Abstract
Curative surgery for other many cancers requires that the tumor be removed with a zone of normal tissue surrounding the tumor with ‘negative’ margins. Sarcomas, cancers of the bones, muscles, and fat, require WLE for cure. Unfortunately, ‘positive’ margins occur in 20–25% of sarcoma surgeries, associated with cancer recurrence and reduced survival. Our group successfully tested a small-molecule fluorophore (ABY-029) in sarcomas that targets the epidermal growth factor receptor. We sought to evaluate human sarcoma xenografts for epidermal growth factor receptor expression and binding of ABY-029 with and without exposure to standard presurgical chemotherapy and radiation. We inoculated groups of 24 NSG mice with five cell lines (120 mice total). Eight mice from each cell line received: 1) radiation alone; 2) chemotherapy alone; or 3) chemotherapy and radiation. We administered ABY-029 2–4 hours before surgery. Tumor and biopsy portions of background tissues were removed. All tissues were imaged on a LI-COR Odyssey and processed in pathology. There were no significant reductions in epidermal growth factor receptor expression or in ABY-029-mediated fluorescence in tumors exposed to chemotherapy, radiation, or both. fluorescence-guided surgery demonstrates strong promise to improve curative surgical cancer care, particularly for sarcomas where the positive margin rate is substantial. Fluorophore performance must be evaluated under circumstances that duplicate accurately the biological milieu relevant to a particular cancer. This work shows that human sarcoma xenografts subjected to standard therapies do not demonstrate a change in epidermal growth factor receptor expression or in epidermal growth factor receptor-targeted fluorescence, thereby indicating that epidermal growth factor receptor-targeted fluorescence-guided surgery should be feasible under normal therapeutic conditions in the clinic.
Keywords: fluorescence-guided surgery, sarcoma, epidermal growth factor, chemotherapy, radiation
INTRODUCTION:
Fluorescence-guided surgery (fluorescence-guided surgery) is an emerging field using fluorescent markers to label important anatomical structures or tumors. Curative surgery for other many cancers requires that the tumor be removed with a zone of normal tissue surrounding the tumor—termed a wide-local excision (WLE) with ‘negative’ margins. A WLE requires the surgeon to determine the location of the tumor within its surrounding tissues and estimate the margin’s thickness. At present, surgeons rely on radiologic imaging and visual and tactile clues to gauge margin thickness.
Sarcomas, cancers of the bones, muscles, and fat, require WLE for cure. Unfortunately, ‘positive’ margins occur in 20–25% of sarcoma surgeries and are associated with cancer recurrence and reduced survival. Using human sarcoma xenografts, our research group has successfully tested a small-molecule fluorophore (ABY-029) that targets the epidermal growth factor receptor (epidermal growth factor receptor). Approximately 70% of human sarcomas overexpress epidermal growth factor receptor, making it an attractive target for fluorescence-guided surgery. However, most patients with sarcoma undergo preoperative chemotherapy and/or radiation therapy. It is unknown how these therapies alter epidermal growth factor receptor expression and whether expression changes would deleteriously affect the binding of an epidermal growth factor receptor-targeted reporter.
The purpose of this work was therefore to evaluate human sarcoma xenografts for epidermal growth factor receptor expression and binding of ABY-029 with and without exposure to standard presurgical chemo- and radiation therapies. The primary aim was to determine if standard neoadjuvant therapies for human sarcomas reduce the normal expression of the epidermal growth factor receptor and subsequent binding of an epidermal growth factor receptor-targeted probe. Documentation of this finding would potentially jeopardize the advancement of fluorescence-guided surgery using epidermal growth factor receptor-targeted reporters.
METHODS:
IACUC approval was obtained for this study. We inoculated groups of 24 NSG mice (50% female; 50% male) with each of the five cell lines under the dorsal skin (120 mice total) (Fig. 1). Eight mice from each cell line will be assigned by permuted block randomization to a treatment arm: 1) radiation alone; 2) chemotherapy alone; 3) chemotherapy and radiation. Up to 8 weeks are required for tumors to grow to a diameter of 300mm3 (<10% body weight), at which point five of the eight mice from each cell line/treatment group will be assigned randomly to receive therapy and three will be assigned placebo (control). Human patients with soft-tissue sarcoma typically receive 50Gy radiation. Based on an established model with biological equivalence to human dosing, animals received radiation via a Varian 2100c linear accelerator. For chemotherapy, we used an established doxorubicin mouse model. After treatment, the mice had a one-week recovery period before surgery. We administered ABY-029 and begin surgery 2–4 hours later. The tumor and biopsy portions of background tissues (muscle, adipose, fascia, sciatic nerve) were removed. All tissues were imaged on a LI-COR Odyssey. Tissues were then fixed in formalin and underwent epidermal growth factor receptor staining, sectioning, and mounting on slides. A research pathologist blinded to treatment status will review the tumor and background tissue slides and score the specimens for epidermal growth factor receptor staining.
Figure 1 -.
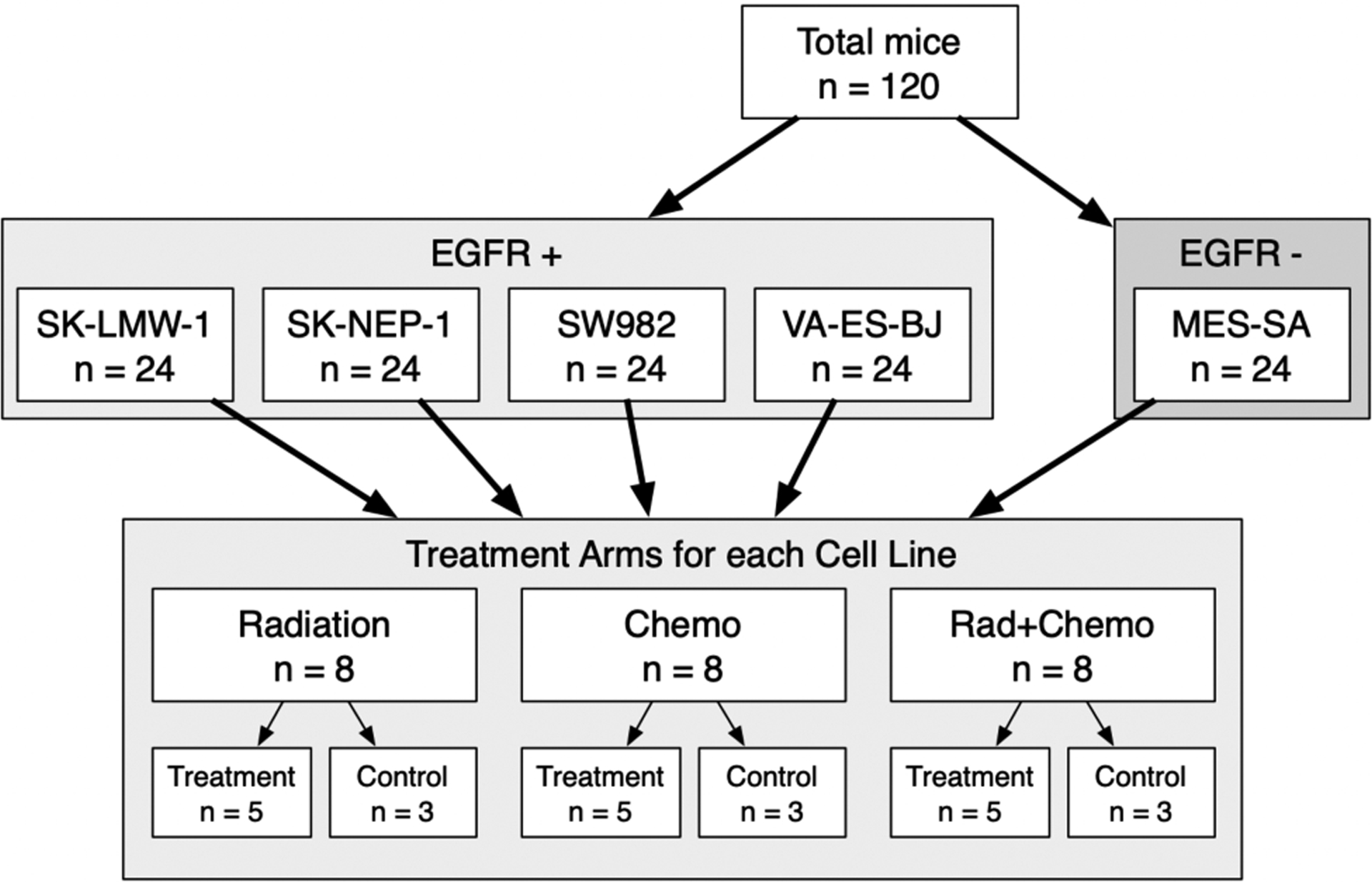
Experimental design for current study
RESULTS:
There were no significant reductions in epidermal growth factor receptor expression in the tumors exposed to chemotherapy, radiation, or both (Fig. 2). These findings indicate that the effect of epidermal growth factor receptor-targeting fluorophores or chemotherapeutic agents would not likely be diminished by the use of conventional preoperative sarcoma therapies.
Figure 2.
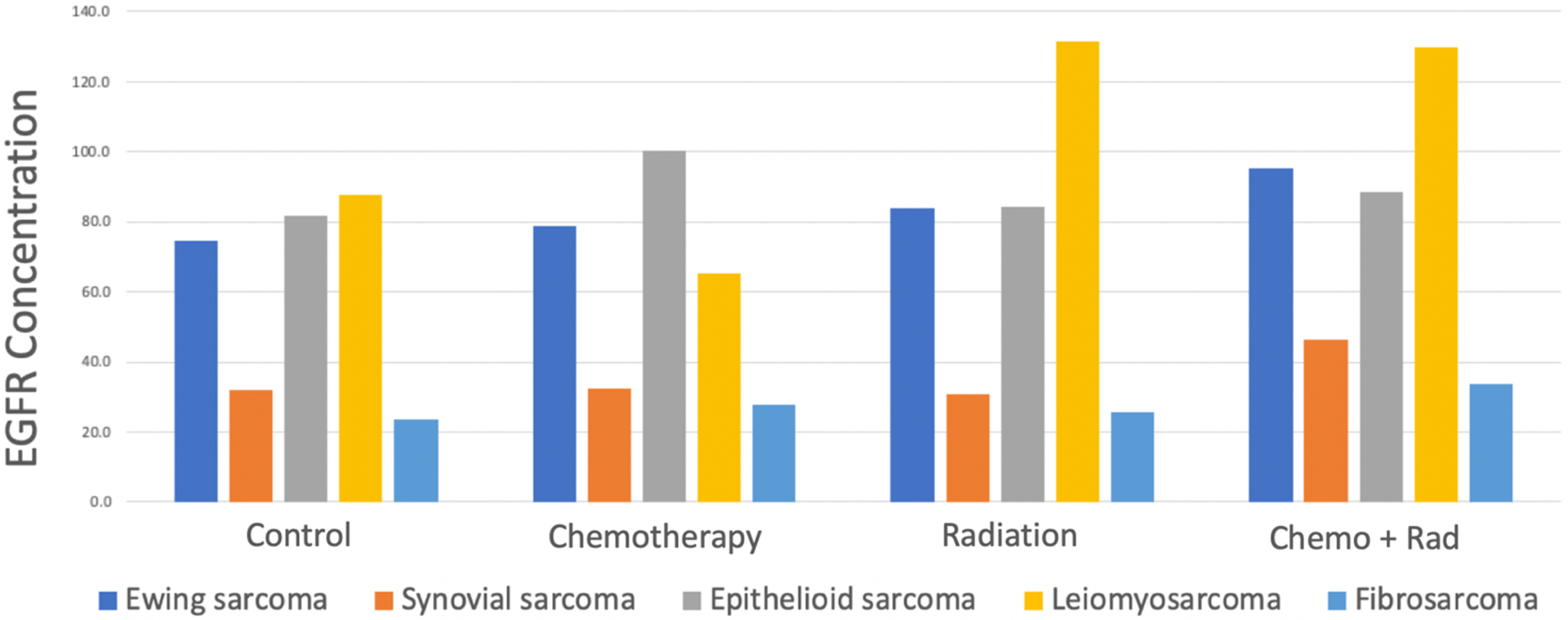
EGFR expression in sarcoma tumors following neoadjuvant therapies
There were no significant reductions in ABY-029-mediated fluorescence in the tumors exposed to chemotherapy, radiation, or both (Fig. 3). These findings indicate that the use of ABY-029 and other epidermal growth factor receptor-targeting fluorophores would not likely be diminished by the use of conventional preoperative sarcoma therapies.
Figure 3.
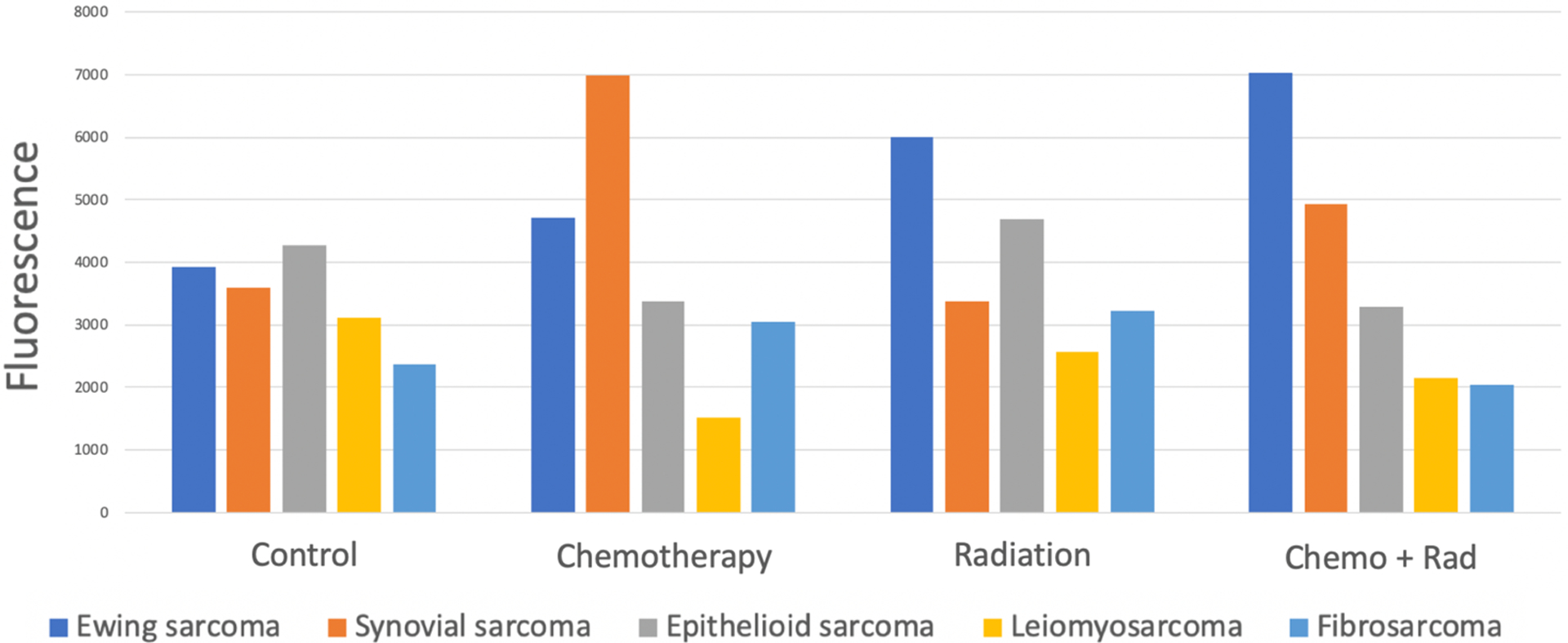
EGFR-targeted fluorescence in sarcoma tumors following neoadjuvant therapies
Tumor growth was affected predictably by therapies with known efficacy, but effective treatment showed no diminution in epidermal growth factor receptor expression or fluorescence.
DISCUSSION:
The results of this study indicate that conventional chemotherapeutic and radiation therapies for soft-tissue sarcomas do not affect the concentration of epidermal growth factor receptor expression and, similarly, do not appear to affect fluorescence from ABY-029, an epidermal growth factor receptor-targeting, small-molecule fluorophore based on an affibody structure.
Fluorescence-guided surgery demonstrates strong promise to improve curative surgical cancer care, particularly for sarcomas where the positive margin rate is substantial. The number of fluorescence reporters in trials is expanding rapidly, with epidermal growth factor receptor-targeted probes receiving substantial attention. epidermal growth factor receptor is commonly overexpressed in many sarcomas, making these agents ideal for use in sarcoma surgery. Anti-cancer therapies, however, can have physical, chemical, and metabolic effects on both tumor and non-tumor tissues, potentially leading to changes in the activity of fluorophores. Fluorophore performance must therefore be evaluated under circumstances that duplicate accurately the biological milieu relevant to a particular cancer. The work described here shows that human sarcoma xenografts subjected to scaled therapy do not demonstrate a change in epidermal growth factor receptor expression or in epidermal growth factor receptor-targeted fluorescence, thereby indicating that epidermal growth factor receptor-targeted fluorescence-guided surgery should be feasible under normal therapeutic conditions in the clinic.


