Abstract
UV resistance of bacterial endospores derives from a unique DNA photochemistry in which the major UV photoproduct is the thymine dimer 5-thyminyl-5,6-dihydrothymine (spore photoproduct [SP]) instead of cyclobutane pyrimidine dimers. Repair of SP during spore germination is due in large part to the activity of the enzyme SP lyase encoded by splB, the second cistron of the splAB operon. Expression of the splAB operon in Bacillus subtilis is transcriptionally activated by the EςG form of RNA polymerase during morphological stage III in the developing forespore compartment, and SP lyase is packaged into the dormant spore. In addition to temporal and compartmental control of splAB expression, a second regulatory circuit which modulates the level of expression of splB-lacZ fusions without altering their developmental timing or compartmentalization is reported here. This second regulatory circuit involves the negative action of the splA gene product, a 79-amino-acid protein with approximately 50% similarity and 17% identity to TRAP, the tryptophan RNA-binding attenuation protein from B. subtilis and Bacillus pumilus.
An important determinant of the high UV resistance of dormant bacterial endospores is repair of DNA damage during germination (reviewed in references 18 and 26). Spores repair the major UV-induced DNA damage, the thymine dimer 5-thyminyl-5,6-dihydrothymine (or spore photoproduct [SP]), during germination in large part by using an SP-specific repair enzyme called SP lyase (16, 17), which is encoded by splB, the second cistron of the splAB operon (8). Expression of the splAB operon is transcriptionally activated at stage III of sporulation in the developing forespore compartment by the sigma-G form of RNA polymerase (EςG) from a major promoter (P1) preceding the splA ribosome binding site (rbs) and a minor promoter (P3) preceding the splB rbs (23). The splB cistron is known to encode SP lyase (7, 8, 19, 25). Although the deduced amino acid sequence of SplA exhibits considerable similarity to a known negative regulatory factor in Bacillus spp., the trp RNA-binding attenuation protein (TRAP) (see Fig. 5), the function of the splA cistron, which encodes a 79-amino-acid, 9.2-kDa protein, has until recently remained obscure. We suspected that the splA cistron is involved in a regulatory circuit which modulates the level of SP lyase produced during sporulation, based on evidence that a deletion from upstream which removed the major P1 promoter and part of splA, or an in-frame deletion of splA itself, caused an increase in the expression of translational splB-lacZ fusions from the SPβ prophage locus, without altering the timing or EςG dependence of fusion expression (24). In this communication, we report the results of cis/trans analyses indicating that SplA functions as a trans-acting negative regulator of splB-lacZ fusion expression during sporulation, possibly via modulation of P1 and P3 utilization by EςG.
FIG. 5.
(A) Comparison of the deduced SplA amino acid sequences from B. subtilis (Bs) (8), B. amyloliquefaciens (Ba) (18) and TRAP from B. subtilis (11) and B. pumilus (Bp) (12). Lysines (K) and arginines (R) forming the KKR motif in TRAP (29) are shown in boldface type. (B) Comparison of the deduced amino acid sequences of SplA from B. subtilis and B. amyloliquefaciens. K and R residues are shown in boldface type. Below both sets of sequences, amino acids are denoted as identical (asterisks), highly similar (colons), or moderately similar (periods). Comparisons were run by using the ClustalW_mp Multiple Sequence Alignment site (http://www2.ebi.ac.uk/clustalw/).
Negative effect of splA in trans upon splB-lacZ fusion expression.
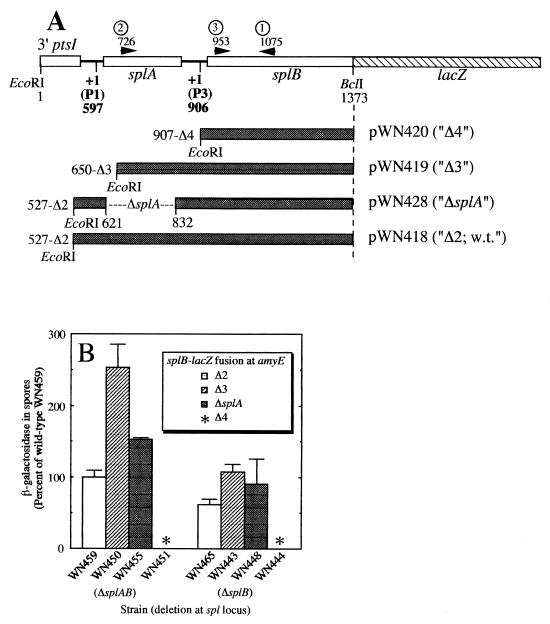
We reconstructed various translational splB-lacZ fusions (24) into the amyE integrative plasmid ptrpBG-1 (27) and introduced the resulting constructs (see Table 2) by transformation (5) into two isogenic Bacillus subtilis strains: WN356, in which the entire splAB operon previously had been deleted, or WN355, in which only splB had previously been deleted, and which therefore expressed splA from its natural locus (19) (Table 1; Fig. 1). Kinetic and compartmentalization experiments (23, 24) confirmed that splB-lacZ fusions placed at amyE were expressed in the forespore at stage III of sporulation in an EςG-dependent manner (P. Fajardo-Cavazos and W. L. Nicholson, unpublished observation). The level of β-galactosidase activity produced by the resulting splB-lacZ fusions from the amyE locus was assayed in purified spores and normalized to spore dipicolinic acid (DPA) content (13, 14, 20). We observed that although the absolute specific activity of β-galactosidase in a given isolate often varied between experiments, the relative specific activities of β-galactosidase in different strains were always reproducible within each experiment and are therefore reported here as normalized values.
TABLE 2.
Plasmids used in this study
| Plasmid | Description | Source or reference |
|---|---|---|
| pMK3 | E. coli-B. subtilis shuttle vector | 28 |
| ptrpBG-1 | lacZ fusion vector, integrates at amyE | 27 |
| pUC18 | Multisite E. coli cloning vector | 31 |
| pWN41 | 2.3-kb EcoRI-HindIII splAB-containing fragment cloned in pBGSC-6 | 23 |
| pWN418 | Wild-type Δ2 splAB-lacZ in ptrpBG-1 | This study |
| pWN419 | Δ3 splB-lacZ in ptrpBG-1 | This study |
| pWN420 | Δ4 splB-lacZ in ptrpBG-1 | This study |
| pWN425 | Down −35 P1 splB-lacZ in ptrpBG-1 | This study |
| pWN426 | Up −35 P1 splB-lacZ in ptrpBG-1 | This study |
| pWN428 | ΔsplA,splB-lacZ in ptrpBG-1 | This study |
| pWN489 | pMK3 carrying the splA cistron | This study |
TABLE 1.
Bacterial strains used in this study
| Strain | Genotype or phenotypea | Source or referenceb |
|---|---|---|
| B. subtilis | ||
| WN355 | metC14 sul thyA1 thyB1 trpC2 uvrB42 ΔsplB::ermC | 19 |
| WN356 | metC14 sul thyA1 thyB1 trpC2 uvrB42 ΔsplAB::ermC | 19 |
| WN443 | As for WN355, Δ3 splB-lacZ at amyE | pWN419→WN355 |
| WN444 | As for WN355, Δ4 splB-lacZ at amyE | pWN420→WN355 |
| WN446 | As for WN355, down −35 P1 splB-lacZ at amyE | pWN425→WN355 |
| WN447 | As for WN355, up −35 P1 splB-lacZ at amyE | pWN426→WN355 |
| WN448 | As for WN355, ΔsplA,splB-lacZ at amyE | pWN428→WN355 |
| WN450 | As for WN356, Δ3 splB-lacZ at amyE | pWN419→WN356 |
| WN451 | As for WN356, Δ4 splB-lacZ at amyE | pWN420→WN356 |
| WN453 | As for WN356, down −35 P1 splB-lacZ at amyE | pWN425→WN356 |
| WN454 | As for WN356, up −35 P1 splB-lacZ at amyE | pWN426→WN356 |
| WN455 | As for WN356, ΔsplA,splB-lacZ at amyE | pWN428→WN356 |
| WN459 | As for WN356, Δ2 splAB-lacZ at amyE | pWN418→WN356 |
| WN465 | As for WN355, Δ2 splAB-lacZ at amyE | pWN418→WN355 |
| WN491 | As for WN459 (pMK3) | pMK3→WN459 |
| WN492 | As for WN459 (pWN489) | pWN489→WN459 |
| WN520 | As for WN450 (pMK3) | pMK3→WN450 |
| WN521 | As for WN453 (pMK3) | pMK3→WN453 |
| WN522 | As for WN455 (pMK3) | pMK3→WN455 |
| WN523 | As for WN454 (pMK3) | pMK3→WN454 |
| WN525 | As WN450 (pWN489) | pWN489→WN450 |
| WN526 | As WN453 (pWN489) | pWN489→WN453 |
| WN527 | As WN454 (pWN489) | pWN489→WN454 |
| WN528 | As WN455 (pWN489) | pWN489→WN455 |
| E. coli JM109 | endA1 recA1 gyrA96 thi hsdR17 (rK− mK+) relA1 supE44 λ− Δ(lac-proAB) [F′ traD36 proA+B+laclqZΔM15] | Laboratory stock (31) |
Plasmids in parentheses are extrachromosomal.
Arrows denote introduction by transformation.
FIG. 1.
(A) The ptsI-splAB region of B. subtilis (open bars) and the endpoints of deletion derivatives cloned in plasmid ptrpBG-1 (shaded bars). Positions and directions of primers 1, 2, and 3 (numbered circles) used in RT-PCR are denoted by arrowheads. Numbers refer to the coordinates of deletion endpoints, restriction sites, transcription start sites, and the endpoints of RT-PCR products in the cloned splAB operon sequence (8). A translational fusion to lacZ (hatched bar) was created at the BclI site at coordinate 1373 of the splB gene (24). (B) Levels of β-galactosidase activity expressed from the amyE locus by the deletion derivatives outlined in panel A in spores of strains either lacking (strain WN356) or carrying (strain WN355) the splA gene at its natural locus. Values of β-galactosidase activity were normalized to those of strain WN459 carrying the wild-type Δ2 splB-lacZ at amyE and a complete deletion of the natural splAB operon. Specific β-galactosidase activity of strain WN459 was 151.1 ± 17.7 U. β-Galactosidase activity expressed by the Δ4 splB-lacZ fusion (asterisks) was not detectable (Fajardo-Cavazos and Nicholson, Unpublished observation). The data are expressed as averages and standard deviations for two independent duplicate determinations.
All of the cis-acting regulatory sequences needed for expression of the splB-lacZ fusion were contained between the Δ2 and Δ4 endpoints located between nucleotides (nt) 527 and 907, encompassing P1, splA, and P3 (Fig. 1A), since deletion to Δ4 immediately upstream from the splB rbs completely abolished splB-lacZ fusion expression (Fig. 1B). The presence of splA at its natural locus resulted in lowered expression of all three splB-lacZ fusions tested [compare β-galactosidase activities in the WN355 (splA+) strain to those in the WN356 (ΔsplA) strain background] (Fig. 1B), consistent with a postulated negative regulatory role for splA. It was also noted that β-galactosidase activity encoded by the splB-lacZ fusion carrying a deletion from upstream which removed the major P1 promoter (Δ3), or an in-frame deletion of the splA coding sequence (ΔsplA), was consistently elevated above wild-type (Δ2) levels in spores, regardless of the presence of the splA gene in trans (Fig. 1B). This observation leads us to suggest that the cis-acting target of SplA action may lie in the vicinity of the splA gene itself, likely between the Δ3 and Δ4 endpoints.
Effect of splA in trans on splB-lacZ expression driven by mutant P1 promoters.
We reasoned that the increase in splB-lacZ expression in the Δ3 deletion mutant in which major promoter P1 was removed (Fig. 1) may have been due to a resultant activation of transcription from the minor P3 promoter and further that switching from P1 to P3 utilization may be a feature of splA-mediated regulation of SP lyase synthesis. To test this hypothesis, we assayed the effects of splA supplied in trans on the expression of the splB-lacZ fusion at amyE driven by P1 promoters containing point mutations in conserved bases within the −35 region (Fig. 2). Based on earlier studies of critical conserved bases in EςG-type promoters (9, 21), a T-to-G transversion at position −32 was predicted to abolish P1 activity, whereas a T-to-G transversion at −35 was predicted to enhance P1 activity by making it conform to the consensus EςG-type promoter sequence (21) (Fig. 2A). The splB-lacZ fusions driven by mutant P1 promoters were placed at the amyE locus of strain WN355 (ΔsplB) or WN356 (ΔsplAB), and β-galactosidase activity was assayed relative to DPA content in dormant spores of the resulting strains. In spores of the background strain WN356 (ΔsplAB), splB-lacZ expression driven by P1 carrying a “down” mutation at −35 produced approximately the same level of β-galactosidase as did the wild-type P1 promoter, and the “up” P1 promoter mutation produced slightly more β-galactosidase activity (Fig. 2B). Placing the splB-lacZ fusion driven by the same three P1 promoter alleles at amyE in strain WN355 (ΔsplB), which expresses the splA gene from its natural locus in trans, resulted in lowered expression of splB-lacZ from both the wild-type and up mutant P1 promoters, consistent with splA encoding a trans-acting negative regulator (Fig. 2B). Interestingly, the β-galactosidase level expressed by the down P1 mutation in spores of strain WN355 was not lowered (Fig. 2B), suggesting that the negative regulatory effect of splA in trans may depend on transcription from P1. Because transcription of the splAB operon is dependent on DNA in the region between nt 527 and 907, including promoters P1 and P3 (Fig. 1), the observation of increased splB-lacZ expression in the P1 down point mutant suggested one of two possibilities: (i) inactivation of P1 led to activation of EςG-mediated transcription from P3, or (ii) that the predicted down mutation engineered at −32 did not in fact inactivate the P1 promoter. To test the second possibility, identical molar quantities of each mutant P1 promoter were used to prime EςG-directed in vitro runoff transcription to the BstNI site at coordinate 1074 of the splB sequence (8), which is predicted to generate a runoff transcript from P1 of 477 nt and one from P3 of 168 nt (23). Quantitation of the resulting autoradiogram revealed that the engineered down mutation at −32 of P1 resulted in abolition of P1 promoter utilization in vitro by EςG (Fig. 2C), while the engineered up mutation at position −35 of P1, which resulted in P1 matching the consensus EςG-type promoter at all critical bases, resulted in a 2.5-fold stimulation of P1 utilization in vitro by EςG over that seen with the wild-type P1 promoter (Fig. 2C). Therefore, the engineered point mutations in P1 behaved in vitro as would be predicted, and presumably P1 utilization in vivo was indeed abolished in the down P1 mutant. However, switching of EςG utilization from P1 to P3, which would be predicted to generate a 168-nt runoff transcript, was not detected in the in vitro runoff assay, even when P1 utilization was abolished by the down mutation at −32 (Fajardo-Cavazos and Nicholson, unpublished observation).
FIG. 2.
(A) Sequence of the splAB P1 promoter and transcription initiation site (bottom line) (23) and the consensus EςG-type promoter (top line) (21). The T-to-G transversions at nt 563 and 566 predicted to enhance or abolish P1 activity are denoted above and below the P1 sequence, respectively. Sequence coordinates are from reference 8. (B) Levels of β-galactosidase activity expressed from the amyE locus by the P1 point mutations outlined in panel A in spores of strains either lacking (strain WN356) or carrying (strain WN355) the splA gene at its natural locus. Values of β-galactosidase activity were normalized to strain WN459 carrying the wild-type Δ2 splB-lacZ fusion at amyE and a complete deletion of the natural splAB operon. Specific β-galactosidase activity of strain WN459 was 151.1 ± 17.7 U. The data are expressed as averages and standard deviations for two independent duplicate determinations. (C) In vitro runoff transcription from the splAB P1 promoter carrying the point mutations described in panel A. RNA polymerase containing sigma G was prepared as described previously (23). Lanes marked G, A, T, and C are DNA sequencing reactions of M13 DNA used as molecular size standards. An arrow denotes the size of the runoff transcript from P1 (477 nt). In vitro transcription initiating from P3, expected to generate a 168-nt runoff transcript, was not detected (data not shown).
Effect of multiple extrachromosomal copies of splA on splB-lacZ fusion expression.
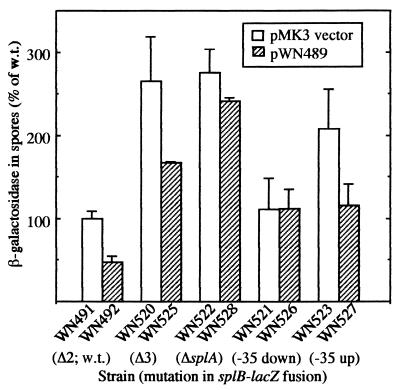
If splA encoded a trans-acting negative regulator, one would predict that supplying splA in trans on a multicopy plasmid would lead to reduced expression of the wild-type (Δ2) splB-lacZ fusion. To test this idea, the splA cistron was inserted into the E. coli-B. subtilis shuttle plasmid pMK3 (28), resulting in plasmid pWN489 (Table 2). Plasmids pMK3 and pWN489 were introduced into strain WN459, which harbors the wild-type Δ2 splB-lacZ fusion at amyE in a ΔsplAB background (Table 1). Plasmid copy number determinations (Fajardo-Cavazos and Nicholson, unpublished observation) indicated that plasmid pWN489 was present in dormant spores at approximately 13 to 14 copies per genome, in close agreement with previous copy number determinations for pUB110-based replicons in B. subtilis spores (13, 14). Dormant spores of the resulting strains, WN491 and WN492 (Table 1), were obtained and assayed for β-galactosidase activity (Fig. 3). The presence of multiple copies of splA in trans on plasmid pWN489 reduced the amount of expression from the wild-type splB-lacZ fusion compared to that of spores carrying vector plasmid pMK3 alone (Fig. 3), strongly supporting the notion that splA encodes a trans-acting negative regulatory factor. In an identical manner, we tested the repressive effect of multiple copies of splA on expression of the splB-lacZ fusion driven by all of the deletion and point mutations which we had generated in the splB-lacZ fusion at amyE (Fig. 1 and 2). As was seen in the experiments testing a single copy of splA in trans (Fig. 1 and 2), supplying splA in multiple extrachromosomal copies was observed to repress expression of the splB-lacZ fusion to some extent in spores of all mutant strains tested except strain WN526, carrying the down mutation in the −35 region of P1 (Fig. 3).
FIG. 3.
β-Galactosidase activity encoded by splB-lacZ fusions in spores of B. subtilis strains carrying either multicopy plasmid pMK3 (open bars) or the splA gene carried on plasmid pMK3 (plasmid pWN489; hatched bars). Values of β-galactosidase activity were normalized to strain WN491 carrying the wild-type Δ2 splB-lacZ fusion at amyE, a complete deletion of the natural splAB operon, and vector plasmid pMK3. Specific β-galactosidase activity of strain WN491 was 341.5 ± 13.2 U. Data are reported as averages ± standard deviation for two independent duplicate determinations.
In vivo transcripts arising from P1 or P3.
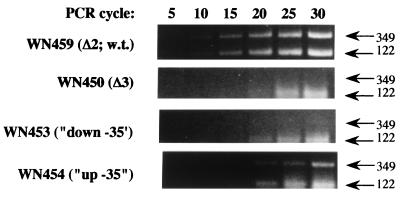
Because the in vitro runoff transcription assay (Fig. 2C) does not completely mimic conditions prevailing in vivo, we tested whether P1-to-P3 switching occurred in vivo by probing for splB-lacZ transcripts originating from P1 or P3 in strains containing wild-type or P1 mutant promoters. Strains were cultivated to sporulation stage III by using expression of the splB-lacZ fusion as a marker (23, 24), total RNA was purified with TRI Reagent (Molecular Research Center, Inc.), and the RNA was treated with DNase I. Total RNA was used as a template for reverse transcriptase (RT)-PCR by using a Master Amp RT-PCR kit (Epicentre Technologies) according to the manufacturer's instructions with an added annealing period of 37°C, 10 min before the reverse transcription step. Primers used to distinguish between transcripts originating from P1 or from P3 were as follows: primer 1, 5′-GGGATATTACGCACCTGATTGTGGG-3′; primer 2, 5′-AGGCGAGCTCTCCCTGTTT-3′; and primer 3, 5′-CTTGTGTATCTAGAACCGA-3′ (see Fig. 1A for coordinates) (8). All three primers were included in each RT-PCR, so that transcripts originating from P1 would yield two RT-PCR products of 349 and 122 bp and transcripts originating from P3 would yield only the 122-bp RT-PCR product. The RT-PCRs were run for 30 cycles, and samples were removed from each reaction mixture at five-cycle intervals and analyzed after electrophoresis through a 1.5% agarose gel (Fig. 4). As expected, RT-PCR of RNAs from strains with the wild-type (WN459) and up −35 (WN454) P1 promoters gave rise to two PCR products of the predicted sizes, whereas strains harboring the Δ3 deletion of P1 (WN450) and down −35 P1 point mutation (WN453) exhibited only the 122-bp RT-PCR products, consistent with only P3 being functional. All products resulted from extension of mRNA by RT, since control PCR of the same templates using Vent-DNA polymerase (New England Bioloabs) did not result in any detectable products (Fajardo-Cavazos and Nicholson, unpublished observation).
FIG. 4.
Detection of splB-lacZ transcripts arising from the P1 or P3 promoter by RT-PCR. Total RNA was isolated from the indicated strains at stage III and subjected to RT-PCR as described in the text.
Homology between SplA and TRAP proteins.
Results from the experiments in this communication indicate that the SplA protein acts in trans as a negative regulator of splB expression. Its deduced amino acid sequence (8) indicates that B. subtilis SplA is a 9.2-kDa 79-amino-acid protein. Although searching of the current databases did not reveal proteins with striking similarity to SplA, a direct comparison of the deduced amino acid sequence of TRAP proteins from B. subtilis and B. pumilus (11, 12) with those of the two SplA proteins characterized to date, cloned from B. subtilis (8) and Bacillus amyloliquefaciens (18), respectively, revealed that these four proteins contain 13 of 75 (17%) identical amino acids and an overall similarity of 38 of 75 amino acids (50%) (Fig. 5). The observed sequence similarity between TRAP and SplA suggests that the two proteins may have evolved from a common ancestral protein and/or may operate by a similar mechanism. The structure of TRAP has been deduced to be an 11-subunit beta wheel (2) in which amino acids K37 from one subunit and K56 and R58 from the adjacent subunit form an RNA-binding KKR motif on the outer surface of the toroid which interacts with trp leader RNA (29). The SplA sequences from B. subtilis and B. amyloliquefaciens do not contain K and R residues at precisely the analogous positions as those found in TRAP (Fig. 5A). However, at four positions (R16, K27, K/R66, and K/R70 using the B. subtilis coordinates), SplA proteins from B. subtilis and B. amyloliquefaciens contain residues which could potentially form a KKR-like motif (Fig. 5B). Current experiments are being directed at deducing the subunit organization of SplA and testing the potential function of the basic residues mentioned above by in vitro mutagenesis experiments.
From the genetic evidence described above, it is clear that the splA gene product functions in trans as a negative regulator of the level of splB-lacZ expression in the developing forespore without altering the timing, compartmentalization, or EςG dependence of fusion expression. Several questions arise from this observation. First, what is the molecular mechanism of splA-mediated regulation of SP lyase production? The sequence similarity between SplA and TRAP (Fig. 5A) suggests that the two proteins may operate by a similar mechanism. TRAP negatively regulates expression of tryptophan biosynthetic genes (encoded by the trpEDCFBA operon and the trpG cistron within the folate operon) in Bacillus spp. (reviewed in reference 3). TRAP monomers form an undecameric complex which is activated when each subunit binds one tryptophan (Trp) molecule (1, 2). The activated TRAP-Trp complex in turn binds to regularly spaced GAG or UAG repeats in the leader RNAs of the target operons through the above-mentioned KKR motif (29). In the trpEDCFBA operon, binding regulates expression via transcriptional attenuation (4, 22). In addition, the TRAP-Trp complex can block translation by binding to GAG or UAG repeats in the vicinity of the rbs in both the trpEDCFBA operon and the trpG cistron (6, 15, 30). Like TRAP, SplA appears to operate in trans as a negative regulator of splB-lacZ expression. The results of the experiments reported here, however, suggest that splA-mediated control of the splAB operon probably differs in some aspects from TRAP attenuation of the trp operon, since (i) the mtrB gene encoding TRAP is situated at a physically distinct locus from its target operons (11), whereas SplA protein and its cis-acting target sequence are likely both encoded within the splAB operon itself (Fig. 1).
Why do sporulating cells need to regulate the amount of SP lyase they produce? The answer may lie in the process of endospore formation itself. Spores are metabolically inactive and therefore accumulate DNA damage during dormant periods of unpredictable length. This cumulative damage must be repaired during early germination, before reactivation of gene expression can occur; hence, SP lyase is produced in the forespore during stage III of sporulation and packaged in the dormant spore (16, 17, 23). In addition to developmental control of SP lyase synthesis, the results described above lead us to speculate that spore-forming microorganisms have evolved an SplA-dependent regulatory circuit within the splAB operon to control the level of SP lyase synthesized during stage III of sporulation and packaged within the dormant spore. It is conceivable that in sporulating microorganisms this circuit serves as a means of sensing the environmental UV flux prevailing at the time of sporulation and, accordingly, of adjusting the amount of SP lyase to be incorporated into the spore. The underlying logic of this proposed mechanism would be that bacteria use the ambient conditions present during sporulation to predict the likely conditions which will prevail during dormancy. This notion of physiological control of spore resistance properties by extrinsic factors has a precedent in experiments showing that several Bacillus spp. produce spores which are more heat resistant when sporulated at their temperature maxima than when they are when sporulated at their temperature optima or minima (reviewed in reference 10). We have in the splAB operon a unique opportunity for studying how this phenomenon may operate at the molecular level. Previous experiments have indicated that EςG-directed transcription of splB-lacZ was initiated mainly from P1, with a minor amount of transcription from P3 (23). The experiments reported here indicate that (i) SplA operates as a trans-acting negative regulatory protein and (ii) at stage III, EςG can initiate transcription of splB-lacZ from either P1 or P3 and that transcription from P3 can be activated under conditions in which P1 is either absent or inactive. Current experiments are directed at elucidating how splA or its product is involved in this regulatory circuit.
Acknowledgments
We thank Mario Pedraza-Reyes and Roberto Rebeil for technical assistance during early parts of this work and Paul Babitzke for helpful discussions.
This research was supported in part by grants from the National Institutes of Health (GM47461) and the Arizona Agricultural Experimental Station (USDA-HATCH-ARZT-136753-H-02-116) to W.L.N.
REFERENCES
- 1.Antson A A, Brzozowski A M, Dodson E J, Dauter Z, Wilson K S, Kurecki T, Otridge J, Gollnick P. 11-fold symmetry of the trp RNA-binding attenuation protein (TRAP) from Bacillus subtilis determined by X-ray analysis. J Mol Biol. 1994;244:1–5. doi: 10.1006/jmbi.1994.1698. [DOI] [PubMed] [Google Scholar]
- 2.Antson A A, Otridge J, Brzozowski A M, Dodson E J, Dodson G G, Wilson K S, Smith T M, Yang M, Kurecki T, Gollnick P. The structure of trp RNA-binding attenuation protein. Nature. 1995;374:693–700. doi: 10.1038/374693a0. [DOI] [PubMed] [Google Scholar]
- 3.Babitzke P. Regulation of tryptophan biosynthesis: trp-ing the TRAP or how Bacillus subtilis reinvented the wheel. Mol Microbiol. 1997;26:1–9. doi: 10.1046/j.1365-2958.1997.5541915.x. [DOI] [PubMed] [Google Scholar]
- 4.Babitzke P, Stults J T, Shire S J, Yanofsky C. TRAP, the trp RNA-binding attenuation protein of Bacillus subtilis, is a multisubunit complex that appears to recognize G/UAG repeats in the trpEDCFBA and trpG transcripts. J Biol Chem. 1994;269:16597–16604. [PubMed] [Google Scholar]
- 5.Boylan R J, Mendelson N H, Brooks D, Young F E. Regulation of the bacterial cell wall: analysis of a mutant of Bacillus subtilis defective in biosynthesis of teichoic acid. J Bacteriol. 1972;110:281–290. doi: 10.1128/jb.110.1.281-290.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du H, Tarpey R, Babitzke P. The trp RNA-binding attenuation protein regulates TrpG synthesis by binding to the trpG ribosome binding site of Bacillus subtilis. J Bacteriol. 1997;179:2582–2586. doi: 10.1128/jb.179.8.2582-2586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fajardo-Cavazos P, Nicholson W L. Molecular dissection of mutations in the Bacillus subtilis spore photoproduct lyase gene which affect repair of spore DNA damage by UV radiation. J Bacteriol. 1995;177:4402–4409. doi: 10.1128/jb.177.15.4402-4409.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fajardo-Cavazos P, Salazar C, Nicholson W L. Molecular cloning and characterization of the Bacillus subtilis spore photoproduct lyase (spl) gene, which is involved in repair of UV radiation-induced DNA damage during spore germination. J Bacteriol. 1993;175:1735–1744. doi: 10.1128/jb.175.6.1735-1744.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fajardo-Cavazos P, Tovar-Rojo F, Setlow P. Effect of promoter mutations and upstream deletions on the expression of genes coding for small, acid-soluble spore proteins of Bacillus subtilis. J Bacteriol. 1991;173:2011–2016. doi: 10.1128/jb.173.6.2011-2016.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerhardt P, Marquis R E. Spore thermoresistance mechanisms. In: Smith I, Slepecky R A, Setlow P, editors. Regulation of procaryotic development. Washington, D.C.: American Society for Microbiology; 1989. pp. 43–63. [Google Scholar]
- 11.Gollnick P, Ishino S, Kuroda M I, Henner D J, Yanofsky C. The mtr locus is a two-gene operon required for transcription attenuation in the trp operon of Bacillus subtilis. Proc Natl Acad Sci USA. 1990;87:8726–8730. doi: 10.1073/pnas.87.22.8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman R J, Gollnick P. The mtrB gene of Bacillus pumilus encodes a protein with sequence and functional homology to the trp RNA-binding attenuation protein (TRAP) of Bacillus subtilis. J Bacteriol. 1995;177:839–842. doi: 10.1128/jb.177.3.839-842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mason J M, Fajardo-Cavazos P, Setlow P. Levels of mRNAs which code for small, acid-soluble spore proteins and their lacZ gene fusions in sporulating cells of Bacillus subtilis. Nucleic Acids Res. 1988a;16:6567–6583. doi: 10.1093/nar/16.14.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mason J M, Hackett R H, Setlow P. Regulation of expression of genes coding for small, acid-soluble proteins of Bacillus subtilis spores: studies using lacZ gene fusions. J Bacteriol. 1988b;170:239–244. doi: 10.1128/jb.170.1.239-244.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merino E, Babitzke P, Yanofsky C. trp RNA-binding attenuation protein (TRAP)-trp leader RNA interactions mediate translational as well as transcriptional regulation of the Bacillus subtilis trp operon. J Bacteriol. 1995;177:6362–6370. doi: 10.1128/jb.177.22.6362-6370.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munakata N, Rupert C S. Genetically controlled removal of “spore photoproduct” from deoxyribonucleic acid of ultraviolet-irradiated Bacillus subtilis spores. J Bacteriol. 1972;111:192–198. doi: 10.1128/jb.111.1.192-198.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munakata N, Rupert C S. Dark repair of DNA containing “spore photoproduct” in Bacillus subtilis. Mol Gen Genet. 1974;130:239–250. doi: 10.1007/BF00268802. [DOI] [PubMed] [Google Scholar]
- 18.Nicholson W L, Fajardo-Cavazos P. DNA repair and the UV resistance of bacterial spores: from the laboratory to the environment. Recent Res Devel Microbiol. 1997;1:125–140. [Google Scholar]
- 19.Nicholson W L, Chooback L, Fajardo-Cavazos P. Analysis of spore photoproduct lyase operon (splAB) function using targeted deletion-insertion mutations spanning the Bacillus subtilis operons ptsHI and splAB. Mol Gen Genet. 1997;255:587–594. doi: 10.1007/s004380050532. [DOI] [PubMed] [Google Scholar]
- 20.Nicholson W L, Setlow P. Sporulation, germination, and outgrowth. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. Sussex, England: John Wiley and Sons; 1990. pp. 391–450. [Google Scholar]
- 21.Nicholson W L, Sun D, Setlow B, Setlow P. Promoter specificity of ςG-containing RNA polymerase from sporulating cells of Bacillus subtilis: identification of a group of forespore-specific promoters. J Bacteriol. 1989;171:2708–2718. doi: 10.1128/jb.171.5.2708-2718.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otridge J, Gollnick P. MtrB from Bacillus subtilis binds specifically to Bacillus subtilis leader RNA in a tryptophan-dependent manner. Proc Natl Acad Sci USA. 1993;90:128–132. doi: 10.1073/pnas.90.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pedraza-Reyes M, Gutiérrez-Corona F, Nicholson W L. Temporal regulation and forespore-specific expression of the spore photoproduct lyase gene by sigma-G RNA polymerase during Bacillus subtilis sporulation. J Bacteriol. 1994;176:3983–3991. doi: 10.1128/jb.176.13.3983-3991.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pedraza-Reyes M, Gutiérrez-Corona F, Nicholson W L. Spore photoproduct lyase operon (splAB) regulation during Bacillus subtilis sporulation: modulation of splB-lacZ fusion expression by P1 promoter mutations and by an in-frame deletion of splA. Curr Microbiol. 1997;34:133–137. doi: 10.1007/s002849900157. [DOI] [PubMed] [Google Scholar]
- 25.Rebeil R, Sun Y, Chooback L, Pedraza-Reyes M, Kinsland C, Begley T P, Nicholson W L. Spore photoproduct lyase from Bacillus subtilis spores is a novel iron-sulfur DNA repair enzyme which shares features with proteins such as class III anaerobic ribonucleotide reductases and pyruvate-formate lyases. J Bacteriol. 1998;180:4879–4885. doi: 10.1128/jb.180.18.4879-4885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Setlow P. Mechanisms for the prevention of damage to DNA in spores of Bacillus species. Annu Rev Microbiol. 1995;49:29–54. doi: 10.1146/annurev.mi.49.100195.000333. [DOI] [PubMed] [Google Scholar]
- 27.Shimotsu H, Henner D J. Construction of a single-copy integration vector and its use in analysis of regulation of the trp operon of Bacillus subtilis. Gene. 1986;43:85–94. doi: 10.1016/0378-1119(86)90011-9. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan M A, Yasbin R E, Young F E. New shuttle vectors for Bacillus subtilis and Escherichia coli which allow rapid detection of inserted fragments. Gene. 1984;29:21–26. doi: 10.1016/0378-1119(84)90161-6. [DOI] [PubMed] [Google Scholar]
- 29.Yang M, Chen X P, Militello K, Hoffman R, Fernandez B, Baumann C, Gollnick P. Alanine-scanning mutagenesis of Bacillus subtilis trp RNA-binding attenuation protein (TRAP) reveals residues involved in tryptophan binding and RNA binding. J Mol Biol. 1997;270:696–710. doi: 10.1006/jmbi.1997.1149. [DOI] [PubMed] [Google Scholar]
- 30.Yang M, de Saizieu A, van Loon A P G M, Gollnick P. Translation of trpG in Bacillus subtilis is regulated by the trp RNA-binding attenuation protein (TRAP) J Bacteriol. 1995;177:4272–4278. doi: 10.1128/jb.177.15.4272-4278.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]