Abstract
Pulmonary disease has been the primary target of inhaled therapeutics for over 50 years. During that period increasing interest has arisen in the use of this route of administration to gain access to the systemic circulation for the treatment of a number of diseases beyond the airways. In order to effectively employ this route the barriers to transport from the lungs following deposition of aerosols must be considered including the nature of the disease whether proximal, as in pulmonary hypertension, or distal, as in diabetes. Delivery to the systemic circulation begins with efficiency of aerosol generation and subsequent deposition in the airways and proceeds to the influence of mechanisms of clearance, including, absorption, metabolism, mucociliary and cell mediated transport, on residence time of drugs in the lungs. The nature of the drug small or large molecular weight, susceptibility to degradation and general physicochemical properties play a role in chemistry of formulation, the physics of aerosol delivery and the biology of disposition.
Keywords: Inhaled therapy, systemic delivery, drug delivery, drug aerosolization, drug release, dissolution rate
The recent US Food and Drug Administration approval of Afrezza [1] will increase interest in the lungs as a route for systemic administration of drugs. Moreover, it should resurrect the enthusiasm for protein and peptide delivery that fueled decades of scientific inquiry and led to many novel technologies but waned upon the withdrawal of Exubera (inhaled insulin, Pfizer) in 2007. A number of diseases with symptoms that manifest beyond the airways including proximally, pulmonary hypertension, and distally, migraine headaches, have been the subject of recent developments [2].
In this context, a review of the considerations in systemic delivery of drugs is timely. There are a number of reasons for selecting the pulmonary route of administration for systemically acting agents. These may be divided into pharmaceutical, metabolic and physiological. Many drugs are not orally bioavailable due to intrinsic physio-chemical properties or chemical instability in the extreme pH conditions of the GI tract. Others are subject to metabolism prior to or following absorption to inactive or toxic metabolites. The disease itself may present physiological advantages to pulmonary delivery due to proximity of the systemic target a notable example being pulmonary hypertension.
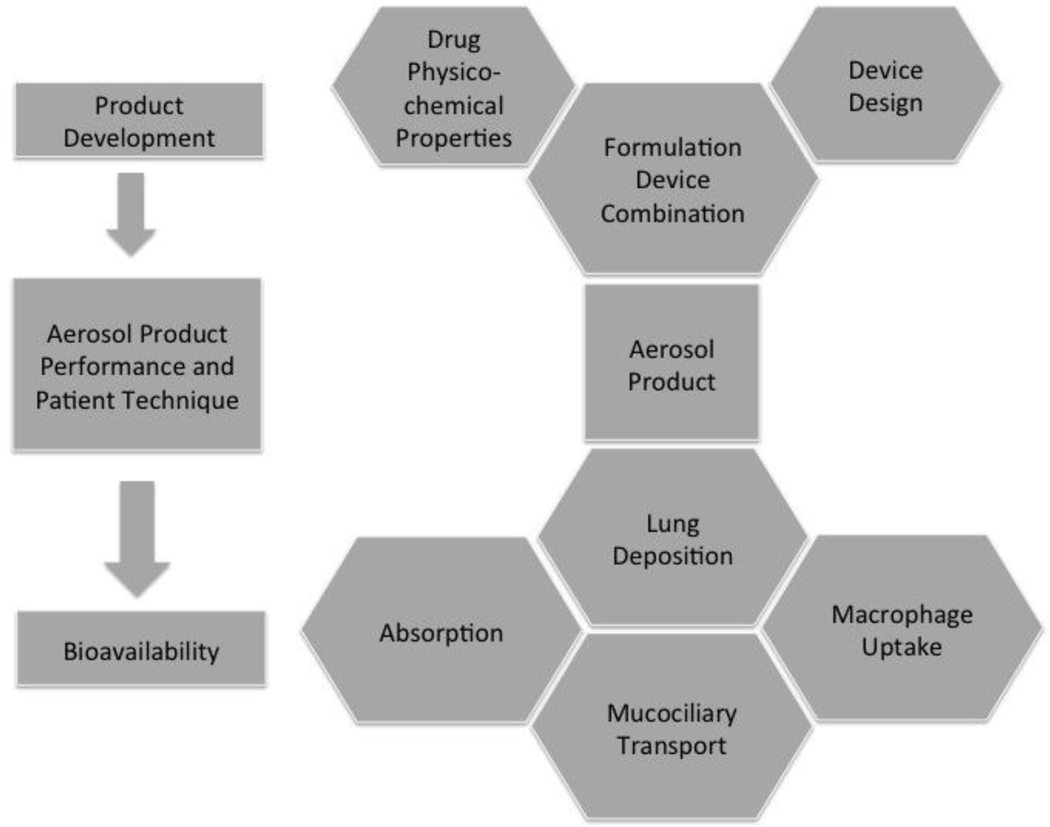
In general inhaled products require the combination of a drug formulation in an inhaler device that accurately meters the drug on the inspiratory flow of the patients resulting in lung deposition and disposition as shown schematically in Figure 1. Product development considerations focus on the formulation development, which depends on the physico-chemical properties of the drug and the design of the device. The combined elements of the formulation, device and metering system allow for the product to dispense and accurate dose in appropriate particle/droplet sizes for deposition in the airways. Upon deposition there are three major mechanisms of disposition absorption, mucociliary and macrophage uptake, cell mediated transport [3]. Each of these mechanisms may be influenced by a variety of biophysical and biochemical phenomena that will inhibit or enhance the bioavailability of the drug and are discussed in greater detail in the following sections.
Figure 1:
Chemical, physical and biological considerations that define the sequence of events from product development through optimal pharmaceutical aerosol product performance to achieve desired bioavailability and therapeutic effect.
Lung Physiology
Pulmonary drug delivery for remote targeting has received less attention than local delivery to treat lung conditions such as asthma and chronic obstructive pulmonary disease. Success for examples such as inhaled administration of human growth hormone [4–6] and insulin [7–13] are strong motivators for exploring this route of drug administration. Inhaled drug delivery can be the answer to increase bioavailability of poor soluble drugs that have poor absorption and bioavailability in the gastrointestinal tract when orally administrated or are highly metabolized by the liver. Pulmonary drug delivery bypasses the first metabolism and has the potential for reduced drug metabolism and high bioavailability. However, pulmonary drug delivery presents the challenge of reaching sufficiently high circulating drug concentrations to achieve therapeutic levels. The biology of the lungs must be taken into consideration when designing inhaled therapies, in conjunction with the physico-chemical properties of the aerosolized drug particles, their disposition in the lungs and drug bioavailability. The design and technical details of the different inhalers available for pulmonary drug delivery are beyond the scope of this review, but have been extensively described elsewhere [14–18].
Aerosol drug delivery to the lungs generally facilitates uptake to the systemic circulation. The optimal region to target for systemic delivery is the periphery (respiratory bronchioles and alveoli) due to the large surface area, the thin epithelial barrier and close proximity to the blood and lymphatic fluid.
The human lungs divide into two functional regions, the conducting airways and the respiratory region. The conducting airways include the nasal cavity and associated sinuses, the pharynx, larynx, trachea, bronchi, and bronchioles. The respiratory region is comprised of the respiratory bronchioles, the alveolar ducts and the alveolar sacs. Together the conducting airways and respiratory regions counts for an estimate 23 to 32 branching or generations [15, 16, 19], all compartments are maintained at high relative humidity which affects the drug particle physico-chemical properties as the drug particle travels though the lung. The role of humidity will be discussed later. The trachea, bronchi and bronchioles are collectively called the airways, and branches out into the alveoli. In an adult the surface area of these airways is only a few m2 in contrast to the alveolar surface area of more than 100 m2.
Airways and Cells
The drug particle traveling to the alveolar space will encounter many different cell types (epithelial, ciliated, goblet, secretory and basal cells), which are adapted to serve the specialized functions of each compartment (Table 1) [16].
Table 1.
Pulmonary cells and their function.
| CELL | PUTATIVE FUNCTION |
|---|---|
|
| |
| Ciliated columnar | Mucus movement |
| Mucus (goblet) | Mucus secretion |
| Serous | Periciliary fluid |
| Clara (nonciliated epithelial) | Surfactant production, metabolism |
| Brush | Transitional form of ciliated epithelial cell |
| Basal | Progenitor for ciliated epithelial cell and goblet cell |
| Dendritic cells | Antigen Presenting Cell |
| Neuroendocrine | Chemoreceptor, paracrine function |
| Alveolar Type I | Alveolar gas exchange |
| Alveolar Type II | Surfactant secretion, differentiation to type I cell |
| Alveolar Macrophages | Pulmonary defense |
| Mast | Immunoregulation |
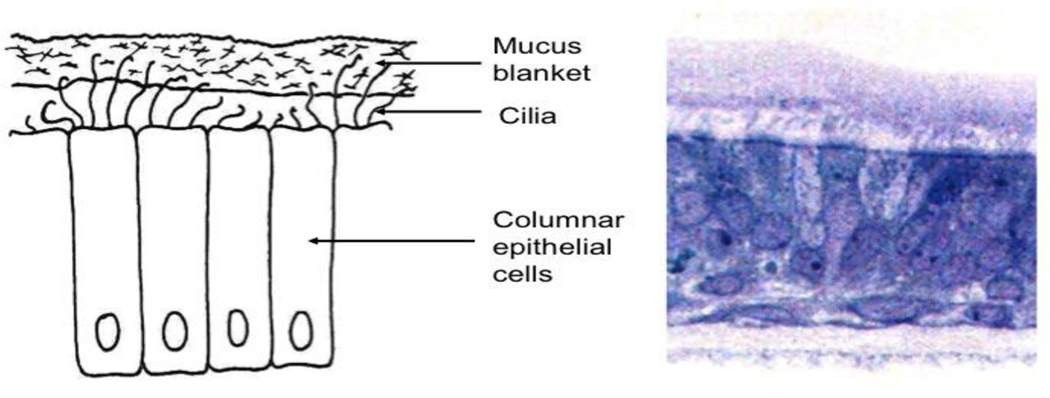
The major cell type of the airways is the epithelial cell, which in the bronchi of the upper airways has a columnar morphology with a height of 58 μm, gradually, thinning in deeper portions of the lungs to a height of 0.1–0.2 m forming a cellular monolayer in the alveoli (Figure 2) [16]. Two important cell types overall in the lungs are mucus producing goblet cells and ciliated cells that together form the mucociliary escalator, which is the ‘housekeeping’ system of the upper airways. If the drug particle deposits in the airway before reaching the alveolar space, it is likely to be carried to the trachea by the mucus escalator before its drug content is released. The protected viscous mucus layer lining the epithelial cells is 8 μm thick in the bronchi transition through the airways into a 0.07 μm thick surfactant layer in the alveoli. The mucus layer is comprised of inorganic salts, proteins, glycoproteins (mucins), lipids and water, whereas the surfactant layer contains phospholipids, cholesterol and proteins [19]. The presence and composition of this protective lining of the lungs is likely to affect both drug solubility and diffusion towards the epithelium and the interaction between drugs and cell surfaces and receptors. Inhaled particles deposited ciliated epithelial regions of the lungs will embed in the mucus layer where they are transported by the ciliated cells into the esophagus, and the material is expectorated and/or swallowed. The kinetics of disposition of particles, as a function of their interaction with these features of lungs, are an important consideration for bioavailability. Mucociliary clearance rates in the absence of unique cell binding phenomena are considered to be in the 12–24 hour range [20].
Figure 2:
(Left) Schematic and (right) histopathological section through the airway depicting cilated epithelial cells.
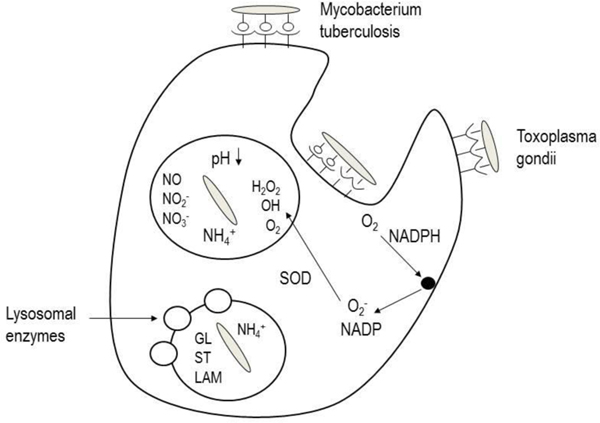
Uptake by alveolar macrophages in the periphery of the lungs is also a challenge to retaining drug particles in the lungs for sufficient time to release their contents unimpeded. Approximately 90% of alveolar macrophages are located at or near the spatial junctions between alveolar epithelial cells [21], and that the air interface of each of the approximately 500 million alveoli are patrolled by 12–14 alveolar macrophages, which phagocytose any insoluble particles that deposit in this location [22]. Delivery of drugs to the lungs can either be designed to avoid phagocytic cells or to take advantage of the phagocytic nature of the macrophages. The benefits that accrue from each of these strategies are important to the action of the drug. Avoiding macrophage uptake is important for drugs, which may be subject to degradation by the enzymes in these cells which are designed to eliminate both endogenous and exogenous substances of biological origin [23]. Conversely, it may be desirable to target macrophages as a target or a depot. Macrophages are frequently the host cells for infectious micro-organisms and co-opting their phagocytic nature to deliver antibiotics allows for lower dose and more potent therapy [24, 25]. Figure 3 illustrates the sequence of events that allows two example intracellular infectious micro-organisms to enter the macrophage and then turn off the lysosomal antimicrobial processes and ultimately disconnect the vesicle from normal intracellular trafficking.
Figure 3:
Disposition of intracellular infectious micro-organisms (Mycobacterium tuberculosis and toxoplasma gondii) in alveolar macrophages.
Pulmonary blood and lymphatic circulation is also essential features of the lungs and require consideration in respiratory drug delivery. In a normal healthy person it is estimated that the pulmonary blood volume is approximately 10% of the bodies total blood volume, with 20–25% being arterial blood volume [26–28]. This presents the opportunity for rapidly achieving a high drug concentration in the blood stream, but also the challenge of avoiding unintended metabolism and clearance of drug. The entire cardiovascular output passes through the lungs, resulting in a high pulmonary blood flow, and the metabolic capacity of the lung should not be ignored [29].
Transport and Metabolism
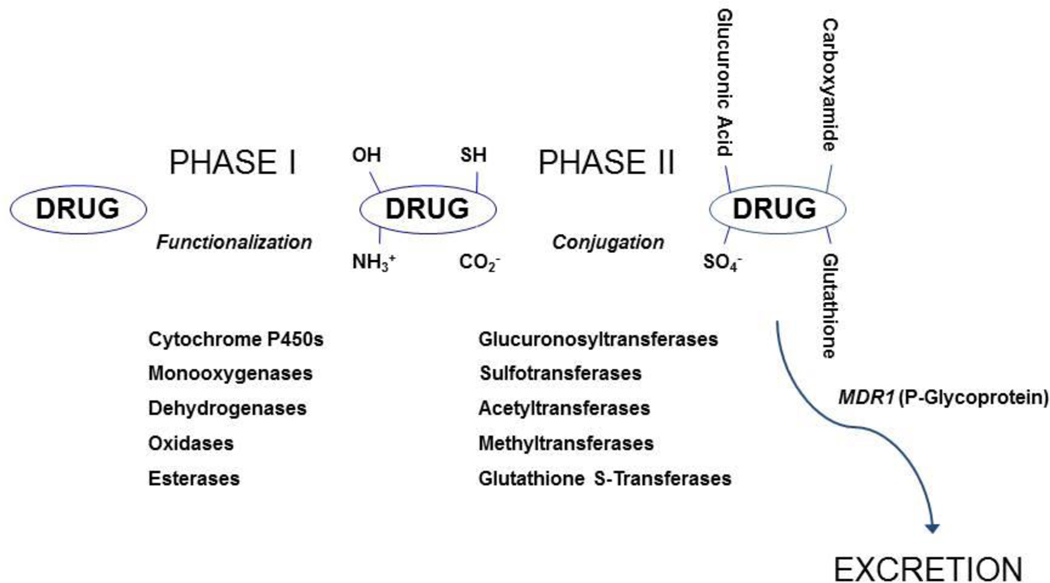
Other biological obstacles in the fate of inhaled drug particles when it has reach the alveolar space are the drug metabolism, efflux transports (such as P-glycoprotein prominent in Type I cells), protein binding and cellular permeability [19]. All metabolic enzymes found in the liver are also present in the lung, but at a lower level. For example cytochrome P450 (CYP450) are 5–20 times lower than in the lung compared to the liver [30–34]. General metabolic enzyme and transporter pathways are illustrated in Figure 4.
Figure 4:
General metabolic pathways and the role of efflux transporters. ( Courtesy of Dr. Matt Redinbo, University of North Carolina at Chapel Hill)
Transporters
Other protein transporters such as breast cancer resistance protein, multidrug resistance associated protein and lung resistance protein are found in the lungs and are responsible for the efflux transport of many drugs, which leads to lower absorption and bioavailability than expected [19]. All the listed biological functions are likely to differ between the various compartments of the lungs and need to be considered when designing inhaled therapies. Many aspects of pulmonary drug-metabolism are not well understood at the present. Also, as a combined entity all the above biological functions will influence the rate and extent of drug absorption and retention in the lungs, which decides the bioavailability of the drug. Where understanding the pulmonary drug metabolism comes in, is that the feasible amount of material delivered to the lungs is about 100–150 mg per does [35], in contrast to the gastrointestinal tract where several grams easily can be administrated. So a lower present of metabolic enzymes, efflux transporters compared to the gastrointestinal tract can still have a significant impact on pulmonary drug delivery.
Metabolism
Lung metabolism for small natural peptides can be very significant unless they are chemically engineered to inhibit peptidases. Beside phase I enzymes discussed above, phase II enzymes such as uridine glucuronosyltransferase (UGT), sulfotransferase (SULT), glutathione S-transferase, and peptidase activity have also been reported [36–40]. Somers et al compared the catalytic activity of both phase I and II enzymes in isolated human lung parenchymal and hepatocyte cells using LC-MS/MS and found that some evidence for substantial sulfation and deesterification capacity in lung parenchymal cells [34]. Transcript profiling of xenobiotic-metabolizing enzymes encoding genes of non-tumoral and tumoral lung tissue from patients with non-small lung cancer also showed activity of several phase I and II enzymes, and furthermore stress the differences in pulmonary metabolism caused by disease [40]. Tables 2 and 3 illustrate the metabolic enzymes which may be found in the lungs.
Table 2:
Phase I metabolic enzymes and their appearance in the lungs.
| Enzymes | mRNA | Protein | Location |
|---|---|---|---|
| Phase I CYPs | |||
|
| |||
| 1A1 | +++ (smokers) | +++ (smokers) | Bronchial epithelial cells, capillary endothelium, alveolar epithelium |
| 1A2 | +/− | +/− | Peripheral tissue |
| 1B1 | ++ | +/− | Alveolar macrophage epithelial cells, bronchial epithelial cells |
| 2A6 | ++ | +/− | Bronchial mucosa, epithelial cells |
| 2A13 | + | ? | Bronchial mucosa, alveolar epithelium |
| 2B6/7 | +++ | +++ | Clara cells, bronchial and peripheral tissue, epithelial cells |
| 2C8/18 | + | +/− | Serous cells of bronchial glands, bronchial and peripheral tissue |
| 2D6 | +/− | +/− | Bronchial mucosa |
| 2E1 | +++ | +++ | Bronchial, bronchiolar and alveolar epithelium, endothelial cells |
| 2F1 | +++ | ? | Alveolar macrophage epithelial cells, endothelial cells |
| 2J2 | + | ++ | Bronchial and vascular smooth muscle cell, vascular endothelium, alveolar macrophages |
| 2S1 | + | + | Epithelial cells |
| 3A4/5 | +++ | ++ | Bronchial, bronchiolar and alveolar epithelium, alveolar endothelium, alveolar macrophages |
| 3A43 | + | ? | ? |
| 4B1 | + | ? | Bronchoalveolar macrophages |
| EHs | ++ | ++ | Bronchial epithelial cells |
| FMOs | ++ | + | ? |
Table 3:
Phase II metabolic enzymes and their appearance in the lungs.
| Enzymes | mRNA | Protein | Location |
|---|---|---|---|
| Phase II | |||
|
| |||
| UGTs | +/− | +/− | Lung tissue |
| GST | |||
| A1 | ++ | + | Bronchial and bronchiolar epithelium |
| A2 | ++ | + | Bronchial and bronchiolar epithelium |
| M1 | ++ | + | Lung tissue |
| M2 | ++ | + | Epithelium of the terminal airways |
| M3 | ++ | + | Ciliated airway epithelium and smooth muscle |
| P1 | ++ | + | Bronchial and bronchiolar epithelium |
| NATs | + | + | Bronchial epithelial cells, alveolar lining cells |
| SULTs | |||
| 1A1 | + | + | Bronchial epithelial cells |
| 2B1b | + | + | Bronchial epithelial cells |
The location of enzymes within the airways and in subcellular organelles may also play a role in disposition since the route that the drug takes from the lungs will have both a spatial and temporal probability of passing through regions of high metabolic activity. Certain cells are known to be highly metabolic such as Clara cells in the airways. Type II alveolar epithelial cells express enzymes required for metabolism such as cytochrome P450 but type I cells are thought to have little capacity for metabolism. Enzymes are distributed in many subcellular compartments but not uniformly as shown in Table 4.
Table 4.
Distribution of Enzymes in subcellular compartments and their function. (Modified from [76]).
| LOCALIZATION | PHASE | ENZYME | REACTION |
|---|---|---|---|
|
| |||
| Cytosol | I | Estarase | Hydrolysis |
| I | Epoxide hydrolase | Hydrolysis | |
| I | Azo- and nitro-reduction | Hydrolysis | |
| I | Carbonyl reduction | Hydrolysis | |
| I | Disulfide reduction | Reduction | |
| I | Sulfoxide reduction | Reduction | |
| I | Quinone reduction | Reduction | |
| I | Alcohol dehydrogenase | Reduction | |
| I | Aldehyde oxidase | Reduction | |
| I | Diamine oxidase | Oxidation | |
| II | Sulfate conjugation | ||
| II | Glutathione conjugation | ||
| II | Acylation | ||
| II | Methylation | ||
|
| |||
| Microsomes | I | Estarase | Hydrolysis |
| I | Epoxide hydrolase | Hydrolysis | |
| I | Azo- and nitro-reduction | Hydrolysis | |
| I | Carbonyl reduction | Hydrolysis | |
| I | Quinone reduction | Reduction | |
| I | Reductive dehalogenation | Reduction | |
| I | Prostaglandin H synthase | Reduction | |
| I | Flavin-monooxygenases | Reduction | |
| I | Cytochome P450 | Reduction | |
| II | Glucuronide conjugation | ||
| II | Glutathione conjugation | ||
| II | Amino acid conjugation | ||
| II | Methylation | ||
|
| |||
| Lysosomes | I | Estarase | Hydrolysis |
| I | Peptidase | Hydrolysis | |
|
| |||
| Mitochondria | I | Alcohol dehydrogenase | Reduction |
| I | Xanthine oxidase | Reduction | |
| II | Amino acid conjugation | ||
| II | Acylation | ||
|
| |||
| Blood | I | Estarase | Hydrolysis |
| I | Peptidase | Hydrolysis | |
| I | Carbonyl reduction | Hydrolysis | |
| II | Methylation | ||
|
| |||
| Microflora | I | Azo- and nitro-reduction | Hydrolysis |
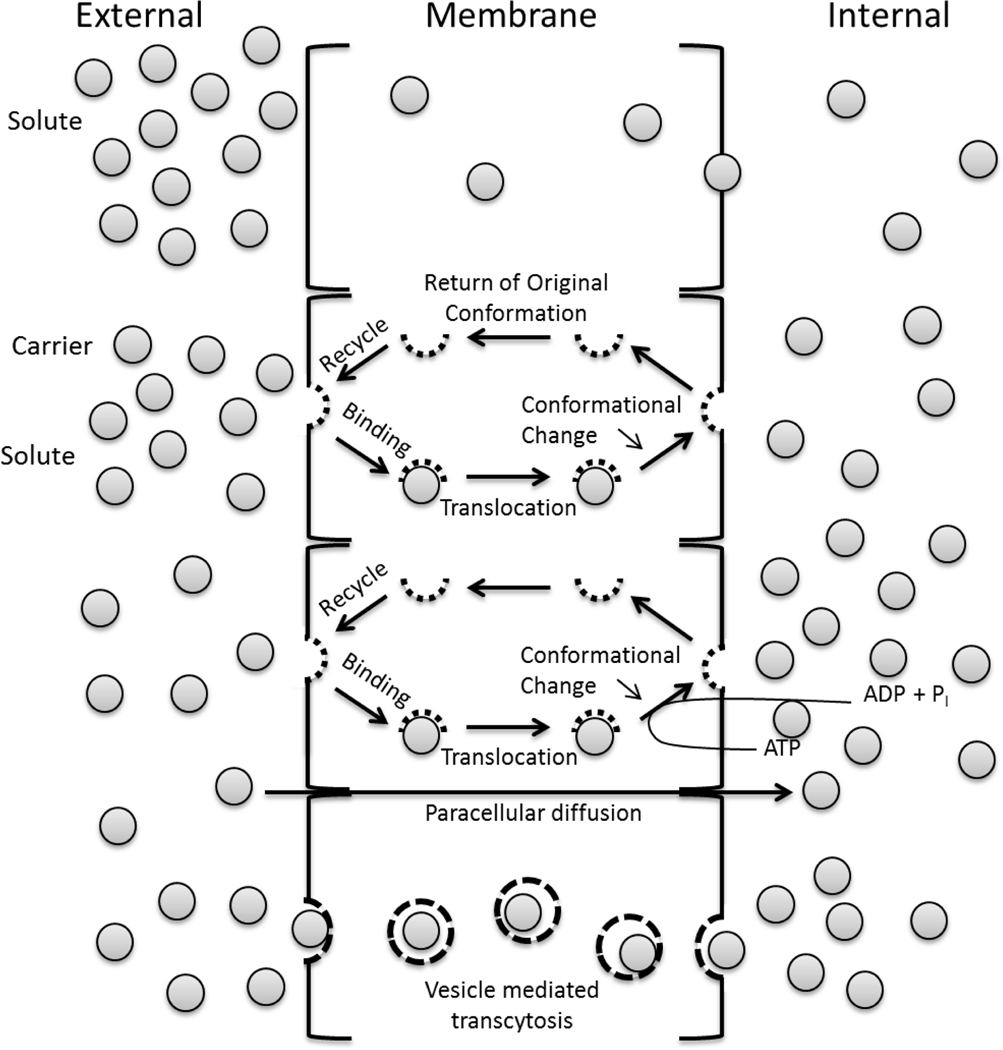
General biochemical principles apply to transport of molecules through or between cells as illustrated in Figure 5. Molecular and peptide absorbance kinetics in the pulmonary space can also be significant influenced by natural occurring glycosylation [7]. A study of Alpha l-antitrypsin showed a fourfold lower tmax if the molecule was naturally glycosylated compared to non-glycosylated [7].
Figure 5.
Mechanisms of transport of drugs across the pulmonary epithelium.
Physical and Biological Barriers
Physical properties of a particle, such as particle size, aerodynamic particle size distribution (mass median aerodynamic diameter (da)), mass distribution, shape and electrostatic charge plays an important role in decide where the drug particles deposit in the lung. Also, the pharmacokinetic properties of drug release, influenced by solubility and dissolution rate, from particles and drug half-life of elimination are important factors to take into consideration for achieving therapeutic concentration.
Pulmonary delivery of nanomedicine has to be designed to deliver the aerosolized drug particle to the intended area of the lung; the deposition is primarily based on particle size. Particle deposition occurs by inertial impaction, sedimentation, diffusion, interception and by electrostatic precipitation [15, 16, 41]. When the particle has sufficient momentum to follow its initial trajectory despite changes in the airstream inertial impaction occurs, and the particle collides with the airway wall, often at or near a bifurcation in the lungs. Gravity also influences the particle route in the airways and in a time-dependent manner causes sedimentation. Particles small enough to be the subject to random motions is subject to diffusion. Impaction and sedimentation are the dominant mechanisms of deposition of therapeutic aerosols.
The particle’s equivalent aerodynamic (da) and volume diameter are the most appropriate descriptors for aerosolized drug particles. The former is particularly important as lung deposition relates to particle aerodynamic behavior. For inhalation therapy the optimal da for a particle is approximately 1–5 μm [42]. Particles larger than 5 μm usually experience inertial impaction early in the airways and are deposited in the oral cavity and pharynx. Particles that are smaller than 0.5 μm are subject to Brownian motion and settle very slowly, and often exhaled before settling occurs. Also influencing the aerodynamic behavior of the drug particles, the shape factor is dependent on particle shape, surface roughness and surface area, and described by the ratio of the drag force of the particle to that of a sphere of equivalent volume. So that the drag force will be greater on a non-spherical particle with high surface roughness when compared to a spherical particle, and as a result the da is smaller than anticipate. Creating porous particles that have a similar mass of drug in a larger volume also increases the drag with respect to a solid particle of the same da [43]. Therefore, particle shape and density can be used to manipulate the aerodynamic properties of aerosols.
The da of a drug particle can be altered when entering the high relative humidity environment in the airways, and thereby alter deposition [30]. Rarely does removal of water occur, more frequently the cause is particle acquisition, which is a function of the initial diameter, so a particle <1 μm may increase fivefold in contrast to the two to three fold increase for a particle >2 μm [44].
The physico-chemical properties of the molecule intended for delivery also plays a critical role in the formulation of inhalation therapy. Crossing the airway epithelial barrier is naturally essentially for the drug to enter the blood stream, and parameters such as molecular weight, lipophilicity (log P), solubility, pKa, protein binding, polar surface area, and charge plays into that [19]. In general lipophilic molecules cross the airway epithelium by passive transport and hydrophilic molecules via extracellular pathways. However molecules are delivered as particles, and particles with low dissolution rate that reach the alveolar space, past the mucociliary escalator, encounter the many alveolar macrophages and are phagocytized or are absorbed into the pulmonary circulation [30]. The particle surface properties, however, is of greater interest than dissolution rate in respect to disposition. Other physico-chemical properties to take into account is Van der Waal’s forces, hydrostatic interaction, mechanical interlocking, electrostatic and capillary forces are important contributors to particle interactions in the lung, which influences aggregation, particle-particle and particle-cell interactions. In pulmonary drug delivery achieving the intended da, and avoiding aggregation, is essential for successful therapeutic results.
As mentioned above, the human airway is coated with a protecting surfactant layer which protein content and composition varies between the compartments of the lungs. Particles will as soon as they interact with protein rich environment acquire a surface protein coating or corona. The corona is dynamic following the Vroman effect, which describes how the more abundant proteins will initially form a looser associated corona, to be replaced with less abundant proteins with higher binding affinity to the surface [45, 46]. The protein corona composition and dynamics has been reported to be influenced by the particle size, surface chemistry and charge [47–50]. The protein composition might play an important role in both macrophage uptake and transport across the epithelial barrier.
Fate of Microparticles and Nanoparticles
It is important to note that lung deposition occurs most effectively for microparticles usually in the 1–5μm size range [51, 52]. The recent interest in nanoparticle delivery poses some important questions in considering aerosol properties. Aerosol particles or droplets are delivered as dry powders alone (from DPIs), from aqueous (nebulizers or SMIs) or non-aqueous (pMDIs) suspension or solution [53]. Regardless of the primary particle size of the formulation, micro- or nano-sized, the particles delivered in these states from these devices are necessarily in micron sizes due to their intrinsic physico-chemical properties and underlying forces of interaction. Thus, micron sized aerosolized droplets may contain many nanoparticles that dry to form aggregates and dry powders are dispersed as micro-agglomerated nanoparticles. Thus, conventional aerosol deposition phenomena occur which for the size range of interest, 1–5 μm, in lung delivery is dominated by inertial impaction and sedimentation as discussed earlier. It is of interest, from both a safety and efficacy standpoint, to consider the extent and rate of deaggregation of nanoparticle agglomerates since this will influence a variety of elements of disposition from dissolution rate to route of elimination or access to target site for therapy or off-target site for toxicity [54]. A micro-agglomerated nanoparticle construct may exhibit low density in which case it may have two potentially desirable properties the first being small aerodynamic particle size in relation to geometric size which is predicted by Stokes’ law [55]. In principle, the longer nanoparticles remain aggregated the more likely they will behave as a microparticle with respect to clearance mechanisms. However, their low density may mean that their geometric size is too large for macrophage uptake [43]. In addition, their large surface area may increase dissolution rate for soluble particles [55]. In turn this will have an effect on the pharmacokinetics of appearance of drug in the systemic circulation. Particles with lower solubility or for other reasons, such as the presence of a hydrophobic additive, exhibit retarded dissolution rate will retain their primary particle structure for a defined time. Assuming deagglomeration occurs within the timeframe of mucociliary clearance, intact nanoparticles will interact differently with mucus and may be retained longer than microparticles by penetrating through the mucus matrix to the epithelial surface [56]. Those particles that deposit in the periphery of the lungs will not be constrained by mucociliary clearance rates. Depending on deagglomeration rates and intermediate, size of fragments of the original particles uptake by both alveolar and airway macrophages and dendritic cells is possible resulting in first contact with the primary elements of the immune system in the lungs [57]. The immune response to this exposure may dictate the safety of the proposed therapy. Certain nanoparticles may also pass directly through the epithelium which has both safety and efficacy implications for systemic exposure depending on the time the particles take to disperse into the molecular state [58, 59].
The most prominent nanoparticles delivered have been macromolecules whether proteins of nucleic acids. Since their hydrodynamic radius is nano-sized these are definitively in the category of nanoparticles. The importance of the molecular weight of these molecules has received considerable attention [60]. In general, as molecular weight increases clearance rates from the lung decrease. It has been suggested that for all practical purposes with some exceptions for functionalized proteins, which may utilize active clearance pathways the molecular weight range suitable for systemic delivery through the lungs is 5–20KDa [7]. Delivery of proteins and peptides may be hindered by the instability of the drug in the presence of lung lining fluid, containing peptidases and other macromolecules (glycoprotein, glycolipid), by which they are degraded or to which they bind, respectively.
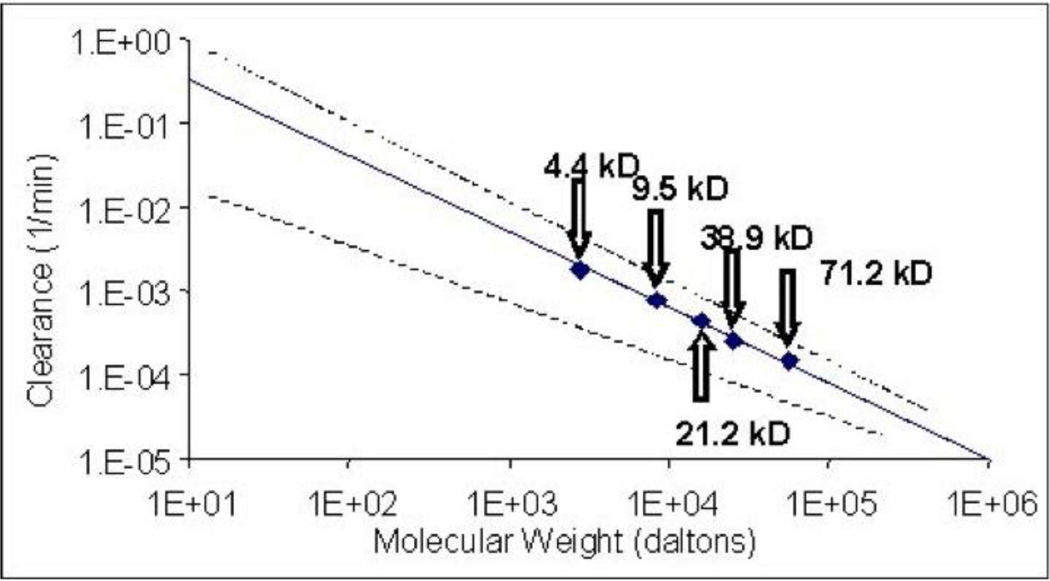
Historically the influence of molecular weight on transport of macromolecules was studied using fluorescent labeled dextrans [61, 62] or other polymers [63]. Clearance has been shown to be linearly and reciprocally related to molecular weight regardless of species [64, 65]. Figure 6 illustrates the phenomenon as observed in a cell culture model using human small airways epithelial cells onto which aerosols were sampled from an inertial impactor to achieve appropriate particle size [66].
Figure 6.
Clearance as a function of molecular weight for fluorescent dextrans. The solid line depicts the mean and the dotted lines the range for data derived from a number of species including man (modified from [65, 75]). The data points represent data points for which permeability in across small airways epithelial cells in culture demonstrated equivalence to in vivo data.
Therapeutic Examples
A wide range of molecules have been considered for delivery to the lungs to treat a variety of systemic diseases. Interest in the lungs as a route of systemic administration existed at the onset of the biotechnology product revolution which began in earnest in the late 1980s. In particular biological molecules that could not be delivered by the oral route and that would otherwise be injected were considered potential candidates [67, 68]. The most prominent of these candidates were insulin for the treatment of diabetes and leuprolide acetate to treat prostate cancer [11, 69]. Insulin posed some unique challenges in that it is required daily and with some discretion by the patient to control circulating glucose levels but in other respects the patient may be healthy. The requirement for exquisite dose control to titrate efficacy safely resulted in the development of formulations and devices (mostly DPI) that propelled pharmaceutical aerosol science into the twenty first century. Leuprolide acetate, a nonapeptide leuteinizing hormone releasing hormone analog, was developed for delivery in a pMDI, and as a relatively small peptide without tertiary secondary or tertiary structure did not pose such a significant formulation and stability challenge. While this product was published widely in the early 1990s it did not proceed to the commercialization. A number of other proteins and peptides, including calcitonin, parathyroid hormone and growth hormone were studied [5, 70]. None were commercialized.
The ability to deliver small molecular weight molecules rapidly through the lungs to relieve symptoms associated with central nervous system (CNS) diseases may be traced back in modern times to an ergotamine pMDI used to treat migraine headaches in the 1960s (3M Pharmaceuticals, MN) [2]. This apparently is an effective strategy since dihydroergotamine is now under development for the same purpose (Levadex, Allergan, CA) [71]. Other agents that act in the CNS have been explored more recently including those to treat agitation in schizophrenia (Loxapine, Alexza, CA) [72] and others used for pain relief [73].
It is not surprising that as systemic delivery has become conceptually more popular that targeting to a proximal site to the airways would be considered. Pulmonary hypertension is a difficult disease to treat since its localization makes systemic therapy problematic. The idea of accessing the target site by delivery directly to the lungs is very attractive. Inhaled prostenoids have been pursued for this purpose [74].
Conclusion
The ongoing success of aerosol therapy for the treatment of pulmonary disease has established a strong foundation of science and technology from which to consider further applications to systemic disease whether proximal, in the case of pulmonary hypertension, or with distal components such as diabetes. Clearly, the physico-chemical properties of the initial dosage form, aerosol physics of leading to lung deposition and the biological barriers to presenting the drug to the circulation all play a role in the likelihood of success in treating systemic disease. All aerosol dosage forms, pressurized metered dose inhalers, dry powder inhalers and nebulizer therapy all have the potential to deliver drugs to the lungs for systemic effects. However, macromolecules are predominantly delivered as nebulized therapy or in dry powder inhalers. These dosage forms more readily accommodate the required doses and stability of biotechnology products. After depositing in the lungs particles must first dissolve during which period they are being transported on the mucociliary escalator or in macrophages depending on their site of deposition and the time taken to completely convert to molecular drug. Once dissolved the drug will have to pass through lung lining fluid where enzyme (e.g. protease) action or binding (protein or lipid) may hinder penetration to the epithelium. Transport through or around epithelial cells may result in metabolism or transporter effects that may influence the availability of drug to the circulation. Assuming that these potential barriers can be circumvented through judicious selection, or design, of molecules the pulmonary route offers great potential for drugs that would otherwise be poorly bioavailable (due to solubility or degradation in gastro-intestinal fluids), toxic (due to large doses), or subject to first pass metabolism. Moreover, the pulmonary route may be considered a topical application of drug which does not require the invasive techniques with requisite oversight and training employed for parenteral administration of drugs. It may be concluded that with appropriate pharmaceutical, biological and disease state considerations aerosol delivery of drugs to the lungs for systemic action may yet meet its full potential.
Acknowledgements:
Dr. Hickey is supported in part by the National Institute of Allergy and Infectious Disease (Grant # R01AI091882) and is principal investigator for the National Institutes of Health Nanomaterial Registry. This Registry has been funded with federal funds from the National Institute of Biomedical Imaging and Bioengineering and the following partners: the National Institute of Environmental and Health Sciences, and the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN268201000022C.
References
- 1.FDA. FDA approves Afrezza to treat diabetes. 2014. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm403122.htm
- 2.Hickey AJ, Back to the future: inhaled drug products. J Pharm Sci, 2013. 102(4): p. 1165–72. [DOI] [PubMed] [Google Scholar]
- 3.Olsson B, et al. , Pulmonary drug metabolism, clearance and absorption, in Controlled Pulmonary Drug Delivery, Smyth HDC and Hickey AJ, Editors. 2012, Springer. p. 21–50. [Google Scholar]
- 4.Soferman R, et al. , Effects of inhaled corticosteroids and inhaled cromolyn sodium on urinary growth hormone excretion in asthmatic children. Pediatr Pulmonol, 1998. 26(5): p. 339–43. [DOI] [PubMed] [Google Scholar]
- 5.Walvoord EC, et al. , Inhaled growth hormone (GH) compared with subcutaneous GH in children with GH deficiency: pharmacokinetics, pharmacodynamics, and safety. J Clin Endocrinol Metab, 2009. 94(6): p. 2052–9. [DOI] [PubMed] [Google Scholar]
- 6.Nelson HS, et al. , Short-term safety of somatropin inhalation powder in adults with mild to moderate asthma. Allergy Asthma Proc, 2009. 30(3): p. 325–32. [DOI] [PubMed] [Google Scholar]
- 7.Byron PR and Patton JS, Drug delivery via the respiratory tract. J Aerosol Med, 1994. 7(1): p. 49–75. [DOI] [PubMed] [Google Scholar]
- 8.Heise T, et al. , PROMAXX inhaled insulin: safe and efficacious administration with a commercially available dry powder inhaler. Diabetes Obes Metab, 2009. 11(5): p. 455–9. [DOI] [PubMed] [Google Scholar]
- 9.Mastrandrea LD, Inhaled insulin: overview of a novel route of insulin administration. Vasc Health Risk Manag, 2010. 6: p. 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mastrandrea LD and Quattrin T, Clinical evaluation of inhaled insulin. Adv Drug Deliv Rev, 2006. 58(9–10): p. 1061–75. [DOI] [PubMed] [Google Scholar]
- 11.Patton JS, Bukar JG, and Eldon MA, Clinical pharmacokinetics and pharmacodynamics of inhaled insulin. Clin Pharmacokinet, 2004. 43(12): p. 781–801. [DOI] [PubMed] [Google Scholar]
- 12.White NH, et al. , Efficacy and safety of inhaled human insulin (Exubera) compared to subcutaneous insulin in children ages 6 to 11 years with type 1 diabetes mellitus: results of a 3-month, randomized, parallel trial. J Pediatr Endocrinol Metab, 2008. 21(6): p. 555–68. [PubMed] [Google Scholar]
- 13.White S, et al. , EXUBERA: pharmaceutical development of a novel product for pulmonary delivery of insulin. Diabetes Technol Ther, 2005. 7(6): p. 896–906. [DOI] [PubMed] [Google Scholar]
- 14.Telko MJ and Hickey AJ, Dry powder inhaler formulation. Respir Care, 2005. 50(9): p. 1209–27. [PubMed] [Google Scholar]
- 15.Hofmann W, Modelling inhaled particle deposition in the human lung-A review. Journal of Aerosol Science, 2011. 42(10): p. 693–724. [Google Scholar]
- 16.Patton JS and Byron PR, Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov, 2007. 6(1): p. 67–74. [DOI] [PubMed] [Google Scholar]
- 17.Misra A, et al. , Inhaled drug therapy for treatment of tuberculosis. Tuberculosis (Edinb), 2011. 91(1): p. 71–81. [DOI] [PubMed] [Google Scholar]
- 18.Hickey AJ, Misra A, and Fourie PB, Dry powder antibiotic aerosol product development: inhaled therapy for tuberculosis. J Pharm Sci, 2013. 102(11): p. 3900–7. [DOI] [PubMed] [Google Scholar]
- 19.Eixarch H, et al. , Drug Delivery to the Lung: Permeability and Physicochemical Characteristics of Drugs as the Basis for a Pulmonary Biopharmaceutical Classification System (pBCS) Journal of Epithelial Biology & Pharmacology, 2010. 3: p. 1–14. [Google Scholar]
- 20.Pillai G, et al. , Generation of concentrated aerosols for inhalation studies. J Aerosol Sci, 1994. 25: p. 10. [Google Scholar]
- 21.Parra SC, et al. , Zonal distribution of alveolar macrophages, type II pneumonocytes, and alveolar septal connective tissue gaps in adult human lungs. Am Rev Respir Dis, 1986. 133(5): p. 908–12. [PubMed] [Google Scholar]
- 22.Stone KC, et al. , Allometric relationships of cell numbers and size in the mammalian lung. Am J Respir Cell Mol Biol, 1992. 6(2): p. 235–43. [DOI] [PubMed] [Google Scholar]
- 23.Lombry C, et al. , Alveolar macrophages are a primary barrier to pulmonary absorption of macromolecules. Am J Physiol Lung Cell Mol Physiol, 2004. 286(5): p. L1002–8. [DOI] [PubMed] [Google Scholar]
- 24.Suarez S, et al. , Airways delivery of rifampicin microparticles for the treatment of tuberculosis. J Antimicrob Chemother, 2001. 48(3): p. 431–4. [DOI] [PubMed] [Google Scholar]
- 25.Suarez S, et al. , Respirable PLGA microspheres containing rifampicin for the treatment of tuberculosis: screening in an infectious disease model. Pharm Res, 2001. 18(9): p. 1315–9. [DOI] [PubMed] [Google Scholar]
- 26.Fishman AP, The volume of blood in the lungs. Circulation, 1966. 33(6): p. 835–8. [DOI] [PubMed] [Google Scholar]
- 27.Lewis ML, et al. , Determinants of pulmonary blood volume. J Clin Invest, 1970. 49(1): p. 170–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dock DS, et al. , The pulmonary blood volume in man. J Clin Invest, 1961. 40: p. 317–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma J, Bhat M, and Rojanasakul Y, Drug metabolism and enzyme kinetics in the lung, in Inhalation Aerosols, Hickey AJ, Editor. 1996, Marcel Dekker, Inc: New York, NY. [Google Scholar]
- 30.Labiris NR and Dolovich MB, Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol, 2003. 56(6): p. 588–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Upton RN and Doolette DJ, Kinetic aspects of drug disposition in the lungs. Clin Exp Pharmacol Physiol, 1999. 26(5–6): p. 381–91. [DOI] [PubMed] [Google Scholar]
- 32.Krishna DR and Klotz U, Extrahepatic metabolism of drugs in humans. Clin Pharmacokinet, 1994. 26(2): p. 144–60. [DOI] [PubMed] [Google Scholar]
- 33.Dahl AR and Lewis JL, Respiratory tract uptake of inhalants and metabolism of xenobiotics. Annu Rev Pharmacol Toxicol, 1993. 33: p. 383–407. [DOI] [PubMed] [Google Scholar]
- 34.Somers GI, et al. , A comparison of the expression and metabolizing activities of phase I and II enzymes in freshly isolated human lung parenchymal cells and cryopreserved human hepatocytes. Drug Metab Dispos, 2007. 35(10): p. 1797–805. [DOI] [PubMed] [Google Scholar]
- 35.Dharmadhikari AS, et al. , Phase I, single-dose, dose-escalating study of inhaled dry powder capreomycin: a new approach to therapy of drug-resistant tuberculosis. Antimicrob Agents Chemother, 2013. 57(6): p. 2613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lorenz J, et al. , Drug metabolism in man and its relationship to that in three rodent species: monooxygenase, epoxide hydrolase, and glutathione S-transferase activities in subcellular fractions of lung and liver. Biochem Med, 1984. 32(1): p. 43–56. [DOI] [PubMed] [Google Scholar]
- 37.Sidorowicz W, et al. , Cleavage of the Arg1-Pro2 bond of bradykinin by a human lung peptidase: isolation, characterization, and inhibition by several beta-lactam antibiotics. Proc Soc Exp Biol Med, 1984. 175(4): p. 503–9. [DOI] [PubMed] [Google Scholar]
- 38.Ekstrom L, Johansson M, and Rane A, Tissue distribution and relative gene expression of UDP-glucuronosyltransferases (2B7, 2B15, 2B17) in the human fetus. Drug Metab Dispos, 2013. 41(2): p. 291–5. [DOI] [PubMed] [Google Scholar]
- 39.Kurogi K, et al. , Sulfation of opioid drugs by human cytosolic sulfotransferases: Metabolic labeling study and enzymatic analysis. Eur J Pharm Sci, 2014. 62C: p. 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leclerc J, et al. , Xenobiotic metabolism and disposition in human lung: transcript profiling in non-tumoral and tumoral tissues. Biochimie, 2011. 93(6): p. 1012–27. [DOI] [PubMed] [Google Scholar]
- 41.Carvalho TC, Peters JI, and Williams RO 3rd, Influence of particle size on regional lung deposition--what evidence is there? Int J Pharm, 2011. 406(1–2): p. 1–10. [DOI] [PubMed] [Google Scholar]
- 42.Goodman DE, et al. , The influence of age, diagnosis, and gender on proper use of metered-dose inhalers. Am J Respir Crit Care Med, 1994. 150(5 Pt 1): p. 1256–61. [DOI] [PubMed] [Google Scholar]
- 43.Edwards DA, et al. , Large porous particles for pulmonary drug delivery. Science, 1997. 276(5320): p. 1868–71. [DOI] [PubMed] [Google Scholar]
- 44.Swift DL, GENERATION AND RESPIRATORY DEPOSITION OF THERAPEUTIC AEROSOLS. American Review of Respiratory Disease, 1980. 122(5): p. 71–77. [DOI] [PubMed] [Google Scholar]
- 45.Vroman L, EFFECT OF ADSORBED PROTEINS ON WETTABILITY OF HYDROPHILIC AND HYDROPHOBIC SOLIDS. Nature, 1962. 196(4853): p. 476-&. [DOI] [PubMed] [Google Scholar]
- 46.Vroman L, et al. , INTERACTION OF HIGH MOLECULAR-WEIGHT KININOGEN, FACTOR-XII, AND FIBRINOGEN IN PLASMA AT INTERFACES. Blood, 1980. 55(1): p. 156–159. [PubMed] [Google Scholar]
- 47.Cedervall T, et al. , Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proceedings of the National Academy of Sciences of the United States of America, 2007. 104(7): p. 2050–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lundqvist M, et al. , Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proceedings of the National Academy of Sciences of the United States of America, 2008. 105(38): p. 14265–14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aggarwal P, et al. , Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Advanced Drug Delivery Reviews, 2009. 61(6): p. 428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mortensen NP, et al. , Dynamic development of the protein corona on silica nanoparticles: composition and role in toxicity. Nanoscale, 2013. 5(14): p. 6372–80. [DOI] [PubMed] [Google Scholar]
- 51.Hickey AJ, Summary of common approaches to pharmaceutical aerosol administration. Second ed. Pharmaceutical Inhalation Aerosol Technology. 2004, New York, NY: Marcel Dekker, Inc. [Google Scholar]
- 52.Hickey AJ, Pulmonary drug delivery - pharmaceutical chemistry and aerosol technology. Drug Delivery Principles and Applications. 2004, New York, NY: John Wiley and Sons. [Google Scholar]
- 53.Hickey AJ, Methods of Aerosol Particle Size Characterization, in Pharmaceutical Inhalation Aerosol Technology, Hickey AJ, Editor. 2004, Marcel Detter, INC.: New York, NY. p. 345–384. [Google Scholar]
- 54.Sayes CM, Staats H, and Hickey AJ, Scale of Health: Indices of Safety and Efficacy in the Evolving Environment of Large Biological Datasets. Pharm Res, 2014. [DOI] [PubMed] [Google Scholar]
- 55.Crowder TM, et al. , Fundamental effects of particle morphology on lung delivery: predictions of Stokes’ law and the particular relevance to dry powder inhaler formulation and development. Pharm Res, 2002. 19(3): p. 239–45. [DOI] [PubMed] [Google Scholar]
- 56.Lai SK, Wang YY, and Hanes J, Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev, 2009. 61(2): p. 158–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walter E, et al. , Hydrophilic poly(DL-lactide-co-glycolide) microspheres for the delivery of DNA to human-derived macrophages and dendritic cells. J Control Release, 2001. 76(1–2): p. 149–68. [DOI] [PubMed] [Google Scholar]
- 58.Geiser M, et al. , Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ Health Perspect, 2005. 113(11): p. 1555–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bharatwaj B, et al. , Polymeric nanocarriers for transport modulation across the pulmonary epithelium: dendrimers, polymeric nanoparticles, and their nanoblends. AAPS J, 2014. 16(3): p. 522–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Effros RM, Solute Transport Following Aerosol Deposition in the Lungs, in Inhalation Aerosols Physical and Biological Basis for Therapy, Hickey AJ, Editor. 2007, Informa Healthcare: New York, NY. p. 127–145. [Google Scholar]
- 61.Effros RM, et al. , Resistance of the pulmonary epithelium to movement of buffer ions. Am J Physiol Lung Cell Mol Physiol, 2003. 285(2): p. L476–83. [DOI] [PubMed] [Google Scholar]
- 62.Bryan R, et al. , The effects of aerosolized dextran in a mouse model of Pseudomonas aeruginosa pulmonary infection. J Infect Dis, 1999. 179(6): p. 1449–58. [DOI] [PubMed] [Google Scholar]
- 63.Niven RW, Rypacek F, and Byron PR, Solute absorption from the airways of the isolated rat lung. III. Absorption of several peptidase-resistant, synthetic polypeptides: poly-(2-hydroxyethyl)aspartamides. Pharm Res, 1990. 7(10): p. 990–4. [DOI] [PubMed] [Google Scholar]
- 64.Matsukawa Y, et al. , Size-dependent dextran transport across rat alveolar epithelial cell monolayers. J Pharm Sci, 1997. 86(3): p. 305–9. [DOI] [PubMed] [Google Scholar]
- 65.Schanker LS, Mitchell EW, and Brown RA Jr., Species comparison of drug absorption from the lung after aerosol inhalation or intratracheal injection. Drug Metab Dispos, 1986. 14(1): p. 79–88. [PubMed] [Google Scholar]
- 66.Cooney D, Kazantseva M, and Hickey AJ, Development of a size-dependent aerosol deposition model utilising human airway epithelial cells for evaluating aerosol drug delivery. Altern Lab Anim, 2004. 32: p. 9. [DOI] [PubMed] [Google Scholar]
- 67.Adjei AL and Gupta PK, Inhalation Delivery of Therapeutic Peptides and Proteins. 1997, New York, NY: Marcel Dekker, Inc. [Google Scholar]
- 68.Patton JS and Platz RM, Routes of Delivery: Case Studies (2) Pulmonary delivery of peptides and proteins for systemic action. Adv Drug Deliv Rev, 1992. 8: p. 17. [Google Scholar]
- 69.Adjei A.and Garren J, Pulmonary delivery of peptide drugs: effect of particle size on bioavailability of leuprolide acetate in healthy male volunteers. Pharm Res, 1990. 7(6): p. 565–9. [DOI] [PubMed] [Google Scholar]
- 70.Patton JS, Trinchero P, and Platz RM, Bioavailability of pulmonary delivered peptides and proteins: Interferon alpha, calcitonins and parathyroid hormones. J. Controlled Release, 1990. 28: p. 6. [Google Scholar]
- 71.Tepper SJ, Orally inhaled dihydroergotamine: a review. Headache, 2013. 53 Suppl 2: p. 43–53. [DOI] [PubMed] [Google Scholar]
- 72.Currier G.and Walsh P, Safety and efficacy review of inhaled loxapine for treatment of agitation. Clin Schizophr Relat Psychoses, 2013. 7(1): p. 25–32. [DOI] [PubMed] [Google Scholar]
- 73.Alexander-Williams JM and Rowbotham DJ, Novel routes of opioid administration. Br J Anaesth, 1998. 81(1): p. 3–7. [DOI] [PubMed] [Google Scholar]
- 74.Gessler T, Seeger W, and Schmehl T, Inhaled prostanoids in the therapy of pulmonary hypertension. J Aerosol Med Pulm Drug Deliv, 2008. 21(1): p. 1–12. [DOI] [PubMed] [Google Scholar]
- 75.Brown RP, et al. , Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health, 1997. 13(4): p. 407–84. [DOI] [PubMed] [Google Scholar]
- 76.Klaassen CD, Casarett & Doull’s Toxicology: The Basic Science of Poisons. 6th ed. 2001, New York, NY: McGraw Hill. [Google Scholar]