Abstract
The virulence regulatory protein ToxR of Vibrio cholerae is unique in that it contains a cytoplasmic DNA-binding–transcriptional activation domain, a transmembrane domain, and a periplasmic domain. Although ToxR and other transmembrane transcriptional activators have been discovered in other bacteria, little is known about their mechanism of activation. Utilizing degenerate oligonucleotides and PCR, we have amplified internal toxR gene sequences from seven Vibrio and Photobacterium species and subspecies, demonstrating that toxR is an ancestral gene of the family Vibrionaceae. Sequence alignment of all available ToxR amino acid sequences revealed a region between the transcriptional activation and transmembrane domains that displays wide divergence among Vibrio species. We hypothesize that this region merely tethers the transcriptional activation domain to the cytoplasmic membrane and thus can tolerate wide divergence and multiple insertions and deletions. The divergence in the tether region at the nucleotide level may provide a useful tool for the distinction of Vibrio and Photobacterium species.
The bacterium Vibrio cholerae expresses virulence factors that allow it to colonize the human intestine and cause the disease cholera. The transmembrane transcriptional activator protein ToxR of V. cholerae is required for coordinate expression of virulence factors, including cholera toxin and the toxin-coregulated pilus (13, 17). When discovered, ToxR was unique in that it was the first example of a transcriptional activating protein that also contained a transmembrane segment and periplasmic domain in addition to the cytoplasmic DNA-binding domain. It was hypothesized that the periplasmic domain allowed ToxR to directly sense the extracellular environment and activate the transcription of virulence genes only under inducing conditions. However, since the discovery of ToxR, it has become clear that induction of virulence factor expression in V. cholerae is much more complicated. ToxR requires another transmembrane transcriptional activator, TcpP, in order to synergistically activate transcription of toxT, which encodes yet a third transcriptional activator that is entirely cytoplasmically located (5, 7). ToxT is the direct transcriptional activator of the ctx and tcp genes, which encode the cholera toxin and toxin-coregulated pilus proteins.
This complicated virulence cascade apparently is the result of the acquisition of multiple mobile genetic elements in V. cholerae: the ctx genes are encoded in a filamentous bacteriophage (18), while the toxT and tcp genes (including tcpP) are located on a large, recently acquired pathogenicity island, which may also be a filamentous bacteriophage (8, 9). The toxR gene, however, was apparently present in the ancestral chromosome because it has been found in three other closely related species, V. fischeri, V. parahaemolyticus, and Photobacterium profundum (11, 16, 19). Thus, the recently acquired genetic elements in V. cholerae appear to have coerced an ancestral regulatory protein into controlling virulence factor expression. The ancestral role of ToxR was likely as a regulator of outer membrane porins, because V. cholerae ToxR still controls expression of the porins OmpU and OmpT in a ToxT- and TcpP-independent manner (1). ToxR of P. profundum has also been demonstrated to control outer membrane porin expression (19).
Given the central role ToxR plays in the virulence of V. cholerae and the interesting topology of the protein itself, we wished to compare various ToxR sequences to determine (i) if toxR is, in fact, an ancestral Vibrio gene (i.e., is it widespread throughout Vibrio and Photobacterium species?), (ii) whether any clues to ToxR function can be drawn from a comparative analysis of multiple ToxR sequences, and (iii) if differences in the toxR sequence can be used to distinguish various Vibrio and/or Photobacterium species.
Identification of toxR in seven Vibrio and Photobacterium species.
We designed degenerate oligonucleotides based on conserved regions of the four ToxR protein sequences available. These oligonucleotides, recognizing the coding sequences for EQGFEVDD (located within the transcription activation domain) and VIATGGQN (located in the periplasmic domain), were used to amplify internal toxR fragments from an additional seven Vibrio or Photobacterium species by PCR; the primers also incorporated restriction sites for EcoRI and BamHI. The PCR with Taq DNA polymerase consisted of 92°C for 45 s, 42°C for 1 min, and 72°C for 1.5 min for 30 cycles. The resulting fragments were first digested with EcoRI and BamHI and then ligated into pTZ19U (12) that had been similarly digested.
We amplified partial toxR sequences from the human pathogens V. alginolyticus, V. mimicus, V. fluvialis, V. hollisae, and V. vulnificus and from the fish pathogens Photobacterium damselae subsp. damselae and P. damselae subsp. piscicida. Clearly, toxR is an ancestral gene of the Vibrio-Photobacterium lineage since it is present in all of these species and subspecies.
Alignment of ToxR amino acid sequences reveals a degenerate tether region.
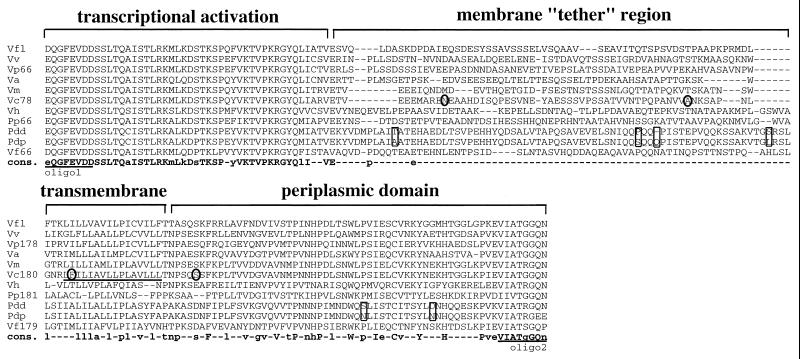
The amino acid sequences deduced from these seven partial toxR genes were aligned with the previously described ToxR amino acid sequences of V. cholerae, V. fischeri, V. parahaemolyticus, and P. profundum (Fig. 1). Interestingly, while there is a high level of homology between the transcriptional activation domains of the ToxR proteins and relatively conserved homology between the transmembrane and periplasmic domains, there is essentially no homology within the region between the transcriptional activation domain and the transmembrane domain (see the consensus sequence [cons.] in Fig. 1). This region tethers the transcriptional activation domain to the cytoplasmic membrane, and we have therefore named it the membrane tether. We utilized several different alignment programs (CLUSTALW, MULTALIN, and BLAST), but none assigned any significant homology within this region between the Vibrio and Photobacterium ToxR sequences. Also noticeable in the alignment are multiple deletions and/or insertions within this membrane tether region.
FIG. 1.
Alignment of ToxR amino acid sequences from 11 Vibrio and Photobacterium species. The partial toxR genes from V. fluvialis (Vfl), V. vulnificus (Vv), V. alginolyticus (Va), V. mimicus (Vm), V. hollisae (Vh), P. damselae subsp. damselae (Pdd), and P. damselae subsp. piscicida (Pdp) were amplified by using degenerate oligonucleotides recognizing the sequences EQGFEVDD and VIATGGQN (underlined; oligo1 and oligo2). The deduced amino acid sequences are shown aligned with the ToxR amino acid sequences from V. cholerae (Vc78 and Vc180; accession no. M21249), V. parahaemolyticus (Vp66 and Vp178; accession no. L11929), V. fischeri (Vf66 and Vf179; accession no. L29053), and P. profundum (Pp66 and Pp181; accession no. U77060); the numerical part of each designation is the starting amino acid residue of the ToxR sequence. The alignment shown was created by MULTALIN, which depicts gaps as dashes (2). The consensus (cons.) sequence is shown at the bottom in boldface letters, residues identical in at least 10 of the 11 sequences are shown in uppercase letters, and residues identical in at least 6 of the 11 sequences are shown in lowercase letters. Residues identical in five or fewer sequences are depicted as dashes. The defined transmembrane domain of V. cholerae ToxR is underlined; all other ToxR sequences were predicted to have transmembrane domains in this region by the dense alignment surface method of transmembrane prediction (3). The four amino acids which differ between the classical and El Tor V. cholerae biotype ToxR proteins are circled, and the six amino acid differences between P. damselae subsp. damselae and P. damselae subsp. piscicida are boxed.
The differences between the ToxR proteins is particularly noticeable in closely related bacteria. The closely related subspecies P. damselae subsp. damselae and P. damselae subsp. piscicida, which have 100% identical 16S RNA genes, have six amino acid differences in their deduced ToxR sequences, and four of the six differences lie in the membrane tether region (including a 3-bp deletion in P. damselae subsp. damselae which results in a one-amino-acid deletion [Fig. 1, boxes]). The even more closely related classical and El Tor V. cholerae biotype ToxR proteins differ by four amino acids (4), and two of these four differences lie within the membrane tether region (Fig. 1, circles).
The high level of divergence within this membrane tether region between closely related species, subspecies, and biotypes indicates either a strong selective pressure toward divergence of function or an absolute lack of critical function in this region. We favor the latter hypothesis, given the apparent randomness of the amino acid sequences in this region and the insertions and/or deletions occurring here. If this region serves no function besides acting to tether the transcriptional activation domain to the transmembrane domain, then the amino acid sequence would be irrelevant and deletions or insertions would be tolerated. It has been shown that the isolated cytoplasmic portion of V. cholerae ToxR is inactive, but fusing the cytoplasmic portion to a heterologous transmembrane domain restores activity (15), consistent with the idea that tethering the cytoplasmic portion to the membrane is critical for ToxR activity. Our prediction that the amino acid sequence of the membrane tether region is irrelevant awaits further structure-function studies.
Use of toxR nucleotide divergence for identification of Vibrio and Photobacterium species.
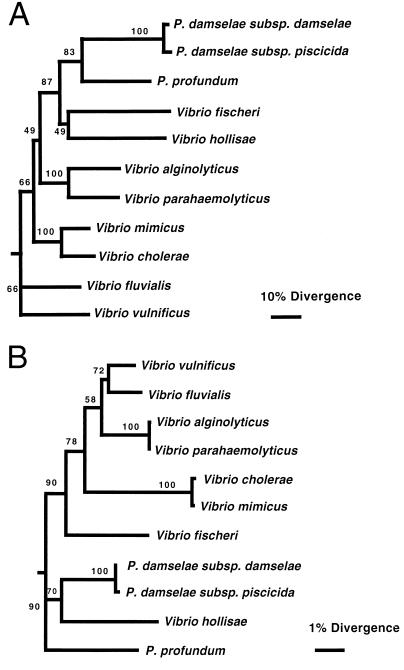
The membrane tether region may serve as a useful diagnostic tool for the distinction of various Vibrio and Photobacterium species, given the high level of divergence within this region. Using the nucleotide sequences of the partial toxR genes, we constructed a phylogenetic tree (Fig. 2A) which demonstrates much greater divergence (based on nucleotide differences) than a phylogenetic tree constructed with the 16S genes from the same species and subspecies (Fig. 2B). For example, V. cholerae and V. mimicus, which have 99.6% identical 16S genes, have only 71.2% identical nucleotides within the partial toxR sequence described here and V. alginolyticus and V. parahaemolyticus, which have 99.8% identical 16S genes, have only 61.7% identical nucleotides within the partial toxR sequence. This may be a useful means of distinguishing between these species, as suggested by Kim et al. (10). Finally, P. damselae subsp. damselae and P. damselae subsp. piscicida have 100% identical 16S genes, as mentioned above (14), but have only 91% identical nucleotides within the partial toxR sequence. These Photobacterium subspecies have different host specificities, and new molecular techniques to distinguish them would benefit fish disease management strategies. Perhaps divergence within the membrane tether of ToxR could provide the basis of the distinction of other Vibrio and Photobacterium species.
FIG. 2.
Unrooted trees constructed by the neighbor-joining method showing the phylogenetic interrelationships of the different Vibrio and Photobacterium species used in the present study, based on toxR gene nucleotide sequences (A) and 16S rRNA genes (B). Bootstrap values (from 1,000 tree replicates generated by using the programs SEQBOOT, DNADIST, and CONSENSE of the PHYLIP package) are given at the branching points. The bars show sequence divergence (6) (note the difference between the scales). Accession numbers for 16S genes: P. damselae subsp. damselae, X74700; P. damselae subsp. piscicida, Y18496; V. alginolyticus, X74690; V. fischeri, X74702; V. fluvialis, X76335; V. hollisae, X74707; V. mimicus, X74713; V. parahaemolyticus, X74720; V. vulnificus, X76334; V. cholerae, X76337; P. profundum, D21226. Accession numbers for toxR sequences are given in the text and the legend to Fig. 1.
Nucleotide sequence accession numbers.
The nucleotide sequences reported in this paper were deposited in the GenBank database under accession no. AF170885 (V. fluvialis), AF170883 (V. vulnificus), AF170882 (V. alginolyticus), AF170881 (V. mimicus), AF170884 (V. hollisae), AF170886 (P. damselae subsp. damselae), and AF170887 (P. damselae subsp. piscicida).
Acknowledgments
We thank Cristina Pascual Ramos for assistance with phylogenetic trees.
This work was supported by an Institutional New Faculty Award of the Howard Hughes Medical Institute to K.E.K. and a predoctoral fellowship of the Spanish Ministry of Education and Science to C.R.O.
REFERENCES
- 1.Champion G A, Neely M N, Brennan M A, DiRita V J. A branch in the ToxR regulatory cascade of Vibrio cholerae revealed by characterization of toxT mutant strains. Mol Microbiol. 1997;23:323–331. doi: 10.1046/j.1365-2958.1997.2191585.x. [DOI] [PubMed] [Google Scholar]
- 2.Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988;16:10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cserzo M, Wallin E, Simon I, von Heijne G, Elofsson A. Prediction of transmembrane alpha-helices in prokaryotic membrane proteins: the dense alignment surface method. Protein Eng. 1997;10:673–676. doi: 10.1093/protein/10.6.673. [DOI] [PubMed] [Google Scholar]
- 4.DiRita V J, Neely M, Taylor R K, Bruss P M. Differential expression of the ToxR regulon in classical and El Tor biotypes of Vibrio cholerae is due to biotype-specific control over toxT expression. Proc Natl Acad Sci USA. 1996;93:7991–7995. doi: 10.1073/pnas.93.15.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiRita V J, Parsot C, Jander G, Mekalanos J J. Regulatory cascade controls virulence in Vibrio cholerae. Proc Natl Acad Sci USA. 1991;88:5403–5407. doi: 10.1073/pnas.88.12.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felsenstein J. Inferring phylogenies from protein sequences by parsimony, distance, and likelihood methods. Methods Enzymol. 1996;266:418–427. doi: 10.1016/s0076-6879(96)66026-1. [DOI] [PubMed] [Google Scholar]
- 7.Hase C C, Mekalanos J J. TcpP protein is a positive regulator of virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci USA. 1998;95:730–734. doi: 10.1073/pnas.95.2.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karaolis D K, Johnson J A, Bailey C C, Boedeker E C, Kaper J B, Reeves P R. A Vibrio cholerae pathogenicity island associated with epidemic and pandemic strains. Proc Natl Acad Sci USA. 1998;95:3134–3139. doi: 10.1073/pnas.95.6.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karaolis D K, Somara S, Maneval D R J, Johnson J A, Kaper J B. A bacteriophage encoding a pathogenicity island, a type-IV pilus and a phage receptor in cholera bacteria. Nature. 1999;399:375–379. doi: 10.1038/20715. [DOI] [PubMed] [Google Scholar]
- 10.Kim Y B, Okuda J, Matsumoto C, Takahashi N, Hashimoto S, Nishibuchi M. Identification of Vibrio parahaemolyticus strains at the species level by PCR targeted to the toxR gene. J Clin Microbiol. 1999;37:1173–1177. doi: 10.1128/jcm.37.4.1173-1177.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin Z, Kumagai K, Baba K, Mekalanos J J, Nishibuchi M. Vibrio parahaemolyticus has a homolog of the Vibrio cholerae toxRS operon that mediates environmentally induced regulation of the thermostable direct hemolysin gene. J Bacteriol. 1993;175:3844–3855. doi: 10.1128/jb.175.12.3844-3855.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mead D A, Szczesna-Skorupa E, Kemper B. Single-stranded DNA ‘blue’ T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986;1:67–74. doi: 10.1093/protein/1.1.67. [DOI] [PubMed] [Google Scholar]
- 13.Miller V L, Taylor R K, Mekalanos J J. Cholera toxin transcriptional activator ToxR is a transmembrane DNA binding protein. Cell. 1987;48:271–279. doi: 10.1016/0092-8674(87)90430-2. [DOI] [PubMed] [Google Scholar]
- 14.Osorio C R, Collins M D, Toranzo A E, Barja J L, Romalde J L. 16S rRNA gene sequence analysis of Photobacterium damselae and nested PCR method for rapid detection of the causative agent of fish pasteurellosis. Appl Environ Microbiol. 1999;65:2942–2946. doi: 10.1128/aem.65.7.2942-2946.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ottemann K M, Mekalanos J J. Analysis of Vibrio cholerae ToxR function by construction of novel fusion proteins. Mol Microbiol. 1995;15:719–731. doi: 10.1111/j.1365-2958.1995.tb02380.x. [DOI] [PubMed] [Google Scholar]
- 16.Reich K A, Schoolnik G K. The light organ symbiont Vibrio fischeri possesses a homolog of the Vibrio cholerae transmembrane transcriptional activator ToxR. J Bacteriol. 1994;176:3085–3088. doi: 10.1128/jb.176.10.3085-3088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor R K, Miller V L, Furlong D B, Mekalanos J J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci USA. 1987;84:2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waldor M K, Mekalanos J J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 19.Welch T J, Bartlett D H. Identification of a regulatory protein required for pressure-responsive gene expression in the deep-sea bacterium Photobacterium species strain SS9. Mol Microbiol. 1998;27:977–985. doi: 10.1046/j.1365-2958.1998.00742.x. [DOI] [PubMed] [Google Scholar]