Abstract
Cells of Escherichia coli undergo a metabolic switch associated with the production and utilization of acetate. During exponential growth on tryptone broth, these cells excrete acetate via the phosphotransacetylase-acetate kinase (Pta-AckA) pathway. As they begin the transition to stationary phase, they instead resorb acetate, activate it to acetyl coenzyme A (acetyl-CoA) by means of the enzyme acetyl-CoA synthetase (Acs) and utilize it to generate energy and biosynthetic components via the tricarboxylic acid cycle and the glyoxylate shunt, respectively. This metabolic switch depends upon the induction of Acs. As part of our effort to dissect the mechanism(s) underlying induction and to identify the signal(s) that triggers that induction, we sought the sigma factor most responsible for acs expression. Using isogenic strains that carry a temperature sensitivity allele of the gene that encodes ς70 and either a wild-type or null allele of the gene that encodes ςS, we determined by immunoblotting, reverse transcriptase PCR, and acs::lacZ transcriptional fusion analyses that ς70 is the sigma factor primarily responsible for the acs transcription that cells induce during mid-exponential phase. In contrast, ςS partially inhibits that transcription as cells enter stationary phase.
During exponential growth on a mixture of amino acids, such as tryptone broth (TB), cells of Escherichia coli consume l-serine and l-aspartate in a strictly preferential order while simultaneously excreting acetate. Once they have consumed both serine and aspartate, these cells undergo a metabolic switch. Instead of excreting acetate, they consume it. This acetate-associated metabolic switch occurs just as the cells begin to decelerate their growth rate, i.e., as they begin the transition to stationary phase (14). The resorption and utilization of excreted acetate depends upon the induction of acetyl coenzyme A (acetyl-CoA) synthetase (Acs) [acetate:CoA ligase (AMP-forming), EC 6.2.1.1], the enzyme that catalyzes the reaction
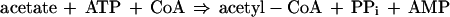 |
which proceeds through an enzyme-bound acetyladenylate intermediate (acetylAMP) (1, 6, 10).
This acetate switch occurs because cells induce acs transcription (A. J. Wolfe, C. Beatty, and S. Kumari, unpublished data). To help focus our efforts to identify and characterize the signal(s) and underlying mechanism(s) that trigger this induction, we sought the sigma factor responsible for acs transcription. We suspected the housekeeping ς70 because it is present and active during the period in question. We also suspected the stationary-phase-associated ςS because its induction approximates that of Acs (9, 13, 17, 20; Wolfe et al., unpublished data). Other evidence also supported the possibility that acs transcription might depend, at least in part, on ςS: incubation in spent medium, a condition that stimulates transcription by ςS (13), also induces acs transcription (17), and cells lacking both ςS and the glyoxylate bypass repressor IclR transcribe acs at a lower level than do wild-type cells (17).
To determine whether ς70, ςS, or both play a role in controlling acs expression, we used strains UQ285 and AJW1404. Cells of UQ285, an E. coli K-12 derivative, carry a temperature sensitivity allele of rpoD, which encodes ς70 (8, 21). This strain had been used previously by Yim et al. (21) to support their contention that osmY transcription was dependent upon ςS and not ς70. Cells of AJW1404, a UQ285 derivative, carry both the temperature sensitivity rpoD allele and a null allele of rpoS, which encodes ςS. AJW1404 was constructed by P1 generalized transduction (18), using strain ZK1000 (rpoS::Km) (3) as the source of donor DNA.
The following temperature shift protocol formed the basis for all experiments described below. Cells were grown at 32°C in TB (1% [wt/vol] tryptone and 0.5% [wt/vol] sodium chloride) until the culture reached mid-exponential phase. At this point, the culture was divided: half the culture was further incubated at 32°C (permissive temperature for ς70 function), while the other half was incubated at 42°C (restrictive temperature for ς70 function). At the indicated times or optical densities (monitored at 610 nm [OD610]), cells were harvested and subjected to immunoblot analysis (11) with an anti-E. coli Acs antiserum (10), β-galactosidase assays (12), or reverse transcriptase (RT)-PCR analysis (5) while the supernatant liquid was assayed for acetate content (2).
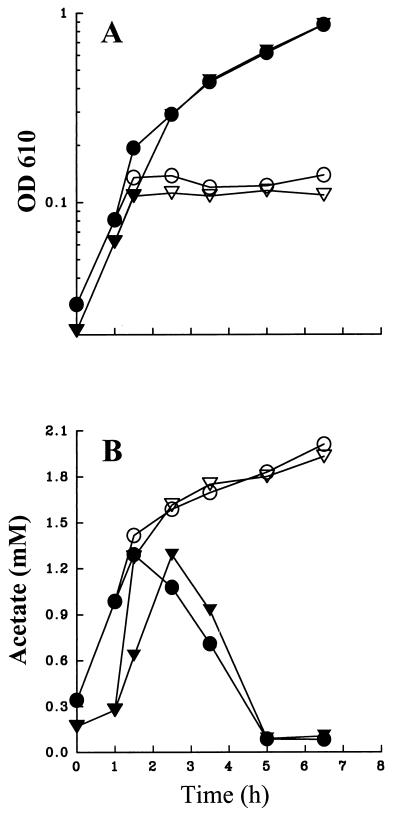
Within 30 min of the shift from permissive (32°C) to restrictive (42°C) temperature, cultures of wild-type cells (strain UQ285) or cells deficient for ςS (strain AJW1404) stopped growing (Fig. 1A), indicating that ς70 no longer functioned. After this shift, the extracellular concentration of acetate produced by cells of both strains continued to increase (Fig. 1B) as if these cells lacked Acs (10). In contrast, cells grown exclusively at the permissive temperature resorbed extracellular acetate at about the same rate regardless of whether ςS was present (Fig. 1B). Intriguingly, ςS seemed to play some role in regulating the production of acetate: acetate excretion, and, therefore, the beginning of its resorption by cells lacking ςS, was delayed about 1 h relative to that by wild-type cells with ςS (Fig. 1B).
FIG. 1.
OD (A) and extracellular acetate concentration (B) plotted as a function of time for cells of strains UQ285 [rpoD(Ts) rpoS+] and AJW1404 [rpoD(Ts) rpoS::Km] grown at 32°C in TB until the culture reached mid-exponential phase (OD610, ∼0.1), at which point the cultures were divided. Half the culture was further incubated at 32°C (closed symbols) while the other half was incubated at 42°C (open symbols). Circles, strain UQ285; triangles, strain AJW1404. Bars for the standard errors of the mean of triplicate experiments are small relative to the size of the symbols.
To determine whether the temperature shift exerted its own effect upon the ability of cells to resorb extracellular acetate, the acs+ rpoD+ rpoS+ strain CP875 (15), its rpoS::Km derivative (strain AJW1015; constructed by P1 generalized transduction with strain ZK1000 as the source of donor DNA), and its acs::Km derivative (strain AJW803) (10) were subjected to the temperature shift protocol. When shifted to the restrictive temperature, cultures of all three strains accumulated about 30% more extracellular acetate than did identical cultures incubated at 32°C (data not shown), a result consistent with that made previously by Prüß and Wolfe (15). The temperature increase, however, did not affect the ability of any of these three strains to remove acetate from the media. Whereas cells wild type for Acs (strains CP875 and AJW1015) resorbed acetate, those deficient for Acs (strain AJW803) did not (data not shown). As observed for cells with the UQ285 genetic background (Fig. 1), the excretion of acetate but not its resorption by cells lacking ςS (strain AJW1015) was delayed relative to that exhibited by cells wild type for ςS (strain CP875).
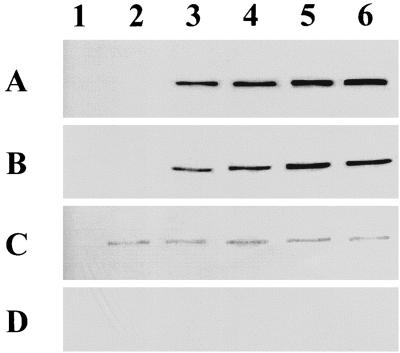
To determine whether the inability to resorb extracellular acetate at a restrictive temperature results from the lack of Acs, immunoblot analysis was performed (Fig. 2). The relative timing of Acs synthesis by wild-type cells (Fig. 2A, strain UQ285) or cells deficient for ςS (Fig. 2B, strain AJW1404) grown exclusively at the permissive temperature was similar. Steady-state levels became detectable just prior to the transition from exponential growth to stationary phase (OD610, between ∼0.2 and ∼0.4) and continued to increase at least until entry into stationary phase (OD610, ∼0.8). The amount of Acs exhibited by ςS-deficient cells, however, was invariably about 25 to 40% less than that displayed by cells wild type for ςS. At the restrictive temperature where ς70 no longer functions, wild-type cells with ςS expressed very low Acs levels (Fig. 2C), while those also deficient for ςS did not exhibit levels above the limit of detection (Fig. 2D).
FIG. 2.
Immunoblot analyses with rabbit anti-E. coli Acs polyclonal antibody after sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% polyacrylamide). Cells of strains UQ285 [rpoD(Ts) rpoS+] (A and C) and AJW1404 [rpoD(Ts) rpoS::Km] (B and D) were subjected to the temperature shift protocol described in the legend to Fig. 1. Lane 1, cells harvested just prior to the culture split. Cells grown at 32°C (A and B) were harvested at the following mean OD610s ± standard error of the mean: lanes 2, 0.10 ± 0.01; lanes 3, 0.25 ± 0.01; lanes 4, 0.45 ± 0.01; lanes 5, 0.60 ± 0.02; lanes 6, 0.70 ± 0.03. Cells grown at 42°C (C and D) were harvested at the same time as those of the same strain grown at 32°C.
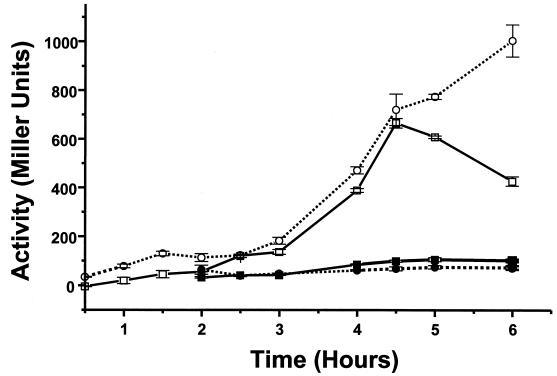
To determine whether the effects of ς70 and ςS on Acs synthesis operate at the level of transcription, strains UQ285 and AJW1404 were lysogenized with acs::lacZ transcriptional fusions that carried various portions of the regulatory region (Fig. 3) and verified as single lysogens (19). Each resultant lysogen or transformant was subjected to the temperature shift protocol, and its β-galactosidase activity was assayed. At the permissive temperature, i.e., in the presence of functional ς70, ςS partially inhibited the activity of the λCB7 fusion (Fig. 4). At the restrictive temperature, i.e., in the absence of functional ς70, this fusion exhibited very low activity regardless of the presence or absence of ςS. We obtained qualitatively similar results for the λCB5 and λCB6 fusions (data not shown). Activities of the fusions lacP::lacZ and bolA::lacZ were entirely dependent upon ς70 and ςS, respectively (data not shown).
FIG. 3.
Schematic representation of the intergenic nrfA-acs region depicting the approximate extent and/or location of the nrfA promoter, the acs promoters P1 and P2 (A. J. Wolfe et al., unpublished data), selected restriction sites [M, MunI; S, SphI; (A), ApoI introduced by site-directed mutagenesis], primers used for RT-PCR analyses, and acs::lacZ transcription fusions used in this study. The nucleotide immediately 5′ to the ATG codon that encodes the translation initiation site of the acs open reading frame was arbitrarily chosen as −1.
FIG. 4.
β-Galactosidase activity exhibited by the acs::lacZ fusion carried by λCB7 as a lysogen of strains UQ285 [rpoD(Ts) rpoS+] and AJW1404 [rpoD(Ts) rpoS::Km]. Each lysogen was subjected to the temperature shift protocol described in the legend to Fig. 1. Open symbols, 32°C; closed symbols, 42°C; squares, strain UQ285; circles, strain AJW1404. Values are the mean ± standard error of the mean of at least three independent determinations.
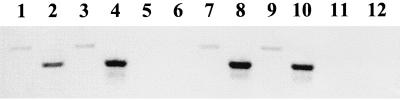
To determine whether the low-level promoter activity observed at the restrictive temperature was physiologically relevant, RT-PCR analysis was performed on cells of strains UQ285 and AJW1404 (Fig. 5). Prior to the temperature shift, PCR products consistent with acs transcription were observed from cells that expressed ςS (Fig. 5, lanes 1 and 2 [strain UQ285]) and also from those that did not (Fig. 5, lanes 7 and 8 [strain AJW1404]). Similar results were observed after further incubation at the permissive temperature (Fig. 5, lanes 3 and 4 [strain UQ285] and 9 and 10 [strain AJW1404]). In contrast, the cultures shifted to and incubated at the restrictive temperature did not yield detectable PCR products regardless of whether ςS was present (Fig. 5, lanes 5 and 6 [strain UQ285] and 11 and 12 [strain AJW1404]).
FIG. 5.
RT-PCR analysis. Cells of strains UQ285 [rpoD(Ts) rpoS+] and AJW1404 [rpoD(Ts) rpoS::Km] were subjected to the temperature shift protocol described in the legend to Fig. 1. Lanes 1 to 6, strain UQ285; lanes 7 to 12, strain AJW1404; lanes 1, 2, 7, and 8; cells grown at 32°C and harvested during mid-exponential growth just prior to dividing the culture; lanes 3, 4, 9, and 10, cells further incubated at 32°C and harvested upon entry into stationary phase; lanes 5, 6, 11, and 12, cells further incubated at 42°C and harvested at the same time as those incubated at 32°C; lanes 1, 3, 5, 7, 9, 11, primer pair F5 and R1, which amplifies a 563-bp region, was used (see Fig. 3); lanes 2, 4, 6, 8, 10, and 12, primer pair F6 and R1, which amplifies a 460-bp region, was used (see Fig. 3).
We conclude that acs transcription occurs as a function of ς70 activity. We base this conclusion on our observation that cells lacking functional ς70 did not resorb extracellular acetate, exhibited barely detectable levels of Acs protein, synthesized no detectable acs transcript, and displayed little acs promoter activity. Because ςS clearly did not contribute to the activation of acs transcription, we posit that the small amount of seemingly ςS-dependent Acs protein observed by immunoblot analysis results from some indirect mechanism, perhaps by transcribing some accessory transcription factor that participates in the control of ς70-dependent initiation of acs transcription. We also conclude that this ςS-dependent protein plays, at most, a secondary role since cells lacking ςS grow on small concentrations of acetate about as well as those that synthesize ςS (reference 10 and data not shown).
Intriguingly, ςS inhibits acs transcription during the transition to stationary phase, a period through which steady-state levels of ςS increase. Thus, it would seem that as extracellular acetate concentrations rise during late exponential growth, some ς70-dependent mechanism activates acs transcription. This would ensure the presence of sufficient Acs to rapidly and efficiently resorb acetate. Once this has been accomplished, the rising pool of ςS would inhibit acs transcription, presumably by competing with ς70 for core polymerase (7). This would reduce acs transcription to levels appropriate for the stationary phase.
Interestingly, the lack of ςS delays the excretion of extracellular acetate. This delay likely results from a decrease in the flux of acetate through the phosphotransacetylase-acetate kinase pathway that converts acetyl-CoA to acetate through an acetyl-phosphate intermediate (16). This decreased flux could result from reduced levels of its substrate, acetyl-CoA, or as a result of altered synthesis, stability, or activity of phosphotransacetylase and/or acetate kinase. Alternatively, the delay may result from the loss of some other acetate-producing enzyme whose transcription depends upon ςS, e.g., PoxB (4).
Acknowledgments
We thank R. Kolter and M. Villarejo for strains, R. W. Simons for the tools necessary to construct the acs::lacZ fusions, C. Beatty for λacs::lacZ fusions, and H. Minges for technical assistance.
This work was supported by grant MCB-9630647 from the National Science Foundation.
REFERENCES
- 1.Berg P. Acyl adenylates: an enzymatic mechanism of acetate activation. J Biol Chem. 1956;222:991–1013. [PubMed] [Google Scholar]
- 2.Bergmeyer H U, Möllering H. Enzymatische Bestimmung von Acetate. Biochem Z. 1966;344:167–189. [PubMed] [Google Scholar]
- 3.Bohannon D E, Connell N, Keener J, Tormo A, Espinosa-Urgel M, Zambrano M M, Kolter R. Stationary-phase-inducible “Gearbox” promoters: differentiatial effects of KatF mutations and role of ς70. J Bacteriol. 1991;173:4482–4492. doi: 10.1128/jb.173.14.4482-4492.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang Y Y, Wang A Y, Cronan J E., Jr Expression of Escherichia coli pyruvate oxidase (PoxB) depends on the sigma factor encoded by the rpoS (katF) gene. Mol Microbiol. 1994;11:1019–1028. doi: 10.1111/j.1365-2958.1994.tb00380.x. [DOI] [PubMed] [Google Scholar]
- 5.Davis L D, Kuehl W M, Battey J F. Basic methods in molecular biology. 2nd ed. Norwalk, Conn: Appleton and Lange; 1994. [Google Scholar]
- 6.el-Mansi E M, Holms W H. Control of carbon flux to acetate excretion during growth of Escherichia coli in batch and continuous cultures. J Gen Microbiol. 1989;135:2875–2883. doi: 10.1099/00221287-135-11-2875. [DOI] [PubMed] [Google Scholar]
- 7.Farewell A, Kvint K, Nystrom T. Negative regulation by RpoS: a case of sigma factor competition. Mol Microbiol. 1998;29:1039–1051. doi: 10.1046/j.1365-2958.1998.00990.x. [DOI] [PubMed] [Google Scholar]
- 8.Isaksson L A, Skold S-E, Skoldebrand J, Takata R. A procedure for isolation of spontaneous mutants with temperature sensitive synthesis of RNA and/or protein. Mol Gen Genet. 1977;156:233–237. doi: 10.1007/BF00267177. [DOI] [PubMed] [Google Scholar]
- 9.Jishage M, Ishihama A. Regulation of RNA polymerase sigma subunit synthesis in Escherichia coli: intracellular levels of ς70 and ς38. J Bacteriol. 1995;177:6832–6835. doi: 10.1128/jb.177.23.6832-6835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumari S, Tishel R, Eisenbach M, Wolfe A J. Cloning, characterization, and functional expression of acs, the gene which encodes acetyl coenzyme A synthetase in Escherichia coli. J Bacteriol. 1995;177:2878–2886. doi: 10.1128/jb.177.10.2878-2886.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 12.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 13.Mulvey M R, Switala J, Borys A, Loewen P C. Regulation of transcription of katE and katF in Escherichia coli. J Bacteriol. 1990;172:6713–6720. doi: 10.1128/jb.172.12.6713-6720.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prüß B M, Nelms J M, Park C, Wolfe A J. Mutations in NADH:ubiquinone oxidoreductase of Escherichia coli affect growth on mixed amino acids. J Bacteriol. 1994;176:2143–2150. doi: 10.1128/jb.176.8.2143-2150.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prüß B M, Wolfe A J. Regulation of acetyl phosphate synthesis and degradation, and the control of flagellar expression in Escherichia coli. Mol Microbiol. 1994;12:973–984. doi: 10.1111/j.1365-2958.1994.tb01085.x. [DOI] [PubMed] [Google Scholar]
- 16.Rose I A, Grunsberg-Manago M, Korey S R, Ochoa S. Enzymatic phosphorylation of acetate. J Biol Chem. 1954;211:737–756. [PubMed] [Google Scholar]
- 17.Shin S, Song S G, Lee D S, Pan J G, Park C. Involvement of iclR and rpoS in the induction of acs, the gene for acetyl coenzyme A synthetase of Escherichia coli K-12. FEMS Microbiol Lett. 1997;146:103–108. doi: 10.1111/j.1574-6968.1997.tb10178.x. [DOI] [PubMed] [Google Scholar]
- 18.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1984. [Google Scholar]
- 19.Simons R W, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka K, Takayanagi Y, Fujita F, Ishihama A, Takahashi H. Heterogeneity of the principal ς factor in Escherichia coli: the rpoS gene product, ς38, is a second principal ς factor of RNA polymerase in stationary phase Escherichia coli. Proc Natl Acad Sci USA. 1993;90:3511–3515. doi: 10.1073/pnas.90.8.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yim H H, Brems R L, Villarejo M. Molecular characterization of the promoter of osmY, an rpoS-dependent gene. J Bacteriol. 1994;176:100–107. doi: 10.1128/jb.176.1.100-107.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]