ABSTRACT
The wound healing assay is a simple and inexpensive method that allows researchers to experimentally mimic cell growth and migration leading to wound healing. In this assay, a wound is created on a monolayer of cultured mammalian cells and cell migration is monitored. Micrographs are captured at regular intervals during the duration of the experiment. These microscopy images are analyzed to compare cell migration and wound closure under different conditions. Introduction of different cytotoxic treatments into a wound healing assay can provide information as to whether a particular drug or compound of interest has the ability to affect cell migration. This type of analysis is important when assessing the ability of a particular cancer cell line to display invasive and metastatic behaviors. One of the challenges of this assay is to create the original wound in a way that is consistent across plates or treatments, facilitating comparisons across experimental groups. This is a particular challenge when using the wound healing assay in the context of an undergraduate biology class to expose students to a distinct form of mammalian cell culture and help them apply scientific knowledge and research skills. We found an easy way to overcome this obstacle by using ibidi plates. In this article, we provide a simple protocol to use ibidi plates and HeLa cells to set up wound healing assays. This laboratory exercise allows undergraduate students to utilize different skills developed through cell culture experience, such as growing, treating, and imaging mammalian cells.
KEYWORDS: wound healing assay, ibidi plates, cytotoxic agents, HeLa cells, cancer invasion
INTRODUCTION
Cell migration is an essential process in all multicellular organisms and a hallmark of tissue development, cancer invasion, and wound repair. The wound healing assay is a diverse technique used in a range of disciplines to study the coordinated movement of a cell population in vitro (1). Wound healing is a complex and complicated cellular process that involves migration of cells. The wound healing assay in this study was performed using a specific type of 4-well plates called ibidi plates (ibidi catalog number 80466). These plates feature a removable silicone insert that separates wells where cells are grown. After the cells have successfully attached, the insert can be removed to reveal four 500-μm-wide cell-free gaps and one 1,000-μm center gap which act as artificial wounds. The advantage of this method over the classical method, which involves manual scratching a confluent cell monolayer with a micropipette tip to generate a wound, is that it provides reproducible gap sizes. More importantly, scratching removes surface coating and alters cell adherence and migration in that area. These challenges can be overcome by using ibidi plates.
In this article, we describe how to use ibidi plates and HeLa cells to teach undergraduate students to perform wound healing assays. For analysis, students captured images at regular intervals during the progression of the assays to assess the effects of different treatments on the rate of mammalian cell migration (2).
PROCEDURE
Safety considerations and precautions
Prior to the first laboratory session, students were required to complete online Collaborative Institutional Training Initiative training, a hepatitis B declination form, and an on-site blood-borne pathogen training checklist, to read over laboratory safety guidelines, and to sign a document confirming that they agreed to comply. Students were also required to watch a video on laboratory safety (North Carolina Community Colleges BioNetwork lab safety video series, https://www.youtube.com/playlist?list=PL4qaj9envIYnBaQSPpcOMUqWiQUAgPoMq) and complete a quiz with a passing grade to demonstrate their understanding. While inside the lab, students must wear protective eyewear, lab coats, closed-toe shoes, and gloves. Disposal of biosafety level 2 materials (such as HeLa cells) and reagents should be contained in designated biohazard containers within the laboratory. Waste should be autoclaved or bleached before disposal. It is critical for students to maintain a clean workspace using 70% ethanol and utilize a handwashing station before entering and exiting the lab. Details about the safety considerations and precautions can be found in a previous publication (3).
Materials and methods
Establishing and maintaining mammalian cell culture. HeLa cells were obtained from the American Type Culture Collection. All cell culture-related experiments were performed in a tissue culture hood and by using aseptic technique. Before students could conduct cell culture, the instructor familiarized students with basic cell culture techniques through lab demonstrations. Students then mirrored the technique independently, ultimately generating their own HeLa cell starter plate. Detailed information about HeLa cell culture can be found in a previous publication (4).
Implementation of experiments in the undergraduate laboratory. Students grew a population of HeLa cells in ibidi plates until they reached 100% confluence. Each plate was properly labeled with student initials, quadrant numbers, and type of treatment, and growth was monitored. The culture insert was removed from the plate using a pair of sterilized tweezers. The ibidi plates were then tilted to collect and remove spent medium with a micropipette. Cells were rinsed several times with 2 mL of Dulbecco's modified Eagle medium (DMEM) supplemented with 1% fetal bovine serum (FBS). Washing the plate is a very important step, as this prevents settling of detached and floating cells in the wound which eventually fills the gap.
After the ibidi plates were rid of the culture insert and nonadherent cells, treatments were added. A control plate included 2 mL of 1% FBS–DMEM containing dimethyl sulfoxide (DMSO), while the experimental plate contained 2 mL of 1% FBS–DMEM with one of the cytotoxic agents, fisetin or cycloheximide, dissolved in DMSO. FBS was used at 1% to maximize migration of cells, rather than proliferation, for wound healing. A microscope was used to determine if the cell quadrants were well-defined and to image the plate immediately after the wound creation. This initial image served as a starting reference that was compared to future images to determine the progress of gap closure, i.e., wound healing. Both ibidi plates were incubated at 37°C in a humidified atmosphere consisting of 5% CO2. After 24 and 48 h, students returned to the lab to image the plates and compare cell migration to the starting reference. Schematic representation of experimental workflow for a wound healing assay using the ibidi plate is shown in Fig. 1.
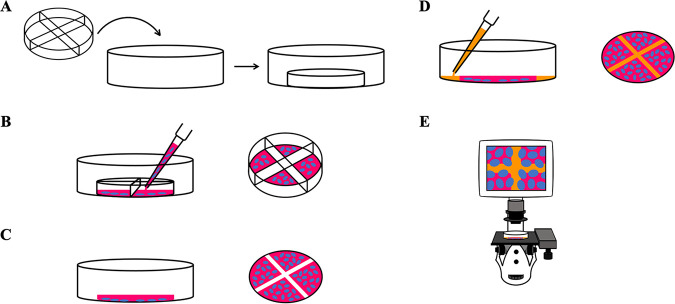
FIG 1.
Principle for the wound healing assay using ibidi plates. (A) The 4-well silicone insert with defined cell-free gaps is placed inside the 35-mm μ-dish. (B) Appropriate numbers of cells (blue) with medium (red) are seeded in chambers and incubated for cell attachment (left). A closer view of the 4-well silicone insert with cells is shown (right). (C) The culture insert and dividers are removed after cell attachment (left). Removal of silicone insert reveals four 0.5-mm cell-free gaps and one center area with a 1-mm diagonal gap (right). (D) After removal of the old medium and multiple rinses, new medium is added, with or without the treatment drug (left). Gaps are covered by medium (orange), allowing cells to migrate and close the wound (right). (E) A microscope is used to observe cells migrating into cell-free gaps and closing the wound.
Data analysis in the undergraduate laboratory. To analyze the data, image analysis software such as ImageJ (5) or Motic Images Plus (6) can be used. Also, ibidi offers a FastTrack AI image analysis service, which is a web-based quantitative image analysis solution. We used ImageJ, which is freely available from NIH (https://imagej.nih.gov/ij/) to calculate percent wound closure at time x by using the following equation (7): wound closure = {[(wound area at time 0) − (wound area at time x)/(wound area at time 0)] × 100}. For details, see Appendix 2 in the supplemental material.
Micrographs after treating the cells with DMSO and cycloheximide (50 μg/mL) are shown in Fig. 2A. As expected, we observed less migration and wound closure when the cells were treated with the anticancer agent cycloheximide. DMSO-treated control cells showed 33.9% and 74.5% wound closure, while cycloheximide-treated cells displayed only 11.2% and 23.5% wound closure at 24 and 48 h, respectively (Fig. 2B). When HeLa cells were treated with fisetin (60 μM), the percentage wound closure dropped to −8.3% in 24 h (Fig. 3). This might have been due to the fact that fisetin is known to inhibit proliferation and induce apoptotic cell death in several cancer cell lines. We chose cycloheximide and fisetin as our choices of treatment because these anticancer drugs showed promising effects in our clonogenic assays reported previously (3).
FIG 2.
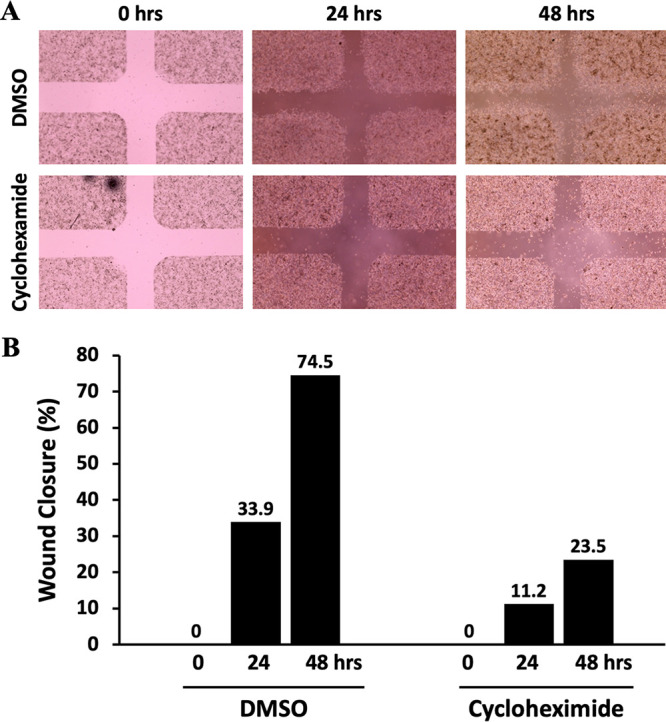
Analysis of HeLa cell migration in the in vitro wound healing assay. (A) Micrograph of wound healing assays with DMSO and cycloheximide. Assays were performed with an ibidi culture insert in 4-well 35-mm dishes. (Top) Microscopy images of wound closure of DMSO-treated control cells at 0, 24, and 48 h after culture insert removal. (Bottom) Images were also taken at 0, 24, and 48 h after cycloheximide treatment. Cycloheximide-treated cells showed less migration than DMSO-treated counterparts. (B) Percentage of wound closure at 0, 24, and 48 h postinjury. The center wound areas were selected from micrographs, and wound closure was quantified using ImageJ software.
FIG 3.
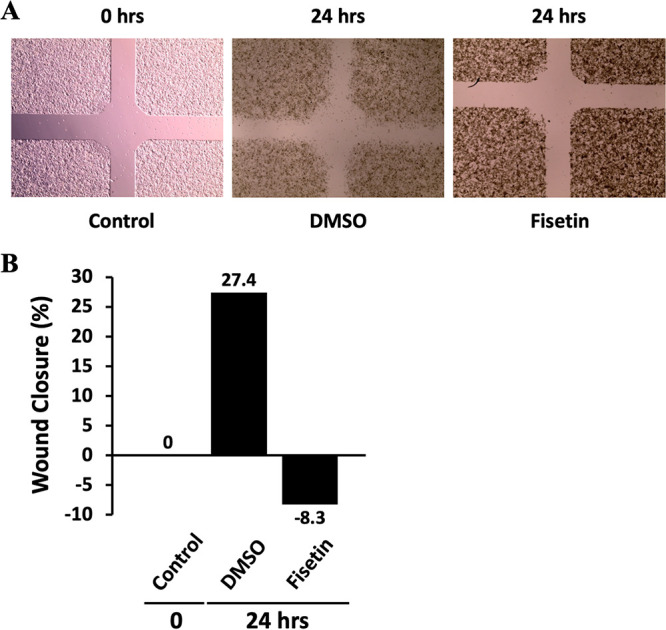
Effect of fisetin on HeLa cell migration. (A) Micrograph of wound healing assays with DMSO and fisetin. Assays were performed with ibidi plates with culture inserts. Microscopy images of wound closure by HeLa cells before treatment (control) and after DMSO or fisetin treatment are shown. Fisetin-treated cells showed less wound closure than the control or DMSO-treated cells. (B) The center wound areas were selected from micrographs, and the percentage wound closure at 0 and 24 h was quantified using ImageJ software.
Intended audience
This laboratory exercise is intended for undergraduate students in college or university. At High Point University, this exercise was used in an upper-level cancer biology class to explore concepts related to cancer metastasis, mammalian cell culture, cell migration, and contact inhibition.
Alternative applications
Wound healing assay results can be complemented with other phenotypic assays for cellular behaviors, such as invasion and colony formation assays (3). In addition to using this assay as a readout for a drug treatment, it can be used in the context of genetic inhibition or overexpression of genes of interest.
CONCLUSION
This laboratory activity is an effective way for undergraduate students to explore cell migration and wound healing, reinforcing student understanding of cell biology.
ACKNOWLEDGMENTS
No external funding was used for this educational research.
We declare that the research was conducted in the absence of any relationships that could be construed as a potential conflict of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Verónica A. Segarra, Email: veronica.segarra@goucher.edu.
Laura J. MacDonald, Hendrix College
REFERENCES
- 1.Jonkman JE, Cathcart JA, Xu F, Bartolini ME, Amon JE, Stevens KM, Colarusso P. 2014. An introduction to the wound healing assay using live-cell microscopy. Cell Adh Migr 8:440–451. doi: 10.4161/cam.36224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayr C, Beyreis M, Dobias H, Gaisberger M, Fuchs J, Pichler M, Ritter M, Jakab M, Helm K, Neureiter D, Kiesslich T. 2019. Continuous, label-free, 96-well-based determination of cell migration using confluence measurement. Cell Adh Migr 13:76–82. doi: 10.1080/19336918.2018.1526612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson AM, Crow MM, Kratz A, Ritts T, Suh YK, Segarra VA. 2021. Introducing mammalian cell colony formation in the undergraduate biology laboratory. J Microbiol Biol Educ 22:22.1.15. doi: 10.1128/jmbe.v22i1.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowey-Dellinger K, Dixon L, Ackerman K, Vigueira C, Suh YK, Lyda T, Sapp K, Grider M, Crater D, Russell T, Elias M, Coffield VM, Segarra VA. 2017. Introducing mammalian cell culture and cell viability techniques in the undergraduate biology laboratory. J Microbiol Biol Educ 18:18.2.38. doi: 10.1128/jmbe.v18i2.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venter C, Niesler CU. 2019. Rapid quantification of cellular proliferation and migration using ImageJ. Biotechniques 66:99–102. doi: 10.2144/btn-2018-0132. [DOI] [PubMed] [Google Scholar]
- 6.Goetsch KP, Niesler CU. 2011. Optimization of the scratch assay for in vitro skeletal muscle wound healing analysis. Anal Biochem 411:158–160. doi: 10.1016/j.ab.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Grada A, Otero-Vinas M, Prieto-Castrillo F, Obagi Z, Falanga V. 2017. Research techniques made simple: analysis of collective cell migration using the wound healing assay. J Invest Dermatol 137:e11–e16. doi: 10.1016/j.jid.2016.11.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendices S1 and S2. Download jmbe.00061-22-s0001.pdf, PDF file, 0.10 MB (100.4KB, pdf)