Abstract
Pirfenidone (PFN) is an anti-fibrotic drug with significant anti-inflammatory property used for treatment of fibrotic conditions such as idiopathic pulmonary fibrosis (IPF). In the coronavirus disease 2019 (Covid-19) era, severe acute respiratory syndrome 2 (SARS-CoV-2) could initially lead to acute lung injury (ALI) and in severe cases may cause acute respiratory distress syndrome (ARDS) which is usually resolved with normal lung function. However, some cases of ALI and ARDS are progressed to the more severe critical stage of pulmonary fibrosis commonly named post-Covid-19 pulmonary fibrosis which needs an urgent address and proper management. Therefore, the objective of the present study was to highlight the potential role of PFN in the management of post-Covid-19 pulmonary fibrosis. The precise mechanism of post-Covid-19 pulmonary fibrosis is related to the activation of transforming growth factor beta (TGF-β1), which activates the release of extracellular proteins, fibroblast proliferation, fibroblast migration and myofibroblast conversion. PFN inhibits accumulation and recruitment of inflammatory cells, fibroblast proliferation, deposition of extracellular matrix in response to TGFβ1 and other pro-inflammatory cytokines. In addition, PFN suppresses furin (TGFβ1 convertase activator) a protein effector involved in the entry of SARS-CoV-2 and activation of TGFβ1, and thus PFN reduces the pathogenesis of SARS-CoV-2. Besides, PFN modulates signaling pathways such as Wingless/Int (Wnt/β-catenin), Yes-Associated Protein (YAP)/Transcription Co-Activator PDZ Binding Motif (TAZ) and Hippo Signaling Pathways that are involved in the pathogenesis of post-Covid-19 pulmonary fibrosis. In conclusion, the anti-inflammatory and anti-fibrotic properties of PFN may attenuate post-Covid-19 pulmonary fibrosis.
Keywords: Pirfenidone, Pulmonary Fibrosis, Covid-19, Anti-Inflammatory, Anti-Fibrotic
Introduction
Pirfenidone (PFN) is an anti-fibrotic drug with significant anti-inflammatory property used for the treatment of fibrotic conditions such as idiopathic pulmonary fibrosis (IPF) (Taniguchi et al. 2010). PFN acts through inhibition the production of fibroblast, suppression release of transforming growth factor beta (TGF-β1), and inhibition release and deposition of collagen (Carter 2011). Besides, PFN reduces the synthesis of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) (Takeda et al. 2014). It was first used in Japan in 2008 after a successful clinical trial for IPF. PFN in 2014 was approved for clinical uses in the USA (Maher et al. 2020). Anti-fibrotic drugs are commonly indicated in the management of IPF, liver fibrosis, and as an adjuvant with chemotherapy against lung cancer (Ley et al. 2017).
In the coronavirus disease 2019 (Covid-19) era, severe acute respiratory syndrome 2 (SARS-CoV-2) could initially lead to acute lung injury (ALI) and in severe cases may cause acute respiratory distress syndrome (ARDS) that are usually resolved with normal lung function (Al-Kuraishy et al. 2021a; Mostafa-Hedeab et al. 2022). However, some cases of ALI and ARDS are progressed to the more severe critical stage of pulmonary fibrosis commonly named post-Covid-19 pulmonary fibrosis which needs an urgent address and proper management. Therefore, the objective of the present study was to review the potential role of PFN in the management of post-Covid-19 pulmonary fibrosis (Ali and Ghonimy 2021).
Covid-19 and risk of pulmonary fibrosis
By the end of April 2022, over one billion subjects had been established infected all over the world with more than six million deaths. Most of the Covid-19 patients presented with mild respiratory symptoms in near 85% to ARDS in about 5% (Al-Kuraishy et al. 2021b). Furthermost Covid-19 patients are cured without lung injury, though numbers of affected patients still have residual serious sequelae (Lugnier et al. 2021). The crucial risk factors for Covid-19 severity are old age, male sex, and comorbidities such as hypertension and diabetes mellitus that might augment the risk of post-Covid-19 squeals (Al-Kuraishy et al. 2021c). These squeals ranged from mild such as fatigue or severe such as pulmonary fibrosis, which requires prolonged oxygen supplementation or lung transplantation (Mahmud et al. 2021). Therefore, long-term follow-up of patients with severe Covid-19 is compulsory to find early and late complications in post-Covid-19 recovery.
It has been reported that severe SARS-CoV-2 infection is linked with the development of significant pulmonary fibrotic changes (Schwensen et al. 2020). However, these fibrotic changes are uncommon with most respiratory viral infections and have never been reported following the HINI influenza epidemic (Bridevaux et al. 2014). Into the bargain, pulmonary fibrotic disorders have been reported in 8% of SARS patients and 20% in patients with H7N9 influenza (Udwadia et al. 2021b). Previously, Ajlan et al (2014) reported that Middle East Respiratory Syndrome coronavirus (MERS-CoV) is associated with the progression of interstitial pulmonary fibrosis. Likewise, pulmonary fibrosis may develop in numbers of patients who recovered from MERS-CoV pneumonia mainly in old age patients' undergone admission to the intensive care unit (ICU) with high lactate dehydrogenase (LDH) levels (Das et al. 2017). Both SARS-CoV and MERS-CoV are genetically similar to that of SARS-CoV-2 and can cause pulmonary disorders similar to that of SARS-CoV-2 (Al-Kuraishy et al. 2020a). SARS-CoV lung radiological manifestations are observed following 2 weeks of infection and may persist for 4 weeks. Though, 15 years of follow-up of 71 patients who recovered from SARS-CoV infection, about 4.6% develop bilateral pulmonary interstitial abnormality (Zhang et al. 2020a, b).
In a pre-Covid-19 era, approximately 30% of patients that developed ARDS regardless of etiology showed pulmonary fibrosis and residual lung abnormality (Osterholzer et al. 2012). Taken together, the older age group appears to be predominant among hospitalized Covid-19 patients who develop ARDS due to underlying autoimmune inflammatory disorders that eventually predispose them to the development of pulmonary fibrosis (Al-kuraishy et al. 2021d). Post-Covid-19 pulmonary fibrosis might be irreversible and characterized by a significant reduction in lung function and early mortality (Fila et al. 2021). Thus, urgent recognition of the pathophysiology of post-Covid-19 pulmonary fibrosis is mandatory to highlight therapeutic lines for this serious late complication.
In Covid-19, the development of pulmonary fibrosis is so differed due to the large affected population and using of steroid therapy as a standard treatment, which might reduce the incidence of this squeal. However, steroid dose in the management of Covid-19 does not prevent post-Covid-19 pulmonary fibrosis adequately (Udwadia et al. 2021a). Moreover, recovered Covid-19 patients experienced a restrictive pulmonary abnormality, abnormal diffusion capacity, pulmonary microangiopathy, alveolar dysfunctions and abnormal lung functions two weeks following hospital discharge (Zhao et al. 2020; Mo et al. 2020). Udwadia et al (2021b) observed that most discharged severe Covid-19 patients within six weeks have an abnormal lung CT scan, insistent dyspnea, and abnormal diffusion capacity, and these pulmonary abnormalities declined significantly following 12 weeks. It is suggested that pulmonary abnormalities in Covid-19 patients are not progressive and might be resolved with time.
In contrast, retrospective studies illustrated that Covid-19 patients with ARDS on mechanical ventilation may develop pulmonary fibrosis, interstitial thickening, diffuse alveolar injury and honeycomb appearance similar to that of IPF within four weeks despite intensive antiviral and steroid therapies (Kayhan and Kocakoç 2020). As well, lung interstitial thickening and fibrosis may follow mild SARS-CoV-2 infections; also irregular reticular changes and formation of parenchymal bands might be predictors for post-Covid-19 pulmonary fibrosis (Xue et al. 2021). It has been revealed that smoking, alcoholism, prolonged duration of mechanical ventilation, severe ALI and cytokine storm are correlated high risk of pulmonary fibrosis (McDonald 2020). Besides, high interleukin (IL)-6, C reactive protein (CRP) and LDH levels in SARS-CoV-2-induced pneumonia are linked with a high risk of the development of pulmonary fibrosis (Leeming et al. 2021). Therefore, clinical review and frequent biochemical investigations mainly LDH serum levels in recovered Covid-19 patients are mandatory to follow the risk of pulmonary fibrosis.
Pathogenesis of post-Covid-19 pulmonary fibrosis
The precise mechanism of post-Covid-19 pulmonary fibrosis is related to the activation of TGF-β1, which activates the release of extracellular proteins, fibroblast proliferation, fibroblast migration, and myofibroblast conversion (Yo et al. 2021). Both influenza pneumonia and SARS infections are connected with stimulation and elevation of TGF-β1 (Denney et al. 2018; Wang et al. 2017). Li et al (2016) illustrated that SARS-CoV-2 papain-like protease (PLpro) up-regulates TGF-β1 through activation of reactive oxygen species (ROS), p38 mitogen-activated protein kinase (p38 MAPK) and signal transducer and activator of transcription 3 (STAT3) pathways. In SRAS-CoV-2 infection, TGF-β1 serum level is correlated with Covid-19 severity and development of pulmonary fibrosis (Hamer et al. 2020). SRAS-CoV-2 induces an adaptive immune response via stimulation of TGF-β1 gene expression, which engaged with neutrophils recruitment to the site of pulmonary infection (Barnes et al. 2020). TGF-β1 is tangled in the development of systemic inflammation and cytokine storm through the initiation release of IL-6 (Turner et al. 1990). In addition, TGF-β1 may induce vasculitis and endothelial dysfunction via switching of IgA production (Yu et al. 2020). Of note, TGF-β1 mediates its action through the triggering of Smad 3 protein, which is also stimulated by angiotensin II (AngII), CRP and advanced glycation end-product (AGEP) in patients with metabolic disorders (Nath et al. 2011). Therefore, TGF-β1-induced hypercytokinemia and pulmonary vascular dysfunction may predispose to severe ALI and subsequent progression for post-Covid-19 pulmonary fibrosis.
On the other hand, the binding of SRAS-CoV-2 to the angiotensin-converting enzyme 2 (ACE2) induces metabolic sequences through elevation of circulating Ang II and reduction of vasodilator Ang1-9 (Al-Kuraishy et al. 2020b). High circulating AngII induces pulmonary inflammation and fibrosis through different pathways including activation release of TGF-β1, induction formation of pulmonary ROS, induction apoptosis of pulmonary epithelial cells, acceleration release of extracellular matrix, promotion of fibro-proliferations, and stimulation release of IL-6 that together differentiation, migration and proliferation of pulmonary fibrosis (Konigshoff et al. 2007). AngII-induced pulmonary fibrosis is mainly mediated through stimulation of AngII receptor type 1(ART1); however, Rathinasabapathy et al (2018) showed that activation of ART2 attenuates ALI and pulmonary fibrosis. Zhang et al. observed that recombinant ACE2 protects from ARDS in patients with viral infections through reduction of AngII and elevation of Ang1-7 (Zhang and Baker 2017). Therefore, in SARS-CoV-2 there is dominancy of AngII/ATR1/ACE axis over that of Ang1-7/ATR2/ACE2 axis leading to ALI and progressive pulmonary fibrosis (Kai and Kai 2020). Previously, Marshal et al. (2000) illustrated that AngII has a prolonged mitogenic effect on the lung by promoting the effect of TGF-β1 and platelets-derived growth factor (PDGF). Indeed, AngII-induced pulmonary PDGF activation is linked with the progression of pulmonary vascular endothelial dysfunction and the development of pulmonary hypertension that exacerbates hypoxia and pulmonary fibrosis (Li et al. 2018).
Furthermore, most patients with post-Covid-19 pulmonary fibrosis were subjected to mechanical ventilation and high oxygen concentration that per se may cause pulmonary fibrosis, since oxygen toxicity is correlated with lung injury and fibrosis (Lee et al. 2020). Combet et al (2020) reported a rapid onset development of pulmonary fibrosis in Covid-19 patients who were never subjected to mechanical ventilation which might be due to aberrant immunological response on both pulmonary endothelial and alveolar cells with subsequent development of honeycomb pulmonary fibrosis. However, a retrospective study involving 107 ARDS patients found a positive correlation between the power of mechanical ventilation and high levels of TGF-β1 and connective tissue growth factors as well as pulmonary structural remodeling (Xie et al. 2019). Likewise, the use of high oxygen concentration in the management of Covid-19 patients with ARDS is linked with the production of ROS and the progression of oxidative stress-induced pulmonary fibrosis (Amarelle et al. 2021).
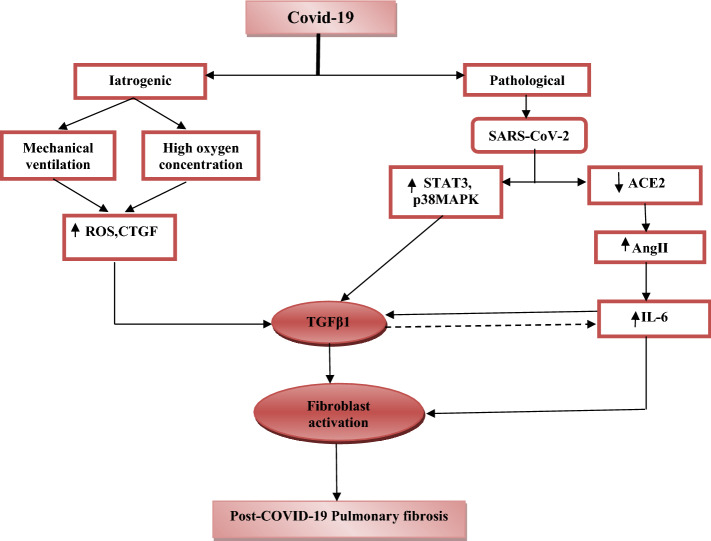
Therefore, the causal relationship between SARS-CoV-2 infection and the risk of pulmonary fibrosis is so complex but mainly related to the pathological and iatrogenic factors that may interrelate together in susceptible high-risk Covid-19 patients (Fig. 1).
Fig. 1.
Pathogenesis of post-Covid-19 pulmonary fibrosis: Covid-19 leads to pulmonary fibrosis through iatrogenic pathway, therapy with high oxygen concentration or mechanical ventilation cause oxygen toxicity, which induces the production of reactive oxygen species (ROS) and connective tissue growth factors (CTGFs). The pathological pathway through SARS-CoV-2 induces downregulation of angiotensin-converting enzyme 2 (ACE2) with subsequent activation of AngII, p38 mitogen-activated protein kinase (MAPK) and signal transducer and activator of transcription 3 (STAT3). Together, AngII, MAPK and STAT3 activate the production of transforming growth factor beta1 (TGFβ1). Both AngII and TGFβ1 activate fibroblast activation with subsequent development of pulmonary fibrosis
Role of pirfenidone in post-Covid-19 pulmonary fibrosis
PFN has potent anti-inflammatory and anti-fibrotic effects in various pulmonary disorders that are induced by different stimuli in animal and human studies as in bleomycin-induced pulmonary fibrosis (Liu et al. 2017). PFN was approved in 2013 in china for the treatment of IPF and unclassified interstitial pulmonary diseases; it inhibits the accumulation and recruitment of inflammatory cells as well as fibroblast proliferation and deposition of extracellular matrix in response to TGFβ1, PDGF and other pro-inflammatory cytokines (Lancaster et al. 2017). PFN in different clinical trials has been proposed to be effective against pro-fibrotic changes induced by SARS-CoV-2 and related inflammatory deregulation as observed in previous SARS pandemic (Rosa and Santos 2020). It has been shown that early use of PFN in Covid-19 may reduce and prevent subsequent immunological and inflammatory complications that trigger pulmonary fibrotic changes (Ferrara et al. 2020). Therefore, timing administration of PFN reflects its efficacy in the prevention of pulmonary fibrosis; however, PFN can cure residual pulmonary fibrotic changes in recovered Covid-19 patients with severe SARS-CoV-2 pneumonia (Seifirad 2020). Thus, PFN has potential therapeutic effects against Covid-19 both in the early hyperinflammatory phase through inhibition of pro-inflammatory cytokines, and in the late phase through anti-fibrotic mechanism since it is effective for post-inflammatory pulmonary fibrosis (Kayarat et al. 2021).
In silico study by Artigas et al (2020) confirmed that PFN in combination with melatonin leads to a significant reduction of SARS-CoV-2 viral load. PFN suppresses furin (TGFβ1 convertase activator) a protein effector involved in the entry of SARS-CoV-2 and activation of TGFβ1, and thus PFN reduces the pathogenesis of SARS-CoV-2 (Quay et al.). It has been verified that PFN inhibits the activation of NLR family pyrin domain containing 3 (NLRP3) inflammasome in acute kidney injury (Sharawy and Serrya 2020). NLRP3 inflammasome is activated by SARS-CoV-2 via the NF-κB pathway and is involved in the release of pro-inflammatory cytokines (Rodrigues et al. 2020; Al-kuraishy and Al-Gareeb 2021). Similarly, PFN attenuates lung fibrosis through inhibition of the NF-κB pathway and related chemokines and pro-inflammatory cytokines such as IL-1β, IL-8, IL-6, CCL5 and CXCL10 (Liu et al. 2019). Herein, PFN might have noteworthy anti-inflammatory and anti-SARS-CoV-2 effects through modulation of NLRP3 inflammasome/ NF-κB axis, and inhibition of cellular furin activity.
Besides, the antioxidant effect of PFN may mitigate acute inflammatory reactions and late pulmonary fibrosis in Covid-19 patients through inhibition of NLRP3 inflammasome and NF-κB signaling pathway (Shah et al. 2021). Rasooli et al (2020) disclosed that antioxidant agents like vitamin E intensify the antioxidant effect of PFN in the mitigation of pulmonary fibrosis caused by parquets.
Of note, activation of p38 MAPK is engaged with SARS-CoV-2 infection, and p38 MAPK pro-inflammatory pathway is activated directly by SARS-CoV-2 viral proteins or indirectly by high circulating AngII (Grimes and Grimes 2020; Al-kuraishy et al. 2022). Over-activated p38 MAPK is linked with the progression of endothelial dysfunction, vasoconstriction, thrombosis, systemic inflammation and development of pulmonary fibrosis (Goda et al. 2020). It has been documented that PFN attenuates and controls the activation of p38 MAPK by the AngII/ART1 axis (Li et al. 2017). Therefore, PFN down-streaming of p38 MAPK activation in early and late Covid-19 by SARS-CoV-2 viral proteins and AngII respectively. Thus, PFN role in Covid-19 seems to be highly effective against the progression of hypercytokinemia in the acute phase, and useful in the late phase and recovery period via attenuation development of pulmonary fibrosis (Malik et al. 2021).
Different studies have observed that residual pulmonary lesions persist for years and take a long time for complete resolution mainly in older subjects following recovery from SARS-CoV-2 infection. In addition, total viral eradication does not entirely prevent the development of pulmonary fibrosis, which might be irreversible that could cause prolonged morbidity and early mortality (Grillo et al. 2021). These findings suggest an ill-defined mechanism for post-Covid-19 pulmonary fibrosis; herein, cohort-retrospective and long-term prospective studies are needed to determine the precise mechanism and to find drugs related to these mechanistic mechanisms. Consequently, more sophisticated pathways have been searched in relation to post-Covid-19 pulmonary fibrosis including; Wingless/Int (Wnt/β-catenin), Yes-Associated Protein (YAP)/Transcription Co-Activator PDZ Binding Motif (TAZ) and Hippo Signaling Pathways (Zhang et al. 2020a, b).
YAP/TAZ pathway is a key controller and regulator of matrix formation and fibroblast activation. The activity of this pathway is correlated with the commotion of extracellular matrix stimulation and cell adhesion (Boopathy and Hong 2019). Activated YAP/TAZ signaling pathway leads to prompting of nuclear transcription factors for TGF-β1, CTGF, type I receptor TGF (TGFB1R), type II receptor TGF (TGFB2R), collagen type I alpha 1 chain (COL1A1), collagen type III alpha 1 chain (COL3A1), tissue inhibitor of metalloproteinase 1(TIMP1) and fibronectin (Noguchi et al. 2018). Zhu et al (2020) showed that activation of YAP/TAZ pathway is allied with the development of pulmonary fibrosis and pharmacological targeting of this pathway may attenuate inflammatory-induced pulmonary fibrosis. Selective YAP/TAZ pathway inhibitors such as dopamine type 1 receptor agonists inhibit pulmonary fibrosis by restraining the conversion of pro-fibrotic pulmonary extracellular matrix to fibrotic state (Haak et al. 2019). Besides, Zmajkovicova et al (2019) revealed that prostacyclin receptor agonist inhibits the conversion of fibroblast to myofibroblast, synthesis of extracellular matrix and release of IL-6 through inhibition of YAP/TAZ pathway in IPF. A placebo-controlled clinical trial showed that prostacyclin improves clinical outcomes in Covid-19 patients on mechanical ventilation, which might be due to modulation of YAP/TAZ pathway (Johansson et al. 2020). Walsh primer study for Covid-19 showed that YAP/TAZ pathway is activated in patients with SARS-CoV-2 pneumonia on mechanical ventilation, and this activation predisposes for post-Covid-19 pulmonary fibrosis (Walsh 2020). Recently, PFN inhibits fibroblast activation through suppression of the lung fibroblast YAP/TAZ pathway (Aravamudhan et al. 2020).
Wnt/β-catenin pathway is a signaling pathway in which Wnt protein binds Frizzled receptor inside the cells that regulate cell cytoskeleton and involved with cell proliferation, migration and differentiation as well as tissue regeneration. Mutation of the Wnt/β-catenin pathway is associated with the development of breast cancer and type II diabetes mellitus (Tabatabai et al. 2017). Activation of the Wnt/β-catenin pathway is linked with the progression and development of pulmonary fibrosis through the proliferation of myofibroblast and mesenchymal stem cells as well as prevention of lung resolution through stimulation of macrophages (Sennello et al. 2017). It has been shown that Wnt/β-catenin pathway inhibitors such as pyrvinium attenuate cardiac remodeling (Saraswati et al. 2010). Karamian et al (2021) illustrated that pyrvinium inhibits the release of IL-6 and IL-8, so it may prevent the development of pulmonary fibrosis. As well, vitamin D attenuates the activation of the Wnt/β-catenin pathway in melanoma (Muralidhar et al. 2019). Gu et al (2020) revealed that net-cellular signal transduction during SARS-CoV-2 is associated with activation of the Wnt/β-catenin pathway and subsequent lung injury. Therefore, Wnt/β-catenin inhibitors might reduce extensive lung injury in SARS-CoV-2 infection and may attenuate post-Covid-19 pulmonary fibrosis. Of note, pyrvinium blocks the replication of SARS-CoV-2 and prevents the progression of associated inflammations (Balaramnavar et al. 2020). Therefore, Wnt/β-catenin pathway inhibitors might be effective in the prevention of post-Covid-19 pulmonary fibrosis through attenuation of SARS-CoV-2-induced ALI. It has been reported that PFN inhibits the Wnt/β-catenin pathway in cell lines and abrogates fibrotic reactions (Zou et al. 2017).
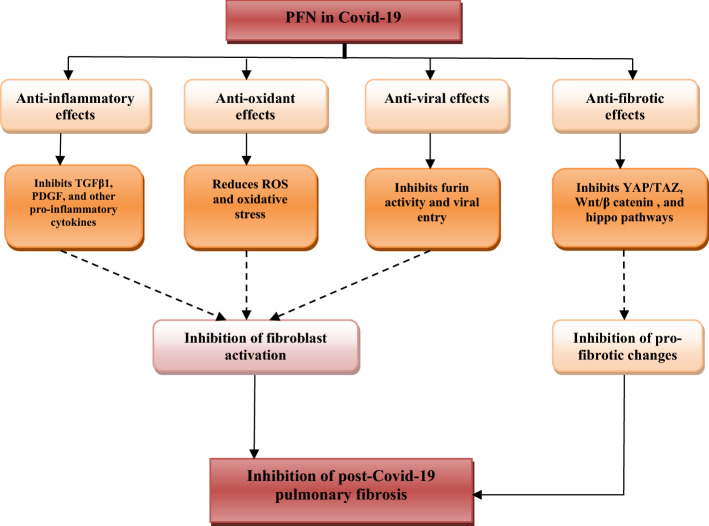
Moreover, hippo pathway consists of different kinase cascades involved in the regulation of cell proliferation through promoting apoptosis, so this pathway controls organ size, and its mutation may lead to overgrowth of tissues and the creation of the hippopotamus phenotype (Ma et al. 2019). The hippo pathway is regulated by cell polarity, cellular tension, and signaling of G-protein coupled receptors. Besides, the hippo pathway controls and modulates YAP/TAZ activity as well as regulates critical physiological functions (Yu and Guan 2013). Gokey et al (2018) found that the hippo pathway is connected with the pathogenesis of pulmonary fibrosis via induction proliferation and migration of pulmonary fibroblasts. Similarly, melatonin attenuates pulmonary fibrosis via inhibition translocation of YAP from the cytoplasm to the nucleus, an effector of the hippo pathway (Zhao et al. 2018). Shneider et al (2020) inaugurate that melatonin reduces Covid-19 severity through attenuation of lung immune-pathological reaction to SARS-CoV-2 invasion. Thus, melatonin might be a potential candidate for the prevention of post-Covid-19 pulmonary fibrosis. Furthermore, PFN inhibits fibroblast and myofibroblast activations and profibrogenic T cells through modulation of the hippo pathway signaling pathway (Latella and Viscido 2020). Finally, Chen et al (2019) PFN and other anti-fibrotic agents prevent pulmonary fibroblast activation and fibrogenesis through the restoration of the miR-15a/YAP/Hippo pathway. The net effect of PFN on development of post-Covid-19 pulmonary fibrosis is summarized (Fig. 2).
Fig. 2.
Role of Pirfenidone in post-Covid-19 pulmonary fibrosis: Pirfenidone (PFN) has anti-inflammatory effects through inhibition of transforming growth factor beta (TGF-β1), platelet-derived growth factor (PDGF) and pro-inflammatory cytokines, antioxidant effect through inhibition production of reactive oxygen species (ROS) and oxidative stress, antiviral effect through inhibition of cellular furin activity and viral entry, anti-fibrotic effect through suppression of Yes-Associated Protein (YAP)/Transcription Co-Activator PDZ Binding Motif (TAZ), Wingless/Int (Wnt/β-catenin), and Hippo Signaling Pathways. These effects by PFN reduce pro-fibrotic changes and fibroblast activation with subsequent attenuation of post-Covid-19 pulmonary fibrosis
Despite these findings, Ferrara et al (2020) demonstrated that the therapeutic efficacy of PFN in pulmonary fibrosis induced by SARS-CoV-2 is still being demonstrated in clinical trials. A recent single center retrospective study involved case analysis of patients with post-Covid-19 pulmonary fibrosis who were treated with PFN 2400 mg/day for 12–24 weeks showed that PFN reduced risk of development of pulmonary fibrosis (Co et al. 2022). Therefore, PFN adds-on standard anti-Covid-19 therapy for 12–24 week could be effective in reduction of oxygen requirement and lung radiological abnormalities (Co et al. 2022). A clinical trial illustrated that PFN was effective in the reduction of D-dimer and risk of thrombotic events in Covid-19 patients (Zhang et al. 2022). Tanvir et al (2022) revealed in an open label pilot trial involved 60 Covid-19 patients, of them 17 received PFN and 19 received corticosteroids. The study parameters were evaluated at the baseline, and after six weeks, the anti-fibrotic effects of PFN was more than corticosteroids (P < 0.001). These observations suggest that early treatment with PFN in severely affected Covid-19 patients may reduce risk of development of post-Covid-19 pulmonary fibrosis.
It seems from recent findings and according to the disturbances at molecular levels that post-Covid-19 pulmonary fibrosis is not simply related to the post-SARS-CoV-2 inflammatory. Therefore, deep molecular investigations in relation to the initiation and development of post-Covid-19 pulmonary fibrosis are warranted to find an effective drug that prevents and treats this challenged critical condition.
Conclusion
In Covid-19 era, SARS-CoV-2 could initially lead to ALI and in severe cases may cause ARDS that are usually resolved. However, some cases of ALI and ARDS may progress to a more severe critical stage of pulmonary fibrosis commonly named post-Covid-19 pulmonary fibrosis. Anti-inflammatory, antiviral, antioxidant and anti-fibrotic properties of PFN may attenuate post-Covid-19 pulmonary fibrosis. It seems from recent findings and according to the disturbances at molecular levels that post-Covid-19 pulmonary fibrosis is not simply related to the post-SARS-CoV-2 inflammatory. Therefore, deep molecular investigations in relation to the initiation and development of post-Covid-19 pulmonary fibrosis are warranted to find an effective drug that prevents and treats this challenged critical condition.
Author contributions
The authors equally participated in the development of the manuscript and provided their final approval of all content and submission for publication.
Funding
Funding for open access charge provided by University of Vigo/CISUG.
Data availability
Enquiries about data availability should be directed to the authors.
Declarations
Competing interest
The authors have not disclosed any competing interests.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hayder M. Al-kuraishy, Email: Hayderm36@yahoo.com
Gaber El-Saber Batiha, Email: gaberbatiha@gmail.com.
Hani Faidah, Email: hsfaidah@uqu.edu.sa.
Ali I. Al-Gareeb, Email: Dr.alialgareeb78@yahoo.com
Hebatallah M. Saad, Email: heba.magdy@mau.edu.eg
Jesus Simal-Gandara, Email: jsimal@uvigo.es.
References
- Ajlan AM, Ahyad RA, Jamjoom LG, Alharthy A, Madani TA. Middle East respiratory syndrome coronavirus (MERS-CoV) infection: chest CT findings. AJR Am J Roentgenol. 2014;203(4):782–787. doi: 10.2214/AJR.14.13021. [DOI] [PubMed] [Google Scholar]
- Ali RM, Ghonimy MB. Post-COVID-19 pneumonia lung fibrosis: a worrisome sequelae in surviving patients. Egypt J Radiol Nucl Med. 2021;52(1):1–8. doi: 10.1186/s43055-021-00484-3. [DOI] [Google Scholar]
- Al-Kuraishy HM, Al-Gareeb AI. Acute kidney injury and COVID-19. Egypt J Intern Med. 2021;33(1):1–5. doi: 10.1186/s43162-021-00064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kuraishy HM, Al-Naimi MS, Lungnier CM, Al-Gareeb AI. Macrolides and COVID-19: an optimum premise. Biomed Biotechnol Res J. 2020;4(3):189. doi: 10.4103/bbrj.bbrj_103_20. [DOI] [Google Scholar]
- Al-Kuraishy HM, Al-Niemi MS, Hussain NR, Al-Gareeb AI, Al-Harchan NA, Al-Kurashi AH. The Potential Role of Renin Angiotensin System (RAS) and Dipeptidyl Peptidase-4 (DPP-4) in COVID-19: Navigating the Uncharted. In: Kibel A, editor. Selected chapters from the reninangiotensin system. London: IntechOpen; 2020. pp. 151–165. [Google Scholar]
- Al-Kuraishy HM, Al-Gareeb AI, Alblihed M, Cruz-Martins N, Batiha GE. COVID-19 and risk of acute ischemic stroke and acute lung injury in patients with type II diabetes mellitus: the anti-inflammatory role of metformin. Front Med. 2021;8:110. doi: 10.3389/fmed.2021.644295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kuraishy HM, Al-Gareeb AI, Faidah H, Al-Maiahy TJ, Cruz-Martins N, Batiha GE. The looming effects of estrogen in Covid-19: a rocky rollout. Front Nutr. 2021;8:649128. doi: 10.3389/fnut.2021.649128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kuraishy HM, Al-Gareeb AI, Qusty N, Cruz-Martins N, Batiha GE. Sequential doxycycline and colchicine combination therapy in Covid-19: the salutary effects. Pulm Pharmacol Ther. 2021;67:102008. doi: 10.1016/j.pupt.2021.102008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kuraishy HM, Al-Gareeb AI, Alkazmi L, Habotta OA, Batiha GE. High-mobility group box 1 (HMGB1) in COVID-19: extrapolation of dangerous liaisons. Inflammopharmacology. 2022;26:1. doi: 10.1007/s10787-022-00988-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-kuraishy H, Al-Gareeb AI, Guerreiro SG, Cruz-Martins N, Batiha GE. COVID-19 in relation to hyperglycemia and diabetes mellitus. Front Cardiovasc Med. 2021;8:335. doi: 10.3389/fcvm.2021.644095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarelle L, Quintela L, Hurtado J, Malacrida L. Hyperoxia and lungs: what we have learned from animal models. Front Med. 2021;8:235. doi: 10.3389/fmed.2021.606678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravamudhan A, Haak AJ, Choi KM, Meridew JA, Caporarello N, Jones DL, Tan Q, Ligresti G, Tschumperlin DJ. TBK1 regulates YAP/TAZ and fibrogenic fibroblast activation. Am J Physiol Lung Cell Mol Physiol. 2020;318(5):L852–L863. doi: 10.1152/ajplung.00324.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artigas L, Coma M, Matos-Filipe P, Aguirre-Plans J, Farrés J, Valls R, Fernandez-Fuentes N, de la Haba-Rodriguez J, Olvera A, Barbera J, Morales R. In-silico drug repurposing study predicts the combination of pirfenidone and melatonin as a promising candidate therapy to reduce SARS-CoV-2 infection progression and respiratory distress caused by cytokine storm. PLoS One. 2020;15(10):e0240149. doi: 10.1371/journal.pone.0240149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaramnavar VM, Ahmad K, Saeed M, Ahmad I, Kamal M, Jawed T. Pharmacophore-based approaches in the rational repurposing technique for FDA approved drugs targeting SARS-CoV-2 Mpro. RSC Adv. 2020;10(66):40264–40275. doi: 10.1039/D0RA06038K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM, Daßler-Plenker J, Guerci P, Huynh C, Knight JS, Loda M. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J Exp Med. 2020;217(6):e20200652. doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boopathy GT, Hong W. Role of hippo pathway-YAP/TAZ signaling in angiogenesis. Front Cell Dev Biol. 2019;7:49. doi: 10.3389/fcell.2019.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridevaux PO, Aubert JD, Soccal PM, Mazza-Stalder J, Berutto C, Rochat T, Turin L, Van Belle S, Nicod L, Meylan P, Wagner G. Incidence and outcomes of respiratory viral infections in lung transplant recipients: a prospective study. Thorax. 2014;69(1):32–38. doi: 10.1136/thoraxjnl-2013-203581. [DOI] [PubMed] [Google Scholar]
- Carter NJ. Pirfenidone Drugs. 2011;71(13):1721–1732. doi: 10.2165/11207710-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhao X, Sun J, Su W, Zhang L, Li Y, Liu Y, Zhang L, Lu Y, Shan H, Liang H. YAP1/twist promotes fibroblast activation and lung fibrosis that conferred by miR-15a loss in IPF. Cell Death Differ. 2019;26(9):1832–1844. doi: 10.1038/s41418-018-0250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Co JL, Badilles FA, Halun CB, Dy-Agra GN, Campomanes CM, Pagcatipunan RS, Divinagracia RM (2022) Pirfenidone for COVID-19 Associated Pulmonary Fibrosis: A Case Series. InC38. RARE AND COMMON ILDS: EPIDEMIOLOGY, DIAGNOSIS, AND MANAGEMENT 2022 May (pp. A4046-A4046). American Thoracic Society.
- Combet M, Pavot A, Savale L, Humbert M, Monnet X. Rapid onset honeycombing fibrosis in spontaneously breathing patient with Covid-19. Eur Respir J. 2020;56(2):2001808. doi: 10.1183/13993003.01808-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das KM, Lee EY, Singh R, Enani MA, Al Dossari K, Van Gorkom K, Larsson SG, Langer RD. Follow-up chest radiographic findings in patients with MERS-CoV after recovery. Indian J Radiol Imaging. 2017;27(3):342. doi: 10.4103/ijri.IJRI_469_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denney L, Branchett W, Gregory LG, Oliver RA, Lloyd CM. Epithelial-derived TGF-β1 acts as a pro-viral factor in the lung during influenza A infection. Mucosal Immunol. 2018;11(2):523–535. doi: 10.1038/mi.2017.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara F, Granata G, Pelliccia C, La Porta R, Vitiello A. The added value of pirfenidone to fight inflammation and fibrotic state induced by SARS-CoV-2. Eur J Clin Pharmacol. 2020;76(11):1615–1618. doi: 10.1007/s00228-020-02947-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fila E, Rocco I, Rocco G, Zuccon U, Ruberti E. Recommendations for the respiratory rehabilitation of hospitalized and discharged COVID-19 patients: a sistematic review. G Ital Med Lav Erg. 2021;43(1):56–65. [Google Scholar]
- Goda C, Balli D, Black M, Milewski D, Le T, Ustiyan V, Ren X, Kalinichenko VV, Kalin TV. Loss of FOXM1 in macrophages promotes pulmonary fibrosis by activating p38 MAPK signaling pathway. PLoS Genet. 2020;16(4):e1008692. doi: 10.1371/journal.pgen.1008692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokey JJ, Sridharan A, Xu Y, Green J, Carraro G, Stripp BR, Perl AK, Whitsett JA. Active epithelial Hippo signaling in idiopathic pulmonary fibrosis. JCI Insight. 2018;3(6):e98738. doi: 10.1172/jci.insight.98738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo F, Barisione E, Ball L, Mastracci L, Fiocca R. Lung fibrosis: an undervalued finding in COVID-19 pathological series. Lancet Infect Dis. 2021;21(4):e72. doi: 10.1016/S1473-3099(20)30582-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes JM, Grimes KV. p38 MAPK inhibition: a promising therapeutic approach for COVID-19. J Mol Cell Cardiol. 2020;144:63–65. doi: 10.1016/j.yjmcc.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Cao J, Zhang X, Gao H, Wang Y, Wang J, Zhang J, Shen G, Jiang X, Yang J, Zheng X (2020) Interaction network of SARS-CoV-2 with host receptome through spike protein. 10.1101/2020.09.09.287508
- Haak AJ, Kostallari E, Sicard D, Ligresti G, Choi KM, Caporarello N, Jones DL, Tan Q, Meridew J, Espinosa AM, Aravamudhan A. Selective YAP/TAZ inhibition in fibroblasts via dopamine receptor D1 agonism reverses fibrosis. Sci Transl Med. 2019;11(516):eaau6296. doi: 10.1126/scitranslmed.aau6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer M, Kivimäki M, Gale CR, Batty GD. Lifestyle risk factors, inflammatory mechanisms, and COVID-19 hospitalization: a community-based cohort study of 387,109 adults in UK. Brain Behav Immun. 2020;87:184–187. doi: 10.1016/j.bbi.2020.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson PI, Bestle M, Søe-Jensen P, Kristiansen KT, Stensballe J, Clausen NE, Perner A. The effect of prostacyclin (Iloprost) infusion at a dose of 1 ng/kg/min for 72 hours compared to placebo in mechanically ventilated patients with COVID-19: A structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21:746. doi: 10.1186/s13063-020-04696-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai H, Kai M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors-lessons from available evidence and insights into COVID-19. Hypertens Res. 2020;43:648–654. doi: 10.1038/s41440-020-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamian A, Paktinat S, Esfandyari S, Nazarian H, Ziai SA, Zarnani AH, Salehpour S, Hosseinirad H, Karamian A, Novin MG. Pyrvinium pamoate induces in-vitro suppression of IL-6 and IL-8 produced by human endometriotic stromal cells. Hum Exp Toxicol. 2021;40(4):649–660. doi: 10.1177/0960327120964543. [DOI] [PubMed] [Google Scholar]
- Kayarat B, Khanna P, Sarkar S. Pulmonary fibrosis in COVID-19 recovered patients: problem and potential management. Indian J Crit Care Med. 2021;25(2):242–244. doi: 10.5005/jp-journals-10071-23733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayhan S, Kocakoç E. Pulmonary fibrosis due to COVID-19 pneumonia. Korean J Radiol. 2020;21(11):1273. doi: 10.3348/kjr.2020.0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konigshoff M, Wilhelm A, Jahn A, Sedding D, Amarie OV, Eul B, Seeger W, Fink L, Gunther A, Eickelberg O, Rose F. The angiotensin II receptor 2 is expressed and mediates angiotensin II signaling in lung fibrosis. Am J Respir Cell Mol Biol. 2007;37(6):640–650. doi: 10.1165/rcmb.2006-0379TR. [DOI] [PubMed] [Google Scholar]
- Lancaster LH, de Andrade JA, Zibrak JD, Padilla ML, Albera C, Nathan SD, Wijsenbeek MS, Stauffer JL, Kirchgaessler KU, Costabel U. Pirfenidone safety and adverse event management in idiopathic pulmonary fibrosis. Eur Respir Rev. 2017;26(146):170057. doi: 10.1183/16000617.0057-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latella G, Viscido A. Could pirfenidone also be effective in treating intestinal fibrosis? Cells. 2020;9(8):1762. doi: 10.3390/cells9081762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Lim CM, Koh Y, Hong SB, Song JW, Huh JW. High-flow nasal cannula oxygen therapy in idiopathic pulmonary fibrosis patients with respiratory failure. J Thorac Dis. 2020;12(3):966. doi: 10.21037/jtd.2019.12.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeming DJ, Genovese F, Sand JM, Rasmussen DG, Christiansen C, Jenkins G, Maher TM, Vestbo J, Karsdal MA. Can biomarkers of extracellular matrix remodelling and wound healing be used to identify high risk patients infected with SARS-CoV-2?: lessons learned from pulmonary fibrosis. Respir Res. 2021;22(1):1–7. doi: 10.1186/s12931-020-01590-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley B, Swigris J, Day BM, Stauffer JL, Raimundo K, Chou W, Collard HR. Pirfenidone reduces respiratory-related hospitalizations in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2017;196(6):756–761. doi: 10.1164/rccm.201701-0091OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SW, Wang CY, Jou YJ, Yang TC, Huang SH, Wan L, Lin YJ, Lin CW. SARS coronavirus papain-like protease induces Egr-1-dependent up-regulation of TGF-β1 via ROS/p38 MAPK/STAT3 pathway. Sci Rep. 2016;6(1):1–3. doi: 10.1038/srep25754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Han R, Kang L, Wang J, Gao Y, Li Y, He J, Tian J. Pirfenidone controls the feedback loop of the AT1R/p38 MAPK/renin-angiotensin system axis by regulating liver X receptor-α in myocardial infarction-induced cardiac fibrosis. Sci Rep. 2017;7(1):1–1. doi: 10.1038/srep40523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li L, Qian Z, Lin B, Chen J, Luo Y, Qu J, Raj JU, Gou D. Phosphatidylinositol 3-Kinase–DNA Methyltransferase 1–miR-1281–histone deacetylase 4 regulatory axis mediates platelet-derived growth factor-induced proliferation and migration of pulmonary artery smooth muscle cells. J Am Heart Assoc. 2018;7(6):e007572. doi: 10.1161/JAHA.117.007572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lu F, Kang L, Wang Z, Wang Y. Pirfenidone attenuates bleomycin-induced pulmonary fibrosis in mice by regulating Nrf2/Bach1 equilibrium. BMC Pulm Med. 2017;17(1):1–1. doi: 10.1186/s12890-017-0405-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Rong Y, Sun D, Li W, Chen H, Cao B, Wang T. Costunolide inhibits pulmonary fibrosis via regulating NF-kB and TGF-β1/Smad2/Nrf2-NOX4 signaling pathways. Biochem Biophys Res Commun. 2019;510(2):329–333. doi: 10.1016/j.bbrc.2019.01.104. [DOI] [PubMed] [Google Scholar]
- Lugnier C, Al-Kuraishy HM, Rousseau E. PDE4 inhibition as a therapeutic strategy for improvement of pulmonary dysfunctions in Covid-19 and cigarette smoking. Biochem Pharmacol. 2021;185:114431. doi: 10.1016/j.bcp.2021.114431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Meng Z, Chen R, Guan KL. The Hippo pathway: biology and pathophysiology. Annu Rev Biochem. 2019;88:577–604. doi: 10.1146/annurev-biochem-013118-111829. [DOI] [PubMed] [Google Scholar]
- Maher TM, Corte TJ, Fischer A, Kreuter M, Lederer DJ, Molina-Molina M, Axmann J, Kirchgaessler KU, Samara K, Gilberg F, Cottin V. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 2020;8(2):147–157. doi: 10.1016/S2213-2600(19)30341-8. [DOI] [PubMed] [Google Scholar]
- Mahmud R, Rahman MM, Rassel MA, Monayem FB, Sayeed SJ, Islam MS, Islam MM. Post-COVID-19 syndrome among symptomatic COVID-19 patients: a prospective cohort study in a tertiary care center of Bangladesh. PLoS One. 2021;16(4):e0249644. doi: 10.1371/journal.pone.0249644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik B, Abdelazeem B, Ghatol A. Pulmonary fibrosis after COVID-19 Pneumonia. Cureus. 2021;13(3):e13923. doi: 10.7759/cureus.13923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall RP, McANULTY RJ, Laurent GJ. Angiotensin II is mitogenic for human lung fibroblasts via activation of the type 1 receptor. Am J Respir Crit Care Med. 2000;161(6):1999–2004. doi: 10.1164/ajrccm.161.6.9907004. [DOI] [PubMed] [Google Scholar]
- McDonald LT. Healing after Covid-19: are survivors at risk for development of pulmonary fibrosis? Am J Physiol Lung Cell Mol Physiol. 2020;320(2):L257–L265. doi: 10.1152/ajplung.00238.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo X, Jian W, Su Z, Chen M, Peng H, Peng P, Lei C, Chen R, Zhong N, Li S. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55(6):2001217. doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa-Hedeab G, Al-Kuraishy HM, Al-Gareeb AI, Jeandet P, Saad HM, Batiha GES. A raising dawn of pentoxifylline in management of inflammatory disorders in Covid-19. Inflammopharmacology. 2022;30:1–11. doi: 10.1007/s10787-022-00993-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidhar S, Filia A, Nsengimana J, Poźniak J, O'Shea SJ, Diaz JM, Harland M, Randerson-Moor JA, Reichrath J, Laye JP, van der Weyden L. Vitamin D-VDR signaling inhibits Wnt/β-catenin–mediated melanoma progression and promotes antitumor immunity. Cancer Res. 2019;79(23):5986–5998. doi: 10.1158/0008-5472.CAN-18-3927. [DOI] [PubMed] [Google Scholar]
- Nath KA, Croatt AJ, Warner GM, Grande JP. Genetic deficiency of Smad3 protects against murine ischemic acute kidney injury. Am J Physiol Renal Physiol. 2011;301(2):F436–F442. doi: 10.1152/ajprenal.00162.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi S, Saito A, Nagase T. YAP/TAZ signaling as a molecular link between fibrosis and cancer. Int J Mol Sci. 2018;19(11):3674. doi: 10.3390/ijms19113674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterholzer JJ, Christensen PJ, Lama V, Horowitz JC, Hattori N, Subbotina N, Cunningham A, Lin Y, Murdock BJ, Morey RE, Olszewski MA. PAI-1 promotes the accumulation of exudate macrophages and worsens pulmonary fibrosis following type II alveolar epithelial cell injury. J Pathol. 2012;228(2):170–180. doi: 10.1002/path.3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quay SC, Chen SC, Rea J, Remmel HL. AT-H201 constituents collectively are the most potent inhibitors of SARS-CoV-2 infectivity in VERO cells identified and mechanistically act as a chemical vaccine: Human safety data support rapid clinical development as inhaled therapy for COVID-19. Virol J. 2019;16(69):11–19. [Google Scholar]
- Rasooli R, Kamali Y, Mandegary A. Effects of pirfenidone, vitamin E, and pirfenidone–vitamin E combination in paraquat-induced pulmonary fibrosis. Comp Clin Path. 2020;29(3):667–673. doi: 10.1007/s00580-020-03104-0. [DOI] [Google Scholar]
- Rathinasabapathy A, Horowitz A, Horton K, Kumar A, Gladson S, Unger T, Martinez D, Bedse G, West J, Raizada MK, Steckelings UM. The selective angiotensin II type 2 receptor agonist, compound 21, attenuates the progression of lung fibrosis and pulmonary hypertension in an experimental model of bleomycin-induced lung injury. Front Physiol. 2018;9:180. doi: 10.3389/fphys.2018.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues TS, de Sá KS, Ishimoto AY, Becerra A, Oliveira S, Almeida L, Gonçalves AV, Perucello DB, Andrade WA, Castro R, Veras FP. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J Exp Med. 2020;218(3):e20201707. doi: 10.1084/jem.20201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa SG, Santos WC. Clinical trials on drug repositioning for COVID-19 treatment. Rev Panam Salud Publica. 2020;44:e40. doi: 10.26633/RPSP.2020.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraswati S, Alfaro MP, Thorne CA, Atkinson J, Lee E, Young PP. Pyrvinium, a potent small molecule Wnt inhibitor, promotes wound repair and post-MI cardiac remodeling. PLoS One. 2010;5(11):e15521. doi: 10.1371/journal.pone.0015521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwensen HF, Borreschmidt LK, Storgaard M, Redsted S, Christensen S, Madsen LB. Fatal pulmonary fibrosis: a post-COVID-19 autopsy case. J Clin Pathol. 2020;74(6):400–402. doi: 10.1136/jclinpath-2020-206879. [DOI] [PubMed] [Google Scholar]
- Seifirad S. Pirfenidone: a novel hypothetical treatment for COVID-19. Med Hypotheses. 2020;144:110005. doi: 10.1016/j.mehy.2020.110005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sennello JA, Misharin AV, Flozak AS, Berdnikovs S, Cheresh P, Varga J, Kamp DW, Budinger GS, Gottardi CJ, Lam AP. Lrp5/β-catenin signaling controls lung macrophage differentiation and inhibits resolution of fibrosis. Am J Respir Cell Mol Biol. 2017;56(2):191–201. doi: 10.1165/rcmb.2016-0147OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PV, Balani P, Lopez AR, Nobleza CM, Siddiqui M, Khan S. A review of pirfenidone as an anti-fibrotic in idiopathic pulmonary fibrosis and its probable role in other diseases. Cureus. 2021;13(1):e12482. doi: 10.7759/cureus.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharawy MH, Serrya MS. Pirfenidone attenuates gentamicin-induced acute kidney injury by inhibiting inflammasome-dependent NLRP3 pathway in rats. Life Sci. 2020;260:118454. doi: 10.1016/j.lfs.2020.118454. [DOI] [PubMed] [Google Scholar]
- Shneider A, Kudriavtsev A, Vakhrusheva A. Can melatonin reduce the severity of COVID-19 pandemic? Int Rev Immunol. 2020;39(4):153–162. doi: 10.1080/08830185.2020.1756284. [DOI] [PubMed] [Google Scholar]
- Tabatabai R, Linhares Y, Bolos D, Mita M, Mita A. Targeting the Wnt pathway in cancer: a review of novel therapeutics. Target Oncol. 2017;12(5):623–641. doi: 10.1007/s11523-017-0507-4. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Tsujino K, Kijima T, Kumanogoh A. Efficacy and safety of pirfenidone for idiopathic pulmonary fibrosis. Patient Prefer Adherence. 2014;8:361. doi: 10.2147/PPA.S37233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, Taguchi Y, Takahashi H, Nakata K, Sato A, Takeuchi M. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35(4):821–829. doi: 10.1183/09031936.00005209. [DOI] [PubMed] [Google Scholar]
- Tanvir M, Wagay I, Nisar S, Ahmed RN, Maqbool M, Kareem O, Muzaffer U. 2022 Early intervention with anti-fibrotic pirfenidone is effective than corticosteroids in preventing pulmonary fibrosis in severe COVID pneumonia patients. Curr Med Res Pract. 2022;12(2):53. doi: 10.4103/cmrp.cmrp_110_21. [DOI] [Google Scholar]
- Turner M, Chantry D, Feldmann M. Transforming growth factor β induces the production of interleukin 6 by human peripheral blood mononuclear cells. Cytokine. 1990;2(3):211–216. doi: 10.1016/1043-4666(90)90018-O. [DOI] [PubMed] [Google Scholar]
- Udwadia ZF, Koul PA, Richeldi L. a) Post-COVID lung fibrosis: the tsunami that will follow the earthquake. Lung India. 2021;38(7):41. doi: 10.4103/lungindia.lungindia_818_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udwadia ZF, Pokhariyal PK, Tripathi AK, Kohli A. Fibrotic interstitial lung disease occurring as sequelae of COVID-19 pneumonia despite concomitant steroids. Lung India. 2021;38(7):61. doi: 10.4103/lungindia.lungindia_533_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh TJ. Primer on the pathogenesis of Severe COVID-19: part two. EMJ. 2020 doi: 10.33590/emj/20-00159. [DOI] [Google Scholar]
- Wang CY, Lu CY, Li SW, Lai CC, Hua CH, Huang SH, Lin YJ, Hour MJ, Lin CW. SARS coronavirus papain-like protease up-regulates the collagen expression through non-Samd TGF-β1 signaling. Virus Res. 2017;235:58–66. doi: 10.1016/j.virusres.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Wang Y, Liu K, Li X. Correlation analysis between mechanical power, transforming growth factor-β1, and connective tissue growth factor levels in acute respiratory distress syndrome patients and their clinical significance in pulmonary structural remodeling. Medicine. 2019;98(29):e16531. doi: 10.1097/MD.0000000000016531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Zhang T, Chen H, Zeng Y, Lin R, Zhen Y, Li N, Huang Z, Hu H, Zhou L, Wang H. Krebs Von den Lungen-6 as a predictive indicator for the risk of secondary pulmonary fibrosis and its reversibility in COVID-19 patients. Int J Biol Sci. 2021;17(6):1565–1573. doi: 10.7150/ijbs.58825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yo EC, Kadharusman MM, Karman AP, Louisa M, Arozal W. Potential pharmacological options and new avenues using inhaled curcumin nanoformulations for treatment of post-COVID-19 fibrosis. Syst Rev Pharm. 2021;12(1):1119–1128. [Google Scholar]
- Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27(4):355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HQ, Sun BQ, Fang ZF, Zhao JC, Liu XY, Li YM, Sun XZ, Liang HF, Zhong B, Huang ZF, Zheng PY. Distinct features of SARS-CoV-2-specific IgA response in COVID-19 patients. Eur Respir J. 2020;56(2):2001526. doi: 10.1183/13993003.01526-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Baker A. Recombinant human ACE2: acing out angiotensin II in ARDS therapy. Crit Care. 2017;21(1):305. doi: 10.1186/s13054-017-1882-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Wu Z, Li JW, Tan K, Yang W, Zhao H, Wang GQ. Discharge may not be the end of treatment: pay attention to pulmonary fibrosis caused by severe COVID-19. J Med Virol. 2020;93(3):1378–1386. doi: 10.1002/jmv.26634. [DOI] [PubMed] [Google Scholar]
- Zhang P, Li J, Liu H, Han N, Ju J, Kou Y, Chen L, Jiang M, Pan F, Zheng Y, Gao Z. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Res. 2020;8(1):1–8. doi: 10.1038/s41413-020-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wei Y, He L, Zhang H, Hu Q, Yue H, He J, Dai H. A trial of pirfenidone in hospitalized adult patients with severe coronavirus disease 2019. Chin Med J. 2022;135(03):368–370. doi: 10.1097/CM9.0000000000001614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Sun J, Su W, Shan H, Zhang B, Wang Y, Shabanova A, Shan H, Liang H. Melatonin protects against lung fibrosis by regulating the Hippo/YAP pathway. Int J Mol Sci. 2018;19(4):1118. doi: 10.3390/ijms19041118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YM, Shang YM, Song WB, Li QQ, Xie H, Xu QF, Jia JL, Li LM, Mao HL, Zhou XM, Luo H. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T, Ma Z, Wang H, Jia X, Wu Y, Fu L, Li Z, Zhang C, Yu G. YAP/TAZ affects the development of pulmonary fibrosis by regulating multiple signaling pathways. Mol Cell Biochem. 2020;475(1):137–149. doi: 10.1007/s11010-020-03866-9. [DOI] [PubMed] [Google Scholar]
- Zmajkovicova K, Menyhart K, Bauer Y, Studer R, Renault B, Schnoebelen M, Bolinger M, Nayler O, Gatfield J. The antifibrotic activity of prostacyclin receptor agonism is mediated through inhibition of YAP/TAZ. Am J Respir Cell Mol Biol. 2019;60(5):578–591. doi: 10.1165/rcmb.2018-0142OC. [DOI] [PubMed] [Google Scholar]
- Zou WJ, Huang Z, Jiang TP, Shen YP, Zhao AS, Zhou S, Zhang S. Pirfenidone inhibits proliferation and promotes apoptosis of hepatocellular carcinoma cells by inhibiting the Wnt/β-catenin signaling pathway. Med Sci Monit. 2017;23:6107. doi: 10.12659/MSM.907891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Enquiries about data availability should be directed to the authors.