME6 construct, we tested filamentation of the SC5314-derived efg1Δ/Δ PRBT5-UME6/PRBT5-UME6 strain under conditions of progressive iron limitation. Iron levels were modulated through chelation with bathophenanthrolinedisulfonic acid (BPS) (23). In YPD medium at 37°C, addition of BPS increased the frequency and length of hyphal filaments (Fig. 3A and B). In an efg1Δ/Δ mutant without the PRBT5-UME6 construct, BPS did not induce filamentation (Fig. 3A). Therefore, iron limitation increases RBT5 promoter activity in the PRBT5-UME6 allele.
FIG 3.
Effect of iron chelation on RBT5 promoter constructs. (A) Filamentation assays of efg1Δ/Δ, efg1Δ/Δ PRBT5-UME6/PRBT5-UME6, and efg1Δ/Δ PRBT5-HGC1/PRBT5-HGC1 strains were grown in YPD plus 0, 20, 50, 100, 200, 300, or 400 μM BPS at 37°C for 4 h with shaking. Fixed cells were stained with calcofluor white for confocal microscopy. The white scale bar (top left panel) represents 50 μm. (B) Cell body lengths were quantified with ImageJ, with a minimum of 100 cells for each BPS concentration. Values shown are means with SD. Pairs of means connected by a horizontal bar are significantly different (Tukey-Kramer test: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
To establish that the RBT5 promoter can be applied to additional genes, we turned to the HGC1 gene. HGC1 encodes a hypha-associated cyclin whose overexpression is sufficient to drive filamentation in a variety of noninducing conditions (24, 25). An efg1Δ/Δ PRBT5-HGC1/PRBT5-HGC1 strain grew as yeast in YPD culture and produced filaments in RPMI with serum (Fig. 2A). A control efg1Δ/Δ PTDH3-HGC1/PTDH3-HGC1 strain produced elongated cells in YPD culture and hyphae in RPMI with serum culture (Fig. 2A). Constitutive HGC1 expression has been reported previously not to override all filament-inducing signals (24), and the results in YPD at 30°C are consistent with those reports. Importantly, PRBT5-HGC1 can bypass the dependence of filamentation on Efg1, as expected given that Hgc1 acts downstream of Efg1 (20) and that it is expressed independently of Efg1 from the PRBT5-HGC1 allele.
We used BPS titration in YPD at 37°C to confirm that iron levels regulate PRBT5-HGC1 activity. Increasing BPS concentrations resulted in increasing filamentation of the efg1Δ/Δ PRBT5-HGC1/PRBT5-HGC1 strain (Fig. 3A and B). The level of filamentation driven by PRBT5-HGC1 was less than that driven by PRBT5-UME6 (Fig. 3A and B), a recapitulation of observations with the TDH3 promoter-driven alleles in YPD (Fig. 2A). These results confirmed that iron limitation increases RBT5 promoter activity in the PRBT5-HGC1 allele.
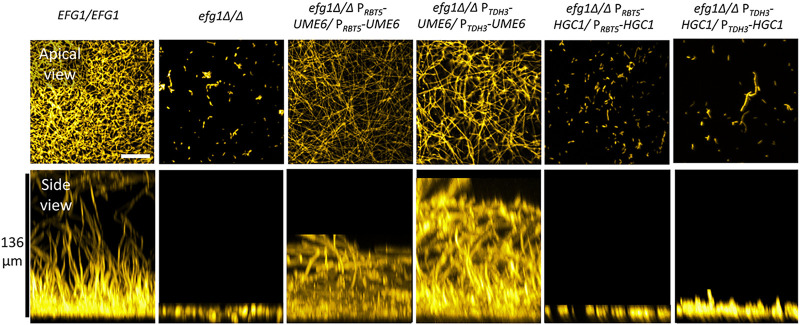
UME6 and HGC1 govern biofilm formation as well as hypha formation (20). To assess the utility of RBT5 promoter activity under biofilm growth conditions, we assayed biofilm formation by wild-type, efg1Δ/Δ, efg1Δ/Δ PRBT5-UME6/PRBT5-UME6 and efg1Δ/Δ PRBT5-HGC1/PRBT5-HGC1 strains. Biofilms were grown in RPMI with serum at 37°C. The wild-type strain produced biofilm and the efg1Δ/Δ strain did not, as expected (Fig. 4). The efg1Δ/Δ PRBT5-UME6/PRBT5-UME6 strain produced biofilm at levels comparable to an efg1Δ/Δ PTDH3-UME6/PTDH3-UME6 strain, assayed side-by-side (Fig. 4). The efg1Δ/Δ PRBT5-HGC1/PRBT5-HGC1 strain failed to produce biofilm, as did a side-by-side efg1Δ/Δ PTDH3-HGC1/PTDH3-HGC1 strain (Fig. 4). Given that either of two promoter fusions enables UME6 to bypass the efg1Δ/Δ mutation and that neither enables HGC1 to do so, we infer that the RBT5 promoter is active under these biofilm growth conditions. The difference in outcomes with PRBT5-UME6 and PRBT5-HGC1 likely reflects differences in functional activity of Ume6 and Hgc1.
FIG 4.
Biofilm assays. Cells of genotype EFG1/EFG1 or efg1Δ/Δ and otherwise wild type or homozygous for PRBT5-UME6, PTDH3-UME6, PRBT5-HGC1, or PTDH3-HGC1 as indicated were assayed for biofilm formation after growth in a 96-well plate in RPMI with 10% serum at 37°C for 24 h. Fixed biofilms were stained using calcofluor white, then imaged by confocal microscopy. Representative apical and side views are shown. For apical views, the white scale bar represents 50 μm. For side views, the vertical black scale bar represents 136 μm.
To test expression levels driven by the RBT5 promoter, we conducted reverse transcription-quantitative PCR (RT-qPCR) assays with cells grown in RPMI with serum medium. RNA levels of PRBT5-UME6 or PRBT5-HGC1 alleles were 20- to 40-fold higher than for the native alleles in the parent efg1Δ/Δ strain (Fig. 5A). RNA levels were similar in magnitude for alleles driven by the RBT5 and TDH3 promoters (Fig. 5A). In a wild-type background, RNA levels driven by the RBT5 promoter and the native UME6 or HGC1 promoter were comparable (Fig. 5B). These results confirmed that the RBT5 promoter can drive high-level expression of C. albicans genes.
FIG 5.
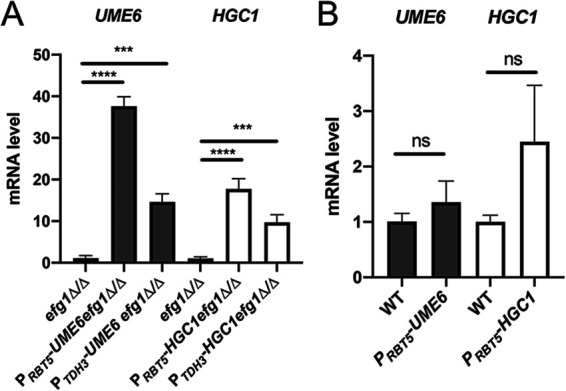
Expression levels of RBT5 promoter constructs. Strains were grown in RPMI with 10% serum at 37°C for 4 h with shaking. qRT-PCR analysis for RNA levels of UME6 or HGC1 under control of their native promoters, the RBT5 promoter, or the TDH3 promoter were measured and normalized to the level of ACT1 mRNA. Results from three independent experiments are shown. Values shown are means (+SD). Pairs of means connected by a horizontal bar are significantly different (Tukey-Kramer test: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001). (A) Strains were genotype efg1Δ/Δ and homozygous for the hybrid UME6 or HGC1 alleles indicated. (B) Strains were genotype EFG1/EFG1 and homozygous for the hybrid UME6 or HGC1 alleles indicated.
RBT5 promoter activity during infection.
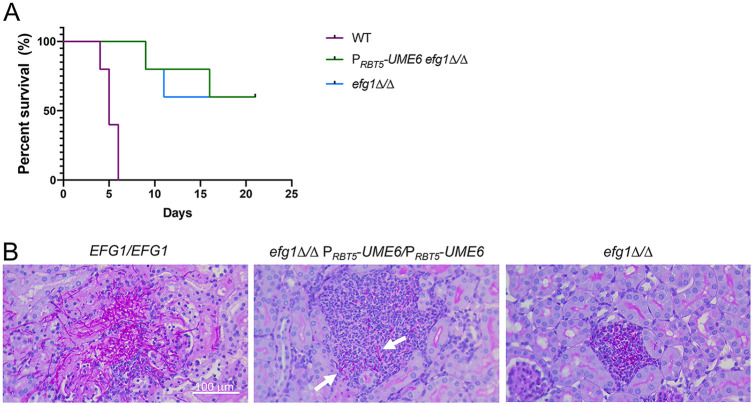
To determine whether the RBT5 promoter can drive gene expression during infection, we used a mouse model of hematogenously disseminated infection (26). We compared mouse survival after infection with wild-type, efg1Δ/Δ, and efg1Δ/Δ PRBT5-UME6/PRBT5-UME6 strains. Based on host survival times, the wild-type strain was virulent and the efg1Δ/Δ mutant was attenuated (Fig. 6A), as expected from previous studies (27). The efg1Δ/Δ PRBT5-UME6/PRBT5-UME6 strain was no more virulent than the efg1Δ/Δ strain (Fig. 6A). To determine whether the PRBT5-UME6 allele may drive filamentation during infection, we conducted histopathological examination of kidney sections from mice at 2 days postinfection. The wild-type strain produced abundant filaments in the kidney, while the efg1Δ/Δ mutant produced none (Fig. 6B). The efg1Δ/Δ PRBT5-UME6/PRBT5-UME6 strain produced short filaments (Fig. 6B). The ability of the PRBT5-UME6 allele to modify the efg1Δ/Δ mutant phenotype indicates that the PRBT5-UME6 allele is expressed and functional during infection. It will be interesting in future detailed studies to define the spectrum of genes that are expressed by the efg1Δ/Δ PRBT5-UME6/PRBT5-UME6 strain during infection. Prior in vitro studies indicated that UME6 overexpression in an efg1Δ/Δ mutant is not sufficient to activate many known virulence-related genes, including ECE1, ALS3, and HWP1 (20). The efg1Δ/Δ PRBT5-UME6/PRBT5-UME6 strain may be a useful platform to define Efg1-dependent genes that promote virulence.
FIG 6.
Virulence tests. (A) Survival of mice infected with the indicated strains. (B) Histology sections of infected mouse kidneys at 2 days postinfection with the strains indicated. White arrows point to filamentous cells produced by the efg1Δ/Δ PRBT5-UME6/PRBT5-UME6 strain. White scale bar, 100 μm.
MATERIALS AND METHODS
Strains and culture conditions.
All strains are listed in Table 1. C. albicans strains SC5314, P37037, P87, L26, and 19F (21, 22) and their derived efg1Δ/Δ mutants were used as transformation recipients. Construction of the efg1Δ/Δ mutants in clinical isolates has been described previously (17) and will be detailed for strains P37037, L26, and 19F separately. Fungal strains were grown at 30°C in YPD (2% Bacto peptone, 2% dextrose, 1% yeast extract) with shaking. For phenotypic assays and biofilm assays, strains were grown in liquid RPMI 1640 medium (Sigma-Aldrich, St. Louis, MO) adjusted to pH 7.4 and supplemented with 10% fetal bovine serum (Atlanta Biologicals, Inc., Flowery Branch, GA). C. albicans transformants were selected on YPD plus NAT (2% Bacto peptone, 2% dextrose, 1% yeast extract and 400 μg/mL [Werner BioAgents]) for nourseothricin-resistant (NatR) isolates. All strains were stored as glycerol stocks at −80°C.
TABLE 1.
Strains
| Strain no. | Strain | Phenotype | Genotype |
|---|---|---|---|
| YM1 | SC5314 | His+ NatS | SC5314 wild type |
| MC84 | SC5314 efg1Δ/Δ | His+ NatS | efg1Δ::r1HIS1r1/efg1Δ::r1HIS1r1 his1Δ::r3/his1Δ::r3 |
| MC431 | SC5314 efg1Δ/Δ PTDH3-UME6 | His+ NatR | efg1Δ::r1HIS1r1/efg1Δ::r1HIS1r1 UME6::PTDH3-UME6/UME6::PTDH3-UME6 his1Δ::r3/his1Δ::r3 |
| MC153 | P37037 efg1Δ/Δ | His+ NatS | efg1Δ::r1HIS1r1/efg1Δ::r1HIS1r1 his1Δ::r3/his1Δ::r3 |
| MC144 | P87 efg1Δ/Δ | His+ NatS | efg1Δ::r1HIS1r1/efg1Δ::r1HIS1r1 his1Δ::r3/his1Δ::r3 |
| MC147 | L26 efg1Δ/Δ | His+ NatS | efg1Δ::r1HIS1r1/efg1Δ::r1HIS1r1 his1Δ::r3/his1Δ::r3 |
| MC150 | 19F efg1Δ/Δ | His+ NatS | efg1Δ::r1HIS1r1/efg1Δ::r1HIS1r1 his1Δ::r3/his1Δ::r3 |
| YM150 | SC5314 efg1Δ/Δ PRBT5-UME6 | His+ NatR | efg1Δ::r1HIS1r1/efg1Δ::r1HIS1r1 UME6::PRBT5-UME6/UME6::PRBT5-UME6 his1Δ::r3/his1Δ::r3 |
| YM154 | SC5314 PRBT5-UME6 | His+ NatR | UME6::PRBT5-UME6/UME6::PRBT5-UME6 |
| YM155 | P37037 efg1Δ/Δ PRBT5-UME6 | His+ NatR | efg1Δ::r1HIS1r1/efg1Δ::r1HIS1r1 UME6::PRBT5-UME6/UME6::PRBT5-UME6 his1Δ::r3/his1Δ::r3 |
| YM156 | P87 efg1Δ/Δ PRBT5-UME6 | His+ NatR | efg1Δ::r1HIS1r1/efg1Δ::r1HIS1r1 UME6::PRBT5-UME6/UME6::PRBT5-UME6 his1Δ::r3/his1Δ::r3 |
| YM157 | L26 efg1Δ/Δ PRBT5-UME6 | His+ NatR | efg1Δ::r1HIS1r1/efg1Δ::r1HIS1r1, UME6::PRBT5-UME6/UME6::PRBT5-UME6 his1Δ::r3/his1Δ::r3 |
| YM158 | 19F efg1Δ/Δ PRBT5-UME6 | His+ NatR | efg1Δ::r1HIS1r1/efg1Δ::r1HIS1r1 UME6::PRBT5-UME6/UME6::PRBT5-UME6 his1Δ::r3/his1Δ::r3 |
| YM160 | SC5314 PRBT5-HGC1 | His+ NatR | HGC1::PRBT5-HGC1/HGC1::PRBT5-HGC1 |
| YM161 | SC5314 efg1Δ/Δ PRBT5-HGC1 | His+ NatR | efg1Δ::r1HIS1r1/efg1Δ::r1HIS1r1 HGC1::PRBT5-HGC1/HGC1::PRBT5-HGC1 his1Δ::r3/his1Δ::r3 |
| ASM350 | SC5314 efg1Δ/Δ PTDH3-HGC1 | His+ NatR | efg1Δ::r1HIS1r1/efg1Δ::r1HIS1r1 HGC1::PTDH3-HGC1/HGC1::PTDH3-HGC1 his1Δ::r3/his1Δ::r3 |
Plasmids and DNA.
Primers are listed in Table 2 and plasmids are listed in Table 3.
TABLE 2.
Primers
| Primer name | Sequencea |
|---|---|
| RBT5/F | cgattcgatactaacgcaatcactatttttaatgatcctacttctatcccgaaaaag |
| RBT5/R | aacgcgttgggagctctcccatatggtagtattagttattagtgatagttagtgaagaattag |
| OE backbone/F | atatgggagagctcccaac |
| OE backbone/R | tagtgattgcgttagtatcgaatc |
| Multipromoter UME6/F | aaaaaagaattcttcgtaatatctatatagatatcttcatttaattttcttggttgtttgatattactttgttgtactttATCAAGCTTGCCTCGTCCCC |
| Multipromoter HGC1/F | cccaaactatacttcccaataaaagatagaaactcgcttacaacaacacaatcctgaagattattaaatctctaattttcATCAAGCTTGCCTCGTCCCC |
| Multipromoter UME6/R | ttcaactttattgtatcttctccataaggcgaatttggtgctgaagaagttgaatcgggtgtaaccatatgggtaatcatCGTTGGGAGCTCTCCCAATG |
| Multipromoter HGC1/R | atgtttttgtatggatgttgttgttgttgtttttgttgtgaaattgattttggagttaatggtttagttatatttatcatCGTTGGGAGCTCTCCCAATG |
| CaCas9/for | Atctcattagatttggaacttgtgggtt |
| CaCas9/rev | Ttcgagcgtcccaaaaccttct |
| UME6p sgRNA/F | caagaaaattatcaaattctgttttagagctagaaatagcaagttaaa |
| UME6p SNR52/R | agaatttgataattttcttgcaaattaaaaatagtttacgcaagtc |
| HGC1p sgRNA/F | gtgtgtatagtgtagtatccgtgttttagagctagaaatagcaagttaaa |
| HGC1p SNR52/R | acggatactacactatacacaccaaattaaaaatagtttacgcaagtc |
| UME6 qRT/F | tccactttaccattatccaagtctactc |
| UME6 qRT/R | gtgttgataatgaatgaactaaatttgccc |
| HGC1 qRT/F | caccaccacaaatgcattctca |
| HGC1 qRT/R | atgaggtgcaggaagctgac |
| UME6p OE NAT1 TDH3/F | gggaaaaaagaattcttcgtaatatctatatagatatcttcatttaattttcttggttgtttgatattactttgttgtacATCAAGCTTGCCTCGTCCCC |
| UME6p OE TDH3 NAT1/R | ttcaactttattgtatcttctccataaggcgaatttggtgctgaagaagttgaatcgggtgtaaccatatgggtaatcatTGTTAATTAATTTGATTGTAAAGTTTGTTGATG |
| UME6p check up/F | gagagttttaatcaattagaaaccaacagagg |
| UME6p check int/R | cgaatgacaaagttaagtcaaaaattggacc |
| NAT1 check/R | tcaatggtggatcaactggaacttc |
| HGC1 OE/F | cccaaactatacttcccaataaaagatagaaactcgcttacaacaacacaatcctgaagattattaaatctctaattttcATCAAGCTTGCCTCGTCCCC |
| HGC1 OE/R | atgtttttgtatggatgttgttgttgttgtttttgttgtgaaattgattttggagttaatggtttagttatatttatcatTGTTAATTAATTTGATTGTAAAGTTTGTTGATG |
| HGC1 check/F | cttacattttagacgaccaacggatactaca |
| HGC1 check/R | cttcgattgaaggatcatttaaagaccattctaaa |
Nucleotides in bold uppercase letters are complementary to the pTH10 plasmid template.
TABLE 3.
Plasmids
| Plasmid name | Description | Marker | Reference |
|---|---|---|---|
| pNAT | NAT1 marker | ampR | 19 |
| pCJN542 | NAT1-TDH3 promoter | ampR | 16 |
| pV1093 | CaCas9/sgRNA expression vector | ampR | 28 |
| pMH01 | pRS424 carrying C.d.HIS1 from pSN52 at KpnI site | ampR | 30 |
| pMH02 | pRS424 carrying C.d.HIS1 from pSN52 at SapI site | ampR | 30 |
| pTH10 | RBT5 promoter cassette | ampR | This study |
Generation of plasmid pTH10.
To construct plasmid pTH10, we modified plasmid pCJN542 (16) by replacing the TDH3 promoter with the RBT5 promoter. The 1-kb RBT5 promoter was amplified by PCR from SC5314 genomic DNA using primers RBT5/F and RBT5/R. The two primers were flanked by a 25-bp segment of homology upstream or downstream of the TDH3 promoter in order to make ligation with the other parts of plasmid. The 4.2-kb backbone fragment containing the ORI sequence, AmpR, and NatR was amplified by PCR from pCJN542 using primers OE backbone/F and OE backbone/R. PCR products of the RBT5 promoter and backbone were purified and ligated by using a NEBuilder HiFi DNA assembly cloning kit (NEB, USA) to yield plasmid pTH10.
PRBT5 cassettes.
The PRBT5 cassette for UME6 overexpression containing NatR and the RBT5 promoter was amplified by PCR using primers multipromoter UME6/F and multipromoter UME6/R, containing 80 bp of homology upstream or downstream of the UME6 promoter region. The PRBT5 cassette for HGC1 overexpression containing NatR and the RBT5 promoter was amplified by PCR using primers multipromoter HGC1/F and multipromoter HGC1/R, containing 80 bp of homology upstream or downstream of the HGC1 promoter region.
PTDH3 cassettes.
The PTDH3 cassette for UME6 overexpression containing NatR and the TDH3 promoter was amplified from plasmid pCJN542 by PCR using primers UME6p OE NAT1 TDH3/F and UME6p OE TDH3 NAT1/R, containing 80 bp of homology upstream or downstream of the UME6 promoter region. The PTDH3 cassette for HGC1 overexpression containing NatR and the TDH3 promoter was amplified from plasmid pCJN542 by PCR using primers multi HGC1 OE/F and HGC1 OE/R, containing 80 bp of homology upstream or downstream of the HGC1 promoter region.
Other DNA cassettes.
The approximately 5-kb CaCas9 cassette containing an ENO1 promoter, the CaCas9 open reading frame (ORF), and a CYC1 terminator was amplified from pV1093 (28) using primers CaCas9/for and CaCas9/rev. The single guide RNA (sgRNA) cassettes for the UME6 or HGC1 5′ region, containing the SNR52 promoter, guide sequence, and sgRNA scaffold sequence, were amplified via split-joint PCR as previously described (19) using primer pairs UME6p-sgRNA/F and UME6p-sgRNA/R and HGC1p1-sgRNA/F and HGC1p-sgRNA/R, respectively. PCR products were purified and concentrated with the GeneJET PCR purification kit (Thermo Fisher Scientific, Inc.).
C. albicans transformation.
Transformation was done via the transient CRISPR system (19). The PRBT5 cassette (2.2 μg) was cotransformed with the CaCas9 cassette (1.5 μg) and sgRNA cassette (1.5 μg), using the lithium acetate transformation method (28). NatR transformants were selected and genotyped. The UME6 overexpression cassette was verified by PCR from genomic DNA using primers UME6p check up/F and UME6p check int/R for absence of the UME6 promoter and primers UME6p check up/F and NAT1 Check/R for presence of the NAT1 marker. The HGC1 overexpression cassette was verified by PCR from genomic DNA using primers HGC1 check/F and HGC1 check/R for absence of the HGC1 promoter and using primers HGC1 check/F and NAT1 Check/R for presence of the NAT1 marker.
Filamentation assay.
To assay hyphal formation, strains were inoculated from YPD overnight cultures to an OD600 of 0.4 into 5 mL of RPMI with 10% serum in glass test tubes. Cells were grown for 4 h at 37°C with shaking, then collected by centrifugation and fixed with 4% formaldehyde for 15 min. Fixed cells were washed in phosphate-buffered saline (PBS), stained with 200 ng/μL calcofluor white, and imaged using a slit-scan confocal optical unit on a Zeiss Axiovert 200 microscope with a Zeiss C-Apochromat 40×, 1.2 numerical aperture water immersion objective. Lengths of hyphal units, i.e., the distance between septa on hyphae, were quantified using ImageJ. At least 100 interseptal distance measurements were taken from 3 separate views.
Biofilm assay.
Biofilm formation was assayed in 96-well plates (Greiner 96 wells, catalog number 655090). Cells were transferred to 100 μL of prewarmed RPMI with 10% serum to an OD600 of 0.3 from YPD overnight cultures. Cells were incubated at 37°C for 90 min with mild shaking (60 rpm) to allow for adherence to the bottom of 96-well plates, and then each well was gently washed twice with PBS to remove nonadhered cells. Next, cells were incubated in 100 μL of prewarmed RPMI with 10% serum at 37°C for 24 h with mild shaking. Then, the medium was removed and biofilms were fixed by incubation with 100 μL of 4% formaldehyde in PBS solution for 1 h and then gently washed twice with PBS. Biofilms were stained with 200 μg/mL calcofluor white overnight at room temperature with mild shaking, and then each well was gently washed twice with PBS. Finally, we used 100% thiodiethanol (TDE) followed by 50% TDE in PBS to clarify biofilms. Biofilms were imaged using a Zeiss LSM 710 inverted confocal microscope and analyzed with Fiji ImageJ.
RNA extraction and qPCR.
RNA extractions were conducted as previously described (29). Briefly, strains were inoculated in triplicate from overnight cultures cells into 25 mL of RPMI with 10% serum at 37°C with shaking for 4 h to an initial OD600 of 0.2. Cells were harvested by vacuum with filtration. Then, cells were lysed using a BeadBeater and a Qiagen RNeasy minikit (catalog number 74104). RNA was isolated with the RNeasy kit and reverse transcribed to cDNA using the iScript gDNA clear cDNA synthesis kit (catalog number 172-5034). Then, qPCR was performed using iQ SYBR green supermix (catalog number 170-8880). UME6 and HGC1 expression levels were normalized to the ACT1 gene and compared using the threshold cycle ΔΔCT method. Differences between strains were analyzed with the Tukey-Kramer test.
Animal studies.
Virulence of C. albicans strains was determined using the mouse model of hematogenously disseminated candidiasis (HDC) in 5- to 6-week-old male BALB/c mice (Taconics). Mice were injected via the lateral tail vein with 5 × 105 yeast and monitored for survival over a 3-week period. For histology, two additional mice were infected via the tail vein with 1 × 106 yeast and sacrificed after 2 days, after which a kidney from each mouse was fixed in zinc-buffered formalin and embedded in paraffin. Thin sections were cut and then stained with Periodic acid-Schiff and imaged by light microscopy. Animal work was approved by the Lundquist Institute for Biomedical Research at Harbor-UCLA Medical Center and carried out in accordance with the National Institutes of Health guidelines for the ethical treatment of animals.
Data availability.
Plasmid pTH10 and its sequence have been deposited with Addgene.
ACKNOWLEDGMENTS
We thank Max Kuhr for expert technical support and lab management and Trevor Haskins for help with the early stages of this project. This work was supported by NIH grant 1R01AI146103 (A.P.M.) and by startup funds from the University of Georgia (A.P.M.).
Contributor Information
Aaron P. Mitchell, Email: Aaron.Mitchell@uga.edu.
Michael Lorenz, University of Texas Health Science Center.
REFERENCES
- 1.Prelich G. 2012. Gene overexpression: uses, mechanisms, and interpretation. Genetics 190:841–854. doi: 10.1534/genetics.111.136911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang YC, Amon A. 2013. Gene copy-number alterations: a cost-benefit analysis. Cell 152:394–405. doi: 10.1016/j.cell.2012.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rai LS, van Wijlick L, Chauvel M, d'Enfert C, Legrand M, Bachellier-Bassi S. 2022. Overexpression approaches to advance understanding of Candida albicans. Mol Microbiol 117:589–599. doi: 10.1111/mmi.14818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fay JC. 2013. The molecular basis of phenotypic variation in yeast. Curr Opin Genet Dev 23:672–677. doi: 10.1016/j.gde.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skelly DA, Magwene PM. 2016. Population perspectives on functional genomic variation in yeast. Brief Funct Genomics 15:138–146. doi: 10.1093/bfgp/elv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson MZ, Bennett RJ. 2016. Budding off: bringing functional genomics to Candida albicans. Brief Funct Genomics 15:85–94. doi: 10.1093/bfgp/elv035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delgado ML, Gil ML, Gozalbo D. 2003. Candida albicans TDH3 gene promotes secretion of internal invertase when expressed in Saccharomyces cerevisiae as a glyceraldehyde-3-phosphate dehydrogenase-invertase fusion protein. Yeast 20:713–722. doi: 10.1002/yea.993. [DOI] [PubMed] [Google Scholar]
- 9.Eckert SE, Muhlschlegel FA. 2009. Promoter regulation in Candida albicans and related species. FEMS Yeast Res 9:2–15. doi: 10.1111/j.1567-1364.2008.00455.x. [DOI] [PubMed] [Google Scholar]
- 10.Leuker CE, Sonneborn A, Delbruck S, Ernst JF. 1997. Sequence and promoter regulation of the PCK1 gene encoding phosphoenolpyruvate carboxykinase of the fungal pathogen Candida albicans. Gene 192:235–240. doi: 10.1016/s0378-1119(97)00069-3. [DOI] [PubMed] [Google Scholar]
- 11.Care RS, Trevethick J, Binley KM, Sudbery PE. 1999. The MET3 promoter: a new tool for Candida albicans molecular genetics. Mol Microbiol 34:792–798. doi: 10.1046/j.1365-2958.1999.01641.x. [DOI] [PubMed] [Google Scholar]
- 12.Backen AC, Broadbent ID, Fetherston RW, Rosamond JD, Schnell NF, Stark MJ. 2000. Evaluation of the CaMAL2 promoter for regulated expression of genes in Candida albicans. Yeast 16:1121–1129. doi:. [DOI] [PubMed] [Google Scholar]
- 13.Park YN, Morschhauser J. 2005. Tetracycline-inducible gene expression and gene deletion in Candida albicans. Eukaryot Cell 4:1328–1342. doi: 10.1128/EC.4.8.1328-1342.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weissman Z, Kornitzer D. 2004. A family of Candida cell surface haem-binding proteins involved in haemin and haemoglobin-iron utilization. Mol Microbiol 53:1209–1220. doi: 10.1111/j.1365-2958.2004.04199.x. [DOI] [PubMed] [Google Scholar]
- 15.Xu W, Solis NV, Ehrlich RL, Woolford CA, Filler SG, Mitchell AP. 2015. Activation and alliance of regulatory pathways in C. albicans during mammalian infection. PLoS Biol 13:e1002076. doi: 10.1371/journal.pbio.1002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nobile CJ, Solis N, Myers CL, Fay AJ, Deneault JS, Nantel A, Mitchell AP, Filler SG. 2008. Candida albicans transcription factor Rim101 mediates pathogenic interactions through cell wall functions. Cell Microbiol 10:2180–2196. doi: 10.1111/j.1462-5822.2008.01198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang MY, Woolford CA, May G, McManus CJ, Mitchell AP. 2019. Circuit diversification in a biofilm regulatory network. PLoS Pathog 15:e1007787. doi: 10.1371/journal.ppat.1007787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banerjee M, Thompson DS, Lazzell A, Carlisle PL, Pierce C, Monteagudo C, Lopez-Ribot JL, Kadosh D. 2008. UME6, a novel filament-specific regulator of Candida albicans hyphal extension and virulence. Mol Biol Cell 19:1354–1365. doi: 10.1091/mbc.e07-11-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Min K, Ichikawa Y, Woolford CA, Mitchell AP. 2016. Candida albicans gene deletion with a transient CRISPR-Cas9 system. mSphere 1. doi: 10.1128/mSphere.00130-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banerjee M, Uppuluri P, Zhao XR, Carlisle PL, Vipulanandan G, Villar CC, Lopez-Ribot JL, Kadosh D. 2013. Expression of UME6, a key regulator of Candida albicans hyphal development, enhances biofilm formation via Hgc1- and Sun41-dependent mechanisms. Eukaryot Cell 12:224–232. doi: 10.1128/EC.00163-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu W, Lockhart SR, Pujol C, Srikantha T, Soll DR. 2007. Heterozygosity of genes on the sex chromosome regulates Candida albicans virulence. Mol Microbiol 64:1587–1604. doi: 10.1111/j.1365-2958.2007.05759.x. [DOI] [PubMed] [Google Scholar]
- 22.Hirakawa MP, Martinez DA, Sakthikumar S, Anderson MZ, Berlin A, Gujja S, Zeng Q, Zisson E, Wang JM, Greenberg JM, Berman J, Bennett RJ, Cuomo CA. 2015. Genetic and phenotypic intra-species variation in Candida albicans. Genome Res 25:413–425. doi: 10.1101/gr.174623.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cowart RE, Singleton FL, Hind JS. 1993. A comparison of bathophenanthrolinedisulfonic acid and ferrozine as chelators of iron(II) in reduction reactions. Anal Biochem 211:151–155. doi: 10.1006/abio.1993.1246. [DOI] [PubMed] [Google Scholar]
- 24.Zheng X, Wang Y, Wang Y. 2004. Hgc1, a novel hypha-specific G1 cyclin-related protein regulates Candida albicans hyphal morphogenesis. EMBO J 23:1845–1856. doi: 10.1038/sj.emboj.7600195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y. 2016. Hgc1-Cdc28: how much does a single protein kinase do in the regulation of hyphal development in Candida albicans? J Microbiol 54:170–177. doi: 10.1007/s12275-016-5550-9. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez AA, Johnston DA, Myers C, Edwards JE, Jr, Mitchell AP, Filler SG. 2004. Relationship between Candida albicans virulence during experimental hematogenously disseminated infection and endothelial cell damage in vitro. Infect Immun 72:598–601. doi: 10.1128/IAI.72.1.598-601.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glazier VE. 2022. EFG1, everyone's favorite gene in Candida albicans: a comprehensive literature review. Front Cell Infect Microbiol 12:855229. doi: 10.3389/fcimb.2022.855229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vyas VK, Barrasa MI, Fink GR. 2015. A Candida albicans CRISPR system permits genetic engineering of essential genes and gene families. Sci Adv 1:e1500248. doi: 10.1126/sciadv.1500248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cravener MV, Mitchell AP. 2020. Candida albicans culture, cell harvesting, and total RNA extraction. Bio Protoc 10:e3803. doi: 10.21769/BioProtoc.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang MY, Mitchell AP. 2017. Marker recycling in Candida albicans through CRISPR-Cas9-induced marker excision. mSphere 2. doi: 10.1128/mSphere.00050-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Plasmid pTH10 and its sequence have been deposited with Addgene.