ABSTRACT
The COVID-19 shutdown forced many institutions of higher education to shift in-person teaching to emergency remote teaching. This was particularly challenging for laboratory courses, where students are expected to learn hands-on skills needed for their career goals. Here, we describe the transformation of an upper-division microbiology laboratory to a course that seamlessly integrates online simulations with safe, hands-on experiences that can be done from home. This blended lab course helped students attain learning outcomes similar to those achieved in the in-person class. We illustrate the implementation of Unknown Portfolios to help students gain the data analysis and critical thinking skills needed to identify an unknown microorganism. Our data show that students who took these online courses mastered material as well as students who took the lab in person, demonstrating proficiency in laboratory safety skills, hands-on techniques, and theoretical class content. Last, we explore online adaptations to enhance in-person lab classes, aiming at reducing the accessibility and equity gaps inherited in many courses, as well as discussing challenges that instructors might experience in this process.
KEYWORDS: COVID-19, hands-on experience, microbiology laboratory, online class, unknown identification
INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic forced educational institutions to shift instruction from in-person delivery to emergency remote teaching (ERT) (1). This was particularly challenging for laboratory courses, where students learn hand-on skills. In-person microbiology lab courses emphasize mastery of hands-on skills that are challenging to teach remotely, including aseptic technique, microbial plating techniques, microscope skills, and microbial stains (2, 3). For microbiology students, it is imperative to demonstrate mastery of these techniques and skills beyond their conceptual and theoretical understanding.
Teaching hands-on techniques usually involves demonstrations by faculty and graduate assistants, utilizing equipment readily available in teaching labs. Lab courses designed for remote delivery incorporate class content and experiences intentionally orchestrated to provide students with an experience similar to that obtained in the in-person lab. This is often done by using videos or simulations (4–8). However, the experience of being physically in the lab, as well as the acquisition of hands-on skills needed to perform microbiological techniques, is challenging to replicate remotely with ERT (3, 5). The principal challenge of ERT is that most faculty have neither the training nor the time to prepare lab course content for effective remote instruction (9). Under ERT conditions, how do instructors translate the in-person lab experience to an online environment?
In this article, we describe the adaptation of an upper-division microbiology laboratory course for remote delivery in the fall 2020 and spring 2021 semesters at a Ph.D.-granting research university in California. This lab incorporated simulations and hands-on experiments that could be safely performed at home. Also, we created Unknown Portfolios, providing students the opportunity to analyze biochemical data to identify an unknown pseudomonad. We hypothesized that our adapted online course would prepare students as well as the in-person laboratory class. Here, we analyzed data from these blended, online courses and compared it to labs taught in person. We conclude with the lessons learned from this experience and discuss modifications that could be incorporated into future lab courses.
BACKGROUND
The transition to ERT in spring 2020 affected institutions of learning worldwide. Although there has been a trend to expand courses to online delivery, doing so during a global crisis posed challenges for instructors, many lacking training, time, and resources (1). Online courses are carefully orchestrated and intentionally designed for online delivery. They help accommodate students’ schedules (10), help mitigate the challenges of increase enrollments, and free lab funding for other needs (11). Some online laboratory models rely exclusively on online content, using homemade or commercial simulations to emulate the in-person experience (12). Labster, for example, provides a large selection of simulations covering multiple microbiology topics (13–15). However, institutional access to these simulations is costly and therefore not readily available to all institutions.
Some online courses incorporate remote hands-on activities to provide students with the skills usually experienced in an in-person lab. These might come at a considerable cost to the student—over $300 for courses taught via extension programs. Science Interactive (https://www.scienceinteractive.com/), for example, provides kits for microbiology courses that can be customized to accommodate different microbiology curricula. Students have reported enjoying the hands-on component in these mixed-methods courses and expressed their desire for more courses to incorporate hands-on experiences (16).
The General Microbiology Laboratory
The General Microbiology Laboratory is a 3-unit, upper-division course that meets twice a week for a 2-h 50-min lab and once a week for a 50-min lecture. This format is similar to that of other upper-division microbiology lab courses taught at our sister institutions. This course, taken by juniors and seniors, must be taken in conjunction with (or after) the General Microbiology Lecture class (a 4-unit upper-division course), as its lab activities are designed to reinforce the material covered in the lecture course. To take these microbiology classes, students must have passed introductory biology, introductory molecular biology (both lower-division courses), and the upper-division cell biology course.
During the 15-week semester, this lab incorporates 11 experimental activities covering microscopy, bacterial quantification, regulation of the lac operon in Escherichia coli, bacterial transformation, isolation and characterization of auxotrophic mutants, the Ames mutagenicity test, and transmission of drug resistance. Through the delivery of these laboratory experiences, the course also instills general microbiology laboratory skills, such as aseptic technique, microscopy, microbial sample preparation and staining, various cell plating methods, and serial dilutions. The capstone project of the course is an inquiry-based experiment where students enrich, isolate, and characterize, both biochemically and molecularly, a pseudomonad species obtained from soil as well as a provided unknown pseudomonad. Students are taught to keep a laboratory notebook following good laboratory practice (GLP) principles associated with the biotech industry. Assessment for the class includes two exams that evaluate theoretical, quantitative, and practical knowledge and skills. Learning is evaluated also by six quizzes, six notebook checks, homework assignments, lab effort and participation, and the capstone project paper.
Transforming the General Microbiology Laboratory for remote delivery during ERT
The COVID-19 shutdown forced us to move our courses online during the 2020-2021 academic year. During summer 2020, we reorganized the General Microbiology Laboratory course following the principles described by Herzog and Mawn (17). We created a class where students would get practical experience in microbiological techniques at home as well as the theory and virtual practice afforded in laboratory simulations. Students received a lab kit at home, at no charge, to perform hands-on experiments. We also transformed the class’s unknown-pseudomonad capstone project for remote delivery. We removed the soil bacterium enrichment and isolation portion of the lab and provided students microscopic and biochemical data organized in an Unknown Portfolio. We adapted our exams to be delivered online while retaining the streak plate technique as the hands-on practical assessment. Moreover, lab notebooks were moved online using platforms like Google Docs. We hypothesized that our online course, like the in-person class, provided the theoretical and hands-on components required to achieve the course’s learning objectives. The course’s learning objectives can be found in supplemental material 1.
Creation of a fully online course incorporating homemade and commercial simulations
We took a blended-model approach when redesigning the General Microbiology lab for ERT that included Labster and homemade simulations. We incorporated 18 Labster simulations that aligned with the topics taught in the General Microbiology lecture course or which reinforced the topics covered in the lab experiments. Also, we incorporated the following non-Labster simulations: (i) streak plate technique (18), (ii) the Bacterial Identification Lab by BioInteractive (19), and (iii) “Advanced concepts in lac operon transcription regulation” by Cell Collective (cellcollective.org) (20, 21). A list of the simulations used, including their URL addresses, is available in supplemental material 2.
Retained labs that could be easily adapted to the online environment
The online laboratory incorporated various experiments previously used during the in-person course. For example, experiment 4 (plate counts) and experiment 5 (growth rates) explore the concepts of serial dilutions, as well as the math of calculating dilutions and dilution factors. They also teach students the math associated with exponential growth. To transition these labs for online delivery, we provided students with data from previous iterations of the class, allowing them to analyze the data and generate growth curves.
Home video demonstrations
We created videos to guide students through the hands-on skills they would learn in the lab and reinforce lab course concepts. We also used videos available online free of charge. We made videos illustrating (i) pouring agar into petri dishes, (ii) streak plate technique, and (iii) Gram staining at home. For an example of a home video demonstration, see supplemental material 3.
Hands-on skills at home
We provided students with lab materials to recreate experimental procedures at home. Students received a beginner’s microscope capable of ×1,200 magnification from AmScope (https://amscope.com/products/c-m30-abs-kt2). From Home Science Tools, students received a bacteria growing kit containing nutrient agar and 20 petri dishes (https://www.homesciencetools.com/product/bacteria-growing-kit/), a Gram staining kit (https://www.homesciencetools.com/product/gram-stain-lab-kit/), and an inoculating loop (https://www.homesciencetools.com/product/inoculating-needle-looped-end/). In total, the cost of these materials was $104.04 per student. At home, students would need their personal protective equipment (PPE), a candle to sterilize their inoculating loop and heat-fix their Gram stain slides, matches, disinfectant solution to clean their work area, 10% bleach solution to treat used nutrient agar plates before disposal, and a large plastic disposable container to incubate their plates.
Growth of kombucha microbes
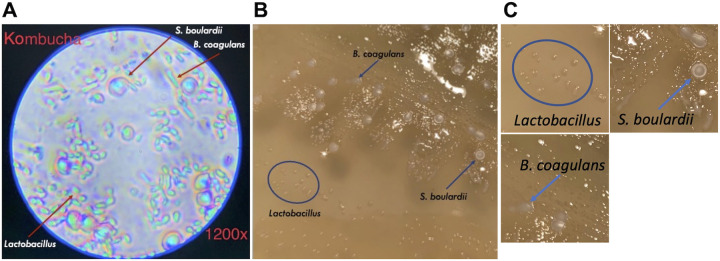
We wanted to provide students with experience handling microorganisms, keeping in mind the safety considerations of performing these activities at home. Kombucha, a fermented tea, provides a model microbial community that is safe for students to handle outside the teaching lab (22, 23). GT’s kombucha contains 3 probiotics: Bacillus coagulans, Saccharomyces boulardii, and lactobacilli. These are easily distinguishable from one another (Fig. 1) using microscopy and colony morphology (24, 25). These microbes settle at the bottom of a kombucha bottle, creating a slurry that students used for plating.
FIG 1.
Illustration of the microbial community in GT’s kombucha. (A) Microscopic image, at ×1,200 magnification, showing the cell morphologies for presumed Bacillus coagulans, Saccharomyces boulardii, and Lactobacillus. The image was taken with the microscope provided to students. (B) Colony morphology of kombucha microbes grown in homemade petri dishes. (C) Enlargement of selected colonies to enhance visualization.
Probiotics are considered safe for human consumption and manipulation (26–28). Kombucha microbes are classified by default as risk group 1 (RG1) agents in Appendix B of the NIH’s “Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules” (29), as they are agents not associated with disease in healthy adult humans. However, even RG1 microbes may pose health risks if mishandled or if handled by immunocompromised individuals. Therefore, students were instructed to treat the kombucha microorganisms carefully, always using aseptic technique and wearing appropriate PPE when handling them.
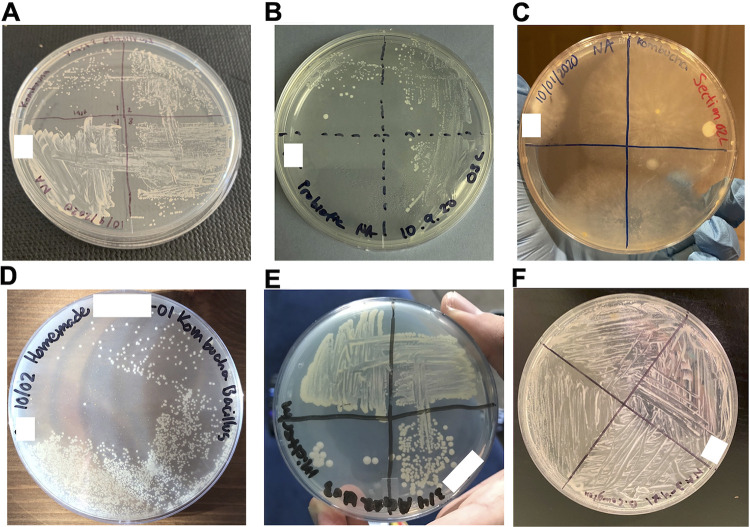
To isolate individual colonies from kombucha microbes, students started by pouring their own nutrient agar petri dishes. Subsequently, they plated the kombucha slurry onto nutrient agar plates. After a 48-h incubation at room temperature, students examined their plates for growth and submitted pictures for feedback. Students were instructed to place their plates in a plastic, disposable secondary container during incubation, to prevent potential exposure and minimize contamination. Students then selected colonies of either B. coagulans, S. boulardii, or lactobacilli and used the streak plate technique to restreak the individual colonies onto nutrient agar plates, evaluating purity after a 48-h incubation at room temperature (Fig. 2). They also performed Gram stains on their selected colonies (data not shown). To discard used nutrient agar plates, students covered the plates with 10% bleach for 10 min before discarding them in the trash.
FIG 2.
Students’ streak plating technique for the isolation of single colonies from kombucha as part of exam 1. (A and D) Representative streak plates demonstrating a high level of mastery of the streak plating technique. Note the single colonies in the fourth quadrant. (B and E) Plates demonstrating intermediate levels of mastery of the streak plating technique. Note the absence of colonies in the fourth quadrant (B) and the lack of separation of colonies (E). (C and F) Representative samples of unsuccessful streak technique. Note the presence of contamination (C) and the absence of sample dilution (F). White squares hide students’ identifying information.
Laboratory safety instruction
A critical subject taught in any microbiology course is lab safety. Instructional labs are usually biosafety level 1 (BSL-1) facilities and have equipment designed to contain the potential spread of microorganisms and mitigate accidents (fire extinguishers, eye wash stations, shower stations, first-aid kits, and chemical spill containment kits) (30). These are largely missing from nonclassroom instructional environments, like a student’s home or dorm. Therefore, instructors must be deliberate in teaching laboratory safety principles remotely as well as cognizant of designing laboratory experiences to be performed safely outside of the lab.
We took a multiassignment approach to teach students the theory of laboratory safety. First, we required that students pass the institution’s environmental health and safety (EHS) course “Lab Safety Fundamentals W/O HazMat.” This course is required for any person, irrespective of rank, to perform bench science research at our institution. After passing the course, students submitted a copy of their certificate to earn credit. Second, students in the fall 2020 and spring 2021 semesters performed and passed Labster’s Lab Safety and Biosafety simulations, which reinforced the principles of safety needed to perform the experiments at home. These simulations were assigned at the beginning of the course, before starting any hands-on manipulations. Lastly, students read and signed the Laboratory Safety Institute (labsafety.org) “Safe Science at Home Science Safety Rules Agreement,” to emphasize the practice of laboratory safety principles at home. We hypothesized that these activities increased students’ awareness of the potential safety issues of performing microbiology laboratory experiments at home.
Identification of an unknown pseudomonad
Like in many microbiology labs (31–33), identification of an unknown bacterium is a capstone project of our class. Our class project involves the enrichment and isolation of a pseudomonad from soil, followed by morphological, biochemical, and molecular characterization of the isolate. During ERT, we transitioned our unknown pseudomonad capstone project to a fully online experience. First, we removed the soil bacterium enrichment and isolation portion of the lab. Second, we produced 7 Unknown Portfolios, consisting of student-generated microscopic and biochemical data images from previous semesters, designed to help characterize the unknown pseudomonad.
Students were provided background content about pseudomonads and then randomly assigned one of 7 Unknown Portfolios. These contain student-generated images of the results of a wet mount, Gram stain, oxidation-fermentation tests, and the oxidase test. Students were asked to interpret these test results and determine whether these data were consistent with the enrichment of a pseudomonad. Following this, students received a number and were provided a corresponding Unknown Portfolio with images of results for the following tests: starch hydrolysis, gelatin hydrolysis, lecithin hydrolysis, pigment production, nitrate reduction, and growth at 4°C, 30°C, and 42°C. These images included labeled positive and negative controls and were generated from data collected by students in previous semesters. The Unknown Portfolio also contained catabolic data in the form of optical density readings at 600 nm (OD600) meant to represent their unknown’s ability to utilize 10 different carbon sources. The Unknown Portfolio also included a key containing the results of the biochemical tests and catabolic data for all potential Pseudomonas species. For an example of the Unknown Portfolio for P. aeruginosa, see supplemental material 4.
Students interpreted the results of their biochemical assays, identified their unknown organism and reported their findings in a lab report following a detailed rubric. Students earned 10% of the grade for accurately identifying their unknown pseudomonad based on the correct interpretation of the results in the Unknown Portfolio. Students were given points for correctly interpreted results even if they were unable to correctly identify their unknown organism.
Molecular characterization of the unknown pseudomonad
Prior to ERT, students PCR-amplified the 16S rRNA gene of their pseudomonad isolate and unknown to obtain sequence data. The sequence would then be analyzed by BLAST, corroborating the biochemical identification of their pseudomonad species. In lieu of this, during ERT, students received 16S rRNA gene sequences for their assigned unknown pseudomonads and performed BLAST analysis to identify it. This analysis was done in conjunction with the BioInteractive simulation. Together, the molecular and biochemical data were used to identify the unknown pseudomonad. We hypothesized that the Unknown Portfolio would be as effective as the in-person project at providing students the necessarily experience and data to correctly identify their unknown pseudomonad.
METHODS
Context
For this study, we evaluated data from an upper-division general microbiology laboratory course taught during the 15-week spring 2019, fall 2019, spring 2020, fall 2020, and spring 2021 semesters at an R2 Carnegie Classification (34) research university in California. The spring 2019 and fall 2019 semesters were taught fully in person (IP); the first 10 weeks of the spring 2020 semester were taught in person, and classes transitioned to ERT after spring break. The fall 2020 and spring 2021 semesters were taught remotely (RMT) (Table 1). The spring 2019, fall 2019, and spring 2020 semesters had 3 teaching assistants (TAs), while both the fall 2020 and spring 2021 semesters had 2 TAs. Each week, the lab course met twice for a 2-h 50-min lab session and once for a 50-min lecture.
TABLE 1.
Description of teaching and exam delivery mode for each semester
| Semester | No. of participants (N = 228) | No. of wks (range) |
Delivery modea |
||
|---|---|---|---|---|---|
| In person | Remote | Exam 1 | Exam 2 | ||
| Spring 2019 | 56 | 1–15 | IP | IP | |
| Fall 2019 | 34 | 1–15 | IP | IP | |
| Spring 2020 | 58 | 1–9 | 10–15 | IP | RMT |
| Fall 2020 | 39 | 1–15 | RMT | RMT | |
| Spring 2021 | 41 | 1–15 | RMT | RMT | |
IP, in person; RMT, remote.
The institution has a diverse student population: the university has over 8,321 undergraduate students and is designated as both a Hispanic-serving institution (HSI) and an Asian American/Native American/Pacific Islander-serving institution (AANAPISI). In fall 2021, 70% of the institution’s undergraduates were first-generation college students, 61% were Pell grant eligible, and 71% came from traditionally underrepresented communities. STEM (science, technology, engineering, and math) majors constitute 55% of the undergraduate students.
Participants
The student population in this study included 56 students in spring 2019, 34 students in fall 2019, 58 students in spring 2020, 40 students in fall 2020, and 41 students in spring 2020 (N = 229). The predominant racial and ethnic makeup of the combined cohorts was 37.6% Asian and 43.8% Hispanic students, reflecting the university’s HSI and AANAPISI designations. The remaining population comprised 2.2% Black, 10.9% white, 2.2% multiracial, and 4.4% international students. The cohort is primarily composed of female students (64.2%) and seniors (94.8%). The average student age was 21.3 years (standard deviation [SD] = 1.2; range, 19 to 31). A one-way analysis of variance (ANOVA) showed that students’ grade point average (GPA) did not differ significantly between the five cohorts [F(4,223) = 1.27, P = 0.282]. Demographics and grade data came from institutional records. These data are summarized in Table 2.
TABLE 2.
Participants’ demographic information
| Characteristic | Value for: |
|||||
|---|---|---|---|---|---|---|
| Spring 2019 (n = 56) | Fall 2019 (n = 34) | Spring 2020 (n = 58) | Fall 2020 (n = 40) | Spring 2021 (n = 41) | Overall (N = 229) | |
| Gender [no. (%)] | ||||||
| Male | 20 (35.7) | 11 (32.4) | 20 (34) | 17 (42.5) | 13 (31.7) | 81 (35.4) |
| Female | 35 (62.5) | 23 (67.6) | 38 (66) | 23 (57.5) | 28 (68.3) | 147 (64.2) |
| Race/ethnicity [no. (%)] | ||||||
| Asian | 26 (46.4) | 11 (32.4) | 20 (34.5) | 15 (37.5) | 14 (34.1) | 86 (37.6) |
| Black | 1 (1.8) | 0 (0) | 1 (1.7) | 2 (5.0) | 1 (2.4) | 5 (2.2) |
| Hispanic | 23 (41.1) | 15 (44.1) | 28 (48.3) | 12 (30) | 20 (48.8) | 98 (42.8) |
| White | 4 (7.1) | 6 (17.6) | 5 (8.6) | 6 (15) | 4 (9.8) | 25 (10.9) |
| Multiracial | 0 (0) | 0 (0) | 1 (1.7) | 2 (5.0) | 0 (0) | 3 (2.2) |
| International | 1 (1.8) | 2 (5.9) | 3 (5.2) | 3 (7.5) | 1 (2.4) | 10 (4.4) |
| Unknowna | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.4) | 1 (0.4) |
| Age (yr) [M (range)] | 21.4 (20–24) | 21.6 (20–31) | 21.4 (20–29) | 21 (20–22) | 21.1 (19–24) | 21.3 (19–31) |
| Class (%) | ||||||
| Junior | 0 | 2.9 | 5.2 | 10 | 9.8 | 5.2 |
| Senior | 100 | 97.1 | 94.8 | 90 | 90.2 | 94.8 |
| GPA (M ± SD) | 3.06 ± 0.35 | 3.18 ± 0.36 | 3.02 ± 0.39 | 3.13 ± 0.43 | 3.13 ± 0.36 | 3.09 ± 0.38 |
Students who declined to report race/ethnicity.
Student assessment data acquisition, analysis, and statistics
All students’ assessment data were obtained from Canvas, the courses’ learning management system (LMS). This included overall course performance, scores from questions evaluating mastery of laboratory safety procedures, homework, streak plate scores, and results from the laboratory project on the identification of an unknown pseudomonad. Data were deidentified before analysis and are presented as means (M) ± SD or as raw total numbers.
(i) Exams
The lab class has a midterm (exam 1) and a final (exam 2). The exams given in the in-person and online courses were isometric, similar exams with slight modifications to questions assessing identical learning outcomes between semesters. These changes include using different values for calculation questions, changing colony images to evaluate knowledge of colony morphology terminology, and asking different questions within the same Bloom’s taxonomic hierarchy. This was done to dissuade sharing of exams or knowledge of exams between semesters and sections. An example of these types of isometric questions can be found in supplemental material 5. The final exam was not comprehensive and was identical in all instances, as the students did not get the opportunity to keep this assessment.
(ii) Discouraging academic dishonesty
Exams were proctored carefully to minimize the opportunity for cheating. All exams were taken during the lab period, irrespective of the semester taken. Exams taken in person were proctored by the teaching assistants. Exams given remotely through the LMS were proctored by using the Respondus LockDown browser. The LockDown browser creates a secure browser page that opens the assessment and prevents activities such as printing, copying/pasting, opening unauthorized web pages, or accessing other unauthorized applications.
To further minimize the opportunity for cheating during the online exam, isometric exam questions from previous semesters were placed in a question group, and the LMS randomly assigned a question from each group to a student. For an example of questions found in a group, see supplemental material 5.
Statistical analysis was done in Microsoft Excel (version 16.54) or using the Social Science Statistics website (35–39). The chi-square test of independence (37) was used to compare the number of correctly identified unknowns versus the mode of data presentation (Unknown Portfolio versus in-person data collection). One-tailed t tests (36) were used to compare student performance on exams between in-person semesters and semesters with remote instruction. Hedges’ g statistic was used to measure the effect size between semesters (35), while confidence intervals (38) were calculated to estimate the probability that the values calculated lie within the calculated means. One-way ANOVAs were used to find statistical differences between exam performance across semesters as well as to compare overall course performance between semesters. Tukey’s honestly significant difference (HSD) test was used to identify which semesters’ exam performance were significantly different (39). Statistical analyses were considered significant at a P value of <0.05.
RESULTS
Overall course performance
We designed an online lab that blended the theoretical and hands-on components associated with an in-person microbiology laboratory class. We hypothesized that students in the online course would perform similarly to students in an in-person laboratory class, as they were provided with similar course content and the courses had the same learning outcomes. To evaluate this, we examined the overall course performance in semesters taught remotely (RMT) and compared it to that in semesters taught in person (IP). The spring 2019 and fall 2019 semesters were taught fully in person, the spring 2020 semester was partially taught in person, and the fall 2020 and spring 2021 semesters were taught fully remotely (Table 1). Figure 3 shows a box-and-whisker plot summarizing these data. A one-way ANOVA on these final course scores yielded significant variation among semesters F(4,223) = 6.03; P = 0.0001. A post hoc Tukey’s HSD test showed that the IP spring 2019 semester (M = 88.7, SD = 4.2, P = 0.019), the IP fall 2019 semester (M = 91.8, SD = 3.9, P = 0.00001), and the (partially RMT) spring 2020 semester (M = 89.9, SD = 7.5, P = 0.002) differed significantly from the RMT fall 2020 semester (M = 83.7, SD = 11.7). The partially RMT spring 2020 semester was not significantly different from the IP spring 2019 or IP fall 2019 semester. The RMT spring 2021 semester (M = 88.1, SD = 8.8) was not significantly different from the other four semesters.
FIG 3.
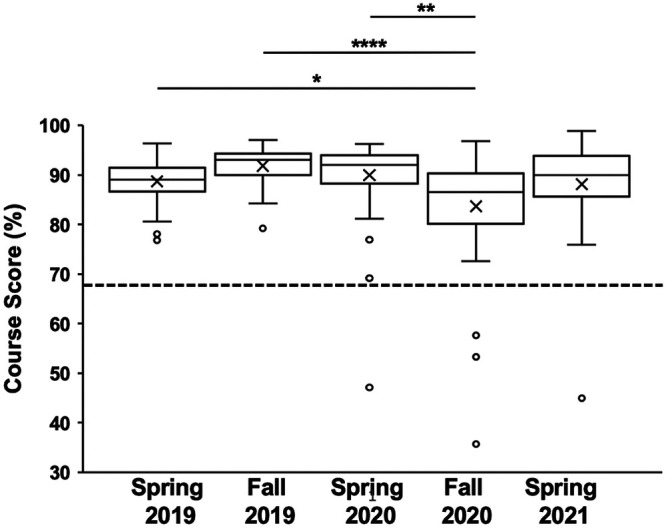
Box-and-whisker graph of the overall course score (in percent) for each semester examined. The “×” in each box represents the average score of the class, while the horizontal line in the box represents the median score. The dashed line at 68% represents a C−, the passing threshold grade for the class; Students with scores of ≥68% passed the class. Outliers are shown as open dots. Spring 2019, n = 56; fall 2019, n = 34; spring 2020, n = 58; fall 2020, n = 40; spring 2021, n = 41. *, P ≤ 0.05; **, P ≤ 0.01; ****, P ≤ 0.0001.
Exam performance
We hypothesized that our blended, online course prepared students as well as the in-person laboratory class. We therefore compared the exam performance for the students who took the class in person to the students who took the lab remotely. The lab class has a midterm (exam 1) and a noncomprehensive final (exam 2). As shown in Table 1, exam 1 was administered in person in spring 2019, fall 2019, and spring 2020, whereas it was administered online in fall 2020 and spring 2021. Exam 2 was administered in person only in spring 2019 and fall 2019, whereas it was administered online in spring 2020, fall 2020, and spring 2021.
Table 3 summarizes the performance data for exam 1 and exam 2. Since exam 1 in different semesters is isometric and evaluates the same learning outcomes, we compared the raw scores of exam 1 delivered in person (n = 148) and remotely (n = 80). We found no difference in scores between students who took exam 1 in person (M = 120.6, SD = 13.8) or remotely (M = 120.9, SD = 14.7), as determined by a one-tailed t test [t(226) = −0.15; P > 0.05; 95% confidence interval (CI), −3.57 to 4.16; Hedges’ g = 0.02].
TABLE 3.
Assessment score comparison between semesters
| Semester or mode | Exam 1 |
Exam 2 |
HW 4/5a |
Final score (%)b |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Score |
n | Score |
M | SD | M | SD | |||
| M | SD | M | SD | |||||||
| Spring 2019 | 56 | 121.6 | 12.9 | 121.5 | 12.9 | 29.4 | 1.6 | 88.7 | 4.2 | |
| Fall 2019 | 34 | 125.5 | 12.8 | 127.3 | 12.7 | 28.5 | 1.8 | 91.8 | 3.9 | |
| Spring 2020 | 58 | 116.8 | 14.3 | 123.8 | 13.3 | 25.7 | 4.3 | 89.9 | 7.7 | |
| Fall 2020 | 39 | 118.7 | 15.4 | 105.0 | 22.1 | 20.8 | 7.9 | 83.7 | 11.8 | |
| Spring 2021 | 41 | 123.0 | 13.9 | 116.3 | 19.0 | 24.9 | 7.4 | 88.1 | 8.8 | |
| In personc | 148 | 120.6 | 13.8 | 90 | 123.7 | 13.1 | 29.0 | 1.7 | 89.9 | 86.0 |
| Remote | 80 | 120.9 | 14.7 | 138 | 116.2 | 19.4 | 24.1 | 6.7 | 5.8 | 10.5 |
Lab 4/5 homework score.
Final score data for spring 2020 were included in the in-person combined average and standard deviation calculations, as most of the semester was taught in person.
For in-person evaluation (n = 148), exam 1 data from spring 2019 (n = 56), fall 2019 (n = 34) and spring 2020 (n = 58) were aggregated, while remote data (n = 80) included fall 2020 (n = 39) and spring 2021 (n = 41) data. For exam 2 and HW 4/5, in-person data (n = 90) included spring 2019 and fall 2019 data only, while remote data (n = 138) included spring 2020, fall 2020 and spring 2021 data. Exam 1 and exam 2 had a maximum score of 150 points, HW 4/5 had a maximum score of 30 points, and the final score had a maximum of 100%.
We then compared the raw scores of exam 2 deployed in person (n = 90) and remotely (n = 138). Students who took exam 2 in person (M = 123.7, SD = 13.1) performed better than students who took the exam remotely (M = 116.2, SD = 19.4). This difference was determined to be significant by a one-tailed t test [t(226) = 3.21; P < 0.001; 95% CI, 2.88 to 12.04; Hedges’ g = 0.43].
To further elucidate the source of variation in exam 2 performance, we disaggregated the exam 2 data and compared individual exam performance for all 5 semesters (Table 3). A one-way ANOVA for exam 2 scores confirmed that there was a significant difference between scores: F(4, 223) = 11.58, P < 0.00001. Tukey’s HSD test indicated that exam 2 scores for IP spring 2019 (M = 121.5, SD = 12.9), IP fall 2019 (M = 127.3, SD = 12.7), and partially RMT spring 2020 (M = 123.8, SD = 13.3) were significantly higher (P < 0.00003) than the RMT fall 2020 scores (M = 105, SD = 22.1). The exam 2 scores for IP fall 2019 were also significantly higher (P = 0.014) than RMT spring 2021 scores (M = 116.3, SD = 19.0).
Experiment 4/5 homework
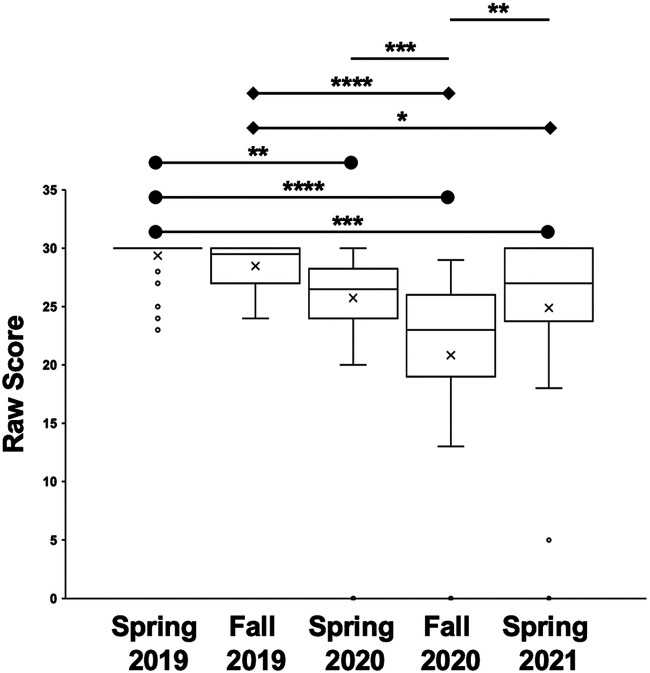
We compared student performance in another assessment, the combined homework for experiment 4 (plate counts) and experiment 5 (growth rates). These labs explored the concepts of serial dilutions, as well as the math of calculating dilutions, dilution factors, and exponential growth. The homework score for students who took the class in person (M = 29.0, SD = 1.7) was significantly higher than the score for students who took the class remotely (M = 24.1, SD = 6.7), as calculated by a one-tailed t test [t(226) = 6.78; P < 0.00001; 95% CI, 3.49 to 6.35; Hedges’ g = 0.92].
We disassociated the experiment 4/5 homework data to determine the source of variation. A one-tailed ANOVA confirmed the results of the t test F(4,223) = 18.4, P < 0.00001. The post hoc Tukey HSD revealed multiple differences (Fig. 4). The IP spring 2019 homework score (M = 29.4, SD = 1.6) was significantly higher (P = 0.01) than the partially RMT spring 2020 homework score (M = 25.7, SD = 4.3), significantly higher (P < 0.000001) than the RMT fall 2020 homework score (M = 20.8, SD = 7.9), and significantly higher (P = 0.00062) than the homework score for RMT spring 2021 (M = 24.9, SD = 7.4). Moreover, the IP fall 2019 homework score (M = 28.5, SD = 1.8) was significantly higher than the RMT fall 2020 homework score (P < 0.000001) and the RMT spring 2021 homework score (P = 0.011). Last, the RMT fall 2020 homework score was significantly lower than those for the partially RMT spring 2020 (P = 0.00012) and the RMT spring 2021 (P = 0.0025) homework.
FIG 4.
Box-and-whisker graph illustrating the experiment 4/5 homework scores for each semester examined. The “×” in each box represents the average score of the class, while the horizontal line in the box represents the median score. Outliers are shown as open dots. Horizontal lines with circles compare IP spring 2019 scores, while horizontal lines with diamonds compare the IP fall 2019 scores. This assignment had a maximum value of 30 points. Spring 2019, n = 56; fall 2019, n = 34; spring 2020, n = 58; fall 2020, n = 39; and spring 2021, n = 41. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001.
Lab safety questions
We hypothesized that the lab safety training provided in our fall 2020 and spring 2021 online courses would be as effective as the training provided in-person. In both semesters, students were required to pass the institution’s environmental health and safety course Lab Safety Fundamentals W/O HazMat. They also experienced two Labster simulations: Lab Safety and Biosafety. As part of exam 1, students were asked the following 2 lab safety essay questions, each worth 10 points.
Question 8: How aware are you about laboratory safety risks in the microbiology lab? List 4 lab safety risks in our lab, and what are the measures to address them.
Question 21: List 5 safety concerns illustrated in this image [see Fig. S1].
We compared the raw scores for lab safety question 8 and question 21 in the RMT fall 2020 (n = 40) and RMT spring 2021 (n = 41) semesters. Students performed equally well on question 8 independent of semester (fall 2020, M = 8.0, SD = 2.6; spring 2021, M = 8.2, SD = 2.3), as determined by a one-tailed t test [t(78) = 0.35; P > 0.05; 95% CI, −0.89 to 1.29; Hedges’ g = 0.08]. Similarly, a one-tailed t test showed that there was no difference in score for question 21 between the two semesters (fall 2020: M = 9.8, SD = 0.5; spring 2021, M = 9.7, SD = 0.8) [t(78) = −0.87; P > 0.05; 95% CI, −0.2 to 0.4; Hedges’ g = 0.15] (Fig. 5).
FIG 5.
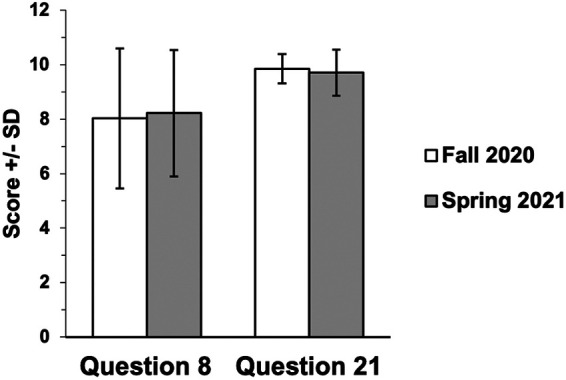
Bar graph comparing the mean value scores on two biosafety questions from exam 1 from the fall 2020 and spring 2021 semesters. Students were asked to identify hazards in a microbiology lab for question 8 and point out bad lab practices from an image in question 21. The maximum score in both questions is 10 points. Fall 2020, n = 40; spring 2021, n = 41.
Unknown Portfolios
The capstone project of many microbiology laboratory courses is the identification of an unknown (31–33). For the semesters taught remotely, we provided students with Unknown Portfolios, containing data images from various microscopic and biochemical tests for 7 different unknown pseudomonads. We hypothesized that the Unknown Portfolio would be as effective as the in-person project at providing students the necessarily experience and data to correctly identify their unknown pseudomonad. Therefore, we evaluated whether students correctly or incorrectly identified their unknown pseudomonads when the experiment was performed in person (n = 146) or remotely using the Unknown Portfolio (n = 71). These calculations were based on students who submitted the assignment, as 11 students did not submit it (Table 4). Students who submitted a paper but failed to include their unknown number were assigned to the incorrectly identified group. A chi-square test of independence showed that there was no significant association between the correct identification of the unknown pseudomonad and mode of data presentation [χ2(1, N = 217) = 0.05, P > 0.05].
TABLE 4.
Numbers of correctly identified unknown pseudomonads based on semester and mode of class delivery
| Semester or mode | No. |
Percenta |
||||
|---|---|---|---|---|---|---|
| Total | Submitted |
Unsubmitted | ||||
| Correct | Incorrect | Correct | Incorrect | |||
| Spring 2019 | 56 | 35 | 21 | 0 | 63 | 38 |
| Fall 2019 | 34 | 19 | 15 | 0 | 56 | 44 |
| Spring 2020 | 58 | 32 | 24 | 2 | 57 | 43 |
| Fall 2020 | 39 | 22 | 11 | 6 | 67 | 33 |
| Spring 2021 | 41 | 21 | 17 | 3 | 55 | 45 |
| In personb | 148 | 86 | 60 | 2 | 59 | 41 |
| Portfolio | 80 | 43 | 28 | 9 | 61 | 39 |
Unsubmitted assignments were not used to calculate percentages.
For in-person evaluation (n = 148), unknown-pseudomonad data from spring 2019 (n = 56), fall 2019 (n = 34), and spring 2020 (n = 58) were aggregated, while remote data (n = 80) included fall 2020 (n = 39) and spring 2021 (n = 41) data.
Streak plate technique
We hypothesized that students in the blended, online course would gain good hands-on techniques remotely. The streak plate technique is an important skill for any microbiology student to isolate independent colonies. Therefore, we evaluated the scores obtained in the streak plate assessments submitted as part of exam 1 (see Fig. 2 for sample plates). We compared the scores for the fall 2020 and spring 2021 remote semesters only, as we did not have access to scores from previous in-person semesters. Based on our analysis, the streak plate scores for the fall 2020 semester (M = 3.9, SD = 1.7) and spring 2021 semester (M = 4.1, SD = 1.5) were not statistically different, as determined by a one-tailed t test [t(78) = −0.31; P = 0.38; 95% CI, −0.56 to 0.86; Hedges’ g = 0.12] (Fig. S2).
DISCUSSION
The COVID-19 pandemic shutdown forced us to switch our in-person microbiology lab to online delivery. Over the 2020 summer, we developed an online microbiology laboratory that blended online simulations with hands-on laboratory experiments. We examined performance in various assessments to evaluate how students taking the blended, online lab compared to students who took the equivalent lab in-person. Taken together, our data indicate that the blended, online lab was as effective as the in-person class at helping students master course content and achieve course learning outcomes. Students in the blended, remote lab, for example, were able to isolate individual microbial colonies from a microbial community in kombucha, demonstrating mastery of the streak plating technique, a hands-on skill primarily taught in in-person lab courses. Moreover, students performed equally well on lab safety questions, indicating that our delivery of lab safety content was effective across platforms. Last, students in the online lab were as able to correctly identify an unknown pseudomonad as students taking the lab in person. This suggests that the Unknown Portfolios used in the online course provided students with a similar experience to collecting and interpreting data in person, allowing them to use their critical thinking skills to identify their unknown microorganisms.
Intriguingly, students in the RMT fall 2020 lab had the lowest performance of the labs evaluated. This lab class cohort had the second lowest exam 1 score average (79%), the lowest exam 2 score average (70%), and the lowest lab 4/5 homework score average (69%). These scores contributed to this cohort having the lowest final course score average of the labs examined (83.7%). Although students in this cohort had the lowest performance among the cohorts studied, it is worth noting that only 3 of 39 (7.7%) students did not pass the class (Fig. 3), demonstrating that most students earned scores to successfully achieve a passing grade.
What factors could have contributed to this reduction in class performance in fall 2020? We think that the stress associated with the COVID-19 pandemic was exacerbated by the 2020 California’s wildfire season (40) and the growing sociopolitical tension associated with the 2020 presidential election (41), resulting in a cognitive load increase that impacted academic performance.
The COVID-19 pandemic impacted student performance and deepened achievement gaps among students in kindergarten through 12th grade (42, 43) and college students (44) across the United States, especially in students of color (45–47). Our students, who are overwhelmingly first-generation college students and come from underserved communities, often experience additional stressors, including housing and food insecurity, lack of adequate spaces for learning at home, and challenges with access to technology for remote learning and connectivity to the Internet (48). Academic uncertainty, brought out by the challenges in online class delivery, might have led to high levels of stress, which made it difficult for students to deal with the academic demands and disruptions caused by the pandemic (49).
Over 70% of our students are first-generation college students, and more than 80% come from minoritized communities (particularly Hispanic, Asian, and Black). Many of our students are the older children of frontline workers (50), which often increased their domestic and financial obligations associated with returning to live at home. The combined effect of these stressors potentially reduced the cognitive load capacity of our students, reducing their ability to focus on academic tasks (51). For example, 6 students of 39 did not submit their unknown pseudomonad lab report in fall 2020 (Table 4), and other assignments were similarly affected. This was the highest number of missing assignments in the 14 years that we have taught this lab.
Students in the RMT spring 2021 semester performed better. By this time, the stress related to the California wildfire season and the presidential election were no longer part of the equation. Students adapted to remote instruction and developed coping mechanisms that helped mitigate the stressors. Moreover, we modified our course by incorporating lessons learned from the fall 2020 semester, improving the delivery of class material. It is probable that the combination of these adaptive factors increased the cognitive load capacity of our students, resulting in increased performance in this semester compared to students in the RMT fall 2020 semester. This is a topic that merits more structured research.
Two recent publications evaluated the effect of the COVID-19 pandemic on student performance, finding that it increased in the spring 2020 academic term (52, 53). Our spring 2020 data align with these findings, as the overall performance for this semester was not different from that of the IP spring 2019 and IP fall 2019 semesters (Fig. 3 and Table 3). There are, however, differences between our work and these studies. The most relevant difference is study design. Both studies compared performance of over 25 different courses taught by different faculty, involving thousands of students, while our study examined performance for a single, upper-division lab course taught by one faculty, involving 229 students. Our study also included data from the fall 2020 and spring 2021 semesters, which were completely online, while these studies examined the spring 2020 term, when the transition to online teaching occurred. Another difference lies in the demographic distribution of our students: the cohort studied by Zuckerman and colleagues (53) included 40% students designated PEER (persons excluded because of their ethnicity or race) (54). PEER were calculated as nonwhite and non-Asian students. Using the same formula, the PEER population in our study is 51.5%. On the other hand, the student population studied by Supriya and colleagues was 49% white, 14% Asian, 5% Black, and 25% Hispanic (52), while our student population was 11% white, 38% Asian, 2% Black, and 43% Hispanic (Table 2). It would be interesting to evaluate performance in STEM courses at those institutions for the fall 2020 and spring 2021 semesters. Moreover, it would be important to evaluate the performance across STEM courses taken by biology majors at our institution by the methods used in these studies.
CONCLUSIONS
Lessons learned
The COVID-19 pandemic irreversibly changed the way we see online teaching, especially for laboratory courses. In the future, it is imperative that we incorporate successful elements of online pedagogy used during ERT in our in-person courses (3, 5, 55–57). The primary lesson learned from adapting a microbiology laboratory course to the online learning environment is that we must be flexible in all aspects of our teaching.
Flexibility in the way material is covered is the lesson we learned from transitioning our course to the online environment during the COVID-19 pandemic shutdown. This calls for the integration of simulations in future in-person lab courses, giving students the opportunity to experience material from multiple platforms. A blended lab course would also expose students to laboratory techniques unavailable in their lab courses, reducing the accessibility gap and expanding students’ knowledge of state-of-the-art experimental procedures. For example, by training with the Labster simulation Next Generation Sequence, students would learn how sequence technology works to obtain gene sequence data. Without the simulation, their understanding of gene sequencing would have remained in the realm of theory, as our teaching lab does not have access to next-generation sequencing technology. As technology moves forward faster than the budget for teaching laboratories, simulations help make knowledge accessible to all students, making the learning lab more equitable and better preparing them for their careers (13). Conversely, students in online lab classes would benefit from experiencing hands-on components. The Crafty techniques (58), or the hands-on activities described here, would improve any online microbiology lab course, better preparing students. We were horrified at the thought of one of our students going to a job or graduate school interview and stating that they did not get to handle microbes in their microbiology lab course. Moreover, providing students the hands-on experience of a lab gives them a more realistic understanding of the challenges of experimental microbiology and ensures that our students are prepared for their future. For example, many students experienced difficulties preparing their bacterial plates, which provided a glimpse of the efforts lab technicians make to ensure that all lab materials are ready for a lab session.
Limitations of the experimental materials for at-home experiments
The microscope and media sent to students were adequate to perform the experimental procedures at home. However, these had limitations: for example, the student’s microscope allows the visualization of microbial samples at a ×1,200 magnification, but images suffer from chromatic aberrations that make it challenging to discern the results of a Gram stain. The solidified nutrient agar was delicate and could be easily perforated while practicing the streak plate technique. Since the bacterium growing kit did not display the exact content of the nutrient agar, we could not determine how much agar was present in the medium. Instructors should keep these limitations in mind when designing their experiments.
Cost
Students who took the laboratory course during the COVID-19 pandemic received the lab materials free of charge. The cost of course reagents is estimated to be $104.04 per student (Table S1). The cost of the Labster simulations depends on the type of license the institution purchases (https://www.labster.com/pricing/). Assuming that the university purchased an institutional license, the per-student cost for the Labster simulation would range from $4 to $20. For our calculation, we assumed the highest Labster simulation cost of $20 per student. Based on this assumption, the cost of delivering the course per student would be $124.04 ($104.04 for materials and $20 for Labster). Therefore, the cost for delivering the course to 40 students in fall 2020 was $4,961.60, while the cost in spring 2021 to 41 students was $5085.64 (Table S3). Both these semesters had a course fee of $85, but this fee was not collected during remote offering.
Students might also incur other expenses associated with purchasing of PPE. This might include the price of lab coats, safety goggles, and nitrile gloves, as well as the cost of a lab notebook. This cost is estimated to amount to about $55 per student (Table S2). Future remote offerings of this lab may require that the full costs of the supplies and PPE equipment be passed on to the students.
At-home laboratory safety
Lab safety precautions for at-home labs is of the utmost importance to ensure the safety of students, housemates, pets, and family members. As in the in-person laboratory, students must follow safety precautions and protocols while performing experiments at home. Modeling good safety practices can be as simple as instructors wearing full PPE while recording themselves performing demonstrations from home and discussing the important aspects of safety while performing these demonstrations. However, monitoring lab safety at home is challenging. Having students sign a lab safety agreement (labsafety.org) is an important tool to ensure that students (and their parents if the students are minors) have read and understand the lab safety precautions and agree to follow these rules.
Students could not be directly monitored to ensure that they were following proper lab safety protocols. In both semesters taught online, students reported neither accidents nor any adverse incidents while performing the lab procedures from home. Future iterations of the remote lab would incorporate having students take videos of themselves while performing the lab procedures. This would allow us to (i) evaluate the techniques used by students, (ii) ascertain the use of proper PPE, and (iii) examine the use and implementation of proper lab safety practices.
Last, students require guidance for the disposal of chemical and biohazard material. To discard bacterial plates, students were instructed to flood their plates with a 10% bleach solution for 10 min. Following treatment, the bleach solution would be discarded in the drain and the plate could be safely discarded in the trash.
Gram stain reagents were discarded down the drain, following the manufacturer’s indications. This poses a challenge, as different states and municipalities might have different disposal requirements. Therefore, having students check local and state regulations will help determine the appropriate way of disposing of these reagents. Gram stain reagents can be disposed of down the drain with 20-fold excess water when the drain is connected to the sanitary sewer system (https://www.wardsci.com/store/product/8871107/crystal-violet).
Increasing students’ job marketability
We want to ensure that our students are prepared for their careers, especially meeting the demands of biotechnology, which requires skills not usually associated with higher education (59). A blended lab course could be an excellent venue to provide students with these career skills. By incorporating lab safety simulations with the EHS lab safety course, we provided the students with a marketable skill that they can put in their resumes, increasing their potential to be hired in a microbiology or any research lab.
In conclusion, we created an online laboratory experience that proved effective at transmitting both theoretical and practical knowledge to our students. Elements of this class could be adapted to other microbiology courses, irrespective of their level or mode of delivery. In the future, online components of this course ought to be incorporated into the in-person class, creating a blended course that will serve all students equally.
ACKNOWLEDGMENTS
First, we acknowledge the students from the general microbiology laboratory, for providing the results and data used to create the Unknown Portfolios. We thank Evin Doscher, Ruihao Li, and Eli Maciel for their role in the initial transition of the microbiology lab course to ERT in spring 2020. Eli Maciel was also instrumental in the delivery of the course in the fall 2020 and spring 2021 semesters. This course would not be possible without the hard work and dedication of the Life Sciences Instructional Lab team, managed by James Whalen.
We acknowledge the dean of the School of Natural Sciences for funding the cost of the reagents and supplies sent to students, making it possible for them to engage in hands-on laboratory learning at home.
We have no conflicts of interest to declare.
Footnotes
Supplemental material is available online only.
Contributor Information
Marcos E. García-Ojeda, Email: mgarcia-ojeda@ucmerced.edu.
Jennifer Herzog, Herkimer County Community College.
REFERENCES
- 1.Hodges C, Moore S, Lockee B, Trust T, Bond A. 2020. The difference between emergency remote teaching and online learning. Educause. https://er.educause.edu/articles/2020/3/the-difference-between-emergency-remote-teaching-and-online-learning. Retrieved 3 December 2021. [Google Scholar]
- 2.ASM. 2021. ASM curriculum guidelines for undergraduate microbiology. https://asm.org/Guideline/ASM-Curriculum-Guidelines-for-Undergraduate-Microb.
- 3.Noel TC, Rubin JE, Acebo Guerrero Y, Davis MC, Dietz H, Libertucci J, Sukdeo N. 2020. Keeping the microbiology lab alive: essential microbiology lab skill development in the wake of COVID-19. Can J Microbiol 66:603–604. doi: 10.1139/cjm-2020-0373. [DOI] [PubMed] [Google Scholar]
- 4.Yap WH, Teoh ML, Tang YQ, Goh BH. 2021. Exploring the use of virtual laboratory simulations before, during, and post COVID-19 recovery phase: an animal biotechnology case study. Biochem Mol Biol Educ 49:685–691. doi: 10.1002/bmb.21562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen TE, Barker SD. 2021. BME labs in the era of COVID-19: transitioning a hands-on integrative lab experience to remote instruction using gamified lab simulations. Biomed Eng Educ 1:99–104. doi: 10.1007/s43683-020-00015-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones N. 2018. Simulated labs are booming. Nature 562:S5–S7. doi: 10.1038/d41586-018-06831-1. [DOI] [PubMed] [Google Scholar]
- 7.Darrah M, Humbert R, Finstein J, Simon M, Hopkins J. 2014. Are virtual labs as effective as hands-on labs for undergraduate physics? A comparative study at two major universities. J Sci Educ Technol 23:803–814. doi: 10.1007/s10956-014-9513-9. [DOI] [Google Scholar]
- 8.Alvarez KS. 2021. Using virtual simulations in online laboratory instruction and active learning exercises as a response to instructional challenges during COVID-19. J Microbiol Biol Educ 22:ev22i1.2503. doi: 10.1128/jmbe.v22i1.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson N, Veletsianos G, Seaman J. 2020. U.S. faculty and administrators’ experiences and approaches in the early weeks of the COVID-19 pandemic. OLJ 24:6–21. doi: 10.24059/olj.v24i2.2285. [DOI] [Google Scholar]
- 10.Flint S, Stewart T. 2010. Food microbiology—design and testing of a virtual laboratory exercise. J Food Science Education 9:84–89. doi: 10.1111/j.1541-4329.2010.00108.x. [DOI] [Google Scholar]
- 11.Brown P, Peterson J. 2021. The effect of online instruction in an introductory anatomy and physiology course and implications for online laboratory instruction in health field prerequisites. J College Sci Teaching 50. [Google Scholar]
- 12.Ma J, Nickerson JV. 2006. Hands-on, simulated, and remote laboratories. ACM Comput Surv 38:7. doi: 10.1145/1132960.1132961. [DOI] [Google Scholar]
- 13.Bonde MT, Makransky G, Wandall J, Larsen M.v, Morsing M, Jarmer H, Sommer MOA. 2014. Improving biotech education through gamified laboratory simulations. Nat Biotechnol 32:694–697. doi: 10.1038/nbt.2955. [DOI] [PubMed] [Google Scholar]
- 14.Makransky G, Petersen GB, Klingenberg S. 2020. Can an immersive virtual reality simulation increase students’ interest and career aspirations in science? Br J Educ Technol 51:2079–2097. doi: 10.1111/bjet.12954. [DOI] [Google Scholar]
- 15.Makransky G, Thisgaard MW, Gadegaard H. 2016. Virtual simulations as preparation for lab exercises: assessing learning of key laboratory skills in microbiology and improvement of essential non-cognitive skills. PLoS One 11:e0155895. doi: 10.1371/journal.pone.0155895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brockman RM, Taylor JM, Segars LW, Selke V, Taylor TAH. 2020. Student perceptions of online and in-person microbiology laboratory experiences in undergraduate medical education. Med Educ Online 25:1710324. doi: 10.1080/10872981.2019.1710324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herzog JA, Mawn M. 2020. Teaching lab-based courses online & remote: from “are you kidding me?” to “this is effective!” https://asm.org/Articles/2020/May/Teaching-Lab-Based-Courses-Online-Remote-From-Are.
- 18.Grove Arvidson C. 2010. Streak plate. https://learn.chm.msu.edu/vibl/content/streakplate.html.
- 19.Amagai S. 2020. Bacterial identification virtual lab. https://www.biointeractive.org/classroom-resources/bacterial-identification-virtual-lab. Retrieved 3 October 2021.
- 20.Helikar T, Kowal B, McClenathan S, Bruckner M, Rowley T, Madrahimov A, Wicks B, Shrestha M, Limbu K, Rogers JA. 2012. The cell collective: toward an open and collaborative approach to systems biology. BMC Syst Biol 6:96. doi: 10.1186/1752-0509-6-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cell Collective. 2021. Advanced concepts in lac operon transcription regulation. http://www.cellcollective.org. Retrieved 3 December 2021.
- 22.Ansari J. 2017. Bottoms up! Discover the microbes in probiotic drinks. American Society for Microbiology. https://asm.org/Articles/2017/August/bottoms-up-discover-the-microbes-in-probiotic-drin. Retrieved 30 September 2021. [Google Scholar]
- 23.Applegate KB, Cheek PR, Inlow JK. 2019. Analysis of kombucha to teach biochemical concepts and techniques to undergraduate students. Biochem Mol Biol Educ 47:459–467. doi: 10.1002/bmb.21240. [DOI] [PubMed] [Google Scholar]
- 24.Sun L, Zhao H, Liu L, Wu X, Gao Q, Zhao Y. 2018. Effects of Lactobacillus on the inhibition of Helicobacter pylori growth. Biotechnol Biotechnol Equip 32:1533–1540. doi: 10.1080/13102818.2018.1515599. [DOI] [Google Scholar]
- 25.Majeed M, Nagabhushanam K, Natarajan S, Sivakumar A, Eshuis-de Ruiter T, Booij-Veurink J, de Vries YP, Ali F. 2016. Evaluation of genetic and phenotypic consistency of Bacillus coagulans MTCC 5856: a commercial probiotic strain. World J Microbiol Biotechnol 32:60. doi: 10.1007/s11274-016-2027-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishibashi N, Yamazaki S. 2001. Probiotics and safety. Am J Clin Nutr 73:465s–470s. doi: 10.1093/ajcn/73.2.465s. [DOI] [PubMed] [Google Scholar]
- 27.EFSA Panel on Biological Hazards (BIOHAZ). 2013. Scientific opinion on the maintenance of the list of QPS biological agents intentionally added to food and feed (2013 update). EFSA J 11:3449. [Google Scholar]
- 28.Rowland IR, Capurso L, Collins K, Cummings J, Delzenne N, Goulet O, Guarner F, Marteau P, Meier R. 2010. Current level of consensus on probiotic science—report of an expert meeting, London, 23 November 2009. Gut Microbes 1:436–439. doi: 10.4161/gmic.1.6.13610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.NIH Department of Health and Human Services. 2019. NIH guidelines for research involving recombinant or synthetic nucleic acid molecules. https://osp.od.nih.gov/wp-content/uploads/NIH_Guidelines.pdf.
- 30.Emmert EAB, ASM Task Committee on Laboratory Biosafety . 2013. Biosafety guidelines for handling microorganisms in the teaching laboratory: development and rationale. J Microbiol Biol Educ 14:78–83. doi: 10.1128/jmbe.v14i1.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhodes DVL, Barshick MR. 2021. Adapting a bacterial unknowns project to online learning: using Microsoft PowerPoint to create an unknowns identification simulation. J Microbiol Biol Educ 22:e00104-21. doi: 10.1128/jmbe.00104-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tawde M, McLaughlin S. 2021. Implementing a virtual midterm to identify unknown bacteria in a microbiology lab course. J Microbiol Biol Educ 22:ev22i1-2579. doi: 10.1128/jmbe.v22i1.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amrein J, Dimond Z, Reboullet J, Hotze E. 2021. Bacterial unknown project in the COVID19 era: transition from in-person lab experience to online environment. J Microbiol Biol Educ 22:ev22i1-2415. doi: 10.1128/jmbe.v22i1.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCormick AC, Zhao C-M. 2005. Rethinking and reframing the carnegie classification. Change Magazine Higher Learning 37:51–57. doi: 10.3200/CHNG.37.5.51-57. [DOI] [Google Scholar]
- 35.Social Science Statistics. 2021. Effect size calculator for T-test. https://www.socscistatistics.com/effectsize/default3.aspx.
- 36.Social Science Statistics. 2021. T-test calculator for 2 independent means. https://www.socscistatistics.com/tests/studentttest/default.aspx.
- 37.Social Science Statistics. 2021. Chi-square calculator. https://www.socscistatistics.com/tests/chisquare/default.aspx.
- 38.Social Science Statistics. 2021. Independent samples confidence interval calculator. https://www.socscistatistics.com/confidenceinterval/default4.aspx.
- 39.Social Science Statistics. 2021. One-way ANOVA calculator, including Tukey HSD. https://www.socscistatistics.com/tests/anova/default2.aspx.
- 40.Freedberg L. 2020. As one University of California campus is evacuated due to fires, others revise fall reopening plans. https://edsource.org/2020/as-one-university-of-california-campus-is-evacuated-due-to-fires-others-revise-fall-reopening-plans/638818.
- 41.Albright JN, Hurd NM. 2020. Marginalized identities, Trump-related distress, and the mental health of underrepresented college students. Am J Community Psychol 65:381–396. doi: 10.1002/ajcp.12407. [DOI] [PubMed] [Google Scholar]
- 42.Bailey DH, Duncan GJ, Murnane RJ, Au Yeung N. 2021. Achievement gaps in the wake of COVID-19. Educ Res 50:266–275. doi: 10.3102/0013189X211011237. [DOI] [Google Scholar]
- 43.García E, Weiss E. 2020. COVID-19 and student performance, equity, and U.S. education policy. Economic Policy Institute. https://www.epi.org/publication/the-consequences-of-the-covid-19-pandemic-for-education-performance-and-equity-in-the-united-states-what-can-we-learn-from-pre-pandemic-research-to-inform-relief-recovery-and-rebuilding/. [Google Scholar]
- 44.Kubrak A. 2021. Did college students perform worse during COVID-19? https://www.ecampusnews.com/2021/01/19/did-college-students-perform-worse-during-covid-19/.
- 45.Molock SD, Parchem B. 2021. The impact of COVID-19 on college students from communities of color. J Am College Health 2021:1–7. doi: 10.1080/07448481.2020.1865380. [DOI] [PubMed] [Google Scholar]
- 46.Goldberg SB. 2021. Education in a pandemic: the disparate impacts of COVID-19 on America’s students. U.S. Department of Education, Washington, DC. [Google Scholar]
- 47.Dorn E, Hancock B, Sarakatsannis J, Viruleg E. 2020. COVID-19 and students learning in the United States: the hurt could last a lifetime. Society for Research in Child Development, Ann Arbor, MI. [Google Scholar]
- 48.Galperin H, Wyatt K, Le T. 2020. COVID-19 and the distance learning gap. University of Southern California, Annenberg Research Network on International Communication, Los Angeles, CA. [Google Scholar]
- 49.Clabaugh A, Duque JF, Fields LJ. 2021. Academic stress and emotional well-being in United States college students following onset of the COVID-19 pandemic. Front Psychol 12:628787. doi: 10.3389/fpsyg.2021.628787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blau FD, Koebe J, Meyerhofer PA. 2021. Who are the essential and frontline workers? Bus Econ 56:168–178. doi: 10.1057/s11369-021-00230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shay JE, Pohan C. 2021. Resilient instructional strategies: helping students cope and thrive in crisis. J Microbiol Biol Educ 22:ev22i1-2405. doi: 10.1128/jmbe.v22i1.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Supriya K, Mead C, Anbar AD, Caulkins JL, Collins JP, Cooper KM, LePore PC, Lewis T, Pate A, Scott RA, Brownell SE. 2021. Undergraduate biology students received higher grades during COVID-19 but perceived negative effects on learning. Front Educ 6. doi: 10.3389/feduc.2021.759624. [DOI] [Google Scholar]
- 53.Zuckerman AL, Hardesty RA, Denaro K, Lo SM, Owens MT. 2021. Effects of remote teaching in a crisis on equity gaps and the constructivist learning environment in an introductory biology course series. J Microbiol Biol Educ 22:ev22i1.2293. doi: 10.1128/jmbe.v22i1.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Asai DJ. 2020. Race matters. Cell 181:754–757. doi: 10.1016/j.cell.2020.03.044. [DOI] [PubMed] [Google Scholar]
- 55.Abriata LA. 2022. How technologies assisted science learning at home during the COVID-19 pandemic. DNA Cell Biol 41:19–24. doi: 10.1089/dna.2021.0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Delgado T, Bhark S-J, Donahue J. 2021. Pandemic teaching: creating and teaching cell biology labs online during COVID-19. Biochem Mol Biol Educ 49:32–37. doi: 10.1002/bmb.21482. [DOI] [PubMed] [Google Scholar]
- 57.Kelley EW. 2020. Reflections on three different high school chemistry lab formats during covid-19 remote learning. J Chem Educ 97:2606–2616. doi: 10.1021/acs.jchemed.0c00814. [DOI] [Google Scholar]
- 58.Estes AM, Jozwick AS, Kerr JE. 2021. Teaching “crafty microbiology”: safely teaching hands-on microbiology skills at home. J Microbiol Biol Educ 22ev22i1-2345. doi: 10.1128/jmbe.v22i1.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Delebecque CJ, Philp J. 2019. Education and training for industrial biotechnology and engineering biology. Eng Biol 3:6–11. doi: 10.1049/enb.2018.0001. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download jmbe.00328-21-s001.pdf, PDF file, 2.3 MB (2.4MB, pdf)