Abstract
Objective
To jointly analyse two food dimensions, the Food Standards Agency Nutrient Profiling System (FSAm-NPS), used to derive the Nutri-Score front-of-pack label, and the NOVA classification in relation to mortality.
Design
Prospective cohort study.
Setting
Moli-sani Study, Italy 2005-10.
Participants
22 895 participants (mean age 55 (SD 12) years; 48% men).
Main outcomes measures
Associations between dietary exposures and mortality risk, assessed using multivariable cause specific Cox proportional hazard models controlled for known risk factors.
Results
A total of 2205 deaths occurred during 272 960 person years of follow-up. In the highest quarter of the FSAm-NPS index compared with the lowest quarter, multivariable adjusted hazard ratios for all cause and cardiovascular mortality were 1.19 (95% confidence interval 1.04 to 1.35; absolute risk difference 4.3%, 95% confidence interval 1.4% to 7.2%) and 1.32 (1.06 to 1.64; 2.6%, 0.3% to 4.9%), respectively. The hazard ratios were 1.19 (1.05 to 1.36; absolute risk difference 9.7%, 5.0% to 14.3%) and 1.27 (1.02 to 1.58; 5.0%, 1.2% to 8.8%), respectively, for all cause and cardiovascular mortality when the two extreme categories of ultra-processed food intake were compared. When these two indices were analysed jointly, the magnitude of the association of the FSAm-NPS dietary index with all cause and cardiovascular mortality was attenuated by 22.3% and 15.4%, respectively, whereas mortality risks associated with high ultra-processed food intake were not altered.
Conclusions
Adults with the lowest quality diet, as measured using the FSAm-NPS dietary index (underpinning the Nutri-Score), and the highest ultra-processed food consumption (NOVA classification) were at the highest risk for all cause and cardiovascular mortality. A significant proportion of the higher mortality risk associated with an elevated intake of nutrient poor foods was explained by a high degree of food processing. In contrast, the relation between a high ultra-processed food intake and mortality was not explained by the poor quality of these foods.
Introduction
Poor diets are responsible for more deaths than any other risk factor globally and are the leading cause of obesity and non-communicable diseases.1 2 Traditional approaches to improve nutrition and health have focused on nutrients as the key determinants of a healthful diet. This nutrient based approach is the basis of the vast majority of food based dietary guidelines and educational programmes worldwide and emphasises the consumption of foods that are natural sources of fibre, vitamins, and minerals and low in saturated fat, cholesterol, and sodium.3 By contrast, the NOVA classification has been proposed as a novel way to rate foods on the basis of the degree of processing rather than their nutrient content; processing of foods might play a role in health beyond their nutritional composition, through a variety of mechanisms triggered by non-nutritional components, such as cosmetic additives, food contact materials, neoformed compounds, and degradation of the food matrix.4 5 Despite large and well conducted cohort studies indicating that increased amounts of ultra-processed food in the food supply are linked to a higher risk of non-communicable diseases, including obesity and mortality,6 7 8 the role of food processing is still largely ignored or minimised.5 9
Governmental strategies to improve diet quality for reducing the burden of non-communicable diseases include the implementation of labelling systems for printing on the front of packages of food products. A variety of front-of-pack labelling systems exist, including both nutrient specific warning labels (for example, to alert consumers when a given food contains an excessive amount of nutrients that should have lower consumption in the diet) and summary indicators that rate the overall nutritional quality of a product, based on algorithms that take both positive and negative attributes of a food’s nutritional composition into account.10 11 Whatever the labelling system used, the main aim of front-of-pack labels is to inform consumers of the overall nutritional value of foods to help them to make healthier dietary choices and, in some cases, to incentivise food companies to reformulate the nutritional composition of their products.10 11 Front-of-pack labels are agreed to be useful tools to improve people’s diet quality, as their use was found to encourage healthier food purchasing.12 13
In May 2020 the European Commission announced the intended adoption of a harmonised and mandatory front-of-pack nutrition labelling scheme at the EU level by the end of 2022, as part of its Farm-to Fork Strategy.10 14 The Nutri-Score,15 developed by French academic researchers and endorsed in seven European countries, is possibly the most well studied nutritional labelling system; several investigations have supported its effectiveness in improving diet quality and associating with health outcomes.10 16 17 18 19 The Nutri-Score is a five colour coded scheme ranging from dark green to dark orange, associated with letters from A to E, to optimise logo accessibility and understanding by consumers of food products; its main goal is to provide the consumer with the information needed to compare the nutritional quality of products in the same category or consumed for the same purpose.10
Although they have gained much attention from the EU authorities, concerns exist about the effectiveness of the front-of-pack nutrition labelling schemes in improving people’s diet quality. Some of the arguments include the fact that the Nutri-Score system, and theoretically all systems focusing only on nutritional composition, does not cover all the dimensions of the food, which cannot be limited to its nutrient balance.20 21 Some foods can be considered nutritionally adequate although being highly processed and containing many additives, as well as other substances that are not typically used in domestic culinary recipes (for example, modified starches, hydrogenated oils, syrups obtained from cereals, flavours).4 5 Moreover, a large scale meta-analysis of nationally representative samples recently found that the classification by degree of food processing correlates with nutritional quality, further complicating the overall understanding of the effect of these two dimensions on human health.22
To contribute to a better understanding of which dimension of foods plays a major role in defining health risk at the population level, we analysed the individual and joint associations of two food classification systems in relation to all cause and cause specific mortality in a large sample of Italian adults from the Moli-sani Study cohort. As a secondary aim, we analysed some biological mechanisms potentially linking these two dietary indicators to the health outcomes investigated. For the purpose of this study, we analysed the Food Standards Agency Nutrient Profiling System (FSAm-NPS), used to compute the Nutri-Score ranking foods according to their nutritional value,23 and the NOVA classification,4 which evaluates the degree of food processing.
Methods
Study population
We analysed data from the Moli-sani Study, a population based cohort study established in 2005-10 with an enrolment of 24 325 men and women (aged ≥35 years) randomly recruited from the general population of Molise, a southern Italian region. The main purpose was to investigate genetic and environmental risk factors in the onset and progression of cardiovascular, cerebrovascular, and cancer diseases. Exclusion criteria were pregnancy at the time of recruitment, disturbances in mental capacity or decision making impairments, current poly-traumas or coma, or refusal to give informed consent. Details of the study are available elsewhere.24
For the purpose of this study, we excluded participants with missing data on diet, implausible energy intakes (<800 or >4000 kcal/d in men and <500 or >3500 kcal/d in women), incomplete dietary or medical questionnaires, or missing data on cause specific death. We finally analysed 22 895 participants. Supplementary figure A shows the flowchart for selection of study participants.
Dietary assessment and calculation of FSAm-NPS dietary index
We assessed food intake during the year before enrolment by an interviewer administered semiquantitative European Prospective Investigation into Cancer and Nutrition (EPIC) food frequency questionnaire (FFQ) validated and adapted to the Italian population.25 The FFQ contains 14 sections (pasta/rice, soup, meat (excluding salami and other cured meats), fish, raw vegetables, cooked vegetables, eggs, sandwiches, salami and other cured meats, cheese, fruit, bread/wine, milk/coffee/cakes, and herbs/spices), with 248 questions concerning 188 different food items. We asked participants to indicate the number of times they consumed a certain food item (per day, week, month, or year), from which we calculated the absolute frequency of consumption of each item. We assessed the quantity of the food consumed by asking the participant to select an image of a food portion or a predefined standard portion when no image was available. We used 17 sets of pictures, each showing a small, medium, and large portion size, with additional quantifiers (for example, “smaller than the small portion” or “between the small and medium portion”).26
We linked frequencies and quantities of each food to Italian food tables by using specifically designed software to obtain estimates of daily intake of macro-nutrients and micro-nutrients plus energy.26 We evaluated adherence to the traditional Mediterranean diet by using the Mediterranean Diet Score developed by Trichopoulou and colleagues.27
The FSAm-NPS is a modified version of the nutrient profiling system (FSA-NPS), which was initially developed in the UK to regulate television food advertising to children.10 15 We calculated the FSAm-NPS score as was previously done in other population cohorts.18 19 23 For all foods and beverages included in the FFQ, on the basis of composition for each 100 g of content, we allocated 0 to 40 points for nutrients that should be consumed in limited amounts (A points; that is, total sugars (g), saturated fats (g), sodium (mg), and energy (kJ)) and 0 to 15 points for nutrients or components that should be promoted (C points; that is, dietary fibre (g), protein (g), and fruit, vegetables, legumes, and nuts (%)). We calculated the total score of the product by subtracting the sum of C points from the sum of A points. Thus, the final FSAm-NPS score for each food/beverage was based on a scale that could theoretically range from −15 (healthiest food) to 40 (least healthy food). On the basis of this overall FSAm-NPS score, the Nutri-Score labelling system categorises food products into five colours, each associated to letters from A (dark green) to E (dark orange) reflecting their nutritional quality (supplementary table A).15
We calculated the FSAm-NPS dietary index at the individual level as an energy weighted mean of the FSAm-NPS scores of all foods and beverages consumed by each participant by using the following equation, in which FSi represents the score of food/beverage i, Ei the energy intake from food/beverage i specific for each participant, and n the total number of foods/beverages consumed. Increasing values of the FSAm-NPS dietary index therefore reflect decreasing diet quality overall.

bonm070688.fa
NOVA classification
To estimate ultra-processed food intake, we used the NOVA classification that groups foods into four categories reflecting different levels of processing: (a) fresh or minimally processed foods (for example, fruit, meat, milk)—that is, foods altered only by processes such as removal of inedible or unwanted parts, drying, crushing, grinding, fractioning, roasting, boiling, pasteurisation, refrigeration, freezing, placing in containers, vacuum packaging, or non-alcoholic fermentation without the addition of salt, sugar, oils or fats, or other food substances to the original food; (b) processed culinary ingredients (for example, oils, butter, sugar); (c) processed foods (for example, canned fish, unpackaged freshly made breads) that are manufactured by adding salt, sugar, oil, or other processed culinary ingredients to minimally processed foods; or (d) ultra-processed foods defined as industrial formulations made mostly or entirely from substances extracted from foods or derived from food constituents often containing added flavours, colours, emulsifiers, and other cosmetic additives and little or no whole foods (for example, carbonated drinks, processed meat, sweet or savoury packaged snacks).4 28 For the purpose of these analyses, we used the fourth ultra-processed foods category. We summed up the amount consumed (g/d) of each food group from the fourth category of NOVA (a total of 22 foods and beverages) and calculated the proportion (%) of ultra-processed food in the total weight of food and beverages consumed (g/d) by creating a weight ratio. Such an approach is more appropriate than an energy ratio, as it better accounts for non-nutritional factors pertaining to food processing (for example, neo-formed contaminants, additives, and alterations to the structure of raw foods).29
We then divided participants into quarters based on the proportion of ultra-processed food consumed over the total food intake. The full list of foods categorised according to the NOVA classification is available as supplementary table B.
Follow-up for vital status
We followed up the Moli-sani Study cohort for mortality from March 2005 to 31 December 2019. We assessed cause specific mortality by using the Italian mortality registry, validated by Italian death certificates (ISTAT form) and coded according to ICD-9 (international classification of diseases, revision 9). Cardiovascular mortality included deaths from diseases of the circulatory system, when the underlying cause of death included ICD-9 codes 390-459. We used ICD-9 codes 430-438 to define specific cause of death for cerebrovascular disease and ICD-9 codes 410-414 and 429 for ischaemic heart disease. Cancer death was when the underlying cause of death included ICD-9 codes 140-208. We included non-cardiovascular/non-cancer causes of death in an “other cause mortality” group.
Baseline covariates assessment
Personal history of cardiovascular disease (angina, myocardial infarction, revascularisation procedures, peripheral artery diseases, and cerebrovascular events) was self-reported and confirmed by medical records and therapy. Personal history of cancer was self-reported and confirmed by medical records. We considered participants to have diabetes, hypertension, or hyperlipidaemia at baseline if they were taking disease specific drugs.
We expressed leisure time physical activity as daily energy expenditure in metabolic equivalent task hours (MET-h/d) for sport, walking, and gardening. Height and weight were measured, and body mass index was calculated as kg/m2. Blood pressure was measured by an automatic device (OMRON-HEM-705CP) three times on the non-dominant arm, with the average of the last two values being taken as the blood pressure. Measurements were made in a quiet room with comfortable temperature, with the participants lying down for at least five minutes.
We classified participants as never, current, or former smokers (reported not having smoked at all over the previous 12 months or more). Educational level was based on the highest qualification attained and categorised as up to lower secondary (approximately ≤8 years of study), upper secondary school (>8 and ≤13 years), and postsecondary education (>13 years). We classified housing tenure as rented, ownership of one dwelling, and ownership of more than one dwelling.
Selection of cardiovascular risk factors
Key biological mechanisms through which nutrient poor diets and ultra-processed foods may adversely affect health include, among others, altered serum lipid concentrations, inflammation, oxidative stress, dysglycaemia, insulin resistance, and hypertension.30 We selected biomarkers reflecting different underlying pathways to incidence and progression of cardiovascular disease,31 32 as potential mediators of an association between diet and mortality, by subject area knowledge according to the following criteria: previously studied for their relevance in pathways predisposing to cardiovascular disease, shown in epidemiological studies to be related to cardiovascular disease or mortality, and already investigated in the Moli-sani Study cohort. Assessment of blood biomarkers of cardiovascular disease risk in the Moli-sani Study cohort is described in the supplementary materials.
Statistical analysis
We report baseline characteristics of the analytical sample across quarters of the FSAm-NPS dietary index (sex specific) and ultra-processed food intake as means (standard deviations) or percentages for continuous and categorical traits, respectively. We calculated differences in the distribution of baseline covariates across quarters of the dietary exposure by using generalised linear models adjusted for age, sex, and energy intake (GENMOD procedure for categorical variables and GLM procedure for continuous variables in SAS software) (table 1 and table 2).
Table 1.
Baseline characteristics of participants from Moli-sani Study cohort (n=22 895) overall and across quarters of the Food Standards Agency nutrient profiling system (FSAm-NPS) dietary index. Values are numbers (percentages) unless stated otherwise
| Characteristics | All (n=22 895) | Quarters of FSAm-NPS dietary index | P value* | |||
|---|---|---|---|---|---|---|
| 1 (n=5723) | 2 (n=5724) | 3 (n=5725) | 4 (n=5723) | |||
| Mean (IQR) FSAm-NPS dietary index | 7.4 (6.7 to 8.4) | 5.5 (5.1 to 6.4) | 7.2 (7.0 to 7.4) | 8.0 (7.8 to 8.2) | 9.0 (8.6 to 9.2) | - |
| Mean (IQR) ultra-processed food intake, weight ratio | 10.8 (6.0 to 13.9) | 8.2 (4.3 to 10.6) | 9.8 (5.7 to 12.4) | 11.6 (6.9 to 14.5) | 13.6 (8.6 to 17.1) | - |
| Mean (SD) age, years | 55.4 (11.7) | 60.2 (11.7) | 56.8 (11.5) | 53.9 (11.1) | 50.8 (10.4) | <0.001 |
| Male sex | 10 922 (47.7) | 2730 (47.7) | 2731 (47.7) | 2731 (47.7) | 2730 (47.7) | 1.00 |
| Educational level: | <0.001 | |||||
| Up to lower secondary | 11 952 (52.2) | 3412 (59.6) | 3172 (55.4) | 2946 (51.5) | 2422 (42.3) | |
| Upper secondary | 7975 (34.8) | 1648 (28.8) | 1866 (32.6) | 2080 (36.3) | 2381 (41.6) | |
| Postsecondary | 2949 (12.9) | 659 (11.5) | 677 (11.8) | 695 (12.1) | 918 (16.0) | |
| Missing data | 19 (0.1) | 4 (0.1) | 9 (0.2) | 4 (0.1) | 2 (<0.1) | |
| Housing: | 0.06 | |||||
| Rent | 2010 (8.8) | 478 (8.4) | 424 (7.4) | 516 (9.0) | 592 (10.3) | |
| 1 dwelling ownership | 18 817 (82.2) | 4721 (82.5) | 4774 (83.4) | 4739 (82.8) | 4583 (80.1) | |
| >1 dwelling ownership | 2026 (8.8) | 513 (9.0) | 511 (8.9) | 464 (8.1) | 538 (9.4) | |
| Missing data | 42 (0.2) | 11 (0.2) | 15 (0.3) | 6 (0.1) | 10 (0.2) | |
| Urban residence | 15 380 (67.2) | 3825 (66.8) | 3775 (66.0) | 3818 (66.7) | 3962 (69.2) | <0.001 |
| Smoking status: | 0.60 | |||||
| Non-smoker | 11 357 (49.6) | 2957 (51.7) | 2871 (50.2) | 2817 (49.2) | 2712 (47.4) | |
| Current smoker | 5259 (23.0) | 1049 (18.3) | 1203 (21.0) | 1385 (24.2) | 1622 (28.3) | |
| Former smoker | 6263 (27.4) | 1713 (29.9) | 1647 (28.8) | 1519 (26.5) | 1384 (24.2) | |
| Missing data | 16 (0.1) | 4 (0.1) | 3 (0.1) | 4 (0.1) | 5 (0.1) | |
| Mean (SD) leisure time physical activity, MET-h/day† | 3.5 (4.0) | 3.9 (4.2) | 3.7 (4.2) | 3.5 (3.9) | 3.2 (3.8) | <0.001 |
| Mean (SD) body mass index† | 28.0 (4.7) | 28.0 (4.8) | 28.1 (4.7) | 28.1 (4.7) | 27.9 (4.7) | 0.03 |
| Cardiovascular disease: | 0.004 | |||||
| No | 21 347 (93.2) | 5120 (89.5) | 5324 (93.0) | 5408 (94.5) | 5495 (96.0) | |
| Yes | 1187 (5.2) | 491 (8.6) | 313 (5.5) | 228 (4.0) | 155 (2.7) | |
| Missing data | 361 (1.6) | 112 (2.0) | 87 (1.5) | 89 (1.6) | 73 (1.3) | |
| Cancer: | 0.70 | |||||
| No | 22 017 (96.2) | 5435 (95.0) | 5492 (95.9) | 5524 (96.5) | 5566 (97.3) | |
| Yes | 794 (3.5) | 261 (4.6) | 208 (3.6) | 183 (3.2) | 142 (2.5) | |
| Missing data | 84 (0.4) | 27 (0.5) | 24 (0.4) | 18 (0.3) | 15 (0.3) | |
| Diabetes: | <0.001 | |||||
| No | 21 517 (94.0) | 5113 (89.3) | 5375 (93.9) | 5480 (95.7) | 5549 (97.0) | |
| Yes | 1095 (4.8) | 525 (9.2) | 276 (4.8) | 178 (3.1) | 116 (2.0) | |
| Missing data | 283 (1.2) | 85 (1.5) | 73 (1.3) | 67 (1.2) | 58 (1.0) | |
| Hypertension: | <0.001 | |||||
| No | 16 260 (71.0) | 3458 (60.4) | 3942 (68.9) | 4272 (74.6) | 4588 (80.2) | |
| Yes | 6474 (28.3) | 2222 (38.8) | 1735 (30.3) | 1419 (24.8) | 1098 (19.2) | |
| Missing data | 161 (0.7) | 43 (0.8) | 47 (0.8) | 34 (0.6) | 37 (0.6) | |
| Hyperlipidaemia: | <0.001 | |||||
| No | 20 929 (91.4) | 4885 (85.4) | 5224 (91.3) | 5361 (93.6) | 5459 (95.4) | |
| Yes | 1759 (7.7) | 771 (13.5) | 440 (7.7) | 321 (5.6) | 227 (4.0) | |
| Missing data | 207 (0.9) | 67 (1.2) | 60 (1.0) | 43 (0.8) | 37 (0.6) | |
IQR=interquartile range; SD=standard deviation.
Means were adjusted for age, sex, and energy intake.
Obtained using generalised linear models for both continuous and categorical dependent variables adjusted for age, sex, and energy intake.
Data for leisure time physical activity and body mass index were missing for 197 and 13 participants, respectively.
Table 2.
Baseline characteristics of participants from Moli-sani Study cohort (n=22 895) across quarters of ultra-processed food intake (weight ratio). Values are numbers (percentages) unless stated otherwise
| Characteristics | Quarters of ultra-processed food intake | P value* | |||
|---|---|---|---|---|---|
| 1 (n=5723) | 2 (n=5724) | 3 (n=5724) | 4 (n=5724) | ||
| Mean (IQR) FSAm-NPS dietary index | 6.7 (6.0 to 7.8) | 7.3 (6.7 to 8.2) | 7.7 (7.1 to 8.5) | 8.0 (7.3 to 8.8) | - |
| Mean (IQR) ultra-processed food intake, weight ratio | 4.1 (3.2 to 5.3) | 7.7 (6.8 to 8.5) | 11.5 (10.3 to 12.6) | 19.9 (15.6 to 22.0) | - |
| Mean (SD) age, years | 61.5 (10.7) | 56.9 (11.3) | 53.2 (10.9) | 50.1 (10.8) | <0.001 |
| Male sex | 3347 (58.5) | 2798 (48.9) | 2488 (43.5) | 2289 (40.0) | <0.001 |
| Educational level: | <0.001 | ||||
| Up to lower secondary | 3664 (64.0) | 3141 (54.9) | 2706 (47.3) | 2441(42.6) | |
| Upper secondary | 1529 (26.7) | 1845 (32.2) | 2212 (38.6) | 2389 (41.7) | |
| Postsecondary | 527 (9.2) | 727 (12.7) | 805 (14.1) | 890 (15.5) | |
| Missing data | 3 (0.1) | 11 (0.2) | 1 (<0.1) | 4 (0.1) | |
| Housing: | 0.04 | ||||
| Rent | 411 (7.2) | 460 (8.0) | 519 (9.1) | 620 (10.8) | |
| 1 dwelling ownership | 4790 (83.7) | 4731 (82.7) | 4678 (81.7) | 4618 (80.7) | |
| >1 dwelling ownership | 510 (8.9) | 518 (9.0) | 517 (9.0) | 481 (8.4) | |
| Missing data | 12 (0.2) | 15 (0.3) | 10 (0.2) | 5 (0.1) | |
| Urban residence | 3722 (65.0) | 3787 (66.2) | 3918 (68.4) | 3953 (69.1) | <0.001 |
| Smoking status: | 0.003 | ||||
| Non-smoker | 2583 (45.1) | 2842 (49.7) | 2912 (50.9) | 3020 (52.8) | |
| Current smoker | 1198 (20.9) | 1240 (21.7) | 1338 (23.4) | 1483 (25.9) | |
| Former smoker | 1938 (33.9) | 1638 (28.6) | 1470 (25.7) | 1217 (21.3) | |
| Missing data | 4 (0.1) | 4 (0.1) | 4 (0.1) | 4 (0.1) | |
| Mean (SD) leisure time physical activity, MET-h/day† | 3.8 (4.5) | 3.6 (4.0) | 3.5 (3.6) | 3.4 (3.8) | <0.001 |
| Mean (SD) body mass index† | 28.2 (4.6) | 28.2 (4.7) | 27.9 (4.7) | 27.6 (4.9) | <0.001 |
| Cardiovascular disease: | 0.3 | ||||
| No | 5145 (89.9) | 5316 (92.9) | 5429 (94.8) | 5457 (95.3) | |
| Yes | 463 (8.1) | 322 (5.6) | 210 (3.7) | 192 (3.4) | |
| Missing data | 115 (2.0) | 86 (1.5) | 85 (1.5) | 75 (1.3) | |
| Cancer: | 0.3 | ||||
| No | 5455 (95.3) | 5508 (96.2) | 5513 (96.3) | 5541 (96.8) | |
| Yes | 239 (4.2) | 190 (3.3) | 201 (3.5) | 164 (2.9) | |
| Missing data | 29 (0.5) | 26 (0.5) | 10 (0.2) | 19 (0.3) | |
| Diabetes: | <0.001 | ||||
| No | 5119 (89.4) | 5351 (93.5) | 5485 (95.8) | 5562 (97.2) | |
| Yes | 516 (9.0) | 308 (5.4) | 170 (3.0) | 101 (1.8) | |
| Missing data | 88 (1.5) | 65 (1.1) | 69 (1.2) | 61 (1.1) | |
| Hypertension: | 0.08 | ||||
| No | 3458 (60.4) | 3896 (68.1) | 4286 (74.9) | 4620 (80.7) | |
| Yes | 2208 (38.6) | 1794 (31.3) | 1405 (24.5) | 1067 (18.6) | |
| Missing data | 57 (1.0) | 34 (0.6) | 33 (0.6) | 37 (0.7) | |
| Hyperlipidaemia: | <0.001 | ||||
| No | 4970 (86.8) | 5163 (90.2) | 5369 (93.8) | 5427 (94.8) | |
| Yes | 683 (11.9) | 497 (8.7) | 317 (5.5) | 262 (4.6) | |
| Missing data | 70 (1.2) | 64 (1.1) | 38 (0.7) | 35 (0.6) | |
FSAm-NPS=Food Standards Agency nutrient profiling system; IQR=interquartile range; SD=standard deviation
Means were adjusted for age, sex, and energy intake.
Obtained using generalised linear models for both continuous and categorical dependent variables adjusted for age, sex, and energy intake.
Data for leisure time physical activity and body mass index were missing for 197 and 13 participants, respectively.
We examined associations of FSAm-NPS dietary index and ultra-processed food intake (quarters and per 1 standard deviation increment) with all cause and cause specific mortality through multivariable cause specific Cox proportional hazards with time on study as the timescale and adjusting for baseline age as a covariate in the model. We visually assessed the proportional hazards assumption (log(−log) plots of survival curves) and identified no violation. We calculated multivariable adjusted hazard ratios across quarters of both dietary exposures (quarter 1 as reference) and included sex, age (continuous), energy intake (continuous), educational level (up to lower secondary, upper secondary, post-secondary), housing tenure (rented, one dwelling ownership, more than one dwelling ownership), smoking (never, current, former smokers), body mass index (continuous), leisure time physical activity (continuous), history of cancer (no/yes), history of cardiovascular disease (no/yes), diabetes (no/yes), hypertension (no/yes), hyperlipidaemia (no/yes), and residence (urban, rural). Participants contributed person time until their date of death, date of emigration, or loss to follow-up or until end of follow-up, whichever occurred first. We included participants who died from a cause other than the one under study and censored them at the date of the competing death event. We defined potential confounders a priori and identified them on the basis of existing literature, rather than deferring to statistical criteria.33
We calculated the absolute risk difference for hazard ratios derived from the previously described models.34 We used the mean value of the included covariates and calculated the difference in absolute risk at the maximum follow-up time. We applied the bootstrap method to derive 95% confidence intervals for absolute risk difference based on 500 bootstrap samples. To maximise data availability, we handled missing data on covariates (see flowchart in supplementary figure A) by using multiple imputation (SAS PROC MI, followed by PROC MIANALYZE; n=10 imputed datasets).
We considered a biomarker as potentially mediating the association of the FSAm-NPS dietary index or ultra-processed food intake with all cause and cause specific mortality if it was on the causal pathway of these associations and if it was associated with both the exposure and the outcome, in accordance with predefined mediation principles.35 We tested these criteria in distinct multivariable regression models for each potential mediator individually (supplementary tables C and D) and through cause specific Cox models including ultra-processed food consumption (continuous) or the FSAm-NPS dietary index (continuous) as a covariate (supplementary tables E and F).
To quantify how much of the association between the FSAm-NPS dietary index (or ultra-processed food intake) and health outcomes was explained by ultra-processed food intake (or by the FSAm-NPS dietary index), we relied on a traditional change-in-estimate method—that is, the quantification of the percentage reduction in the β coefficient for the FSAm-NPS dietary index (or ultra-processed food intake; β0) after inclusion of ultra-processed food intake (or the FSAm-NPS dietary index; β1) to the multivariable model. We calculated the attenuation (%) according to the following equation: 100×(β0–β1)/(β0), where β0=natural log (HR0) and β1=natural log (HR1). HR0 is the multivariable hazard ratio for one dietary exposure (for example, FSAm-NPS dietary index) not adjusted for the other (for example, ultra-processed food intake), and HR1 is the multivariable hazard ratio for the same dietary exposure resulting from the multivariable model further including the other dietary exposure. A negative attenuation indicates no mediation effect (that is, HR1>HR0). We calculated a 95% confidence interval around the percentage attenuation by using a bootstrap method with 1000 re-samplings for each imputed dataset.
The multivariable model also served as the reference for the analysis used to estimate the extent to which selected cardiovascular risk factors explained the association of the FSAm-NPS dietary index or ultra-processed food intake with all cause and cause specific mortality; for this, each marker was alternately, and at the end simultaneously, included into the multivariable adjusted model. For the mediation analysis, we used the publicly available %MEDIATE macro in SAS,36 which calculates the point and interval estimates of the percentage of exposure effect explained by one or more intermediate variables, with 95% confidence intervals and P values.
To test the robustness of the associations, we did sensitivity analyses by excluding participants with a history of cancer, cardiovascular disease, and diabetes; excluding participants with baseline diabetes, hypertension, and hyperlipidaemia (therefore assessing a potential bias resulting from modified habitual dietary intakes due to illness, such as indications to switch to a healthier diet); and using ultra-processed foods as an energy ratio (percentage of calories from ultra-processed foods on the total calories consumed daily), both as exposure and explanatory factors. We used SAS/STAT software, version 9.4, for data analysis.
Patient and public involvement
No patients were involved in setting the research question or the outcome measures; nor were they involved in developing plans for recruitment, design, or implementation of the study. No patients were asked to advise on the interpretation or writing up of results. Experts in communication and scientific dissemination were originally involved in the design of the study in order to develop effective strategies for public dissemination. All participants are periodically informed about the advancements of the study via an annual calendar, which contains information on the research activity related to the study, along with newsletters that are periodically sent to participants or published on the website of the Moli-sani Study (http://www.moli-sani.org/). Regular meetings are also organised with participants from each of the 30 towns/villages from the Molise region, randomly recruited in the cohort, to share the results of the study.
Results
The analytical sample consisted of 11 973 (52.3%) women and 10 922 (47.7%) men, with a mean age at enrolment of 55.4 (SD 11.7) years, a mean FSAm-NPS dietary index of 7.4 (1.4), and a mean ultra-processed food weight ratio of 10.8% (6.7%); the average energy from ultra-processed foods was 18.3% (8.4%; range 8.4-70.0%) of the total calories consumed daily. Although the means of both dietary indices are broadly the same (although in different units), the standard deviations are very different, with the standard deviation for ultra-processed foods being more than four times larger than that for the FSAm-NPS dietary index. As a result, the means for the four quarters are much more different in table 2 than in table 1. The correlation of FSAm-NPS dietary index with ultra-processed food intake was low to moderate (Spearman correlation coefficient=0.34).
Compared with participants in the bottom quarter, those scoring higher on the FSAm-NPS dietary index (quarter 4) were younger and more educated, lived prevalently in urban areas, had lower body mass index, practised less physical activity, and had lower prevalence of chronic conditions at baseline, with the exception of cancer (table 1). Findings were similar for the comparison of the lowest and highest quarters of ultra-processed food intake, with some differences including sex (men tended to consume less ultra-processed food than women), smoking status (current smokers were more represented in the highest quarter of ultra-processed food intake than the lowest), and distribution of baseline cardiovascular disease (observed across FSAm-NPS dietary index quarters but not across ultra-processed food intake quarters) (table 2).
Regarding nutritional factors, the two scores shared many similarities. Higher levels of FSAm-NPS dietary index or ultra-processed food intake were inversely associated with adherence to a Mediterranean diet, monounsaturated-to-saturated fat ratio, and consumption of fruits and nuts, vegetables, cereals, legumes, fish, alcohol, starch, fibre, and protein. Increases in both scores positively correlated with energy intake, total fat, saturated fat, polyunsaturated fat, and dietary cholesterol (table 3).
Table 3.
Associations of Food Standards Agency nutrient profiling system (FSAm-NPS) dietary index and ultra-processed food (weight ratio) with nutritional factors in Moli-sani Study cohort (n=22 895)
| Nutritional factors | Mean* (SD) | Change in nutritional factor | β ratio=β1/β2 (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| FSAm-NPS dietary index | Ultra-processed food | ||||||
| β1 † (SE) | P value‡ | β2 † (SE) | P value‡ | ||||
| Mediterranean diet score | 4.4 (1.6) | −0.38 (0.011) | <0.001 | −0.39 (0.011) | <0.001 | 0.97 (0.90 to 1.04) | |
| Energy intake, kcal/d | 2081 (575) | 151 (3.5) | <0.001 | 87 (3.7) | <0.001 | 1.74 (1.58 to 1.90) | |
| Fruits and nuts, g/d | 355 (200) | −26 (1.4) | <0.001 | −35 (1.3) | <0.001 | 0.74 (0.65 to 0.84) | |
| Vegetables, g/d | 160 (71) | −13 (0.47) | <0.001 | −14 (0.47) | <0.001 | 0.91 (0.83 to 1.00) | |
| Cereals, g/d | 206 (92) | −7.6 (0.47) | <0.001 | −17 (0.46) | <0.001 | 0.44 (0.38 to 0.50) | |
| Legumes, g/d | 27 (21) | −3.6 (0.14) | <0.001 | −1.1 (0.15) | <0.001 | 3.15 (2.30 to 4.01) | |
| Fish, g/d | 45 (26) | −3.6 (0.19) | <0.001 | −1.3 (0.19) | <0.001 | 2.72 (1.91 to 3.52) | |
| MUFA/SFA ratio | 1.4 (0.29) | −0.10 (0.0019) | <0.001 | −0.076 (0.0020) | <0.001 | 1.25 (1.19 to 1.31) | |
| Milk and dairy products, g/d | 186 (122) | 0.89 (0.83) | 0.3 | 5.9 (0.82) | <0.001 | 0.15 (−0.13 to 0.43) | |
| Meat and meat products, g/d | 103 (44) | 0.39 (0.27) | 0.2 | −1.4 (0.27) | <0.001 | −0.28 (−0.68 to 0.11) | |
| Alcohol intake, g/d | 16 (22) | −0.85 (0.13) | <0.001 | −5.6 (0.12) | <0.001 | 0.15 (0.11 to 0.20) | |
| Carbohydrate, % TEI | 49 (7.0) | −0.78 (0.049) | <0.001 | 0.80 (0.049) | <0.001 | −0.98 (−1.15 to −0.80) | |
| Sugar, g/d | 92 (36) | 0.33 (0.20) | 0.1 | 10 (0.19) | <0.001 | 0.03 (−0.01 to 0.07) | |
| Starch, g/d | 161 (64) | −4.0 (0.29) | <0.001 | −5.7 (0.28) | <0.001 | 0.71 (0.59 to 0.83) | |
| Fibre intake, g/d | 20 (6.6) | −1.4 (0.034) | <0.001 | −1.0 (0.034) | <0.001 | 1.32 (1.22 to 1.41) | |
| Protein, % TEI | 16 (2.2) | −0.051 (0.014) | <0.001 | −0.15 (0.014) | <0.001 | 0.35 (0.15 to 0.54) | |
| Fat, % TEI | 33 (5.6) | 0.93 (0.037) | <0.001 | 0.98 (0.037) | <0.001 | 0.95 (0.85 to 1.05) | |
| Saturated fat, % TEI | 12 (2.6) | 0.81 (0.017) | <0.001 | 0.68 (0.017) | <0.001 | 1.19 (1.11 to 1.26) | |
| Saturated fat, g/d | 27 (9.6) | 1.8 (0.041) | <0.001 | 1.7 (0.041) | <0.001 | 1.01 (0.95 to 1.08) | |
| MUFA, % TEI | 16 (3.0) | 0.026 (0.020) | 0.2 | 0.069 (0.020) | 0.001 | 0.38 (−0.22 to 0.98) | |
| PUFA, % TEI | 3.5 (0.64) | 0.019 (0.0044) | <0.001 | 0.099 (0.0043) | <0.001 | 0.19 (0.11 to 0.27) | |
| Dietary cholesterol, mg/d | 318 (108) | 16 (0.53) | <0.001 | 17 (0.53) | <0.001 | 0.93 (0.85 to 1.02) | |
| Sodium, mg/d | 2316 (851) | 61 (3.6) | <0.001 | −16 (3.6) | <0.001 | −3.71 (−5.34 to −2.07) | |
CI=confidence interval; MUFA=monounsaturated fats; PUFA=polyunsaturated fats; SD=standard deviation; SE=standard error; SFA=saturated fats; TEI=total energy intake.
Unadjusted means.
Change for a 1 SD increase in FSAm-NPS dietary index or ultra-processed food intake (weight ratio).
P values were obtained from linear regression models adjusted for sex, age, and energy intake.
We observed divergent association for meats, which were inversely associated with ultra-processed food intake but not with the FSAm-NPS dietary index, and for milk and dairy products, which were directly correlated only with ultra-processed food intake. Energy from carbohydrate decreased according to the FSAm-NPS dietary index but increased with ultra-processed food intake, and sugar was directly associated with ultra-processed food intake but not with the FSAm-NPS dietary index. Monounsaturated fatty acids increased with ultra-processed food intake, and sodium was positively associated with the FSAm-NPS dietary index and inversely with ultra-processed food intake (table 3).
An increase in the FSAm-NPS dietary index was directly associated with higher concentrations of C reactive protein, white blood cell count, granulocyte-to-lymphocyte ratio, insulin, C-peptide, apolipoprotein B100, cystatin C, and diastolic blood pressure, while being inversely linked to blood glucose concentrations, high density lipoprotein cholesterol, triglycerides, lipoprotein (a), and serum vitamin D (supplementary table G). A higher proportion of ultra-processed foods in the diet positively correlated with granulocyte-to-lymphocyte ratio, insulin, cystatin C, creatinine, and heart rate and inversely with blood glucose, blood cholesterol, high density lipoprotein cholesterol, triglycerides, apolipoprotein A, apolipoprotein B100, lipoprotein (a), and systolic and diastolic blood pressure (supplementary table G). Most of these associations withstood multivariable adjusted regression analyses, with some exceptions, such as total blood cholesterol, which became inversely associated with the FSAm-NPS dietary index (supplementary tables C and D).
Of 10 risk factors (insulin, C-peptide, total blood cholesterol, triglycerides, lipoprotein (a), C reactive protein, white blood cell count, granulocyte-to-lymphocyte ratio, diastolic blood pressure, and serum vitamin D) associated with the FSAm-NPS dietary index in cross sectional analysis, eight (insulin, C-peptide, total blood cholesterol, C reactive protein, white blood cell count, granulocyte-to-lymphocyte ratio, diastolic blood pressure, and serum vitamin D) and five (C reactive protein, white blood cell count, granulocyte-to-lymphocyte ratio, diastolic blood pressure, and serum vitamin D) were also associated with all cause and cardiovascular disease mortality, respectively, in a multivariable model also adjusted for the FSAm-NPS dietary index (supplementary table E); we therefore included them in the mediation analysis. For 17 of 18 risk factors associated with ultra-processed food intake (all markers with the exception of serum vitamin D), 12 were associated with all cause mortality (cystatin C, creatinine, blood glucose, insulin, C-peptide, total blood cholesterol, C reactive protein, white blood cell count, granulocyte-to-lymphocyte ratio, diastolic and systolic blood pressure, and heart rate) and eight with cardiovascular disease mortality (cystatin C, creatinine, C reactive protein, white blood cell count, granulocyte-to-lymphocyte ratio, diastolic and systolic blood pressure, and heart rate) in a multivariable adjusted model also including ultra-processed food intake (supplementary table F).
Association with mortality
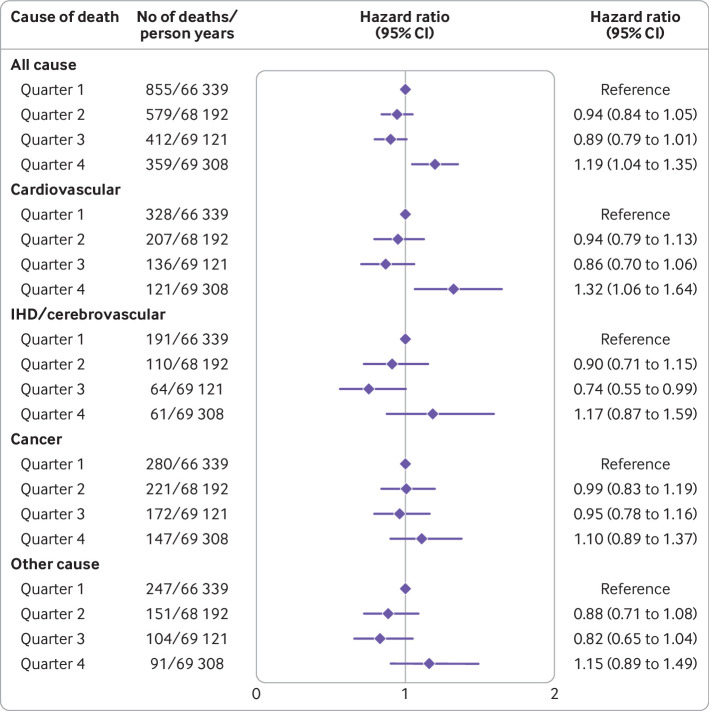
Among 2205 deaths from any cause that occurred over a median follow-up of 12.2 (interquartile range 11.2-13.2) years (272 960 person years), 792 were attributed to cardiovascular disease, of which 426 were due to ischaemic heart disease/cerebrovascular disease, 820 to cancer, and 593 to other causes. In the multivariable adjusted model controlled for sociodemographic and clinical factors, the hazard ratios associated with the highest quarter of the FSAm-NPS dietary index were 1.19 (95% confidence interval 1.04 to 1.35; absolute risk difference 4.3%, 95% confidence interval 1.4% to 7.2%) for all cause mortality and 1.32 (1.06 to 1.64; 2.6%, 0.3% to 4.9%) for cardiovascular disease mortality (fig 1; supplementary table H). We found no associations with the other causes of death (fig 1).
Fig 1.
All cause and cause specific mortality estimates across quarters of Food Standards Agency nutrient profiling system (FSAm-NPS) dietary index in Moli-sani Study cohort (n=22 895), using data obtained from multiple imputation. Hazard ratios with 95% confidence intervals obtained from multivariable cause specific Cox proportional hazards regression models including sex, age (continuous), energy intake (continuous), educational level (up to lower secondary, upper secondary, post-secondary), housing tenure (rent, 1 dwelling ownership, >1 dwelling ownership), smoking (never, current, former smokers), body mass index (continuous), leisure time physical activity (continuous), history of cancer (no/yes), history of cardiovascular disease (no/yes), diabetes (no/yes), hypertension (no/yes), hyperlipidaemia (no/yes), and residence (urban, rural). IHD=ischaemic heart disease
The inclusion of ultra-processed food intake (weight ratio; continuous) into the multivariable model attenuated the association of the FSAm-NPS dietary index with all cause mortality by 22.3% (95% confidence interval 16.4% to 30.2%) and mitigated that with cardiovascular disease mortality by 15.4% (10.5% to 22.6%) (table 4). Consistently, absolute risk differences were also reduced (supplementary table H).
Table 4.
Ultra-processed food (weight ratio) and Food Standards Agency nutrient profiling system (FSAm-NPS) dietary index as explanatory factors of their respective association with all cause and cardiovascular disease mortality
| Outcome | FSAm-NPS dietary index (Q4 v Q1) | FSAm-NPS dietary index (Q4 v Q1) + UPF (continuous) | UPF (Q4 v Q1) | UPF (Q4 v Q1) + FSAm-NPS dietary index (continuous) | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | Attenuation, % (95% CI) | HR (95% CI) | HR (95% CI) | Attenuation, % (95% CI) | |
| All cause mortality | 1.19 (1.04 to 1.35) | 1.14 (1.00 to 1.31) | 22.3 (16.4 to 30.2) | 1.19 (1.05 to 1.36) | 1.20 (1.05 to 1.37) | −3.3 (−7.3 to 0.3) |
| Cardiovascular disease mortality | 1.32 (1.06 to 1.64) | 1.26 (1.01 to 1.58) | 15.4 (10.5 to 22.6) | 1.27 (1.02 to 1.58) | 1.27 (1.02 to 1.59) | 0.0 (−5.0 to 4.9) |
CI=confidence interval; HR=hazard ratio; Q1=quarter 1; Q4=quarter 4; UPF=ultra-processed food.
Hazard ratios with 95% CIs obtained from multivariable cause specific Cox proportional hazards regression models, using data obtained from multiple imputation (SAS PROC MI, followed by PROC MIANALYZE in SAS; n=10 imputed datasets).
Multivariable adjusted model was controlled for sex, age (continuous), energy intake (continuous), educational level (up to lower secondary, upper secondary, post-secondary), housing tenure (rent, 1 dwelling ownership, >1 dwelling ownership), smoking (never, current, former smokers), body mass index (continuous), leisure time physical activity (continuous), history of cancer (no/yes), history of cardiovascular disease (no/yes), diabetes (no/yes), hypertension (no/yes), hyperlipidaemia (no/yes), and residence (urban, rural).
Attenuation represents proportion of FSAm-NPS dietary index (or UPF consumption)-mortality association explained by UPF as weight ratio (or by FSAm-NPS dietary index), and was determined by calculating per cent attenuation in β coefficient for FSAm-NPS dietary index (or UPF intake; β0) after inclusion of UPF (or FSAm-NPS dietary index; β1) to multivariable adjusted model as follows: 100×(β0–β1)/(β0). 95% CI around percentage attenuation was obtained by using bootstrap method with 1000 re-samplings.
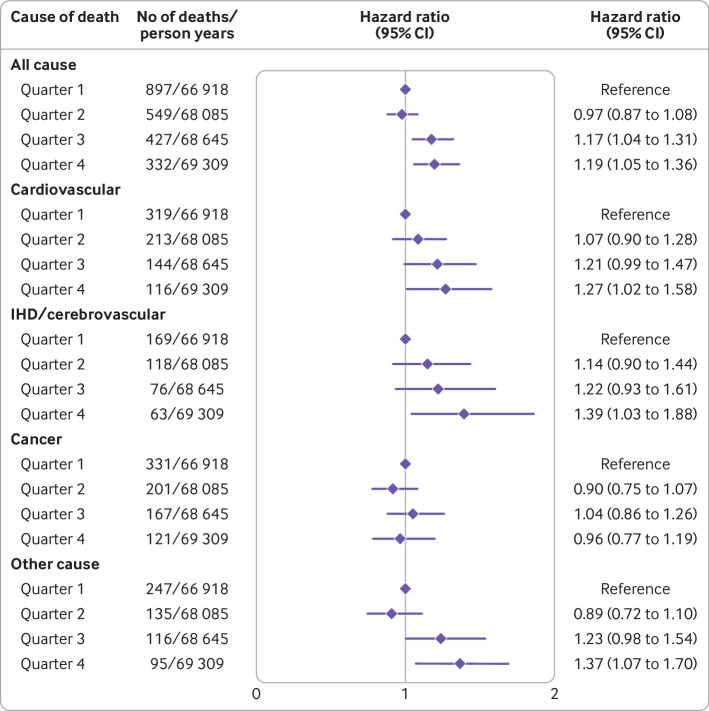
Compared with the lowest quarter, an elevated intake of ultra-processed foods was associated with a higher hazard of all cause mortality (hazard ratio 1.19, 1.05 to 1.36 for quarter 4 versus quarter 1; absolute risk difference 9.7%, 5.0% to 14.3%), cardiovascular disease mortality (1.27, 1.02 to 1.58; 5.0%, 1.2% to 8.8%) (fig 2; supplementary table H), ischaemic heart disease/cerebrovascular disease mortality (1.39, 1.03 to 1.88; 3.0%, 0.1% to 5.9%), and other cause mortality (1.37, 1.07 to 1.70; 7.3%, −2.7% to 17.4%) (fig 2). By contrast with the FSAm-NPS dietary index, for which excess risk was restricted to the highest quarter, these hazard ratios increased close to monotonically across quarters.
Fig 2.
All cause and cause specific mortality estimates across quarters of ultra-processed food intake (weight ratio) as defined by NOVA classification in Moli-sani Study cohort (n=22 895), using data obtained from multiple imputation. Hazard ratios with 95% confidence intervals obtained from multivariable cause specific Cox proportional hazards regression models including sex, age (continuous), energy intake (continuous), educational level (up to lower secondary, upper secondary, post-secondary), housing tenure (rent, 1 dwelling ownership, >1 dwelling ownership), smoking (never, current, former smokers), body mass index (continuous), leisure time physical activity (continuous), history of cancer (no/yes), history of cardiovascular disease (no/yes), diabetes (no/yes), hypertension (no/yes), hyperlipidaemia (no/yes), and residence (urban, rural). IHD=ischaemic heart disease
The inclusion of the FSAm-NPS dietary index (continuous) into the multivariable model did not substantially alter the strength of associations between ultra-processed food intake and mortality risks either as hazard ratios (table 4) or as absolute risk differences (supplementary table H). Analyses using ultra-processed food intake as an energy ratio, both as an exposure and as an explanatory factor, yielded similar results (supplementary table I).
Results did not substantially change in sensitivity analyses, showing that the excess risk of all cause and cardiovascular disease mortality associated with a higher FSAm-NPS dietary index was explained, at least in part, by an elevated degree of food processing, whereas this was not the case for the ultra-processed food-mortality associations (supplementary tables J and K).
Analyses of biological pathways
The excess risk of all cause mortality associated with higher FSAm-NPS dietary index was partly accounted for by altered markers of glucose metabolism and serum cholesterol, which explained 8.9% and 4.2% of this association; all markers explained 18.6% (P<0.001) (table 5). For ultra-processed food intake, the excess of all cause mortality in the highest quarter was largely explained by altered levels of biomarkers reflecting renal function (26.0%; P<0.001) followed by inflammatory markers (10.0%; P=0.007), total blood cholesterol (8.5%; P<0.001), and markers of glucose metabolism (5.8%; P=0.02). Altogether, these factors explained up to 32.7% (P<0.001) of the association of heavy ultra-processed food intake with all cause mortality. The association of high ultra-processed food consumption with cardiovascular disease mortality was mediated by biomarkers of renal function and inflammation (table 5).
Table 5.
Blood biomarkers and established cardiovascular disease risk factors as mediators of association of Food Standards Agency nutrient profiling system (FSAm-NPS) dietary index or ultra-processed food intake (weight ratio) with all cause and cardiovascular disease mortality among 22 895 participants from Moli-sani Study cohort (2005-10)
| Risk factors | FSAm-NPS dietary index | Ultra-processed food | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All cause mortality | Cardiovascular disease mortality | All cause mortality | Cardiovascular disease mortality | ||||||||
| Proportion mediated (95% CI)* | P value | Proportion mediated (95% CI)* | P value | Proportion mediated (95% CI)* | P value | Proportion mediated (95% CI)* | P value | ||||
| Multivariable model + markers of glucose metabolism† | 8.9 (4.0 to 18.5) | <0.001 | - | - | 5.8 (1.9 to 16.9) | 0.02 | - | - | |||
| Multivariable model + markers of renal function‡ | - | - | - | - | 26.0 (12.1 to 47.4) | <0.001 | 21.8 (8.7 to 44.8) | <0.001 | |||
| Multivariable model + blood cholesterol | 4.2 (1.4 to 12.0) | 0.02 | - | - | 8.5 (3.8 to 17.9) | <0.001 | - | - | |||
| Multivariable model + markers of inflammation§ | 3.5 (0.3 to 28.8) | 0.2 | 4.5 (0.7 to 22.7) | 0.1 | 10.0 (3.6 to 25.1) | 0.007 | 10.6 (3.3 to 29.5) | 0.01 | |||
| Multivariable model + systolic and diastolic BP + heart rate | - | - | - | - | 2.1 (0.0 to 58.8) | 0.3 | NM | NM | |||
| Multivariable model + diastolic BP | NM | NM | NM | NM | - | - | - | - | |||
| Multivariable model + serum vitamin D | 2.8 (0.4 to 17.8) | 0.1 | 1.2 (0.1 to 15.7) | 0.2 | - | - | - | - | |||
| Multivariable model + all explanatory factors | 18.6 (7.6 to 38.7) | <0.001 | 3.7 (0.3 to 32.0) | 0.2 | 32.7 (13.9 to 59.3) | <0.001 | 17.4 (5.0 to 45.6) | 0.02 | |||
BP=blood pressure; CI=confidence interval; NM=not mediating.
Proportion of effect explained by intermediate variables with 95% CI and relevant P value as produced by %MEDIATE macro in SAS are reported for each potential mediator, in multivariable adjusted Cox proportional hazards models controlled for sex, age (continuous), energy intake (continuous), educational level (categorical), housing tenure (categorical), smoking (categorical), body mass index (continuous), leisure time physical activity (continuous), history of cancer (no/yes), history of cardiovascular disease (no/yes), diabetes (no/yes), hypertension (no/yes), hyperlipidaemia (no/yes), and residence (categorical). Proportion refers to quarter 4 versus quarter 1 of each dietary index. Mediation analyses were generated using first imputed dataset. Other imputed datasets were similar and thus omitted.
Markers of glucose metabolism include insulin and C-peptide serum concentrations. For analyses of ultra-processed food intake with all cause mortality, blood glucose, insulin, and C-peptide serum concentrations were considered.
Markers of renal function include serum concentrations of cystatin-C and creatinine.
Markers of inflammation include C reactive protein, white blood cell count, and granulocyte-to-lymphocyte ratio.
Discussion
In a large prospective cohort of 22 895 Italian adults, both diets mainly composed of food products with higher FSAm-NPS, which reflects poor nutritional profiles, and those with a large dietary share of ultra-processed foods were associated with higher hazards of all cause and cardiovascular disease mortality. Increased ultra-processed food consumption, but not the FSAm-NPS dietary index, was also an independent risk factor for mortality due to ischaemic heart disease/cerebrovascular disease and other causes. When these two food dimensions (that is, nutrient balance and food processing) were analysed simultaneously, we observed that the associations of the FSAm-NPS dietary index with all cause and cardiovascular disease mortality were significantly attenuated, whereas estimations for ultra-processed food intake remained almost unchanged for all the outcomes under study. These findings suggest that highly processed foods are associated with poor health outcomes independently of their low nutritional composition, but not the other way around.
Comparison with other studies
This is the first study providing a targeted analysis of how nutritional and non-nutritional food dimensions are associated with mortality risk. Although no previous study has specifically investigated this, other investigations have indirectly considered both nutritional quality and food processing in association with a variety of health outcomes. A review of the literature analysing the relative effect of adjusting for diet quality/patterns on the reported associations between ultra-processed food intake and health related outcomes in more than 20 prospective cohort studies found that these adjustments did not explain the association between ultra-processed food intake and health related outcomes, with estimates remaining highly significant.37 On the contrary, none of the large cohort studies evaluating the health impact of the FSAm-NPS dietary index considered the degree of food processing as a potential covariate,18 38 thereby introducing potential sources of bias in these studies.
One exception is the longitudinal analysis from the SUN cohort of Spanish graduates showing that the association between a higher FSAm-NPS dietary index and all cause mortality was stronger among participants with a high intake of ultra-processed foods.19 This may therefore be an underrated common factor in most of the large scale prospective cohort studies that have evaluated the impact of the Nutri-Score on health; much of the research in this field did not consider that food processing and nutritional quality are partially correlated, making disentangling their independent effects on human health difficult. Interestingly, a study comparing the nutritional quality (as assessed by the Nutri-Score) and the degree of processing (as assessed by the NOVA classification) of foods in the Open Food Facts database reported that ultra-processed foods were represented to different extents across all Nutri-Score categories, ranging from 26.1% in nutritional category A (highest nutritional quality) up to 83.7% in nutritional category E (lowest nutritional quality).20 As a result, the authors suggested that front-of-pack labels could benefit from more details besides the Nutri-Score and information on the nutrient content, including a warning on the level of food processing, to really improve people’s diets.20 This position is also endorsed by other experts who call for incorporation of a warning label for ultra-processed foods as an additional measure to guide people towards healthier eating.39
In our study, ultra-processed food consumption was more evidently associated with biomarkers potentially reflecting the biological mechanisms behind its possible effect on mortality risk than the FSAm-NPS dietary index. Moreover, we observed that, although a nutritionally poor diet, as reflected by consumption of foods with higher FSAm-NPS scores, may affect mortality risk through an unfavourable modulation of pathways that are known to be affected by diet (for example, markers of glucose metabolism), the higher mortality hazards associated with a diet rich in ultra-processed foods were ascribed to altered renal function and to a lesser extent to increased markers of inflammation.
Evidence on the potential effect of the overall diet quality on biomarkers of renal function, a well established risk factor for cardiovascular disease, is not robust, with only a few cohort studies supporting an association.40 41 However, diets high in ultra-processed foods have been increasingly shown to be associated with altered renal function and higher inflammation,7 42 43 44 possibly through mechanisms that are triggered by non-nutritional components of the diet, such as food additives and contaminants present in highly processed foods, as well as food processing itself that affects both nutritional composition and the food matrix.45 For instance, the packaging of ultra-processed foods is a major source of synthetic chemicals, such as phthalates and bisphenols, that are among the so-called endocrine disrupting chemicals,46 which could have adverse effects on renal function and might contribute to progressive cumulative renal injury over a lifetime and are also associated with altered concentrations of inflammatory biomarkers.47 48 49 Consistently, evidence shows that acrylamide, which is one of the most relevant contaminants produced when foods are heated to high temperatures (for example, French fries and potato crisps, cereal products, and roasted coffee), has documented nephrotoxic effects and has been linked to increased oxidative stress and inflammation,50 51 as well as some food additives largely used in the food industry.52
Although access to edible, safe, and healthy food is essential, the usefulness of high levels of food processing has been strongly questioned,21 and it has several implications for human health. Modifications to the food matrix during processing can alter nutrient bio-accessibility and absorption kinetics, which may promote an inflammatory gut microbiota that in turn is associated with several cardiometabolic conditions.30 Food processing can also lead to the loss of some protective micronutrients and phytochemicals naturally present in plant foods.21 The differential effect of nutritional quality and food processing on biological pathways represent an additional valuable reason to consider both these two food dimensions as equally important for human health.
Strengths and limitations of study
This is the first study examining the possible health impact of the joint exposure to nutrient poor foods, according to the nutrient profile system underpinning the Nutri-Score front-of-pack label, and to foods characterised by an elevated degree of processing as described by the NOVA classification. Strengths of this analysis include the prospective design, long follow-up, and use of a large dataset, with a careful account for a large number of covariates to minimise confounding. However, the study also has several limitations. Firstly, owing to the observational design, we cannot fully rule out the potential role of residual confounding by unmeasured factors. Dietary data were self-reported, and this may lead to recall bias. Also, potential exists for social desirability bias that might lead to the underreporting of ultra-processed food consumption, which could bias studied associations towards the null. Moreover, the FFQ used in this study, like most FFQs used in large scale prospective cohort studies,18 19 was not originally developed to assess the degree of food processing, so many food items were not included (for example, pre-prepared dishes, energy bars, slimming products).
Although no one gold standard for applying the NOVA categorisation exists,53 we recognise that FFQs may not cover the full spectrum of foods consumed, including ultra-processed foods, owing to the limited number of predefined food lists and the lack of supporting information on cooking methods, ingredients, eating place, and the brand names of the packaged foods,53 54 which would be extremely useful in identifying ultra-processed foods. However, most existing large cohort studies conducted to explore the relation between ultra-processed food consumption and mortality risks have so far used FFQs.55 56 57 Moreover, imprecision in the identification of ultra-processed foods may also pertain to 24 hour diet recalls or diet records.53 Finally, evidence suggests that classification of ultra-processed foods with an FFQ may be valid for the purpose of qualitative comparisons, although less appropriate in absolute intake estimations.54 However, we expect misclassification to be non-systematic, and this would likely lead to non-differential measurement error possibly resulting in an underestimation of the studied associations.
Another weakness is that diet and health data were measured at baseline only, so potential changes occurring over the life course might have modified the strength of the findings; nevertheless, evidence shows that diet in adulthood tends to remain stable over time,58 and most of the biomarkers we tested were not found to vary substantially over time.59 Finally, caution is needed in generalising these findings to other populations.
Conclusions and policy implications
The diet-health relation has been traditionally explored and explained almost exclusively by nutritional composition, leading to recommendations of limiting nutrients to be avoided or reduced (for example, sugar, salt, and fat) while favouring others (for example, fibre) to prevent major nutrition related non-communicable diseases.21 Findings from this large cohort of an Italian general population suggest that part of the mortality risk associated with a nutritionally unbalanced diet is due to an elevated degree of processing that usually characterises nutrient poor foods. Our findings suggest that food processing and the nutritional quality of food cover different but complementary dimensions, which should both be considered when analysing the diet-disease relation. This is in line with the assumption that a food’s health potential is not exclusively associated with its nutritional composition.21
From a public health perspective, this study reinforces the opportunity to reformulate dietary guidelines worldwide, by paying more attention to the degree of processing of foods along with nutrient based recommendations. We acknowledge that some progress has been made in this field; the latest version of the dietary guidelines to improve cardiovascular health released by the American Heart Association recommends choosing minimally processed foods instead of ultra-processed foods,60 in accordance with what has been already done in some countries.61 Finally, our findings will hopefully contribute to the ongoing discussions about the potential implementation of a nutrition labelling system at the European Union level.10 14
What is already known on this topic
The Nutri-Score is an interpretative front-of-pack labelling system grading the nutritional quality of foods and is a candidate for enabling uniform front-of-pack nutrition labelling at EU level
The NOVA classification rates foods according to the degree of processing rather than on their nutrient content
Both systems were separately reported to be associated with poor health outcomes in population cohorts worldwide, but their joint health impact has not been evaluated in large cohorts
What this study adds
In a large Italian population cohort, the Nutri-Score and the NOVA classification were independently associated with all cause and cardiovascular mortality
Part of the excess mortality risk associated with a nutrient poor diet, as reflected by increased values of the Nutri-Score, was significantly explained by a higher degree of food processing
Ultra-processed food intake, by contrast, remained associated with mortality even after the poor nutritional quality of the diet was accounted for
Acknowledgments
We are grateful to the Moli-sani Study participants who enthusiastically joined the study and to the Associazione Cuore Sano ONLUS (Campobasso, Italy) for its support to our research communication activities. ER was supported by Fondazione Umberto Veronesi, which is gratefully acknowledged.
Web extra.
Extra material supplied by authors
Web appendix: Supplementary materials
Contributors: MB, LI, ADiC, GdG, and GG conceived and designed the study. ADeC was in charge of the Moli-sani bio-bank and did laboratory tests. SC stored and managed the data. MB, ER, and ADiC analysed the data. MB and GG drafted the manuscript. CC, MBD, GdG, and LI originally promoted the Moli-sani study and critically revised this manuscript. All authors gave final approval and agree to be accountable for all aspects of the work ensuring integrity and accuracy. MB and LI are the guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: The enrolment phase of the Moli-sani Study was supported by research grants from Pfizer Foundation (Rome, Italy), the Italian Ministry of University and Research (MIUR, Rome, Italy)–Programma Triennale di Ricerca, decreto No1588, and Instrumentation Laboratory, Milan, Italy. The analyses reported here were partially supported by a grant to LI (AIRC individual grant - project code: 25942) and by the Italian Ministry of Health (Ricerca Corrente 2022-2024). The funders had no role in considering the study design or in the collection, analysis, or interpretation of data, the writing of the report, or the decision to submit the article for publication. All authors were and are independent from the funders.
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: support for the submitted work as detailed above; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The lead authors (the manuscript’s guarantors) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: Results from analyses conducted in this paper and their possible impact on real world life will be disseminated to both Moli-sani Study cohort participants and public communities through press releases and social media, as well as by newsletters and regular in-presence meetings.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
Collaborators: Moli-sani Study Investigators, Americo Bonanni, Francesco Gianfagna, Mariarosaria Persichillo, Teresa Di Prospero, Marco Olivieri, Teresa Panzera, Simona Esposito, Alessandro Gialluisi, Sara Magnacca, Benedetta Izzi, Annalisa Marotta, Fabrizia Noro, Roberta Parisi, Alfonsina Tirozzi, Francesca Bracone, Francesca De Lucia, Cristiana Mignogna, and Livia Rago
Ethics statements
Ethical approval
The Moli-sani Study was approved by the Ethics Committee of the Catholic University in Rome, Italy (ID Prot. pdc. P.99 (A.931/03-138-04)/C.E./2004). All participants gave written informed consent.
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author. The data are stored in an institutional repository (https://repository.neuromed.it), and access is restricted by the ethical approvals and the legislation of the European Union.
References
- 1. Champagne B, Arora M, ElSayed A, et al. World Heart Federation Policy Brief: Front-Of-Pack Labelling: Unhealthy Changes in the Global Food System. Glob Heart 2020;15:70. 10.5334/gh.935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GBD 2017 Diet Collaborators . Health effects of dietary risks in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019;393:1958-72. 10.1016/S0140-6736(19)30041-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Waijers PM, Feskens EJ, Ocké MC. A critical review of predefined diet quality scores. Br J Nutr 2007;97:219-31. 10.1017/S0007114507250421 [DOI] [PubMed] [Google Scholar]
- 4. Monteiro CA, Cannon G, Levy R, et al. NOVA. The star shines bright. Food classification. Public Health World Nutr 2016;7:28-38. [Google Scholar]
- 5. Monteiro CA. Nutrition and health. The issue is not food, nor nutrients, so much as processing. Public Health Nutr 2009;12:729-31. 10.1017/S1368980009005291 [DOI] [PubMed] [Google Scholar]
- 6. Suksatan W, Moradi S, Naeini F, et al. Ultra-Processed Food Consumption and Adult Mortality Risk: A Systematic Review and Dose-Response Meta-Analysis of 207,291 Participants. Nutrients 2021;14:174. 10.3390/nu14010174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bonaccio M, Di Castelnuovo A, Costanzo S, et al. Ultra-processed food consumption is associated with increased risk of all-cause and cardiovascular mortality in the Moli-sani Study. Am J Clin Nutr 2021;113:446-55. 10.1093/ajcn/nqaa299 [DOI] [PubMed] [Google Scholar]
- 8. Levy RB, Rauber F, Chang K, et al. Ultra-processed food consumption and type 2 diabetes incidence: A prospective cohort study. Clin Nutr 2021;40:3608-14. 10.1016/j.clnu.2020.12.018 [DOI] [PubMed] [Google Scholar]
- 9. Coutinho JG, Martins APB, Preiss PV, Longhi L, Recine E. UN Food System Summit Fails to Address Real Healthy and Sustainable Diets Challenges. Development (Rome) 2021;64:220-6. 10.1057/s41301-021-00315-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hercberg S, Touvier M, Salas-Salvado J, Group of European scientists supporting the implementation of Nutri-Score in Europe . The Nutri-Score nutrition label. Int J Vitam Nutr Res 2022;92:147-57. 10.1024/0300-9831/a000722 [DOI] [PubMed] [Google Scholar]
- 11. Carruba MO, Caretto A, De Lorenzo A, et al. Front-of-pack (FOP) labelling systems to improve the quality of nutrition information to prevent obesity: NutrInform Battery vs Nutri-Score. Eat Weight Disord 2022;27:1575-84. 10.1007/s40519-021-01316-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Croker H, Packer J, Russell SJ, Stansfield C, Viner RM. Front of pack nutritional labelling schemes: a systematic review and meta-analysis of recent evidence relating to objectively measured consumption and purchasing. J Hum Nutr Diet 2020;33:518-37. 10.1111/jhn.12758 [DOI] [PubMed] [Google Scholar]
- 13. Roberto CA, Ng SW, Ganderats-Fuentes M, et al. The Influence of Front-of-Package Nutrition Labeling on Consumer Behavior and Product Reformulation. Annu Rev Nutr 2021;41:529-50. 10.1146/annurev-nutr-111120-094932 [DOI] [PubMed] [Google Scholar]
- 14. Delhomme V. Front-of-pack nutrition labelling in the European Union: a behavioural, legal and political analysis. Eur J Risk Regul 2021;12:825-48 10.1017/err.2021.5. [DOI] [Google Scholar]
- 15. Julia C, Hercberg S. Development of a new front-of-pack nutrition label in France: the five-Colour Nutri-Score. Public Health Panorama 2017;3:712-25. [Google Scholar]
- 16. Egnell M, Galan P, Farpour-Lambert NJ, et al. Compared to other front-of-pack nutrition labels, the Nutri-Score emerged as the most efficient to inform Swiss consumers on the nutritional quality of food products. PLoS One 2020;15:e0228179. 10.1371/journal.pone.0228179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andreeva VA, Egnell M, Touvier M, Galan P, Julia C, Hercberg S. International evidence for the effectiveness of the front-of-package nutrition label called Nutri-Score. Cent Eur J Public Health 2021;29:76-9. 10.21101/cejph.a6239 [DOI] [PubMed] [Google Scholar]
- 18. Deschasaux M, Huybrechts I, Julia C, et al. Association between nutritional profiles of foods underlying Nutri-Score front-of-pack labels and mortality: EPIC cohort study in 10 European countries. BMJ 2020;370:m3173. 10.1136/bmj.m3173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gómez-Donoso C, Martínez-González MÁ, Perez-Cornago A, Sayón-Orea C, Martínez JA, Bes-Rastrollo M. Association between the nutrient profile system underpinning the Nutri-Score front-of-pack nutrition label and mortality in the SUN project: A prospective cohort study. Clin Nutr 2021;40:1085-94. 10.1016/j.clnu.2020.07.008 [DOI] [PubMed] [Google Scholar]
- 20. Romero Ferreiro C, Lora Pablos D, Gómez de la Cámara A. Two Dimensions of Nutritional Value: Nutri-Score and NOVA. Nutrients 2021;13:2783. 10.3390/nu13082783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fardet A, Rock E. Chronic diseases are first associated with the degradation and artificialization of food matrices rather than with food composition: calorie quality matters more than calorie quantity. Eur J Nutr 2022;61:2239-53. 10.1007/s00394-021-02786-8 [DOI] [PubMed] [Google Scholar]
- 22. Martini D, Godos J, Bonaccio M, Vitaglione P, Grosso G. Ultra-Processed Foods and Nutritional Dietary Profile: A Meta-Analysis of Nationally Representative Samples. Nutrients 2021;13:3390. 10.3390/nu13103390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Julia C, Kesse-Guyot E, Touvier M, Méjean C, Fezeu L, Hercberg S. Application of the British Food Standards Agency nutrient profiling system in a French food composition database. Br J Nutr 2014;112:1699-705. 10.1017/S0007114514002761 [DOI] [PubMed] [Google Scholar]
- 24. Iacoviello L, Bonanni A, Costanzo S, et al. The MOLI-SANI Project, a randomized, prospective cohort study in the Molise region in Italy; design, rationale and objectives. Ital J Public Health 2007;4:110-8. [Google Scholar]
- 25. Pala V, Sieri S, Palli D, et al. Diet in the Italian EPIC cohorts: presentation of data and methodological issues. Tumori 2003;89:594-607. 10.1177/030089160308900603 [DOI] [PubMed] [Google Scholar]
- 26. Salvini S, Parpinel M, Gnagnarella P, Maissoneuve P, Turrini A. Banca dati composizione degli alimenti per studi epidemiologici in Italia. European Institute of Oncology, 1998. [Google Scholar]
- 27. Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med 2003;348:2599-608. 10.1056/NEJMoa025039 [DOI] [PubMed] [Google Scholar]
- 28. Monteiro CA, Cannon G, Levy RB, et al. Ultra-processed foods: what they are and how to identify them. Public Health Nutr 2019;22:936-41. 10.1017/S1368980018003762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Srour B, Fezeu LK, Kesse-Guyot E, et al. Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Santé). BMJ 2019;365:l1451. 10.1136/bmj.l1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Juul F, Vaidean G, Parekh N. Ultra-processed Foods and Cardiovascular Diseases: Potential Mechanisms of Action. Adv Nutr 2021;12:1673-80. 10.1093/advances/nmab049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zeller T, Hughes M, Tuovinen T, et al. BiomarCaRE: rationale and design of the European BiomarCaRE project including 300,000 participants from 13 European countries. Eur J Epidemiol 2014;29:777-90. 10.1007/s10654-014-9952-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Blankenberg S, Zeller T, Saarela O, et al. MORGAM Project . Contribution of 30 biomarkers to 10-year cardiovascular risk estimation in 2 population cohorts: the MONICA, risk, genetics, archiving, and monograph (MORGAM) biomarker project. Circulation 2010;121:2388-97. 10.1161/CIRCULATIONAHA.109.901413 [DOI] [PubMed] [Google Scholar]
- 33. Hernán MA, Hernández-Díaz S, Werler MM, Mitchell AA. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol 2002;155:176-84. 10.1093/aje/155.2.176 [DOI] [PubMed] [Google Scholar]
- 34. Rogawski ET, Westreich DJ, Kang G, Ward HD, Cole SR. Brief Report: Estimating Differences and Ratios in Median Times to Event. Epidemiology 2016;27:848-51. 10.1097/EDE.0000000000000539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986;51:1173-82. 10.1037/0022-3514.51.6.1173 [DOI] [PubMed] [Google Scholar]
- 36.Hertzmark E, Pazaris M, Spiegelman D. The SAS MEDIATE Macro. Harvard T.H. Chan School of Public Health, 2012.
- 37. Dicken SJ, Batterham RL. The Role of Diet Quality in Mediating the Association between Ultra-Processed Food Intake, Obesity and Health-Related Outcomes: A Review of Prospective Cohort Studies. Nutrients 2021;14:23. 10.3390/nu14010023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Donat-Vargas C, Sandoval-Insausti H, Rey-García J, Ramón Banegas J, Rodríguez-Artalejo F, Guallar-Castillón P. Five-color Nutri-Score labeling and mortality risk in a nationwide, population-based cohort in Spain: the Study on Nutrition and Cardiovascular Risk in Spain (ENRICA). Am J Clin Nutr 2021;113:1301-11. 10.1093/ajcn/nqaa389 [DOI] [PubMed] [Google Scholar]
- 39. Cotter T, Kotov A, Wang S, Murukutla N. ‘Warning: ultra-processed’ - A call for warnings on foods that aren’t really foods. BMJ Glob Health 2021;6:e007240. 10.1136/bmjgh-2021-007240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Asghari G, Momenan M, Yuzbashian E, Mirmiran P, Azizi F. Dietary pattern and incidence of chronic kidney disease among adults: a population-based study. Nutr Metab (Lond) 2018;15:88. 10.1186/s12986-018-0322-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cai Q, Dekker LH, Vinke PC, et al. Diet quality and incident chronic kidney disease in the general population: The Lifelines Cohort Study. Clin Nutr 2021;40:5099-105. 10.1016/j.clnu.2021.07.033 [DOI] [PubMed] [Google Scholar]
- 42. Bonaccio M, Costanzo S, Di Castelnuovo A, et al. Ultra-processed food intake and all-cause and cause-specific mortality in individuals with cardiovascular disease: the Moli-sani Study. Eur Heart J 2022;43:213-24. 10.1093/eurheartj/ehab783 [DOI] [PubMed] [Google Scholar]
- 43. Rey-García J, Donat-Vargas C, Sandoval-Insausti H, et al. Ultra-Processed Food Consumption is Associated with Renal Function Decline in Older Adults: A Prospective Cohort Study. Nutrients 2021;13:428. 10.3390/nu13020428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Martins GMDS, França AKTDC, Viola PCAF, et al. Intake of ultra-processed foods is associated with inflammatory markers in Brazilian adolescents. Public Health Nutr 2022;25:591-9. 10.1017/S1368980021004523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fardet A. Minimally processed foods are more satiating and less hyperglycemic than ultra-processed foods: a preliminary study with 98 ready-to-eat foods. Food Funct 2016;7:2338-46. 10.1039/C6FO00107F [DOI] [PubMed] [Google Scholar]
- 46. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev 2009;30:293-342. 10.1210/er.2009-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kataria A, Trasande L, Trachtman H. The effects of environmental chemicals on renal function. Nat Rev Nephrol 2015;11:610-25. 10.1038/nrneph.2015.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Savastano S, Tarantino G, D’Esposito V, et al. Bisphenol-A plasma levels are related to inflammatory markers, visceral obesity and insulin-resistance: a cross-sectional study on adult male population. J Transl Med 2015;13:169. 10.1186/s12967-015-0532-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bai PY, Wittert G, Taylor AW, et al. The association between total phthalate concentration and non-communicable diseases and chronic inflammation in South Australian urban dwelling men. Environ Res 2017;158:366-72. 10.1016/j.envres.2017.06.021 [DOI] [PubMed] [Google Scholar]
- 50. Ghorbel I, Elwej A, Fendri N, et al. Olive oil abrogates acrylamide induced nephrotoxicity by modulating biochemical and histological changes in rats. Ren Fail 2017;39:236-45. 10.1080/0886022X.2016.1256320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Poulsen MW, Hedegaard RV, Andersen JM, et al. Advanced glycation endproducts in food and their effects on health. Food Chem Toxicol 2013;60:10-37. 10.1016/j.fct.2013.06.052 [DOI] [PubMed] [Google Scholar]
- 52. Kramer NI, Hoffmans Y, Wu S, et al. Characterizing the coverage of critical effects relevant in the safety evaluation of food additives by AOPs. Arch Toxicol 2019;93:2115-25. 10.1007/s00204-019-02501-x [DOI] [PubMed] [Google Scholar]
- 53. Khandpur N, Rossato S, Drouin-Chartier JP, et al. Categorising ultra-processed foods in large-scale cohort studies: evidence from the Nurses’ Health Studies, the Health Professionals Follow-up Study, and the Growing Up Today Study. J Nutr Sci 2021;10:e77. 10.1017/jns.2021.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jung S, Park S, Kim JY. Comparison of dietary share of ultra-processed foods assessed with a FFQ against a 24-h dietary recall in adults: results from KNHANES 2016. Public Health Nutr 2022;1-10. 10.1017/S1368980022000179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rico-Campà A, Martínez-González MA, Alvarez-Alvarez I, et al. Association between consumption of ultra-processed foods and all cause mortality: SUN prospective cohort study. BMJ 2019;365:l1949. 10.1136/bmj.l1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Juul F, Vaidean G, Lin Y, Deierlein AL, Parekh N. Ultra-Processed Foods and Incident Cardiovascular Disease in the Framingham Offspring Study. J Am Coll Cardiol 2021;77:1520-31. 10.1016/j.jacc.2021.01.047 [DOI] [PubMed] [Google Scholar]
- 57. Romero Ferreiro C, Martín-Arriscado Arroba C, Cancelas Navia P, Lora Pablos D, Gómez de la Cámara A. Ultra-processed food intake and all-cause mortality: DRECE cohort study. Public Health Nutr 2021;1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Edefonti V, De Vito R, Salvatori A, et al. Reproducibility of A Posteriori Dietary Patterns across Time and Studies: A Scoping Review. Adv Nutr 2020;11:1255-81. 10.1093/advances/nmaa032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Al-Delaimy WK, Jansen EH, Peeters PH, et al. Reliability of biomarkers of iron status, blood lipids, oxidative stress, vitamin D, C-reactive protein and fructosamine in two Dutch cohorts. Biomarkers 2006;11:370-82. 10.1080/13547500600799748 [DOI] [PubMed] [Google Scholar]
- 60. Lichtenstein AH, Appel LJ, Vadiveloo M, et al. 2021 Dietary Guidance to Improve Cardiovascular Health: A Scientific Statement From the American Heart Association. Circulation 2021;144:e472-87. 10.1161/CIR.0000000000001031 [DOI] [PubMed] [Google Scholar]
- 61. Monteiro CA, Cannon G, Moubarac JC, et al. Dietary guidelines to nourish humanity and the planet in the twenty-first century. A blueprint from Brazil. Public Health Nutr 2015;18:2311-22. 10.1017/S1368980015002165 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author. The data are stored in an institutional repository (https://repository.neuromed.it), and access is restricted by the ethical approvals and the legislation of the European Union.