Abstract
Racial residential segregation is associated with multiple adverse health outcomes in Black individuals. Yet, the influence of structural racism and racial residential segregation on brain aging is less understood. In this study, we investigated the association between cumulative exposure to racial residential segregation over 25 years (1985–2010) in young adulthood, as measured by the Getis-Ord Gi* statistic, and year 25 measures of brain volume (cerebral, gray matter, white matter, and hippocampal volumes) in midlife. We studied 290 Black participants with available brain imaging data who were enrolled in the Coronary Artery Risk Development in Young Adults (CARDIA) Study, a prospective cohort study. CARDIA investigators originally recruited 2,637 Black participants aged 18–30 years from 4 field centers across the United States. We conducted analyses using marginal structural models, incorporating inverse probability of treatment weighting and inverse probability of censoring weighting. We found that compared with low/medium segregation, greater cumulative exposure to a high level of racial residential segregation throughout young adulthood was associated with smaller brain volumes in general (e.g., for cerebral volume, β = –0.08, 95% confidence interval: −0.15, −0.02) and with a more pronounced reduction in hippocampal volume, though results were not statistically significant. Our findings suggest that exposure to segregated neighborhoods may be associated with worse brain aging.
Keywords: brain aging, epidemiologic methods, marginal structural models, racism, segregation
Abbreviations
- CARDIA
Coronary Artery Risk Development in Young Adults
- MRI
magnetic resonance imaging
Investigators in several studies have reported racial/ethnic disparities in markers of brain aging (1–3) such that, compared with non-Hispanic Whites, Black individuals in the United States have a greater burden of cerebrovascular disease and Alzheimer disease. These findings are probably largely attributable to the structural racism (4) uniquely experienced by Black Americans, which is a risk factor for most well-established social determinants of brain aging (e.g., education, income, wealth) (5). Structural racism persists in various forms, including racial residential segregation (6). Racial residential segregation has been associated with a variety of adverse health measures, including but not limited to psychosocial factors (7), health behaviors (8), and cardiometabolic disease (9–11), all of which are important determinants of brain aging.
Given that prior studies suggest there is a link between racial segregation and several determinants of brain aging, it is worth examining whether racism, in the form of racial residential segregation, also negatively influences brain integrity, as this could also help explain dementia disparities in older age. Here, we examined the relationship between cumulative exposure to racial residential segregation over 25 years of young adulthood and magnetic resonance imaging (MRI) measures of brain structure in middle age among Black adults in the Coronary Artery Risk Development in Young Adults (CARDIA) Study.
METHODS
Source population and analytical sample
CARDIA is a prospective cohort study of the development and determinants of cardiovascular disease. Beginning in 1985, a total of 5,114 participants aged 18–30 years (51.6% Black and 48.4% White) were recruited into the study from 4 field centers: the University of Alabama at Birmingham (Birmingham, Alabama); the University of Minnesota, Minneapolis (Minneapolis, Minnesota); Northwestern University (Chicago, Illinois); and Kaiser Permanente, a health maintenance organization (Oakland, California) (12). Participants were recruited so as to achieve balance by sex, age, and educational level, and participants provided written informed consent at each visit. The institutional review board at each field site approved the study, and the present analysis was approved by the CARDIA Presentations and Publications Committee.
In 2010, markers of brain aging were measured in a subset of CARDIA participants (n = 719). Participants for this MRI substudy were selected among those who underwent an examination in 2010 and did not have either a contraindication for MRI or a body size that was too large for the MRI tube bore. In addition, participants for the substudy were selected with the aim of achieving balance on ethnicity (Black, White) and sex in the Birmingham, Minneapolis, and Oakland field centers. Separate written consent was needed to participate in the MRI substudy (13).
Given that racial residential segregation in the United States is experienced very differently by Black individuals as compared with other racial groups (since it is often driven by structural forms of racism), our analyses were restricted to Black participants (n = 290; 40%). As such, our final analytical sample consisted of those 290 Black participants who were enrolled in the CARDIA MRI substudy in 2010. The CARDIA study design leading to selection of our MRI analytical sample is presented in Figure 1. A comparison of Black participants who were included in the MRI substudy in 2010 with those excluded from the MRI substudy and thus excluded from the final analytical sample is presented in Web Table 1 (available at https://doi.org/10.1093/aje/kwab297).
Figure 1.
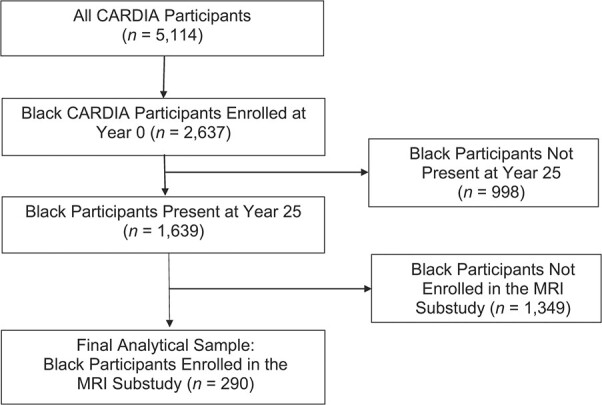
Selection of a sample of Black participants for a magnetic resonance imaging (MRI) substudy of markers of brain aging, Coronary Artery Risk Development in Young Adults (CARDIA) Study, 1985–2010.
Exposure: racial residential segregation during 1985–2010
Neighborhood-level segregation was represented in our study by the Getis-Ord local Gi* statistic (14). The Gi* statistic, which is a widely accepted measure of racial residential segregation, is computed with a spatial weight to account for the racial composition of each US Census tract as compared with neighboring tracts. Compared with other measures of segregation, the Gi* statistic captures information on the racial composition of the geographic region both within and outside of a given census tract, better reflecting both contextual and spatial components of segregation (11). The Gi* statistic produces a z score value representing the number of standard deviations that the racial composition of one’s census tract is from the greater surrounding metropolitan area. Additional details on the Gi* statistic have been published elsewhere (10).
In CARDIA, participants’ geocoded addresses were linked to tract-level Census data at each of the available study visits (1985–1986, 1992–1993, 1995–1996, 2000–2001, 2005–2006, and 2010–2011). Hence, we created a measure of cumulative exposure to racial residential segregation by averaging the Gi* statistic for each person across the follow-up visits and then categorized it into high (Gi* > 1.96), medium (0 ≤ Gi* ≤ 1.96), or low (Gi* < 0) segregation based on critical z score values at the 5% significance level (95% confidence interval (CI)), as is done in the literature (11, 14). The higher the Gi* statistic, the greater the representation of Black residents in the census tract as compared with the larger metropolitan area.
Outcome: structural brain MRI markers in 2010
In 2010, the CARDIA MRI substudy included 3 of the 4 CARDIA sites—Birmingham, Minneapolis, and Oakland—and enrolled a total of 719 participants, of whom 290 were Black (40%). The procedures for the CARDIA MRI substudy have been previously described (12).
Briefly, brain MRIs were acquired on 3-T scanners located proximal to each CARDIA site. MRIs were sent to, quality-controlled at, and analyzed at the CARDIA MRI Reading Center by the Section for Biomedical Image Analysis of the Department of Radiology, Perelman School of Medicine, University of Pennsylvania (Philadelphia, Pennsylvania). To explore associations across a variety of tissues, we examined total brain volumes and both gray matter (encompassing all neuronal cell bodies across brain regions) and white matter (encompassing all connecting tissue) volumes. In addition, we examined hippocampal volumes; the hippocampus is a brain region involved in memory and emotion-regulation processes and has been widely linked to neurocognitive disorders (15–17). Total gray matter and white matter volumes, total cerebral (gray matter + white matter) volumes, and hippocampal volumes were measured from sagittal 3-dimensional T1 images using an automated algorithm that classifies supratentorial brain tissue into gray matter, white matter, and cerebrospinal fluid. Abnormalities in brain tissue increase with age; given the relatively young age of our cohort, tissue abnormalities are few, and thus we focused only on normal tissue volume in our brain volume computations. All volumes were z-scored (representing number of standard deviations from the mean) to facilitate comparison across estimates, with negative values indicating worse brain aging.
Covariates
We included the following as potentially time-varying covariates based on prior literature regarding their relationship with segregation and brain aging (7–11, 18–23). At each visit, CARDIA participants reported their marital status (married vs. not married), duration of education (years), physical activity (in standardized units (24, 25)), income (based on income categories, ranging from ≤$5,000 per year to ≥$100,000 per year), mean alcohol consumption (mL/day), and smoking status (current smoking vs. not smoking). Body mass index (weight (kg)/height (m)2) was calculated using measured weight and height. Blood pressure was measured 3 times, and systolic blood pressure was calculated as the average of the last 2 measurements. Fasting glucose level was measured using the hexokinase ultraviolet method by American BioScience Laboratories (Van Nuys, California). Lastly, depressive symptoms were measured using the Center for Epidemiologic Studies Depression Scale (20-item version). We also included the following covariates as time-invariant confounders only: sex (male/female), age (years) at baseline, and field center (to capture differences by study location).
Statistical analysis
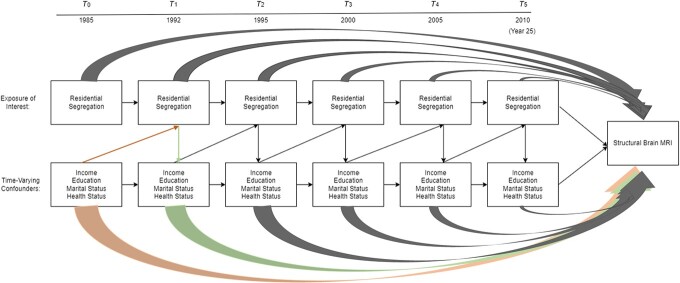
We sought to assess the association of cumulative exposure to racial residential segregation (1985–2010) with structural brain integrity in 2010. Given the longitudinal nature of the study, spanning 25 years, and the repeated measures of racial residential segregation and covariates, we posited that the data structure, illustrated by the directed acyclic graph in Figure 2, reflected time-varying confounding. In other words, we hypothesized that these particular factors acted as both confounders (i.e., a third variable that determines both the exposure and outcome) and mediators (i.e., a third variable that is determined by the exposure and subsequently determines the outcome, which means it is on the proposed pathway) of the associations of interest.
Figure 2.
Directed acyclic graph depicting time-varying confounding of the relationship between racial residential segregation and brain volumes in the Coronary Artery Risk Development in Young Adults (CARDIA) Study, 1985–2010. Health status variables included physical activity, smoking status, body mass index, systolic blood pressure, fasting glucose level, depressive symptoms, and alcohol consumption. To illustrate the time-varying confounding structure, we provide an example of confounding (orange arrow) and an example of mediation (green arrow). MRI, magnetic resonance imaging.
To address the longitudinal nature of the data while appropriately addressing the time-varying confounding structure, we constructed inverse probability of treatment weights at each study visit for each study participant. To do so, we used pooled multinomial logistic regression, combining data across multiple visits per person over time. We first estimated the “numerator” of the weight per person-visit, which is the predicted probability of segregation status (high vs. low/medium) at time T conditional on segregation status at the previous visit (T − 1). Second, using pooled multinomial logistic regression, we estimated the “denominator” of the weight per person-visit, which is the predicted probability of segregation status at time T conditional on segregation status at the previous visit (T − 1) and the time-varying covariates mentioned above measured at T − 1, as well as relevant baseline confounders. Stabilized weights were then computed by dividing the numerator predicted probability by the denominator predicted probability. For each person, these weights were then multiplied across visits. In addition to the inverse probability of treatment weights, we also computed inverse probability of censoring weights to address selective attrition (i.e., loss to follow-up), using the same methodology and the same time-varying confounders. Inverse probability of censoring weights were then truncated (at the 95th percentile) to avoid extreme weights. In a final step, for each participant, we multiplied their cumulative racial residential segregation weight by their cumulative censoring weight to generate their final inverse probability weight, which represented their cumulatively multiplied weight across time.
Marginal structural models (26) were then adjusted for the final weight per person and accounted for clustering by census tract. In these models, we computed robust standard errors to account for the correlation between participants living in the same census tract. In all marginal structural models, we additionally adjusted for the following time-invariant covariates: field center, age at baseline, sex, baseline duration of education, and total intracranial volume (to account for differences in head size). Estimates and 95% CIs are reported, and statistically significant results are indicated by 95% CIs that do not contain the null. Analyses were conducted in SAS 9.4 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Most (83%) of our sample participants were living in neighborhoods with high racial residential segregation at baseline. The mean age at baseline was 24 (standard deviation, 4) years (Table 1). Compared with those living in areas with medium or low racial residential segregation, participants living in areas with high racial residential segregation had a lower educational level and lower household income, were less physically active, and were more likely to be current smokers. At baseline, participants’ clinical factors, such as systolic blood pressure, body mass index, and depressive symptoms, were relatively similar across segregation categories. Finally, compared with Black participants not enrolled in the MRI substudy, those enrolled had lower median household income at baseline and were less likely to be married and to be current smokers, but were more physically active (Web Table 1).
Table 1.
Characteristics of Sample Participants According to Cumulative Racial Residential Segregation Category, CARDIA Study, 1985–2010
| Racial Residential Segregation Category | ||||||
|---|---|---|---|---|---|---|
| Participant Characteristic | High (n = 240) | Low/Medium (n = 50) | ||||
| Mean (SD) | No. | % | Mean (SD) | No. | % | |
| Sociodemographic characteristics at baseline | ||||||
| Age, years | 24 (4) | 26 (4) | ||||
| Duration of education, years | 13 (2) | 14 (2) | ||||
| Annual incomea, thousands of dollars | 20.5 (14.0–30.0) | 30.0 (20.5–42.5) | ||||
| Married | 38 | 16 | 9 | 18 | ||
| Female sex | 137 | 57 | 22 | 44 | ||
| Clinical risk factors at baseline | ||||||
| Systolic blood pressure, mm Hg | 111 (10) | 113 (8) | ||||
| Body mass indexb | 25 (5) | 26 (6) | ||||
| Fasting glucose concentration, mg/dL | 82 (16) | 83 (7) | ||||
| CES-D scorea | 12 (6–16) | 10 (5–13) | ||||
| Health behaviors at baseline | ||||||
| Current smoker | 75 | 31 | 7 | 14 | ||
| Physical activitya, exercise unitsc | 352 (129–487) | 411 (192–536) | ||||
| Mean alcohol consumptiona, mL/day | 11.0 (0.0–13.5) | 10.0 (0.0–12.3) | ||||
| Brain volume at year 25, cm3 | ||||||
| Total cerebral volume | 1,108 (117) | 1,126 (116) | ||||
| Total gray matter volume | 608 (61) | 618 (62) | ||||
| Total white matter volume | 500 (61) | 508 (59) | ||||
| Total hippocampal volume | 7 (1) | 7 (1) | ||||
Abbreviations: CARDIA, Coronary Artery Risk Development in Young Adults; CES-D, Center for Epidemiologic Studies Depression Scale; SD, standard deviation.
a Values are expressed as median (interquartile range).
b Weight (kg)/height (m)2.
Table 2 displays results from marginal structural models examining the relationship between cumulative exposure to racial residential segregation and markers of brain aging, accounting for time-varying confounding and censoring at each visit. In the MRI subsample of 290 subjects, estimates were in the expected direction but did not reach statistical significance for total gray matter volume, total white matter volume, or total hippocampal volume. Only the estimate for total cerebral volume reached statistical significance. Generally, relative to low/medium segregation, greater cumulative exposure to high racial residential segregation throughout young adulthood was associated with smaller cerebral volume (β = −0.08, 95% CI: −0.15, −0.02), total gray matter (β = −0.09, 95% CI: −0.19, 0.01) and total white matter (β = −0.07, 95% CI: −0.14, 0.00) volumes, and hippocampal volume (β = −0.17, 95% CI: −0.36, 0.01) in middle age. Summary measures of racial residential segregation (Gi* statistic) at each visit are reported in Web Table 2.
Table 2.
Association Between High (vs. Low/Medium) Cumulative Exposure to Racial Residential Segregation Throughout Young Adulthood and Midlife Brain Structure in Marginal Structural Modelsa, CARDIA Study, 1985–2010
| Brain Volume Measure b | Effect Estimate | |
|---|---|---|
| β | 95% CI | |
| Total cerebral volume | −0.08 | −0.15, −0.02 |
| Total gray matter volume | −0.09 | −0.19, 0.01 |
| Total white matter volume | −0.07 | −0.14, 0.00 |
| Total hippocampal volume | −0.17 | −0.36, 0.01 |
Abbreviations: CARDIA, Coronary Artery Risk Development in Young Adults; CI, confidence interval.
a The marginal structural models additionally adjusted for time-invariant confounders, including visit, field center, sex, baseline duration of education (years), and total intracranial volume. Estimates are summarized across results from 10 multiply imputed data sets.
b All volumes were z-scored to facilitate comparison of estimates.
DISCUSSION
Overall, our findings suggested that exposure to segregated neighborhoods may be associated with smaller brain volumes, although most of the estimates were imprecise and compatible with both protective and deleterious associations. However, point estimates were consistently in the expected direction when comparing high exposure with low/medium exposure. Furthermore, beyond the statistical significance that was observed for total cerebral volume—which could have been due to the aggregate volume combining both gray matter and white matter (i.e., combining 2 outcomes acting in the same direction)—our results generally point to similar conclusions across different brain tissues. As such, our results do not support making inferences or conclusions about differences in underlying mechanisms.
Our analysis included only Black participants, and as such we used the Gi* statistic as a measure of racial residential segregation in Black participants and not in the White participants. One reason for doing so is the lack of overlap in the distribution of segregation for Black and White participants. More importantly, however, the social processes that influence the sorting of Black individuals into racially segregated neighborhoods tend to be different from those that sort White individuals into these same neighborhoods. The segregation of Black individuals in the United States is often driven by structural forms of racism, such as discriminatory housing and lending practices (27–30) and economic disinvestment (18). Living in underresourced neighborhoods may have adverse health impacts on White individuals, but the additional burden relating to the structural and societal factors that drive racial segregation in Black adults is not the same.
Our results suggest that racial residential segregation may be associated with smaller brain volumes across gray and white matter tissue, and in relatively comparably magnitude, suggesting that this association may be widespread. Furthermore, our effect estimates were observed after adjusting for age and other important mechanisms that are known to influence brain aging. We also found a more pronounced association with smaller hippocampal volume. While not all of the associations reached statistical significance, our results nonetheless suggest that racial residential segregation can be associated with an additional reduction of over one-third of the standard deviation in hippocampal volume, an important brain marker for Alzheimer disease and dementia. We believe these results are important for better understanding of Black-White differences in dementia and neurocognitive pathology, and they indicate venues for future research to better understand the mechanisms underlying the relationship of segregation to brain aging and neurocognitive pathology.
Our findings help to ameliorate the dearth of literature on this topic. Most prior studies have examined individual-level social factors in relation to brain structure, many of which are patterned by segregation (4). Several studies have found that higher socioeconomic status is related to larger brain volume (31–34) and fewer white matter lesions (34). Most recently, using cross-sectional data from 2 cohort studies of Alzheimer disease, Hunt et al. (35) found that higher area-level deprivation was associated with smaller total brain and hippocampal volumes. Evidence also suggests that measures of discrimination are related to greater white matter lesion load (36). Taken together, these data can help generate hypotheses for potential mediators of the association between segregation and brain aging.
There are several mechanisms through which the relationship between segregated neighborhoods and brain integrity can potentially be explained. First, there is evidence which suggests that living in a highly segregated neighborhood is associated with area resource deprivation due to practices of structural racism, such as political disinvestment in predominantly African-American communities (7, 18). Being exposed to limited resources and low socioeconomic conditions can increase one’s risk of being exposed to certain health conditions and behaviors (7–9, 11, 37) that are major determinants of cardiometabolic disease (9, 11) and brain aging (38–42). For example, body mass index, smoking, depressive symptoms, and blood pressure, all of which were included as time-varying confounders in our analysis because of their hypothesized associations with racial segregation, are factors that have been shown to be predictors of cardiovascular disease as well as brain integrity (38–42). Prior research also suggests that segregated neighborhoods are disproportionately affected by concentrated poverty and fewer educational and economic opportunities (18), which in turn have been associated with worse brain aging (43–45). Future studies could confirm these exact mechanisms through formal mediation analyses.
This study had several limitations. First, our study sample consisted of a limited number of Black participants (n = 290) who had MRI data in 2010, so statistical power to detect associations may have been limited. However, we believe there is value in reporting our statistically nonsignificant findings for 3 out of our 4 outcome measures (46, 47), and that using “significance” thresholds to dictate which findings get reported is practicably limiting. For example, this privileges findings from studies with larger sample sizes; yet larger studies with brain imaging outcomes are often clinic-based and lack repeated measures of structural-level social determinants of brain aging. Cohort studies such as CARDIA (n = 290) provide a unique opportunity to examine cumulative exposure to racial residential segregation over time and its relationship with brain integrity—a question we could not have otherwise robustly addressed in a larger clinical sample. Second, although we utilized both a cumulative segregation weight (inverse probability of treatment weighting) and a cumulative censoring weight (inverse probability of censoring weighting) to account for confounding and missingness due to censoring, we had to assume that the variables included in these models were correctly specified. Third, this study focused on brain integrity measures at only 1 time point. Future investigators may want to consider assessing trajectories in brain integrity over time, and their relationship with segregation. Fourth, while we acknowledge that our measure of segregation was the result of both changes in racial composition of neighborhoods over time and changes in participants’ residence, disentangling the two was beyond the scope of this analysis.
Fifth, although we defined our neighborhood using census tract boundaries (which vary in size and are not quite as granular as block groups), prior work has shown that there exist high levels of correlation (range of Spearman’s correlation coefficients, 0.85–0.96) between neighborhood indicators measured at the block group and census tract levels (48); therefore, we can have a relatively strong level of confidence in our use of census tracts for exposure. Sixth, while we did not directly adjust for urbanicity (urban, suburban, or rural) in our analyses, we did control for field center, which likely accounted for urbanicity to some extent. Seventh, although several proposed hypotheses have been described, this study did not assess the specific mechanisms through which racial residential segregation is associated with brain integrity. Eighth, future researchers may want to examine the role of segregation even earlier in the life course (perhaps during childhood) in future brain development and function. Finally, given CARDIA’s study and sampling design, our findings are not generalizable to all US Black adults.
Despite those limitations, this study had some notable strengths. The data used for our analysis contained repeated measures of health and social factors across early adulthood in Black participants enrolled in the CARDIA Study. The longitudinal nature of these data allowed us to account for time-varying confounding and differential censoring over time. In addition, compared with other measures of segregation, the Gi* statistic that we used to ascertain our exposure more closely captured contextual and spatial aspects of segregation. Furthermore, our study on the relationship between racial residential segregation and brain integrity provides a major contribution to an area of research that currently has a dearth of publications. We were able to detect brain structure differences across levels of cumulative racial residential segregation in a relatively young cohort of middle-aged adults. Future investigators will be able to consider our findings when contemplating specific pathways they wish to investigate. Finally, despite limited statistical power, our findings suggest that racial residential segregation can be associated with smaller brain volumes in general and with a more pronounced reduction in hippocampal volume. It is important to pursue these results further to better understand Black-White differences in dementia and neurocognitive pathology (49) and their underlying mechanisms.
Altogether, our findings suggest that racial residential segregation experienced during young adulthood is associated with worse brain structure, although most of the estimates were not statistically significant. Future MRI studies with rich data on measures of structural racism, yet with larger samples, should be conducted to replicate our findings.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, New York, United States (Adina Zeki Al Hazzouri, Neal Jawadekar, Katrina Kezios); Department of Neurology, School of Medicine, University of California, San Francisco, San Francisco, Calfornia, United States (Michelle R. Caunca); Department of Medicine, Miller School of Medicine, University of Miami, Miami, Florida, United States (Tali Elfassy); Department of Health Policy and Management, Mailman School of Public Health, Columbia University, New York, New York, United States (Sebastian Calonico); Division of Epidemiology, Department of Preventative Medicine, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, United States (Kiarri N. Kershaw); Departments of Psychiatry, Neurology, and Epidemiology and Biostatistics, School of Medicine, University of California, San Francisco, San Francisco, California, United States (Kristine Yaffe); Laboratory of Epidemiology and Population Science, National Institute on Aging, National Institutes of Health, Bethesda, Maryland, United States (Lenore Launer); Clinical Research Institute, Department of Internal Medicine, American University of Beirut, Beirut, Lebanon (Martine Elbejjani); Unité Mixte de Recherche 1219, Team VINTAGE, Bordeaux Population Health Research Center, INSERM, Université de Bordeaux, Bordeaux, France (Leslie Grasset); Taub Institute for Research in Alzheimer’s Disease and the Aging Brain, Columbia University, New York, New York, United States (Jennifer Manly); Department of Neurology, Irving Medical Center, Columbia University, New York, New York, United States (Jennifer Manly); Department of Epidemiology and Population Health, School of Medicine, Stanford University, Stanford, California, United States (Michelle C. Odden); and Department of Epidemiology and Biostatistics, School of Medicine, University of California, San Francisco, San Francisco, California, United States (M. Maria Glymour).
M.C.O. and M.M.G. are co–senior authors.
The Coronary Artery Risk Development in Young Adults (CARDIA) Study is supported by contracts HHSN268201800003I, HHSN268201800004I, HHSN268201800005I, HHSN268201800006I, and HHSN268201800007I with the National Heart, Lung, and Blood Institute (NHLBI). CARDIA was also partially supported by the Intramural Research Program of the National Institute on Aging (NIA) and an intraagency agreement between the NIA and the NHLBI (AG0005).
Data can be released and made available to investigators upon request from the CARDIA Publications and Presentations Committee.
The views expressed in this article are those of the authors and do not reflect those of the National Institutes of Health.
Conflict of interest: none declared.
REFERENCES
- 1. Zahodne LB, Manly JJ, Narkhede A, et al. Structural MRI predictors of late-life cognition differ across African Americans, Hispanics, and whites. Curr Alzheimer Res. 2015;12(7):632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brickman AM, Schupf N, Manly JJ, et al. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Arch Neurol. 2008;65(8):1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morris JC, Schindler SE, McCue LM, et al. Assessment of racial disparities in biomarkers for Alzheimer disease. JAMA Neurol. 2019;76(3):264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bailey ZD, Krieger N, Agenor M, et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389(10077):1453–1463. [DOI] [PubMed] [Google Scholar]
- 5. Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic status in health research: one size does not fit all. JAMA. 2005;294(22):2879–2888. [DOI] [PubMed] [Google Scholar]
- 6. DS M. The legacy of the 1968 Fair Housing Act. Sociol Forum (Randolph N J). 2015;30(suppl 1):571–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bécares L, Nazroo J, Jackson J. Ethnic density and depressive symptoms among African Americans: threshold and differential effects across social and demographic subgroups. Am J Public Health. 2014;104(12):2334–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Borrell LN, Kiefe CI, Diez-Roux AV, et al. Racial discrimination, racial/ethnic segregation, and health behaviors in the CARDIA Study. Ethn Health. 2013;18(3):227–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pool LR, Carnethon MR, Goff DC Jr, et al. Longitudinal associations of neighborhood-level racial residential segregation with obesity among blacks. Epidemiology. 2018;29(2):207–214. [DOI] [PubMed] [Google Scholar]
- 10. Kershaw KN, Robinson WR, Gordon-Larsen P, et al. Association of changes in neighborhood-level racial residential segregation with changes in blood pressure among black adults: the CARDIA Study. JAMA Intern Med. 2017;177(7):996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kershaw KN, Osypuk TL, Do DP, et al. Neighborhood-level racial/ethnic residential segregation and incident cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis. Circulation. 2015;131(2):141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. [DOI] [PubMed] [Google Scholar]
- 13. Launer LJ, Lewis CE, Schreiner PJ, et al. Vascular factors and multiple measures of early brain health: CARDIA brain MRI study. PLoS One. 2015;10(3):e0122138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Caunca MR, Odden MC, Glymour MM, et al. Association of racial residential segregation throughout young adulthood and cognitive performance in middle-aged participants in the CARDIA Study. JAMA Neurol. 2020;77(8):1000–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dubois B, Feldman HH, Jacova C, et al. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol. 2010;9(11):1118–1127. [DOI] [PubMed] [Google Scholar]
- 16. Jack CR Jr, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lo RY, Hubbard AE, Shaw LM, et al. Longitudinal change of biomarkers in cognitive decline. Arch Neurol. 2011;68(10):1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116(5):404–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kovalchik SA, Slaughter ME, Miles J, et al. Neighbourhood racial/ethnic composition and segregation and trajectories of cognitive decline among US older adults. J Epidemiol Community Health. 2015;69(10):978–984. [DOI] [PubMed] [Google Scholar]
- 20. Liu H, Zhang Z, Choi SW, et al. Marital status and dementia: evidence from the Health and Retirement Study. J Gerontol B Psychol Sci Soc Sci. 2020;75(8):1783–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Håkansson K, Rovio S, Helkala E-L, et al. Association between mid-life marital status and cognitive function in later life: population based cohort study. BMJ. 2009;339:b2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gardener H, Wright CB, Rundek T, et al. Brain health and shared risk factors for dementia and stroke. Nat Rev Neurol. 2015;11(11):651–657. [DOI] [PubMed] [Google Scholar]
- 23. Glymour MM, Manly JJ. Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychol Rev. 2008;18(3):223–254. [DOI] [PubMed] [Google Scholar]
- 24. Parker ED, Schmitz KH, Jacobs DR Jr, et al. Physical activity in young adults and incident hypertension over 15 years of follow-up: the CARDIA Study. Am J Public Health. 2007;97(4):703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chow L, Eberly LE, Austin E, et al. Fitness change effects on midlife metabolic outcomes: the CARDIA Study. Med Sci Sports Exerc. 2015;47(5):967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. [DOI] [PubMed] [Google Scholar]
- 27. Collin LJ, Gaglioti AH, Beyer KM, et al. Neighborhood-level redlining and lending bias are associated with breast cancer mortality in a large and diverse metropolitan area. Cancer Epidemiol Biomarkers Prev. 2021;30(1):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schwartz E, Onnen N, Craigmile PF, et al. The legacy of redlining: associations between historical neighborhood mapping and contemporary tobacco retailer density in Ohio. Health Place. 2021;68:102529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nardone A, Casey JA, Morello-Frosch R, et al. Associations between historical residential redlining and current age-adjusted rates of emergency department visits due to asthma across eight cities in California: an ecological study. Lancet Planet Health. 2020;4(1):e24–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jacoby SF, Dong B, Beard JH, et al. The enduring impact of historical and structural racism on urban violence in Philadelphia. Soc Sci Med. 2018;199:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chan MY, Na J, Agres PF, et al. Socioeconomic status moderates age-related differences in the brain’s functional network organization and anatomy across the adult lifespan. Proc Natl Acad Sci U S A. 2018;115(22):E5144–E5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Piccolo LR, Merz EC, He X, et al. Age-related differences in cortical thickness vary by socioeconomic status. PLoS One. 2016;11(9):e0162511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Elbejjani M, Fuhrer R, Abrahamowicz M, et al. Life-course socioeconomic position and hippocampal atrophy in a prospective cohort of older adults. Psychosom Med. 2017;79(1):14–23. [DOI] [PubMed] [Google Scholar]
- 34. Waldstein SR, Dore GA, Davatzikos C, et al. Differential associations of socioeconomic status with global brain volumes and white matter lesions in African American and white adults: the HANDLS SCAN study. Psychosom Med. 2017;79(3):327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hunt JFV, Buckingham W, Kim AJ, et al. Association of neighborhood-level disadvantage with cerebral and hippocampal volume. JAMA Neurol. 2020;77(4):451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Beatty Moody DL, Taylor AD, Leibel DK, et al. Lifetime discrimination burden, racial discrimination, and subclinical cerebrovascular disease among African Americans. Health Psychol. 2019;38(1):63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mair C, Diez Roux AV, Osypuk TL, et al. Is neighborhood racial/ethnic composition associated with depressive symptoms? The Multi-Ethnic Study of Atherosclerosis. Soc Sci Med. 2010;71(3):541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Elbejjani M, Auer R, Jacobs DR Jr, et al. Cigarette smoking and gray matter brain volumes in middle age adults: the CARDIA brain MRI sub-study. Transl Psychiatry. 2019;9(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dekkers IA, Jansen PR, Lamb HJ. Obesity, brain volume, and white matter microstructure at MRI: a cross-sectional UK Biobank study. Radiology. 2019;291(3):763–771. [DOI] [PubMed] [Google Scholar]
- 40. Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Harshfield EL, Pennells L, Schwartz JE, et al. Association between depressive symptoms and incident cardiovascular diseases. JAMA. 2020;324(23):2396–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dotson VM, Davatzikos C, Kraut MA, et al. Depressive symptoms and brain volumes in older adults: a longitudinal magnetic resonance imaging study. J Psychiatry Neurosci. 2009;34(5):367–375. [PMC free article] [PubMed] [Google Scholar]
- 43. Zeki Al Hazzouri A, Elfassy T, Sidney S, et al. Sustained economic hardship and cognitive function: the Coronary Artery Risk Development in Young Adults Study. Am J Prev Med. 2017;52(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Grasset L, Glymour MM, Elfassy T, et al. Relation between 20-year income volatility and brain health in midlife: the CARDIA Study. Neurology. 2019;93(20):e1890–e1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sisco S, Gross AL, Shih RA, et al. The role of early-life educational quality and literacy in explaining racial disparities in cognition in late life. J Gerontol B Psychol Sci Soc Sci. 2015;70(4):557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Greenland S, Senn SJ, Rothman KJ, et al. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol. 2016;31(4):337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Alberto A. Statistical nonsignificance in empirical economics. Am Econ Rev. 2(2):193–208. [Google Scholar]
- 48. Diez-Roux AV, Kiefe CI, Jacobs DR Jr, et al. Area characteristics and individual-level socioeconomic position indicators in three population-based epidemiologic studies. Ann Epidemiol. 2001;11(6):395–405. [DOI] [PubMed] [Google Scholar]
- 49. Weuve J, Barnes LL, Mendes de Leon CF, et al. Cognitive aging in black and white Americans: cognition, cognitive decline, and incidence of Alzheimer disease dementia. Epidemiology. 2018;29(1):151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.