ABSTRACT
Inflammatory bowel disease (IBD) is an idiopathic inflammatory disease. Environmental sanitization, modern lifestyles, advanced medicines, ethnic origins, host genetics and immune systems, mucosal barrier function, and the gut microbiota have been delineated to explain how they cause mucosal inflammation. However, the pathogenesis of IBD and its therapeutic targets remain elusive. Recent studies have highlighted the importance of the human gut microbiota in health and disease, suggesting that the pathogenesis of IBD is highly associated with imbalances of the gut microbiota or alterations of epithelial barrier function in the gastrointestinal (GI) tract. Moreover, diet-induced alterations of the gut microbiota in the GI tract modulate immune responses and perturb metabolic homeostasis. This review summarizes recent findings on IBD and its association with diet-induced changes in the gut microbiota; furthermore, it discusses how diets can modulate host gut microbes and immune systems, potentiating the impact of personalized diets on therapeutic targets for IBD.
Keywords: inflammatory bowel disease (IBD), gut microbiota, gastrointestinal tract, diet, immune
The design of appropriate diets based on the microbiota composition is an attractive therapeutic strategy to decrease the risk and severity of IBD.
Introduction
Until the early 1950s, humans suffered greatly from infectious diseases. Since then, many developing countries have undergone industrialization and urbanization, including the introduction of environmental sanitization, adoption of modern lifestyles, and application of advanced medicines, which relieved the burden of bacterial infectious diseases, e.g., cholera and typhoid (1). On the other hand, chronic metabolic diseases have dramatically increased and threaten human health. Besides acute contagious diseases, the pathogenesis of chronic diseases is highly associated with imbalances of the gut microbiota or alterations of epithelial barrier function in the gastrointestinal (GI) tract (2). Indeed, alterations of the gut ecosystem in the GI tract modulate immune responses and perturb metabolic homeostasis (3). Dysbiosis of the gut microbiota might be related to inflammation and metabolic syndromes such as diabetes, obesity, inflammatory bowel disease (IBD), irritable bowel syndrome, autoimmune diseases, and cancer (4, 5).
IBD is the most prevalent chronic disease worldwide in terms of region, culture, environment, and diet type. Diet contents and quantity affect the human microbiota in the human GI tract, highlighting the importance of the human gut microbiome in health and disease (6); therefore, reshaping the composition of the microbiota is an attractive therapeutic strategy to alleviate disease symptoms or prevent chronic diseases. Accordingly, food choices encompassing the use of microbial nutrients (prebiotics), metabolites (postbiotics), and microorganisms (probiotics) have received significant attention for their beneficial or detrimental outcomes in relation to host health. This review investigated recent research (2010–present) focusing on the consequences of diets on complex host–microbe interactions (Supplemental Data). Here we discuss the impact of individuals’ lifestyles and food intakes on the associations of diets and microbiomes with IBD, which will help develop personalized nutrition and preventive food for therapeutic purposes.
IBD
IBD is a chronic relapsing inflammatory disease of the GI tract. Patients with IBD exhibit disorders of the GI tract caused by an aberrant and excessive inflammatory response due to perturbation of intestinal homeostasis encompassing the immune system (7), enterocyte metabolism (8), and gut microbiota (9). The 2 main types of IBD are Crohn's disease (CD) and ulcerative colitis (UC), both of which are characterized by a persistent inflammatory state and have similar pathogenesis. However, they differ in several clinical features, including location, pathology, and complications (7). CD is characterized by goblet cell hypertrophy and lower activity of antimicrobial peptides and affects the digestive tract from the mouth to the anus with discontinuous, patchy gut inflammation. By contrast, UC, which is often characterized by mucus diarrhea although mucin 2 (MUC2) expression is reduced, occurs continuously and only affects regions from the cecum to the rectum (10). Patients with CD can exhibit stenosis, abscess and fistula formation, and colon cancer due to the intestinal barrier dysfunction and impairment of the tight junctions (TJs) in intestinal epithelial cells (IECs) (11). On the other hand, patients with UC can exhibit severe bleeding, toxic megacolon, bowel rupture, and colon cancer (12).
During the last few decades, numerous studies have highlighted associations of the host genotype, environment, gut microbiota, and immunopathogenesis with the pathogenesis of IBD (13). The geographic incidence and epidemiologic study of IBD indicate that environmental factors related to industrialization (i.e., emigration to developed countries) contribute to disease expression and pathogenesis (14). Indeed, case-control studies and meta-analyses have shown that the geographical variability and incidence of IBD are significantly correlated with environmental risk factors (e.g., smoking and appendectomy) and urbanization associated with altered diets and intestinal microbiota, antibiotic use, pollutant and microbial exposures, and socioeconomic and sanitary conditions (15, 16). Notably, changes in environmental factors, such as diet, antibiotic use, and pollution, affect the human gut microbiota composition, which might be associated with an increased risk of IBD (17). These backgrounds suggest that the interaction between environmental risk factors and the gut microbiota plays a vital role in the pathogenesis of IBD. Multiple pathogenic factors relevant to human IBD have been well-reviewed, focusing on the immunopathogenesis of IBD (18). Nevertheless, the etiology of IBD and the correlation between diet and dysbiosis of the gut microbiota in relation to IBD remain unclear. Before a discussion on the association between diet and IBD, in this section, we describe the etiological features of the IBD intestinal environment, current therapies, and their association with gut microbiota, particularly the findings by recent studies.
Therapeutic strategies for the treatment of IBD
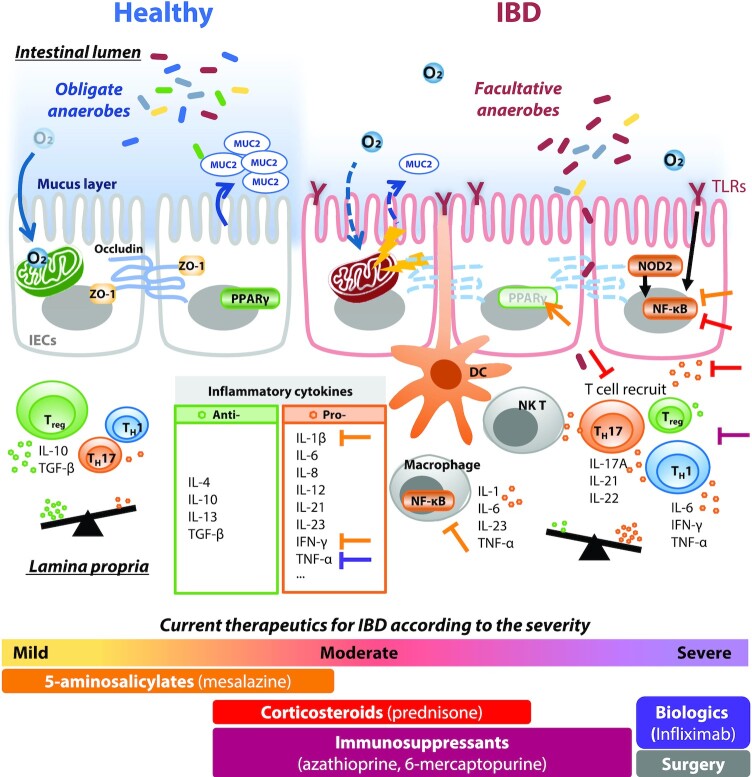
Patients with IBD exhibit dysregulated immune responses, resulting in a cytokine-mediated chronic cycle of inflammation. Such immune abnormalities challenge homeostasis of the gut mucosal environment and affect genetic susceptibility. Loss of intestinal barrier integrity by multifactorial actions causes high gut permeability (i.e., leaky gut). MUC2 expression is reduced in UC patients (19), and patients with CD can exhibit intestinal barrier dysfunction and impairment of the TJs in IECs (11). Subsequently, exposure of impaired IECs to commensal microorganisms as luminal antigens stimulates systemic immune responses through vicious cycles, resulting in chronic inflammation of the GI tract (20). Another feature in the intestinal epithelium of IBD is impaired mitochondrial energy metabolism, leading to IBD-associated dysbiosis (21). The gut microbiota composition in IBD shifts from obligate anaerobes to facultative anaerobes owing to poor oxygen consumption of IBD epithelial mitochondria (22). Overall, the host's immune status can influence the composition of the commensal microbial community in IBD. In addition, commensal bacteria modulate the IEC function underlying crosstalk between luminal microbes in the mucus layer and lymphocytes in the lamina propria (Figure 1). Genome-wide association studies revealed karyotypes with IBD susceptibility loci (23). Variations in genes encoding the Nucleotide-binding and oligomerization domain (NOD)-like receptor (NLR) (24), IL-23 receptor (25), and autophagy-related 16 like 1 gene (ATG16L1) (26) are pathogenic factors for IBD. NOD2 polymorphisms are related to CD susceptibility. Genetic variations in NOD2, which is essential for bacterial recognition through muramyl dipeptide (MDP), suppress the production of the anti-inflammatory cytokine IL-10 (27). Impaired signaling of toll-like receptors (innate immunity) induces inappropriate immune responses that influence oral tolerance (28). This series of events results in an inflammatory response by disrupting the balance of T cell differentiation with inflammatory cytokines in the lamina propria of IBD patients. Therefore, numerous therapeutic drugs have been used to treat these chronic, idiopathic immune diseases, and major therapeutic approaches optimize anti-inflammatory responses in the bowel wall (Figure 1).
FIGURE 1.
Schematic diagram of healthy compared with impaired gut epithelial mucus layers. Dysbiosis of the gut microbiota with a relatively aerobic intestinal lumen is the cause and/or consequence of IBD. Epithelial barrier integrity is lost in IBD, with decreased mucus layer thickness, low expression of MUC2, and abnormal expression of TJ proteins, including ZO-1 and occludin. Increased epithelial permeability due to loss of barrier function activates inflammatory responses by increasing exposure of intestinal epithelial cells and immune cells to pathogens or antigens. DC, dendritic cell; IBD, inflammatory bowel disease; MUC2, mucin 2; NK T, natural killer T cell; NOD2, nucleotide-binding oligomerization domain 2; PPAR, peroxisome proliferator–activated receptor; TGF-β, transforming growth factor-β; TH, helper T cell; TLR, toll-like receptor; Treg, regulatory T cell; ZO-1, zonula occludens-1.
IBD is conventionally treated using surgery or medicinal drugs targeting the downstream signaling pathways of the inflammatory cascades such as 5-aminosalicylic acid derivatives (balsalazide, mesalazine, and sulfasalazine), corticosteroids (budesonide, prednisone, hydrocortisone, and dexamethasone), and immunosuppressants (azathioprine, cyclosporine, 6-mercaptopurine, methotrexate, mycophenolate mofetil, and tacrolimus) to reduce inflammation. However, the remission rate of IBD is low at 37% (29), and the efficacy of treatment depends on the clinical conditions and individual patients (7). Drug therapy and other treatments are not disease-specific and may be ineffective if the patient's diet or lifestyle changes; therefore, many alternative approaches have been tested to treat IBD patients. Antibody-mediated proinflammatory cytokine blockade, including inhibition of TNF, is an effective therapeutic strategy for both CD and UC (30). Still, it also exhibits several adverse effects such as increased risks of pathogenic infection and cancer and severe allergic reaction. Gut-specific anti-integrin therapeutics such as anti-IL-12/IL-23 agents may also be an option for systemic immunosuppression (31). Recently, biologics targeting alternative pathways and small-molecule drugs have attracted attention as a newer category of therapeutics that neutralize proteins involved in inflammation (32). Despite these advances, it is still difficult to control the disease for many IBD patients.
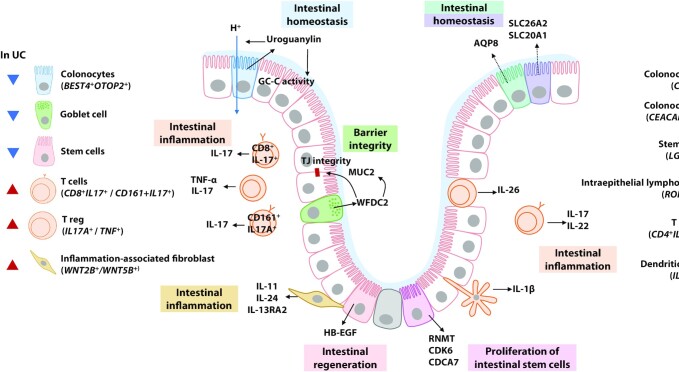
Alternatively, single-cell RNA sequencing (scRNA-Seq) has been exploited for high-resolution analysis of intestinal physiologic characteristics to select more accurate biomarkers and therapeutic targets of IBD. scRNA-Seq can provide information on the gene expression of a small number of cells and observe cellular heterogeneity that has been overlooked in studies using conventional sequencing methods (33, 34). This approach has recently been used to broaden our understanding of the pathogenesis of IBD by characterizing a subset of cells present in the intestinal epithelium and lamina propria (35–37). These studies revealed cellular subtypes of the intestinal epithelium and represented cellular changes in IBD patients compared with healthy individuals (Figure 2). The single colonic epithelial cell profile of intestinal crypts characterized a subtype of bestropin 4 (BEST4) and otopetrin 2 (OTOP2)-expressed absorptive colonocytes (BEST4+OTOP2+) in the crypt-top, revealing that this cell type was depleted in the crypts of UC patients (35, 36). This cell subset activates guanylate cyclase (GC-C) to express uroguanylin, maintaining the epithelial barrier and expressing the OTOP2 (38). It also induces an ion channel that senses pH and transfers protons (39). A recent study found that carbonic anhydrase-1 (CA1+) and carbonicoembryonic antigen cell adhesion molecue 7 (CEACAM7+) colonocytes are found in the intestinal crypt-top of CD patients compared with healthy individuals (40). These cell subtypes had a reduced gene expression of solute carrier family 26 member 2 (SLC26A2) and solute carrier family 20 member 1 (SLC20A1) involved in anion transport and aquaporin 8 (AQP8) involved in water transport, respectively. The signatures of these colono
FIGURE 2.
Colonic cell heterogeneity in IBD revealed by single-cell sequencing analysis. Single-cell RNA sequencing reveals subsets of the intestinal epithelium and immune cells according to gene expression patterns. They can be responsible for the maintenance of intestinal epithelium homeostasis, inflammatory responses, and the epithelial barrier, and are present at differential amounts in IBD patients. AQP8, aquaporin 8; BEST4, bestropin 4; CA1, carbonic anhydrase-1; CD, Crohn’s disease; CDCA7, cell division cycle associated 7; CDK6, cyclin-dependent kinase 6; CEACAM7, carcinoembryonic antigen cell adhesion molecule 7; GC-C, guanylate cyclase; HB-EGF, heparin-binding epidermal growth factor-like growth factor; IBD, inflammatory bowel disease; IL-13RA2, interleukin 13 receptor subunit α 2; LGR5, leucine-rich repeat-containing G-protein coupled receptor 5; OTOP2, otopetrin 2; RNMT, RNA guanine-7 methyltransferase; RORγ, Retineic-acid-receptor-related orphan nuclear receptor gamma; SLC20A1, solute carrier family 20 member 1; SLC26A2, solute carrier family 26 member 2; TJ, tight junction; Treg, regulatory T cell; UC, ulcerative colitis; WFDC2, whey-acidic-protein four-disulfide core domain protein 2; WNT5B, Wnt family member 5B.
cytes revealed in the crypt-top of IBD patients may partly explain the disruption of homeostasis in the IBD intestine. In crypts of UC patients, remodeling of goblet cells has been reported, including depletion of gene encoding whey-acidic-protein (WAP) four-disulfide core ptotein 2 (WFDC2), which is highly expressed by goblet cells under normal conditions (35). This study further established that WFDC2 is necessary for mucus layer formation, TJ integrity, and antimicrobial activity against specific bacteria, suggesting that a WFDC2 defect in UC patients plays a role in impaired barrier function.
In addition, scRNA-Seq has revealed that several types of immune cell subsets responsible for intestinal inflammation with an expression of proinflammatory cytokines were expanded in IBD patients (36, 37, 41). Smillie et al. (36) reported the expansion of Wnt family member 2B and 5B (WNT2B+/WNT5B+) inflammation-associated fibroblasts (IAF) expressing inflammation-related genes including IL-11, IL-24, and IL-13RA2 in the intestine of UC patients. The scRNA-Seq analysis further characterized stem cells at the crypt base of UC patients, confirming downregulated heparin-binding epidermal growth factor-like growth factor (HB-EGF) expression (35). Low concentrations of HB-EGF lead to Wnt signaling inhibition and the failure of intestinal epithelium regeneration (42). Similarly, CD patients exhibited a stem cell signature leading to decreased Wnt signaling (40). Taken together, the accumulation of scRNA-Seq information on IBD pathogenesis could identify more precise therapeutic targets and lead to successful personalized dietary strategies.
Gut microbiota in IBD
The gut microbiota in childhood plays an essential role in the development and maturation of the immune system (43). The abundance of nutrients available for gut bacteria significantly affects neonatal gut colonization (44). Early gut microbial colonization induces tolerance to the commensal microbiota through the postthymic education of colonic Forkhead box P3 (Foxp3)+regulatory T (Treg) cells (45). A healthy and balanced gut microbiota promotes differentiation of naïve gut dendritic cells (DCs), thereby generating Treg cells and the establishment of immune homeostasis. Furthermore, recent studies using scRNA-Seq have reported that the commensal microbiota affected the population of intestinal innate lymphoid cells and mononuclear phagocytes (46, 47). Thus, interactions between the commensal microbiota and host gut epithelial and immune cells are critical for human health and diseases.
Although the gut microbiota and host genetic susceptibility differ among healthy individuals, many risk factors for IBD are responsible for host–microbe interactions, leading to dysregulated immune responses (48). Compositional and temporal changes in the gut microbiota are linked to the disease course of pediatric UC patients (29). Less diversified microorganisms induce immunogenic DCs, which activate effectors, T cells, and subsequent inflammation. Moreover, maternal IBD can influence the dysbiotic microbiota of infants, leading to fewer memory B cells and Treg cells (49). Indeed, increased abundance of the Enterococcus, Lactobacillus, Bifidobacterium, and Escherichia-Shigella genera positively correlated with induced IL-12/23 concentrations in UC (50). Numerous studies have reported the compositional characteristics of the microbiota in patients with IBD (51). Notably, advances in multiomics analysis integrated with information processing indicate that taxonomic dysbiosis can lead to functional dysbiosis, supporting the notion that the gut microbiome is involved in the pathogenesis of IBD (52, 53).
Table 1 summarizes the representative gut microbiota changes and interactions with host cells reported in IBD patients. Firmicutes and Bacteroidetes are the predominant phyla of the human gut microbiota, and changes in their proportions are associated with human disease (54). The gut microbiota of IBD patients exhibits decreased microbial diversity, a decrease in the Firmicutes phylum, and an increase in the Bacteroidetes phylum (55, 56). Firmicutes play a role in butyrate production from oligosaccharides in the human gut, and bacteria belonging to the Bacteroidetes phylum can produce propionate (57). Butyrate is a primary energy source of colon epithelial cells and is mainly used in the colon, whereas propionate and acetate are used systemically in various organs (58). SCFAs such as butyrate contribute to intestinal homeostasis by maintaining the integrity of the intestinal epithelial barrier and regulating the immune response (59).
TABLE 1.
Dysbiosis and its interaction with host cells in IBD1
| Gut microbiota | Changes in IBD | Microbial production | Effects of gut microbes on host cells | References |
|---|---|---|---|---|
| Firmicutes2 | Decreased in CD/UC | Production of SCFAs including butyrate (mainly), propionate, and acetate | • Maintenance of homeostasis of barrier function and immune responses | (55–57) |
| Bacteroidetes2 | Increased in UC | Production of propionate | (56, 57) | |
| Faecalibacterium prausnitzii | Decreased in CD/UC | Production of butyrate | • Maintenance of homeostasis of barrier function and immune responses• Reduction of TH17 differentiation through HDACs inhibition | (60, 62, 63) |
| Roseburia intestinalis | Decreased in CD/UC | Production of butyrate | • Protection of colonic mucosa• Inhibition of IL-17 excretion• Promotion of Treg cells differentiation• Production of propionate | (64, 65) |
| Akkermansia muciniphila | Decreased in CD/UC | Expression of Amuc_1100 | • Increased mucus layer thickness• Induction of TJ proteins expression• Reduction of CD8+ cytotoxic T lymphocytes | (66, 67, 70, 71) |
| Ruminococcus gnavus | Increased in CD/UC | Mucolysis in intestines | • Decomposition of the mucus layer and invasion of pathogens• Induction of TNF-α by dendritic cells | (73, 74, 76) |
| Atopobium parvulum | Increased in CD | Production of H2S | • Induction of colitis and mitochondrial dysfunction | (78) |
| Desulfovibrio 3 | Increased in UC | Production of H2S | • Reduction of mucosal thickness• Induction of apoptosis in colon epithelial cells | (81–83) |
| Bacteroides vulgatus | Increased in UC | Production of proteases | • Induction of intestinal permeability | (84) |
| Bacteroides dorei |
CD, Crohn’s disease; HDAC, histone deacetylase; IBD, inflammatory bowel disease; TH, helper T cell; TJ, tight junction; Treg, regulatory T cell; UC, ulcerative colitis.
Phylum.
Genus.
Moreover, functional analysis of the gut microbiome in patients with IBD revealed deficiencies in the butyrate and propionate pathways in CD patients, whereas deficiencies in the propionate pathways were shown in UC patients (52). At the species level, Faecalibacterium prausnitzii, belonging to the Firmicutes phylum, is representative of butyrate producers with decreasing abundance in CD and UC patients (60). Furthermore, F. prausnitzii–produced butyrate inhibited histone deacetylase (HDAC) 1 or 3, thereby downregulating TH17 differentiation (61, 62). Although the simultaneous reduction of both F. prausnitzii abundance and SCFA concentrations was observed in UC patients, there was no direct correlation between the decrease in butyrate and this species (63). These results suggest that the pathogenesis of IBD accompanies the contribution of different bacterial species. In addition, Roseburia intestinalis, one of the declining butyrate producers in IBD patients, might be involved in the pathogenesis (64). Similarly to F. prausnitzii,R. intestinalis exhibited a protective effect against colitis by promoting Treg differentiation and inhibiting IL-17 secretion (65).
A significant imbalance of the gut microbiota is a hallmark of IBD patients, and the abundance of mucolytic bacteria such as Akkermansia muciniphila was decreased in CD and UC patients (66, 67). Although A. muciniphila is a mucin-degrading bacterium (68), it decreases intestinal permeability by increasing the thickness of the mucin layer (69) and increases intestinal TJ protein expression and goblet cell density, which may also contribute to intestinal barrier integrity (70). Recent studies have reported that Amuc_1100, a membrane protein of A. muciniphila, exerts a beneficial effect against IBD with the reduction of cytotoxic T lymphocytes (70, 71). In addition to its gut barrier function, A. muciniphila contributes to the formation of cross-feeding networks with butyrate-producing bacteria in the human gut belonging to the phylum Firmicutes such as Anaerostipes caccae, Eubacterium hallii, and F. prausnitzii (72). On the other hand, the abundance of mucin-degrading Ruminococcus gnavus was increased in IBD patients, indicating that excessive mucus decomposition facilitates the induction of inflammatory reactions in the intestines (73–75). Furthermore, glucorhamnan produced by R. gnavus might cause inflammation in the host (76). Thus, although the roles of mucin-degrading bacteria in IBD remain to be further investigated, the abundance of some species is critical for maintaining homeostasis of mucin layer thickness, which is tightly coordinated with the presence of prebiotics and the abundance of fiber-degrading bacteria in the human gut microbiota (77).
Studies have shown that crosstalk between microbes and mitochondria in host cells is also involved in the pathology of IBD. Analysis of the gut microbiota in CD patients showed a positive correlation between disease severity and the abundance of H2S producers (e.g., Atopobium,Fusobacterium,Veillonella,Prevotella,Streptococcus, and Leptotrichia) (78). On the other hand, the abundance of butyrate producers (e.g., Blautia, Lachnospiraceae, Roseburia,Eubacterium rectale,Ruminococcus,Clostridium, and Faecalibacterium) was decreased in CD patients. H2S, one of the metabolites produced by gut microbes, inhibits cytochrome oxidase in the intestinal epithelium, thereby inhibiting the mitochondrial tricarboxylic acid (TCA) cycle (79). By contrast, butyrate is used as an energy source for the TCA cycle and activates energy metabolism in mitochondria (80). H2S producers such as Atopobium parvulum contribute to the induction of colitis in an IL-10-deficient mouse model, indicating that the intestinal microbiota causes mitochondrial dysfunction in IBD patients. Similarly, H2S-producing Desulfovibrio was abundant in UC patients (81), and Desulfovibrio induced mucosal thickness reduction and IEC apoptosis (82, 83). Furthermore, a recent multiomics analysis suggested a novel microbial IBD pathogenesis in which a high abundance of protease-producing Bacteroides vulgatus and B. doreiincreases intestinal permeability and induces colitis in UC patients (84).
Remarkably, intestinal microbiota transplantation from IBD individuals into germ-free mice increased the severity of colitis induction compared with healthy individuals (85). Indeed, fecal microbiota transplantation effectively treats IBD. Although its long-term efficacy and safety are still unclear, its therapeutic potential has been highlighted (86). This study suggests that an individual's gut microbiota plays an essential role in regulating the pathogenesis of IBD. In addition to geographical/racial variations in the structure of the microbiome, different eating habits, together with environmental factors such as geography, climate, and urbanization, may cause variations in the gut microbiota (87). Indeed, diet may be an important regulator of IBD progression because it is significantly associated with changes in IBD gut microbiota (73). Therefore, the results described here indicate that the design of more appropriate diets based on the microbiota can decrease the risk and severity of IBD.
Diet-Induced Gut Microbiota Associated with IBD
Individual microbiome communities vary greatly depending on the host's habitats and genetic traits (88). Although knowledge of the association between the gut microbiome and a host with IBD has increased, changes in the composition of the gut microbiota are diverse and challenging to control, and consequently, there is a lack of clarity regarding the interactions between the gut microbiota and IBD in clinical practice. The human gut microbiota is nourished in the GI mucus layer with carbon and nitrogen sources (e.g., O- and N-linked glycans, polysaccharides, proteins, and glutens) from host diets and mucus in the intestinal epithelium (89). The dynamic abundance and availability of dietary and endogenous glycans primarily determine the composition of the human gut microbiota over time, influencing host metabolism in health and disease (90). Thus, the type, quality, and origin of food affect gut microbial ecology, host physiology, and health. In addition, the glycan-degrading activity of mucolytic microbes is critical to maintaining symbiotic, commensal microbiota through cross-feeding networks (72). Consequently, microbial metabolites, such as SCFAs, vitamins, and indole derivatives, together with host-derived molecules (α-defensins, RegIIIγ, and immunoglobulin A), contribute to host nutrition and immune responses through host–microbe interactions (91). The chemical structures of dietary glycans, lipids, and proteins vary in the human gut over time (92). Variations in the foods consumed can affect the composition of microbiota because different bacterial lineages possess different nutrition acquisition strategies (93).
Meta-analyses to establish a link between diet and IBD suggested that westernized diets rich in fats and animal proteins and lacking fruits and vegetables increase the risk of IBD (94). A Western diet is positively correlated with reduced epithelial rigidity, a decrease in Firmicutes, dominance of Bacteroidetes, and intestinal inflammation, consistent with the characteristics of IBD patients (95). Nine prospective cohort studies and case-control studies in IBD cases reported that Western dietary patterns increased the relative risk of CD and UC by 1.72 and 2.15 times, respectively (94). In a mouse model, high-fat and high-sugar diets mimicking a Western diet induced dysbiosis of gut microbiota with an increase in Escherichia coli and significantly reduced concentrations of SCFAs, resulting in intestinal inflammation (95). Remarkably, a retrospective investigation of the dietary habits of 86 CD patients revealed that patients with low CD activity consumed a diet more similar to a Mediterranean diet (96). In UC patients, a decrease in fecal calprotectin, a marker of gut inflammation, was also correlated with the consumption of a Mediterranean diet (97). In addition, a recent meta-analysis suggested that the Mediterranean diet has the potential to prevent IBD through regulation of the gut microbiota, including an increase in Akkermansia and a decrease in Fusobacterium (98). These results indicate that differences in dietary components alter the composition of gut microbiota, resulting in different metabolite profiles, which affect host physiology. This section summarizes recent results from nutritional studies that reported effects on the physiologic properties of IBD and suggests how intestinal microbes may be associated with the pathogenesis of IBD.
Carbohydrate
A Western-style diet characterized by high sugar and low fiber intakes affects the onset of IBD, indicating that carbohydrate consumption can affect the risk and progression of IBD. Indeed, the risk of UC increased with increased consumption of sugar and sweets, and a higher intake of sugar and sweets was positively correlated with the risk of CD (99). These results revealed a correlation between sugar intake and the incidence of CD and UC. In particular, a high-sugar diet containing 50% sucrose induced acute colitis and upregulated proinflammatory cytokines to a greater extent in mice, which might be associated with reduced SCFA concentrations and gut microbiome diversity, and increased gut permeability (100).
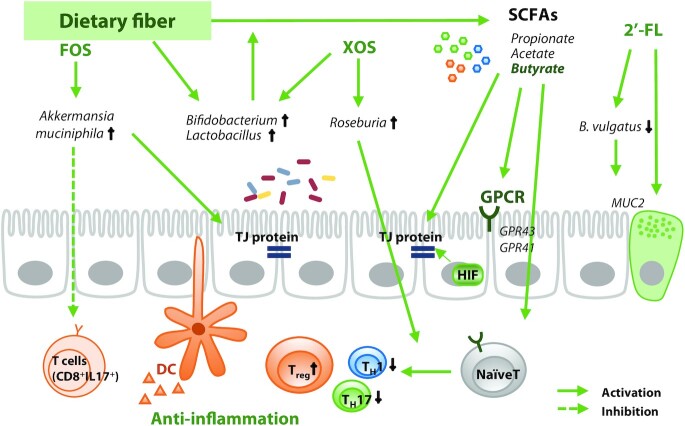
By contrast, case-control studies in IBD patients showed that high fiber intake lowered the risk of CD and UC (101). The crosstalk between fiber intake, the abundance of Bifidobacterium and Lactobacillus, and SCFA production has already been well discussed (102). The abundance of Bifidobacterium and Lactobacillus was increased by fiber intake, and the intake of probiotics including them showed anti-inflammatory activity dependent on fiber (Figure 3). SCFAs regulate the differentiation of T cells by targeting G-protein coupled receptors (GPCRs) such as GPR41 and GPR43, leading to control of the immune response (103, 104). Butyrate produced by intestinal microbes induces expression of hypoxia-inducible factor (HIF) to maintain the integrity of intestinal barriers, thereby mitigating IBD symptoms (105). A population study in middle-aged Danish adults consuming a low-gluten diet revealed that qualitative changes in dietary fiber induced moderate changes in the intestinal microbiome (106). Furthermore, high dietary fiber improved the expression of TJ proteins [e.g., zonula occludens (ZO), occludin, and claudin], which is inhibited by dextran sulfate sodium (DSS), and total SCFAs. Intake of pectin, a water-soluble dietary fiber enriched in fruits, also reduced inflammation by regulating the immune response in colitis models (107). Furthermore, diets with a high pectin content significantly reduced the concentrations of IL-1β and IL-6 with attenuated tissue damage in IL-10-knockout IBD model mice and pectin treatment substantially inhibited IL-6 in Raw264.7 cells, indicating that pectin directly regulates the immune response to relieve colitis (107).
FIGURE 3.
Dietary fiber and its interaction with gut microbiota in regulation of IBD. Intake of dietary fiber increases the abundance of SCFA-producing bacteria, increasing the concentration of SCFAs in the gut. Butyrate activates GPCRs and HIF to maintain barrier integrity and regulates T cell differentiation to promote anti-inflammatory activity. The anti-IBD function of dietary fiber may be dependent on the abundance of SCFA-degrading species in an individual's gut microbiota. DC, dendritic cell; FOS, fructo-oligosaccharide; GPCR, G protein-coupled receptor; HIF, hypoxia-inducible factor; IBD, inflammatory bowel disease; MUC2, mucin 2; TH, helper T cell; TJ, tight junction; Treg, regulatory T cell, XOS, xylo-oligosaccharide; 2’-FL, 2’-fucosyllactose.
Several polysaccharides and oligosaccharides improve IBD in association with the gut microbiota. A diet containing fructo-oligosaccharides (FOSs) and inulin significantly increased the abundance of Bifidobacteria and Lactobacilli in CD patients and colitis models, reducing disease activity in CD patients (108, 109). Intake of FOSs is also one of the potential strategies to enhance the intestinal abundance of A. muciniphila (110), implying that FOSs may relieve IBD pathology by inhibiting CD8+ T cells and enhancing TJ expression. Intake of 2'-fucosyllactose (2'-FL) restored goblet cells and increased MUC2 expression in DSS-induced mice (111), demonstrating that the 2'-FL supplement reduced mucin-degrading bacteria including B. vulgatus. Another prebiotic, xylo-oligosaccharide (XOS), has been shown to promote the growth of Roseburia, Bifidobacterium, and Lactobacillus in UC patients (112), which would be expected to induce an anti-inflammatory effect and Treg differentiation via Roseburia (113).
Taken together, several lines of evidence suggest that supplementation of carbohydrates can control the severity of IBD and risk factors associated with inflammation and barrier function. However, there are gaps in the results, and the mechanisms that mediate this regulation remain unclear. The types of hydrolases and metabolism vary considerably depending on the type of nondigestible carbohydrates. Therefore, variations in microbiota between individuals should be considered to suggest specific diets to alleviate inflammation in IBD patients.
Fat
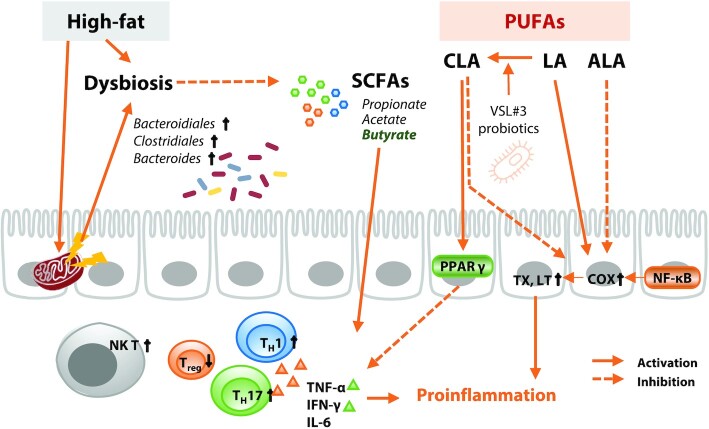
Epidemiologic analyses have shown that high dietary fat intake is a risk factor for IBD (114). Dietary fat can also regulate the physiology of IBD by closely working with the gut microbiota (Figure 4). Cecal samples from high-fat diet–fed mice showed a significant increase in the relative proportion of the Bacteroidiales and Clostridiales orders. Furthermore, intake of a high-fat diet for 6 mo in healthy adults led to a decrease in the total concentration of SCFAs due to dysbiosis of gut microbiota with an increase in Bacteroides, thereby increasing the concentrations of inflammatory factors (115). A high-fat diet led to an increase in NK T cells and a decrease in Treg cells, exacerbating the symptoms of IBD through TNF-α and IFN-γ in mouse models of DSS-induced colitis (116), and accelerated ileal inflammation in mice accompanied by reduced expression of the TJ protein occludin and acceleration of the TH17 immune response, involving TNF and IL-6 (117). Furthermore, a high-fat diet with antibiotic treatment impaired epithelial mitochondrial function, leading to dysbiosis, such as proliferation with Enterobacteriaceae, which aggravates mucosal inflammation (21).
FIGURE 4.
Fat and its interaction with gut microbiota in regulation of inflammatory bowel disease. A high-fat diet causes dysbiosis of the gut microbiota, which reduces production of SCFAs and causes an increase in NK T cells and a decrease in Treg cells, promoting the production of proinflammatory cytokines. Mitochondrial dysfunction in intestinal epithelial cells triggered by a high-fat diet reduces intestinal oxygen consumption and induces dysbiosis. LA and ALA have opposing effects on metabolism of arachidonic acid, regulating TX and LT production. However, LA can be converted to CLA upon ingestion of certain probiotics, leading to inhibition of proinflammatory cytokine production through increased expression of PPARγ. ALA, α-linolenic acid; CLA, conjugated linoleic acid; COX, cyclooxygenase; LA, linoleic acid; LT, leukotriene; NK T, natural killer T cell; PPAR, peroxisome proliferator–activated receptor; TX, thromboxane; TH, helper T cell; Treg, regulatory T cell.
According to Simopoulos, the ratio of ω-6:ω-3 PUFAs recommended for a balanced diet to prevent chronic diseases is 1–4:1; however, this ratio is increased to ∼15:1 in Western diets. Intake of fish oil rich in ω-3 fatty acids relieves DSS-induced colitis through modulation of the cyclooxygenase (COX) pathway (118). This result is consistent with previous evidence collected of a negative correlation between ω-3 PUFA intake and IBD. α-Linolenic acid (ALA; 18:3n–3), one of the ω-3 fatty acids that play an essential role in human physiology, improved intestinal inflammation in an experimental colitis model. Inui et al. (119) demonstrated that an ALA-rich emulsion effectively alleviated histologic damage to the colon in rats with trinitrobenzene sulfonic acid (TNBS)-induced colitis by regulating arachidonic acid (20:4n–6) metabolism. This observation supports the role of ALA in relieving oxidative stress in TNBS-treated rats and modulating NF-κB, leading to lower concentrations of leukotriene B4 (LTB4) and COX, which are inflammatory factors associated with arachidonic acid (120).
Conversely, a prospective cohort study concluded that linoleic acid (LA) (18:2n–6), a type of ω-6 PUFA, contributes to the risk of UC (121). ω-6 PUFA is a precursor of proinflammatory factors such as thromboxanes and leukotrienes, indicating that excessive intake of ω-6 PUFA contributes to the risk of IBD. However, intervention with specific microbiota can reverse the effect of linoleic acid on IBD. Supplementation of the probiotic VSL#3 (e.g., Lactobacillus acidophilus,L. bulgaricus,L. casei,L. plantarum, Bifidobacterium breve, B. infantis,B. longum, and Streptococcus thermophilus) can regulate intestinal inflammation through gut microbial metabolism (122). Remarkably, VSL#3 produces conjugated linoleic acid (CLA) from LA, a dietary risk factor for IBD, and consequently increases peroxisome proliferator–activated receptor (PPAR)-γ expression. This result suggests that CLA produced by probiotics plays a role in the efficacy of VSL#3 to relieve IBD in the presence of LA. CLA, a slightly altered form of LA (ω-6), inhibits the production of inflammatory mediators by regulating arachidonic acid metabolism (123). In a small-scale clinical trial, CLA significantly inhibited the production of proinflammatory cytokines by regulating T cells and attenuated disease activity in CD patients (124). Furthermore, PPARγ mediates the positive effects of CLA to protect the colon from inflammation (125). Although many studies have identified the benefits of specific fatty acids or types of fatty acids for IBD, the results are currently insufficient to conclude there is a clinical advantage.
Protein
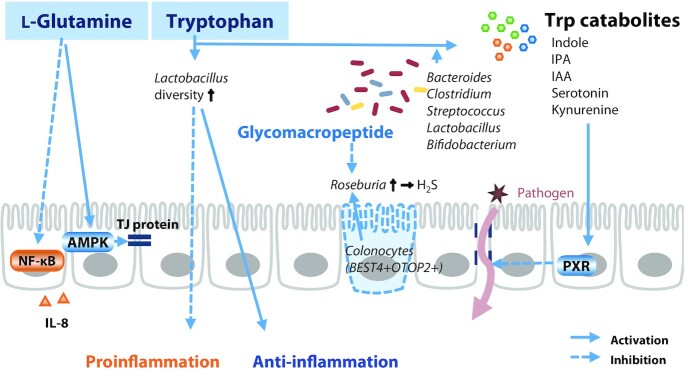
The potential of several amino acids to improve IBD has recently been investigated (Figure 5). l-Glutamine activates Ca2+/calmodulin-dependent protein kinase 2 (CaMKK2)–AMP-activated protein kinase (AMPK) signaling in porcine IECs. CaMKK2–AMPK signaling pathways elevate the abundance of the TJ proteins occludin, claudins, ZO-1, and junction adhesion molecule A (126). Remarkably, l-glutamine treatment decreased the concentration of IL-8 induced by NF-κB in Caco-2 cells and reduced the concentration of IL-8 in HCT-8 cells upon TNF-α-induced inflammation (127). Furthermore, glutamine abolished the cytokine-induced loss of barrier integrity in Caco-2 cells (128). Intriguingly, the low serum concentrations of tryptophan (Trp) in IBD patients revealed a correlation between IBD and Trp (129). In a porcine model of DSS-induced colitis, administration of Trp ameliorated colitis symptoms, lowered intestinal permeability, and inhibited the expression of proinflammatory cytokines (130). In addition, Trp metabolic pathways by interaction with the gut microbiota regulate immune responses and gut barrier function (131). Trp catabolites produced by Bacteroides, Clostridium, Streptococcus, Lactobacillus, and Bifidobacterium (e.g., serotonin, kynurenine, and indole derivatives) affect the activity and severity of IBD (132). Indole, indole propionic acid, and indole acrylic acid produced via Trp catabolism affect mucosal homeostasis by decreasing intestinal permeability through the pregnane X receptor (PXR). Indolealdehyde also affects innate and adaptive immune responses by increasing IL-22 production. In particular, microbial Trp catabolites inhibit inflammation by maintaining the diversity of Lactobacilli in mouse intestines (133). Enterochromaffin cells secrete serotonin [also known as 5-hydroxytryptamine (5-HT)], which has antioxidative and anti-inflammatory activities. However, gut microbiota–dependent Trp metabolism is likely to be perturbed in patients with IBD associated with a westernized diet, leading to impaired 5-HT and IL-22 production (131). These results highlight the link between probiotic intake and host inflammation in the regulation of IBD. Disruption of GC-C activity, identified in IBD epithelial cells by scRNA-Seq analysis, can lead to an imbalance in ion secretion, leading to changes in the gut microbiota, such as increased Desulfovibrio (134). Glycomacropeptide is a dietary ingredient known to decrease the abundance of Desulfovibrio (135). Therefore, the supplementation of glycomacropeptide can alleviate Desulfovibrio-based pathology expected in UC patients with BEST4+OTOP2+ colonocyte deficiency.
FIGURE 5.
Amino acids and their interactions with gut microbiota in regulation of inflammatory bowel disease. l-glutamine induces TJ protein expression through AMPK activation and inhibits proinflammatory cytokine production. Ingestion of Trp regulates the cytokine balance by increasing Lactobacillus diversity. In addition, Trp metabolites produced in the presence of specific gut bacteria activate PXR in intestinal epithelial cells, thereby reducing barrier permeability. AMPK, AMP-activated protein kinase; BEST4, bestropin 4; IAA, indole acrylic acid; IPA, indole propionic acid; OTOP 2, otopetrin 2; PXR, pregnane X receptor; TJ, tight junction.
Personalized Diet Prescription for IBD
We have discussed the impact of the diet-induced gut microbiota and its regulatory role in IBD. There are also significant variations in the composition of each individual's microbiota among patients with CD and UC (136). Different responses to the same diet, failure to reproduce drug efficacy in clinical practice, and a lack of understanding regarding the detailed mechanisms underlying dietary effects make it difficult to design a diet for IBD. Among many other factors (i.e., an individual's genetic characteristics, life patterns, climate, and life cycle), diet primarily affects an individual's gut microbiota, which can influence the regulation of the gut environment by a diet trial (137). Recent data provide insight into the impact of nutritional status and dietary habits on an individual's gut microbiota. For example, supplementation and/or compensation of specific functional microbiota and/or alteration of the host immune system can improve the postprandial distress syndrome score (138). Indeed, spore-forming probiotics lowered the concentrations of proinflammatory IL-17 and Th-17 cytokines in patients and increased the concentrations of beneficial gut microbes, indicating that appropriate microbiota can be used as therapeutic agents to alleviate functional dyspepsia in adult patients. Likewise, clinical phenotypic variations in IBD patients are highly associated with nutrition because the dietary pattern–induced gut microbiota plays a crucial role in inflammation and immunity in individuals with IBD (139). In this regard, the metabolic functions of microbiota and the characteristics of host physiology must be understood to devise a dietary strategy for IBD regulation (140).
Dietary intake patterns influence the composition of an individual's gut microbiota and its association with the immune status, and gut microbiota can also affect the impact of diet on host gut function (141). For example, consumption of nondigestible carbohydrates can increase SCFA production in the intestines; however, in practice, diet-induced increases in fecal butyrate concentrations vary among individuals according to diet intake (142). These differences might be ascribed to the composition of an individual's gut microbiota. It is impossible to use carbohydrates as substrates in individuals with a low abundance of SCFA-producing strains. Individuals with high-fiber diet habits showed more significant gut microbiota changes upon inulin intake (143). Dietary fat alters the gut microbiota depending on the host's gut microbial diversity (144). These individual responses suggest that information on personal dietary habits and customized recommendations based on the gut microbiota are needed when proposing a diet to improve the intestinal environment. European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines recommend supplementing some CD patients with iron and proteins to manage malnutrition (145). Furthermore, primary nutritional therapy is likely to improve CD phenotypes in children, but is inadequate for UC. In addition, probiotics seem to be helpful for UC, but not CD. Although primary nutritional therapy has not yielded promising results in IBD patients, precision nutrition in IBD should be studied using a well-defined patient cohort, and multifactorial metabolomics data such as the host's genetics, microbiome, metabolome, and nutritional status with dietary behavior should be analyzed according to dietary behavior. Indeed, the administration of a specific diet changes the butyrate concentration, enhancing gut integrity and increasing the concentration of Treg cells (146).
Despite the multifactorial complexity of the etiology and pathogenesis of IBD (147), more effective dietary control of IBD necessitates a better understanding of the functional role of the gut microbiota in the host immune responses in the GI tract and its association with dietary intake. Recent multi- and high-throughput omics analyses such as metagenomics, metatranscriptomics, and metaproteomics have advanced our understanding of the functional alterations accompanied by dysbiosis (53, 148, 149). In terms of host cells, pathogenesis understanding at the single-cell level is developing, which can provide insight into the prediction of individual responses to IBD treatment. The abundant IAFs in the patient's mucosa are expected to resist anti-TNF therapy (36). Accumulation of high-resolution data from the host cells and understanding the host–microbe interaction can improve the prediction of the efficacy of dietary interventions in IBD.
There have been several attempts to determine a disease-relieving diet based on individual characteristics of various metabolic diseases. The development of an algorithm to predict postprandial glycemic response by integrating data obtained from monitoring the personal characteristics of 800 individuals, including their dietary habits, blood parameters, anthropometric measurements, physical activity, and gut microbiome, showed the potential of a personalized nutritional intervention proposal to control postprandial blood glucose concentrations (150). Another study identified biomarkers that can predict individual responses to weight loss interventions through multiomics analysis, which is expected to suggest personalized diets (151). The development of computational science, along with the accumulation of multiomics data regarding the regulation of individual genomes, transcriptomes, and gut metagenomes by dietary interventions, could lead to a successful personalized approach for an IBD intervention diet.
Future Perspectives and Conclusions
Diet is an environmental factor that affects microbial composition and function, the intestinal epithelial barrier, and immune cells. In addition, elements that significantly differ among individuals make it challenging to achieve consistent results. Recent personalized mapping studies of drug metabolism support the notion that the unique gut microbiota affects the efficacies of drugs for disease treatment (152). This implies that an individual's nutrient-induced gut microbiota is a potential biomarker for prognosis and a potential therapeutic target for alleviating disease symptoms.
Research on the bidirectional relation between diet and the gut microbiome has been expanded extensively using both in vivo and in vitro/ex vivo models. Udayasuryan et al. (153) summarized the pros and cons of current model systems available for studying host–microbe interactions (e.g., 2D culture system, 3D organoid, gut-on-chip, and mouse). Although current research models provide a broad understanding of the effects of diet on the composition and activity of gut microbiota, there are several limitations to applying diet as a therapeutic tool with respect to reproducibility and controllability, physiologic relevance, and complexity in vivo. In addition, there is currently no standardized approach, and the research results have been obtained using highly heterogeneous models and systems. For example, a significant portion of the studies about gut microbiota has been conducted in mouse models. Unfortunately, there are physiologic and genetic differences between the GI tracts of mice and humans, and thus caution is required when interpreting findings made in mouse models (154). In addition, studies that independently and directly investigated the interactions between diet and microbiota are lacking.
To realize precision nutrition for personalized IBD treatment, a mimetic device that mimics host–microbe interactions is primarily required. However, such devices do not provide a straightforward substitute to reproduce the intestinal environment while enabling strict control of complex interactions. Most of these systems require specifically designed devices or produce interactions in a specific microenvironment, making it difficult to perform various analyses. Unfortunately, it is technically challenging to culture gut microbes under the oxygenic conditions required for IECs because most anaerobic gut microbes are sensitive to oxygen (155). Thus, many studies investigating the interaction between IECs and gut microbes have not performed a direct co-culture but instead used bacterial culture products (76) or an aerobic culture of facultative anaerobes (156). However, this system has a limitation because host cells interact with the gut microbiota by pathways other than the anaerobic metabolic pathways that operate in the gut. Therefore, it is essential to maintain an aerobic–anaerobic interface mimicking the intestinal epithelium to investigate the interactions between colon epithelial cells and gut microbes (157, 158).
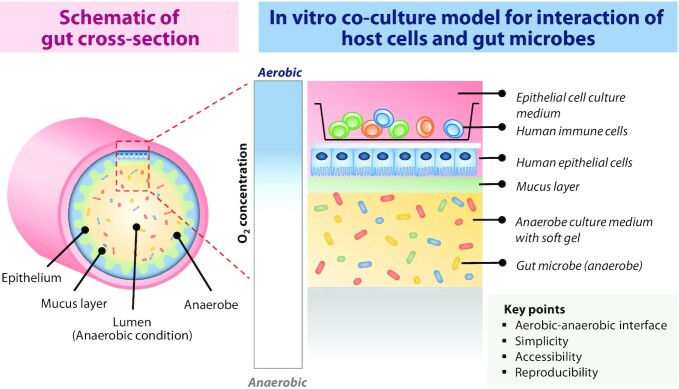
Based on the characteristics of the host–microbe interaction model described here, we propose conditions for the development of models to study these interactions and the effects of diet. The gut is a site that forms the interaction of microbiota with host tissues and the immune system (159). Therefore, the lumen layer inhabited by gut microbes, the lamina propria layer in which immune cells exist, and the intestinal epithelial layer forming a barrier between them should be composed (Figure 6). In addition, the complexity of epithelial composition in the human gut should be reflected, and in particular, the mucus layer produced by goblet cells should be considered (160). The aerobic–anaerobic interface in the human gut should be maintained. Finally, reproducible and controllable models retaining the advantages of 2D models will enable personalized interventions. Development of a co-culture model between the host and microorganisms that can be strictly modulated may help to identify physiologic differences according to the type and severity of IBD, and suggest the optimal intervention according to the particular situation. Furthermore, it will be possible to propose a personalized diet that has been optimized by adjusting the host cell and intestinal environment in the experimental model according to the patient's characteristics.
FIGURE 6.
Schematic diagram of an in vitro model for co-culture of host cells and gut microbes. Co-culture of human epithelial cells and immune cells under aerobic conditions and gut bacteria under anaerobic conditions with the mucin layer as an interface can mimic the physiology of the human gut.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—JEL, DWL, and NJK: conceptualized and designed the review; JEL and KSK: wrote the draft manuscript; HK, DWL, and NJK: revised the first draft; and all authors: read and approved the final manuscript.
Notes
Supported by National Research Foundation of Korea grants 2021R1A2C2007020 (to NJK), 2017M3A9F3043837 (to DWL), 2021M3A9I4021431 (to DWL), and 2021M3A9I4023974 (to NJK), funded by the Ministry of Science and Information & Communications Technology (ICT).
Author disclosures: the authors report no conflicts of interest.
Supplemental Data are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used: ALA, α-linolenic acid; AMPK, AMP-activated protein kinase; CaMKK2, Ca2+/calmodulin-dependent protein kinase 2; CD, Crohn’s disease; CLA, conjugated linoleic acid; COX, cyclooxygenase; DC, dendritic cell; DSS, dextran sulfate sodium; FOS, fructo-oligosaccharide; GC-C, guanylate cyclase; GI, gastrointestinal; GPCR/GPR, G-protein coupled receptor; HB-EGF, heparin-binding epidermal growth factor-like growth factor; IAF, inflammation-associated fibroblast; IBD, inflammatory bowel disease; IEC, intestinal epithelial cell; LA, linoleic acid; MUC2, mucin 2; NOD2, nucleotide-binding oligomerization domain 2; PPAR, peroxisome proliferator–activated receptor; scRNA-Seq, single-cell RNA sequencing; TCA, tricarboxylic acid; TH, helper T cell; TJ, tight junction; TNBS, trinitrobenzene sulfonic acid; Treg, regulatory T cell; UC, ulcerative colitis; WFDC2, whey-acidic-protein four-disulfide core domain protein 2; ZO, zonula occludens; 2'-FL, 2'-fucosyllactose; 5-HT, 5-hydroxytryptamine.
Contributor Information
Jae-Eun Lee, Email: leehicam@yonsei.ac.kr, School of Food Science and Biotechnology, Kyungpook National University, Daegu, South Korea; Department of Biotechnology, Yonsei University, Seoul, South Korea.
Kyoung Su Kim, Department of Biotechnology, Yonsei University, Seoul, South Korea.
Hong Koh, Department of Pediatrics, Yonsei University College of Medicine, Seoul, South Korea.
Dong-Woo Lee, Department of Biotechnology, Yonsei University, Seoul, South Korea.
Nam Joo Kang, Email: njkang@knu.ac.kr, School of Food Science and Biotechnology, Kyungpook National University, Daegu, South Korea.
References
- 1. Saker L, Lee K, Cannito B, Gilmore A, Campbell-Lendrum DH. Globalization and infectious diseases: a review of the linkages. Geneva (Switzerland): World Health Organization; 2004. [Google Scholar]
- 2. Johansson MEV, Sjövall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol. 2013;10(6):352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zeevi D, Korem T, Godneva A, Bar N, Kurilshikov A, Lotan-Pompan Met al. Structural variation in the gut microbiome associates with host health. Nature. 2019;568(7750):43–8. [DOI] [PubMed] [Google Scholar]
- 4. The Integrative HMP (iHMP) Research Network Consortium . The integrative human microbiome project. Nature. 2019;569(7758):641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Vos WM, Tilg H, Van Hul M, Cani PD. Gut microbiome and health: mechanistic insights. Gut. 2022;71(5):1020–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zmora N, Suez J, Elinav E. You are what you eat: diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16(1):35–56. [DOI] [PubMed] [Google Scholar]
- 7. Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14(5):329–42. [DOI] [PubMed] [Google Scholar]
- 8. Litvak Y, Byndloss MX, Baumler AJ. Colonocyte metabolism shapes the gut microbiota. Science. 2018;362(6418):eaat9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Plichta DR, Graham DB, Subramanian S, Xavier RJ. Therapeutic opportunities in inflammatory bowel disease: mechanistic dissection of host-microbiome relationships. Cell. 2019;178(5):1041–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369(9573):1641–57. [DOI] [PubMed] [Google Scholar]
- 11. Baumgart DC, Sandborn WJ. Crohn's disease. Lancet. 2012;380(9853):1590–605. [DOI] [PubMed] [Google Scholar]
- 12. Fiorino G, Cesarini M, Danese S. Biological therapy for ulcerative colitis: what is after anti-TNF. Curr Drug Targets. 2011;12(10):1433–9. [DOI] [PubMed] [Google Scholar]
- 13. Guan Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res. 2019:7247238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EIet al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390(10114):2769–78. [DOI] [PubMed] [Google Scholar]
- 15. Ng SC, Bernstein CN, Vatn MH, Lakatos PL, Loftus EV Jr, Tysk Cet al. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut. 2013;62(4):630–49. [DOI] [PubMed] [Google Scholar]
- 16. Bernstein CN, Shanahan F. Disorders of a modern lifestyle: reconciling the epidemiology of inflammatory bowel diseases. Gut. 2008;57(9):1185–91. [DOI] [PubMed] [Google Scholar]
- 17. Zuo T, Kamm MA, Colombel JF, Ng SC. Urbanization and the gut microbiota in health and inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2018;15(7):440–52. [DOI] [PubMed] [Google Scholar]
- 18. de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13(1):13–27. [DOI] [PubMed] [Google Scholar]
- 19. van der Post S, Jabbar KS, Birchenough G, Arike L, Akhtar N, Sjovall Het al. Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis. Gut. 2019;68(12):2142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roda G, Sartini A, Zambon E, Calafiore A, Marocchi M, Caponi Aet al. Intestinal epithelial cells in inflammatory bowel diseases. World J Gastroenterol. 2010;16(34):4264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee J-Y, Cevallos SA, Byndloss MX, Tiffany CR, Olsan EE, Butler BPet al. High-fat diet and antibiotics cooperatively impair mitochondrial bioenergetics to trigger dysbiosis that exacerbates pre-inflammatory bowel disease. Cell Host Microbe. 2020;28(2):273–84..e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Franzosa EA, Sirota-Madi A, Avila-Pacheco J, Fornelos N, Haiser HJ, Reinker Set al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat Microbiol. 2019;4(2):293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lees CW, Barrett JC, Parkes M, Satsangi J. New IBD genetics: common pathways with other diseases. Gut. 2011;60(12):1739–53. [DOI] [PubMed] [Google Scholar]
- 24. Inoue N, Tamura K, Kinouchi Y, Fukuda Y, Takahashi S, Ogura Yet al. Lack of common NOD2 variants in Japanese patients with Crohn's disease. Gastroenterology. 2002;123(1):86–91. [DOI] [PubMed] [Google Scholar]
- 25. Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJet al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314(5804):1461–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KYet al. Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Noguchi E, Homma Y, Kang X, Netea MG, Ma X. A Crohn's disease–associated NOD2 mutation suppresses transcription of human IL10 by inhibiting activity of the nuclear ribonucleoprotein hnRNP-A1. Nat Immunol. 2009;10(5):471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369(9573):1627–40. [DOI] [PubMed] [Google Scholar]
- 29. Schirmer M, Denson L, Vlamakis H, Franzosa EA, Thomas S, Gotman NMet al. Compositional and temporal changes in the gut microbiome of pediatric ulcerative colitis patients are linked to disease course. Cell Host Microbe. 2018;24(4):600–10..e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Feagan BG, Sandborn WJ, Gasink C, Jacobstein D, Lang Y, Friedman JRet al. Ustekinumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2016;375(20):1946–60. [DOI] [PubMed] [Google Scholar]
- 31. Hazel K, O'Connor A. Emerging treatments for inflammatory bowel disease. Ther Adv Chronic Dis. 2020;11:2040622319899297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sedano R, Almradi A, Ma C, Jairath V, Feagan BG. Novel therapeutics for the treatment of IBD: current status and future directions. Curr Treat Options Gastroenterol. 2020;18(3):442–61. [Google Scholar]
- 33. Tang X, Huang Y, Lei J, Luo H, Zhu X. The single-cell sequencing: new developments and medical applications. Cell Biosci. 2019;9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tang F, Barbacioru C, Wang Y, Nordman E, Lee C, Xu Net al. mRNA-Seq whole-transcriptome analysis of a single cell. Nat Methods. 2009;6(5):377–82. [DOI] [PubMed] [Google Scholar]
- 35. Parikh K, Antanaviciute A, Fawkner-Corbett D, Jagielowicz M, Aulicino A, Lagerholm Cet al. Colonic epithelial cell diversity in health and inflammatory bowel disease. Nature. 2019;567(7746):49–55. [DOI] [PubMed] [Google Scholar]
- 36. Smillie CS, Biton M, Ordovas-Montanes J, Sullivan KM, Burgin G, Graham DBet al. Intra- and inter-cellular rewiring of the human colon during ulcerative colitis. Cell. 2019;178(3):714–30..e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mitsialis V, Wall S, Liu P, Ordovas-Montanes J, Parmet T, Vukovic Met al. Single-cell analyses of colon and blood reveal distinct immune cell signatures of ulcerative colitis and Crohn's disease. Gastroenterology. 2020;159(2):591–608..e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ikpa PT, Sleddens HF, Steinbrecher KA, Peppelenbosch MP, de Jonge HR, Smits Ret al. Guanylin and uroguanylin are produced by mouse intestinal epithelial cells of columnar and secretory lineage. Histochem Cell Biol. 2016;146(4):445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tu Y-H, Cooper AJ, Teng B, Chang RB, Artiga DJ, Turner HNet al. An evolutionarily conserved gene family encodes proton-selective ion channels. Science. 2018;359(6379):1047–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kanke M, Kennedy Ng MM, Connelly S, Singh M, Schaner M, Shanahan MTet al. Single-cell analysis reveals unexpected cellular changes and transposon expression signatures in the colonic epithelium of treatment-naïve adult Crohn's disease patients. Cell Mol Gastroenterol Hepatol. 2022;13(6):1717–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jaeger N, Gamini R, Cella M, Schettini JL, Bugatti M, Zhao Set al. Single-cell analyses of Crohn's disease tissues reveal intestinal intraepithelial T cells heterogeneity and altered subset distributions. Nat Commun. 2021;12(1):1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen C-L, Yang J, James IOA, Zhang H-y, Besner GE. Heparin-binding epidermal growth factor-like growth factor restores Wnt/β-catenin signaling in intestinal stem cells exposed to ischemia/reperfusion injury. Surgery. 2014;155(6):1069–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tamburini S, Shen N, Wu HC, Clemente JC. The microbiome in early life: implications for health outcomes. Nat Med. 2016;22(7):713–22. [DOI] [PubMed] [Google Scholar]
- 44. Dominguez-Bello MG, Blaser MJ, Ley RE, Knight R. Development of the human gastrointestinal microbiota and insights from high-throughput sequencing. Gastroenterology. 2011;140(6):1713–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio C-W, Santacruz Net al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478(7368):250–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gury-BenAri M, Thaiss CA, Serafini N, Winter DR, Giladi A, Lara-Astiaso Det al. The spectrum and regulatory landscape of intestinal innate lymphoid cells are shaped by the microbiome. Cell. 2016;166(5):1231–46..e13. [DOI] [PubMed] [Google Scholar]
- 47. Kang B, Alvarado LJ, Kim T, Lehmann ML, Cho H, He Jet al. Commensal microbiota drive the functional diversification of colon macrophages. Mucosal Immunol. 2020;13(2):216–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huang H, Fang M, Jostins L, Umicevic Mirkov M, Boucher G, Anderson CAet al. Fine-mapping inflammatory bowel disease loci to single-variant resolution. Nature. 2017;547(7662):173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Torres J, Hu J, Seki A, Eisele C, Nair N, Huang Ret al. Infants born to mothers with IBD present with altered gut microbiome that transfers abnormalities of the adaptive immune system to germ-free mice. Gut. 2020;69(1):42–51. [DOI] [PubMed] [Google Scholar]
- 50. Dai ZF, Ma XY, Yang RL, Wang HC, Xu DD, Yang JNet al. Intestinal flora alterations in patients with ulcerative colitis and their association with inflammation. Exp Ther Med. 2021;22(5):1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Waldschmitt N, Metwaly A, Fischer S, Haller D. Microbial signatures as a predictive tool in IBD—pearls and pitfalls. Inflamm Bowel Dis. 2018;24(6):1123–32. [DOI] [PubMed] [Google Scholar]
- 52. Amos GCA, Sergaki C, Logan A, Iriarte R, Bannaga A, Chandrapalan Set al. Exploring how microbiome signatures change across inflammatory bowel disease conditions and disease locations. Sci Rep. 2021;11(1):18699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lloyd-Price J, Arze C, Ananthakrishnan AN, Schirmer M, Avila-Pacheco J, Poon TWet al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569(7758):655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stojanov S, Berlec A, Strukelj B. The influence of probiotics on the Firmicutes/Bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. 2020;8(11):1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul Let al. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut. 2006;55(2):205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Walker AW, Sanderson JD, Churcher C, Parkes GC, Hudspith BN, Rayment Net al. High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol. 2011;11(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol. 2017;19(1):29–41. [DOI] [PubMed] [Google Scholar]
- 58. Pomare EW, Branch WJ, Cummings JH. Carbohydrate fermentation in the human colon and its relation to acetate concentrations in venous blood. J Clin Invest. 1985;75(5):1448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra Get al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhao H, Xu H, Chen S, He J, Zhou Y, Nie Y. Systematic review and meta-analysis of the role of Faecalibacteriumprausnitzii alteration in inflammatory bowel disease. J Gastroenterol Hepatol. 2021;36(2):320–8. [DOI] [PubMed] [Google Scholar]
- 61. Zhang M, Zhou L, Wang Y, Dorfman RG, Tang D, Xu Let al. Faecalibacterium prausnitzii produces butyrate to decrease c-Myc-related metabolism and Th17 differentiation by inhibiting histone deacetylase 3. Int Immunol. 2019;31(8):499–514. [DOI] [PubMed] [Google Scholar]
- 62. Zhou L, Zhang M, Wang Y, Dorfman RG, Liu H, Yu Tet al. Faecalibacterium prausnitzii produces butyrate to maintain Th17/Treg balance and to ameliorate colorectal colitis by inhibiting histone deacetylase 1. Inflamm Bowel Dis. 2018;24(9):1926–40. [DOI] [PubMed] [Google Scholar]
- 63. Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut Vet al. A decrease of the butyrate-producing species Roseburiahominis and Faecalibacteriumprausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63(8):1275–83. [DOI] [PubMed] [Google Scholar]
- 64. Vich Vila A, Imhann F, Collij V, Jankipersadsing SA, Gurry T, Mujagic Zet al. Gut microbiota composition and functional changes in inflammatory bowel disease and irritable bowel syndrome. Sci Transl Med. 2018;10(472):eaap8914. [DOI] [PubMed] [Google Scholar]
- 65. Zhu C, Song K, Shen Z, Quan Y, Tan B, Luo Wet al. Roseburia intestinalis inhibits interleukin-17 excretion and promotes regulatory T cells differentiation in colitis. Mol Med Rep. 2018;17(6):7567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Barberio B, Facchin S, Patuzzi I, Ford AC, Massimi D, Valle Get al. A specific microbiota signature is associated to various degrees of ulcerative colitis as assessed by a machine learning approach. Gut Microbes. 2022;14(1):2028366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang T, Li P, Wu X, Lu G, Marcella C, Ji Xet al. Alterations of Akkermansiamuciniphila in the inflammatory bowel disease patients with washed microbiota transplantation. Appl Microbiol Biotechnol. 2020;104(23):10203–15. [DOI] [PubMed] [Google Scholar]
- 68. Lee J-Y, Jin H-S, Kim KS, Baek J-H, Kim B-S, Lee D-W. Nutrient-specific proteomic analysis of the mucin degrading bacterium Akkermansiamuciniphila. Proteomics. 2022;22(3):e2100125. [DOI] [PubMed] [Google Scholar]
- 69. Li J, Lin S, Vanhoutte PM, Woo CW, Xu A. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe−/− mice. Circulation. 2016;133(24):2434–46. [DOI] [PubMed] [Google Scholar]
- 70. Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts Let al. A purified membrane protein from Akkermansiamuciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23(1):107–13. [DOI] [PubMed] [Google Scholar]
- 71. Wang L, Tang L, Feng Y, Zhao S, Han M, Zhang Cet al. A purified membrane protein from Akkermansiamuciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8+ T cells in mice. Gut. 2020;69(11):1988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Belzer C, Chia LW, Aalvink S, Chamlagain B, Piironen V, Knol Jet al. Microbial metabolic networks at the mucus layer lead to diet-independent butyrate and vitamin B12 production by intestinal symbionts. Mbio. 2017;8(5):e00770–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Clooney AG, Eckenberger J, Laserna-Mendieta E, Sexton KA, Bernstein MT, Vagianos Ket al. Ranking microbiome variance in inflammatory bowel disease: a large longitudinal intercontinental study. Gut. 2021;70(3):499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hall AB, Yassour M, Sauk J, Garner A, Jiang X, Arthur Tet al. A novel Ruminococcusgnavus clade enriched in inflammatory bowel disease patients. Genome Med. 2017;9(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Johansson ME, Gustafsson JK, Holmen-Larsson J, Jabbar KS, Xia L, Xu Het al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2014;63(2):281–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Henke MT, Kenny DJ, Cassilly CD, Vlamakis H, Xavier RJ, Clardy J. Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn's disease, produces an inflammatory polysaccharide. Proc Natl Acad Sci U S A. 2019;116(26):12672–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter Met al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167(5):1339–53..e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mottawea W, Chiang CK, Muhlbauer M, Starr AE, Butcher J, Abujamel Tet al. Altered intestinal microbiota–host mitochondria crosstalk in new onset Crohn's disease. Nat Commun. 2016;7(1):13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Leschelle X, Goubern M, Andriamihaja M, Blottière HM, Couplan E, Gonzalez-Barroso M-D-Met al. Adaptative metabolic response of human colonic epithelial cells to the adverse effects of the luminal compound sulfide. Biochim Biophys Acta. 2005;1725(2):201–12. [DOI] [PubMed] [Google Scholar]
- 80. Donohoe DR, Garge N, Zhang X, Sun W, O'Connell TM, Bunger MKet al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13(5):517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rowan F, Docherty NG, Murphy M, Murphy B, Calvin Coffey J, O'Connell PR. Desulfovibrio bacterial species are increased in ulcerative colitis. Dis Colon Rectum. 2010;53(11):1530–6. [DOI] [PubMed] [Google Scholar]
- 82. Coutinho C, Coutinho-Silva R, Zinkevich V, Pearce CB, Ojcius DM, Beech I. Sulphate-reducing bacteria from ulcerative colitis patients induce apoptosis of gastrointestinal epithelial cells. Microb Pathog. 2017;112:126–34. [DOI] [PubMed] [Google Scholar]
- 83. Håkansson Å, Tormo-Badia N, Baridi A, Xu J, Molin G, Hagslätt M-Let al. Immunological alteration and changes of gut microbiota after dextran sulfate sodium (DSS) administration in mice. Clin Exp Med. 2015;15(1):107–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mills RH, Dulai PS, Vazquez-Baeza Y, Sauceda C, Daniel N, Gerner RRet al. Multi-omics analyses of the ulcerative colitis gut microbiome link Bacteroidesvulgatus proteases with disease severity. Nat Microbiol. 2022;7(2):262–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Britton GJ, Contijoch EJ, Mogno I, Vennaro OH, Llewellyn SR, Ng Ret al. Microbiotas from humans with inflammatory bowel disease alter the balance of gut Th17 and RORγt+ regulatory T cells and exacerbate colitis in mice. Immunity. 2019;50(1):212–24..e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Paramsothy S, Paramsothy R, Rubin DT, Kamm MA, Kaakoush NO, Mitchell HMet al. Faecal microbiota transplantation for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2017;11(10):1180–99. [DOI] [PubMed] [Google Scholar]
- 87. Sonnenburg ED, Sonnenburg JL. The ancestral and industrialized gut microbiota and implications for human health. Nat Rev Microbiol. 2019;17(6):383–90. [DOI] [PubMed] [Google Scholar]
- 88. The Human Microbiome Project Consortium . Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Koropatkin NM, Cameron EA, Martens EC. How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol. 2012;10(5):323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Makki K, Deehan EC, Walter J, Bäckhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23(6):705–15. [DOI] [PubMed] [Google Scholar]
- 91. Campbell C, McKenney PT, Konstantinovsky D, Isaeva OI, Schizas M, Verter Jet al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature. 2020;581(7809):475–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Koppel N, Maini Rekdal V, Balskus EP. Chemical transformation of xenobiotics by the human gut microbiota. Science. 2017;356(6344):eaag2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Reichardt N, Vollmer M, Holtrop G, Farquharson FM, Wefers D, Bunzel Met al. Specific substrate-driven changes in human faecal microbiota composition contrast with functional redundancy in short-chain fatty acid production. ISME J. 2018;12(2):610–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Li T, Qiu Y, Yang HS, Li MY, Zhuang XJ, Zhang SHet al. Systematic review and meta-analysis: association of a pre-illness Western dietary pattern with the risk of developing inflammatory bowel disease. J Digest Dis. 2020;21(7):362–71. [DOI] [PubMed] [Google Scholar]
- 95. Agus A, Denizot J, Thévenot J, Martinez-Medina M, Massier S, Sauvanet Pet al. Western diet induces a shift in microbiota composition enhancing susceptibility to adherent-invasive E. coli infection and intestinal inflammation. Sci Rep. 2016;6(1):19032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Papada E, Amerikanou C, Forbes A, Kaliora AC. Adherence to Mediterranean diet in Crohn's disease. Eur J Nutr. 2020;59(3):1115–21. [DOI] [PubMed] [Google Scholar]
- 97. Godny L, Reshef L, Pfeffer-Gik T, Goren I, Yanai H, Tulchinsky Het al. Adherence to the Mediterranean diet is associated with decreased fecal calprotectin in patients with ulcerative colitis after pouch surgery. Eur J Nutr. 2020;59(7):3183–90. [DOI] [PubMed] [Google Scholar]
- 98. Illescas O, Rodriguez-Sosa M, Gariboldi M. Mediterranean diet to prevent the development of colon diseases: a meta-analysis of gut microbiota studies. Nutrients. 2021;13(7):2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sakamoto N, Kono S, Wakai K, Fukuda Y, Satomi M, Shimoyama Tet al. Dietary risk factors for inflammatory bowel disease: a multicenter case-control study in Japan. Inflamm Bowel Dis. 2005;11(2):154–63. [DOI] [PubMed] [Google Scholar]
- 100. Laffin M, Fedorak R, Zalasky A, Park H, Gill A, Agrawal Aet al. A high-sugar diet rapidly enhances susceptibility to colitis via depletion of luminal short-chain fatty acids in mice. Sci Rep. 2019;9(1):12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hansen TS, Jess T, Vind I, Elkjaer M, Nielsen MF, Gamborg Met al. Environmental factors in inflammatory bowel disease: a case-control study based on a Danish inception cohort. J Crohns Colitis. 2011;5(6):577–84. [DOI] [PubMed] [Google Scholar]
- 102. Kuo S-M. Does modification of the large intestinal microbiome contribute to the anti-inflammatory activity of fermentable fiber?. Curr Dev Nutr. 2018;2(2):nzx004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yang G, Chen S, Deng B, Tan C, Deng J, Zhu Get al. Implication of G protein-coupled receptor 43 in intestinal inflammation: a mini-review. Front Immunol. 2018;9:1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Di Yet al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461(7268):1282–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJet al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17(5):662–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hansen LBS, Roager HM, Søndertoft NB, Gøbel RJ, Kristensen M, Vallès-Colomer Met al. A low-gluten diet induces changes in the intestinal microbiome of healthy Danish adults. Nat Commun. 2018;9(1):4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ishisono K, Mano T, Yabe T, Kitaguchi K. Dietary fiber pectin ameliorates experimental colitis in a neutral sugar side chain-dependent manner. Front Immunol. 2019;10:2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lara-Villoslada F, de Haro O, Camuesco D, Comalada M, Velasco J, Zarzuelo Aet al. Short-chain fructooligosaccharides, in spite of being fermented in the upper part of the large intestine, have anti-inflammatory activity in the TNBS model of colitis. Eur J Nutr. 2006;45(7):418–25. [DOI] [PubMed] [Google Scholar]
- 109. Lindsay JO, Whelan K, Stagg AJ, Gobin P, Al-Hassi HO, Rayment Net al. Clinical, microbiological, and immunological effects of fructo-oligosaccharide in patients with Crohn's disease. Gut. 2006;55(3):348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zhou K. Strategies to promote abundance of Akkermansiamuciniphila, an emerging probiotics in the gut, evidence from dietary intervention studies. J Funct Foods. 2017;33:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Yao Q, Fan L, Zheng N, Blecker C, Delcenserie V, Li Het al. 2'-Fucosyllactose ameliorates inflammatory bowel disease by modulating gut microbiota and promoting MUC2 expression. Front Nutr. 2022;9:822020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Li Z, Li Z, Zhu L, Dai N, Sun G, Peng Let al. Effects of xylo-oligosaccharide on the gut microbiota of patients with ulcerative colitis in clinical remission. Front Nutr. 2021;8:778542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Shen Z, Zhu C, Quan Y, Yang J, Yuan W, Yang Zet al. Insights into Roseburiaintestinalis which alleviates experimental colitis pathology by inducing anti-inflammatory responses. J Gastroenterol Hepatol. 2018;33(10):1751–60. [DOI] [PubMed] [Google Scholar]
- 114. Reif S, Klein I, Lubin F, Farbstein M, Hallak A, Gilat T. Pre-illness dietary factors in inflammatory bowel disease. Gut. 1997;40(6):754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wan Y, Wang F, Yuan J, Li J, Jiang D, Zhang Jet al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: a 6-month randomised controlled-feeding trial. Gut. 2019;68(8):1417–29. [DOI] [PubMed] [Google Scholar]
- 116. Ma X, Torbenson M, Hamad AR, Soloski MJ, Li Z. High-fat diet modulates non-CD1d-restricted natural killer T cells and regulatory T cells in mouse colon and exacerbates experimental colitis. Clin Exp Immunol. 2007;151(1):130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gruber L, Kisling S, Lichti P, Martin F-P, May S, Klingenspor Met al. High fat diet accelerates pathogenesis of murine Crohn's disease-like ileitis independently of obesity. PLoS One. 2013;8(8):e71661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sharma M, Kaur R, Kaushik K, Kaushal N. Redox modulatory protective effects of ω-3 fatty acids rich fish oil against experimental colitis. Toxicol Mech Methods. 2019;29(4):244–54. [DOI] [PubMed] [Google Scholar]
- 119. Inui K, Fukuta Y, Ikeda A, Kameda H, Kokuba Y, Sato M. The effect of α-linolenic acid-rich emulsion on fatty acid metabolism and leukotriene generation of the colon in a rat model with inflammatory bowel disease. Ann Nutr Metab. 1996;40(3):175–82. [DOI] [PubMed] [Google Scholar]
- 120. Hassan A, Ibrahim A, Mbodji K, Coëffier M, Ziegler F, Bounoure Fet al. An α-linolenic acid-rich formula reduces oxidative stress and inflammation by regulating NF-κB in rats with TNBS-induced colitis. J Nutr. 2010;140(10):1714–21. [DOI] [PubMed] [Google Scholar]
- 121. IBD in EPIC Study Investigators, Tjonneland A, Overvad K, Bergmann MM, Nagel G, Linseisen JIBD in EPIC Study Investigators et al. Linoleic acid, a dietary n-6 polyunsaturated fatty acid, and the aetiology of ulcerative colitis: a nested case–control study within a European prospective cohort study. Gut. 2009;58(12):1606–11. [DOI] [PubMed] [Google Scholar]
- 122. Ewaschuk JB, Walker JW, Diaz H, Madsen KL. Bioproduction of conjugated linoleic acid by probiotic bacteria occurs in vitro and in vivo in mice. J Nutr. 2006;136(6):1483–7. [DOI] [PubMed] [Google Scholar]
- 123. Whigham LD, Cook EB, Stahl JL, Saban R, Bjorling DE, Pariza MWet al. CLA reduces antigen-induced histamine and PGE2 release from sensitized guinea pig tracheae. Am J Physiol Regul Integr Comp Physiol. 2001;280(3):R908–12. [DOI] [PubMed] [Google Scholar]
- 124. Bassaganya-Riera J, Hontecillas R, Horne WT, Sandridge M, Herfarth HH, Bloomfeld Ret al. Conjugated linoleic acid modulates immune responses in patients with mild to moderately active Crohn's disease. Clin Nutr. 2012;31(5):721–7. [DOI] [PubMed] [Google Scholar]
- 125. Borniquel S, Jädert C, Lundberg JO. Dietary conjugated linoleic acid activates PPARγ and the intestinal trefoil factor in SW480 cells and mice with dextran sulfate sodium-induced colitis. J Nutr. 2012;142(12):2135–40. [DOI] [PubMed] [Google Scholar]
- 126. Wang B, Wu Z, Ji Y, Sun K, Dai Z, Wu G. L-Glutamine enhances tight junction integrity by activating CaMK kinase 2–AMP-activated protein kinase signaling in intestinal porcine epithelial cells. J Nutr. 2016;146(3):501–8. [DOI] [PubMed] [Google Scholar]
- 127. Hubert-Buron A, Leblond J, Jacquot A, Ducrotté P, Déchelotte P, Coëffier M. Glutamine pretreatment reduces IL-8 production in human intestinal epithelial cells by limiting IκBα ubiquitination. J Nutr. 2006;136(6):1461–5. [DOI] [PubMed] [Google Scholar]
- 128. Li M, Oshima T, Ito C, Yamada M, Tomita T, Fukui Het al. Glutamine blocks interleukin-13-induced intestinal epithelial barrier dysfunction. Digestion. 2021;102(2):170–9. [DOI] [PubMed] [Google Scholar]
- 129. Nikolaus S, Schulte B, Al-Massad N, Thieme F, Schulte DM, Bethge Jet al. Increased tryptophan metabolism is associated with activity of inflammatory bowel diseases. Gastroenterology. 2017;153(6):1504–16..e2. [DOI] [PubMed] [Google Scholar]
- 130. Kim CJ, Kovacs-Nolan JA, Yang C, Archbold T, Fan MZ, Mine Y. l-Tryptophan exhibits therapeutic function in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J Nutr Biochem. 2010;21(6):468–75. [DOI] [PubMed] [Google Scholar]
- 131. Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23(6):716–24. [DOI] [PubMed] [Google Scholar]
- 132. Roager HM, Licht TR. Microbial tryptophan catabolites in health and disease. Nat Commun. 2018;9(1):3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini Get al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39(2):372–85. [DOI] [PubMed] [Google Scholar]
- 134. Mishra V, Bose A, Kiran S, Banerjee S, Shah IA, Chaukimath Pet al. Gut-associated cGMP mediates colitis and dysbiosis in a mouse model of an activating mutation in GUCY2C. J Exp Med. 2021;218(11):e20210479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Sawin EA, De Wolfe TJ, Aktas B, Stroup BM, Murali SG, Steele JLet al. Glycomacropeptide is a prebiotic that reduces Desulfovibrio bacteria, increases cecal short-chain fatty acids, and is anti-inflammatory in mice. Am J Physiol Gastrointest Liver Physiol. 2015;309(7):G590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Halfvarson J, Brislawn CJ, Lamendella R, Vázquez-Baeza Y, Walters WA, Bramer LMet al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat Microbiol. 2017;2(5):17004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi Det al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555(7695):210–5. [DOI] [PubMed] [Google Scholar]
- 138. Wauters L, Slaets H, De Paepe K, Ceulemans M, Wetzels S, Geboers Ket al. Efficacy and safety of spore-forming probiotics in the treatment of functional dyspepsia: a pilot randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol Hepatol. 2021;6(10):784–92. [DOI] [PubMed] [Google Scholar]
- 139. Sasson AN, Ingram RJM, Zhang Z, Taylor LM, Ananthakrishnan AN, Kaplan GGet al. The role of precision nutrition in the modulation of microbial composition and function in people with inflammatory bowel disease. Lancet Gastroenterol Hepatol. 2021;6(9):754–69. [DOI] [PubMed] [Google Scholar]
- 140. Yilmaz B, Juillerat P, Øyås O, Ramon C, Bravo FD, Franc Yet al. Microbial network disturbances in relapsing refractory Crohn's disease. Nat Med. 2019;25(2):323–36. [DOI] [PubMed] [Google Scholar]
- 141. Wastyk HC, Fragiadakis GK, Perelman D, Dahan D, Merrill BD, Yu FBet al. Gut-microbiota-targeted diets modulate human immune status. Cell. 2021;184(16):4137–53..e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. McOrist AL, Miller RB, Bird AR, Keogh JB, Noakes M, Topping DLet al. Fecal butyrate levels vary widely among individuals but are usually increased by a diet high in resistant starch. J Nutr. 2011;141(5):883–9. [DOI] [PubMed] [Google Scholar]
- 143. Healey G, Murphy R, Butts C, Brough L, Whelan K, Coad J. Habitual dietary fibre intake influences gut microbiota response to an inulin-type fructan prebiotic: a randomised, double-blind, placebo-controlled, cross-over, human intervention study. Br J Nutr. 2018;119(2):176–89. [DOI] [PubMed] [Google Scholar]
- 144. Lang JM, Pan C, Cantor RM, Tang WHW, Garcia-Garcia JC, Kurtz Iet al. Impact of individual traits, saturated fat, and protein source on the gut microbiome. Mbio. 2018;9(6):01604–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Forbes A, Escher J, Hebuterne X, Klek S, Krznaric Z, Schneider Set al. ESPEN guideline: clinical nutrition in inflammatory bowel disease. Clin Nutr. 2017;36(2):321–47. [DOI] [PubMed] [Google Scholar]
- 146. Marino E, Richards JL, McLeod KH, Stanley D, Yap YA, Knight Jet al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat Immunol. 2017;18(5):552–62. [DOI] [PubMed] [Google Scholar]
- 147. Bischoff SC, Escher J, Hebuterne X, Klek S, Krznaric Z, Schneider Set al. ESPEN practical guideline: clinical nutrition in inflammatory bowel disease. Clin Nutr. 2020;39(3):632–53. [DOI] [PubMed] [Google Scholar]
- 148. Schirmer M, Franzosa EA, Lloyd-Price J, McIver LJ, Schwager R, Poon TWet al. Dynamics of metatranscription in the inflammatory bowel disease gut microbiome. Nat Microbiol. 2018;3(3):337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Segal JP, Mullish BH, Quraishi MN, Acharjee A, Williams HRT, Iqbal Tet al. The application of omics techniques to understand the role of the gut microbiota in inflammatory bowel disease. Therap Adv Gastroenterol. 2019;12:1756284818822250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger Aet al. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163(5):1079–94. [DOI] [PubMed] [Google Scholar]
- 151. Dao MC, Sokolovska N, Brazeilles R, Affeldt S, Pelloux V, Prifti Eet al. A data integration multi-omics approach to study calorie restriction-induced changes in insulin sensitivity. Front Physiol. 2018;9:1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Javdan B, Lopez JG, Chankhamjon P, Lee Y-CJ, Hull R, Wu Qet al. Personalized mapping of drug metabolism by the human gut microbiome. Cell. 2020;181(7):1661–79..e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Udayasuryan B, Nguyen TTD, Slade DJ, Verbridge SS. Harnessing tissue engineering tools to interrogate host-microbiota crosstalk in cancer. Iscience. 2020;23(12):101878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Hugenholtz F, de Vos WM. Mouse models for human intestinal microbiota research: a critical evaluation. Cell Mol Life Sci. 2018;75(1):149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Browne HP, Forster SC, Anonye BO, Kumar N, Neville BA, Stares MDet al. Culturing of ‘unculturable’ human microbiota reveals novel taxa and extensive sporulation. Nature. 2016;533(7604):543–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Pajarillo EAB, Kim SH, Valeriano VD, Lee JY, Kang D-K. Proteomic view of the crosstalk between Lactobacillusmucosae and intestinal epithelial cells in co-culture revealed by Q exactive-based quantitative proteomics. Front Microbiol. 2017;8:2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Albenberg L, Esipova TV, Judge CP, Bittinger K, Chen J, Laughlin Aet al. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology. 2014;147(5):1055–63..e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Espey MG. Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota. Free Radic Biol Med. 2013;55:130–40. [DOI] [PubMed] [Google Scholar]
- 159. Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Schroeder BO. Fight them or feed them: how the intestinal mucus layer manages the gut microbiota. Gastroenterol Rep (Oxf). 2019;7(1):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.