Abstract
Aims
The combination of bupropion and naltrexone has shown efficacy in reducing binge drinking in animal models. This study assessed the tolerability and potential utility of combined naltrexone and bupropion in reducing binge drinking in human subjects.
Methods
This preliminary study employed an open-label, single-arm, 12-week, prospective design. Twelve men and women who exhibited a minimum of five (men) or three (women) binge drinking episodes per month over the past 3 months were recruited. All subjects received both bupropion-extended release 300 mg/day and naltrexone 50 mg/day and were monitored throughout the 3-month treatment period. Binge drinking was assessed using the timeline follow-back method.
Results
Treatment with combined naltrexone and bupropion reduced the average number of drinks per binge drinking day from 7.8 drinks to 6.4 drinks and reduced the average percentage of binge drinking days per month from 19% (5.7 days/month) to 5% (1.5 days/month). Naltrexone and bupropion were generally well tolerated, with insomnia, headache and nausea/diarrhea being the most common side effects. Six subjects elected to stay on medication after the trial.
Conclusions
This study suggests that combined naltrexone and bupropion therapy should be further investigated for tolerability and efficacy in reducing binge drinking in humans.
This open-label study sought to determine the tolerability and potential utility of naltrexone and bupropion in treating binge drinking in humans. Men and women binge drinkers were given naltrexone and bupropion for 3 months. Bupropion and naltrexone were generally well tolerated and binge drinking frequency decreased.
INTRODUCTION
Binge drinking is a major public health problem in the USA. Defined as a pattern of alcohol consumption leading to a blood alcohol concentration (BAC) of ≥80 mg/dl and acute intoxication, binge drinking usually occurs when a man consumes ≥5 standard drinks (one standard drink being ~14 g of ethanol) or when a woman consumes ≥4 standard drinks in ≤ 2 hours (NIAAA, 2004). Binge drinking is extremely prevalent in the USA, with 26.9% of people ≥18 years reporting binge drinking in the past month (Substance Abuse and Mental Health Services Administration, 2015). Binge drinking is associated with poor health outcomes (Puddey et al., 1999; Hingson et al., 2006; Miller et al., 2007), and individuals who binge drink frequently are at increased risk for developing alcohol dependence (Sacks et al., 2015; Addolorato et al., 2018). Overall, binge drinking contributes to more than half of all deaths attributed to alcohol and to three quarters of the economic cost of excessive alcohol use (Leeman et al., 2008). Binge drinking is a serious public health problem, and one that may exceed traditionally defined alcohol dependence in its cost to society.
Despite the prevalence and impact of binge drinking, this dangerous pattern of behavior has received relatively little attention regarding its pathophysiology or pharmacological treatment. Binge drinking may be a risk factor for more advanced alcohol use disorders (AUDs; Sacks et al., 2015). Indeed, preclinical findings support the idea that binge drinking may lead to neurobiological adaptations that can contribute to the development of alcohol dependence (Sprow and Thiele, 2012; Rinker et al., 2017). Treating binge drinking may therefore represent a therapeutic target in its own right, as well as an approach to disrupt possible progression to alcohol dependence.
The few pharmacological studies that have targeted binge drinking in young adults utilized the opioid receptor antagonist naltrexone (Garbutt, 2009; O’Malley et al., 2015). Naltrexone is approved by the Food and Drug Administration (FDA) in the USA for the treatment of alcohol use disorders (AUDs) and is thought to reduce drinking in some individuals by blocking both the rewarding, euphoric effects of alcohol consumption, and the alcohol cue-induced activation of reward systems (Myrick et al., 2008). These previous studies showed that naltrexone can reduce binge drinking in young adults (Garbutt, 2009; O’Malley et al., 2015). However, the efficacy of naltrexone in reducing binge drinking is modest at best and does not work in some individuals (Garbutt, 2009), prompting efforts to discover new pharmacological therapies.
Pre-clinical studies suggest the melanocortin system (MC) may represent a new target for the pharmacotherapeutic treatment of binge drinking. The MC consists of endogenous peptide hormones and melanocortin receptors. Pre-clinical studies show that melanocortin receptor (MCR) agonists decrease binge-like ethanol consumption, while MCR antagonists increase ethanol consumption (Sprow et al., 2016). This suggests targeting the MC may have therapeutic value in treating binge drinking in humans. Furthermore, the endogenous opioid system and MC may interact to impact binge drinking, as preclinical studies show that opioid antagonists enhance the effect of MCR agonists on reducing binge drinking in mice (Navarro et al., 2015).
In humans, activation of the MC can be achieved using bupropion (Billes et al., 2014), an antidepressant, antinicotine medication. Intriguingly, the combination of bupropion and naltrexone suppresses food intake in rodents (Wright and Rodgers, 2013) and produces weight loss in obese individuals (Greenway et al., 2010), which led to its approval by the FDA for weight loss in 2014. Furthermore, the combination of naltrexone and bupropion was superior to either monotherapy for weight loss (Greenway et al., 2010). We recently extended this work to a pre-clinical model of binge drinking in mice and found that bupropion, alone and in combination with naltrexone, significantly blunted binge-like alcohol intake (Navarro et al., 2019). Taken together, these findings support the hypothesis that combined targeting of the μ-opioid and MC systems in humans may have value in reducing binge drinking. In the current proof-of-concept feasibility and tolerability study, we report results from an open-label trial of naltrexone and bupropion in individuals who binge drink.
METHODS
Participants
Twelve men and women aged 21–44 years who exhibited a minimum of 5 (men) or 3 (women) binge drinking episodes per month over the past 3 months were recruited. A binge drinking episode was defined as the consumption of ≥5 (men) or ≥4 (women) standard drinks (one standard drink being ~ 14 g of ethanol) in about 2 hours or less. Subjects were allowed to meet DSM-V criteria for mild or moderate AUD. Other inclusion criteria include the following: ability to understand and sign written informed consent; having a 0.0 g/dl breathalyzer reading on the day of screening; a BMI ≥18.5; expressing a desire to achieve abstinence or to reduce alcohol consumption and, finally, having a stable residence and being able to identify an individual who could contact the participant if needed.
Exclusion criteria included the following: the presence of physical dependence on alcohol as evidenced by clear tolerance to alcohol or alcohol withdrawal symptoms based on SCID interview or a severe AUD (≥6 DSM-V symptoms); clinically significant medical disease that might interfere with the evaluation of the study medication or present a safety concern (e.g., glaucoma, renal insufficiency, cirrhosis, unstable hypertension, diabetes mellitus or seizure disorder); clinically significant psychiatric illness including any psychotic disorder, bipolar disorder, anorexia/bulimia, severe depression, or suicidal ideation; other substance abuse or dependence disorder other than nicotine or cannabis abuse; concurrent use of anticonvulsants or MAO inhibitors; concurrent use of any psychotropic medication including antidepressants, mood stabilizers, antipsychotics, anxiolytics, stimulants, or hypnotics with the exception of stable doses of antidepressants for 1 month; prior history of adverse reaction to bupropion; AST or ALT > 3.5 X ULN or bilirubin > 1.5 X ULN; positive urine toxicology screen with the exception of cannabis—individuals with positive cannabis screens were excluded only if they had a history of cannabis dependence; pregnant women and women of childbearing potential who do not practice a medically acceptable form of birth control (oral or depot contraceptive, or barrier methods such as diaphragm or condom with spermicide); women who are breastfeeding; individuals requiring inpatient treatment or more intense outpatient treatment for their alcohol problems; participation in any clinical trial within the past 60 days that would have safety concerns for the trial; and finally, court-mandated participation in alcohol treatment or pending incarceration. None of the subjects received any other form of treatment for their alcohol use while participating in this study. Subjects were recruited using the e-mail listserve for UNC students, faculty and staff, as well as Facebook advertisements.
Procedures
The entirety of the study was performed at the University of North Carolina at Chapel Hill in Chapel Hill, NC. This clinical trial is registered on www.clinicaltrials.gov as NCT02842073. This study was approved by the Institutional Review Board at the University of North Carolina at Chapel Hill. Subjects gave written informed consent for inclusion in the trial. An open-label, single-arm, 12-week, prospective study design was used. All subjects received both bupropion and naltrexone. Standard clinical doses of bupropion-XL 300 mg/d and naltrexone 50 mg/d were dispensed by the UNC Investigational Drug Services. Bupropion XL was initiated on Days 1–4 at 150 mg/day and increased to 300 mg/day for Days 5–84. Naltrexone was initiated on Days 7–9 at 25 mg/day and then increased to 50 mg/day for Days 10–84. Bupropion and naltrexone were initiated at different times so subjects could acclimate to one drug at a time and so attribution of initial side effects could be made to a specific drug. Subjects were seen at screening and again at Weeks 1, 3, 5, 8 and 12. Subjects were breathalyzed and received Medical Management counseling (Pettinati et al., 2005) to encourage compliance and progress toward drinking goals. We used the Time-Line Follow-Back Approach (Sobell et al., 1979) to assess alcohol consumption history modified to document if subjects consumed ≥5/4 (male/female) standard drinks in about 2 hours and felt intoxicated, in which case a binge drinking day was documented. Craving for alcohol was assessed using the Penn Alcohol Craving Scale (PACS) (Flannery et al., 1999). We assessed tolerability by probing for adverse effects. Our key outcomes of interest included tolerability and acceptability, as well as drinking behavior including frequency and intensity of binge drinking and craving for alcohol. Because this was an open-label feasibility and tolerability trial, a placebo group was not included.
RESULTS
Study population
Seventeen subjects were screened for participation in the trial. Of these, 12 subjects (mean age: 33 years; age range: 22–43 years; 83% women; 75% Caucasian) were enrolled in the study and five subjects were excluded. Demographics and baseline characteristics are shown in Table 1. The average number of drinks per binge drinking day pre-treatment ranged from 4.3 to 11.3 with an average of 7.8 drinks per binge drinking day. The percentage of binge drinking days pre-treatment ranged from 10% (3.0 days/month) to 37% (11.1 days/month) with an average of 19% (5.7 days/month). The number of drinks per any drinking day (including both binge and non-binge days) ranged from 3.3 to 10.5 with an average of 5.7 drinks per any drinking day. The percentage of total drinking days (including both binge and non-binge days) pre-treatment ranged from 19% (5.7 days/month) to 97% (29.1 days/month) with an average of 49% (14.7 days/month). Finally, the pre-treatment drinks per month ranged from 33 to 133 with an average of 80 drinks per month. On screening, men in the study averaged 9.1 drinks per binge drinking day, while women averaged 7.6 drinks per binge drinking day. The men averaged 24% binge days (7 days/month) while the women averaged 18% binge days (5 days/month). The men also averaged 6.4 drinks per any drinking day, and the women averaged 5.6 drinks per any drinking day. Furthermore, the men averaged 61% total drinking days (18 days/month) pretreatment, while the woman averaged 47% total drinking days (14 days/month) pretreatment. Finally, the men averaged 101 drinks per month, and the women averaged 75 drinks per month pretreatment. Eleven of the 12 patients met criteria for an AUD, with five having a mild disorder and six having a moderate disorder. Two of the participants smoked cigarettes, and the number of cigarettes smoked by these two was not recorded throughout the trial. Furthermore, participants’ weights were not recorded post-treatment.
Table 1.
Demographics and baseline characteristics of patient population
| Demographics | Mean ± SD (n = 12) |
|---|---|
| Age (years) | 33 ± 7 (Range: 22–43) |
| Gender (% female) | 83 |
| Race (% white) | 75 |
| Marital status (% single, never married) | 67 |
| Education (years) | 16 ± 2 |
| Employment (% employed) | 67 |
| Cigarette use (% smokers) | 17 |
| Alcohol use (years) | 13 ± 6 |
| Drinks per binge drinking day | 7.8 ± 2.4 |
| Percent binge drinking days (%) | 19 ± 9 |
| Drinks per any drinking day | 5.7 ± 2.3 |
| Percent total drinking days (%) | 49 ± 27 |
| Drinks per month | 80 ± 38 |
| Alcohol use disorder—mild/moderate (%) | 92 |
| PACS* score | 12 ± 6 |
Twelve men and women who exhibited a minimum of five (men) or three (women) binge drinking episodes per month over the past 3 months were recruited in order to test the effects of combined naltrexone and bupropion on binge drinking. Demographics of these subjects are listed above * = Penn Alcohol Craving Scale.
Of the 12 subjects enrolled in the study, 11 subjects completed the trial. The one subject who dropped out was hypertensive at baseline of 157/98 and dropped out due to further increases in blood pressure to 176/124, possibly related to bupropion. Overall, the participants were largely adherent to their medication regimes, with nine subjects missing zero or one doses, one subject missing four and one subject missing eight.
Tolerability and safety
The vital signs of the participants were measured upon screening, during the trial and upon completion of the trial. At screening, subjects had an average blood pressure of 118/80 and an average heart rate (HR) of 70. Treatment did not significantly impact systolic or diastolic blood pressure (SBP & DBP), as average SBP and DBP did not change by >5 mmHg throughout treatment. Treatment increased average HR, reaching a maximum average of 89 beats/minute on Week 5 and decreasing after this. By the end of the treatment, subjects had an average blood pressure of 116/77 and an average HR of 78.
Basic laboratory studies, including a complete blood count (CBC) with differential, sodium, potassium, chloride, CO2, glucose, blood urea nitrogen (BUN), creatinine, alanine transaminase (ALT), aspartate transaminase (AST), gamma-glutamyl transferase (GGT), bilirubin and urinalysis, were obtained upon screening. Most subjects had unremarkable initial labs. One had moderately low WBCs that remained low throughout treatment. One participant had mildly elevated AST, ALT and GGT upon screening that normalized during treatment. Another subject had elevated total bilirubin that remained stably elevated throughout the study. Direct bilirubin levels were obtained for this subject and were normal throughout the trial suggesting Gilbert’s syndrome. Total bilirubin, AST, ALT and GGT were monitored throughout treatment and upon completing the trial. Overall, there were no marked changes in AST, ALT, GGT or bilirubin for any subject during the trial.
Reported side effects included insomnia/restlessness (n = 5), headache (n = 4), diarrhea/nausea (n = 3), dry mouth (n = 3), dizziness (n = 2) and constipation (n = 2). One subject reported tremor. These side effects are typical of those seen with bupropion and naltrexone. Frequencies of these side effects are reported in Table 2. One subject stopped naltrexone for a few weeks due to a “strange, restless” feeling, then went back on and did well. Another stopped both naltrexone and bupropion due to poor tolerability and stayed off the medications. One participant stopped naltrexone due to feeling lightheaded, dizzy and irritable and stayed off the medication while continuing on bupropion.
Table 2.
Frequency of reported side effects of combined bupropion and naltrexone
| Side effects | Frequency (%) |
|---|---|
| Insomnia/restlessness | 42 |
| Headache | 33 |
| Diarrhea/nausea | 25 |
| Dry mouth | 25 |
| Dizziness | 17 |
| Constipation | 17 |
| Tremor | 8 |
Twelve men and women who exhibited a minimum of five (men) or three (women) binge drinking episodes per month over the past 3 months were recruited and treated with combined bupropion and naltrexone. Side effects of the combined treatment are listed above.
Drinking outcomes
During treatment with combined bupropion and naltrexone, the average number of drinks per binge drinking day did not change significantly (7.8 to 6.4 drinks, Fig. Fig. 1, t test, P = NS). The average percentage of binge drinking days decreased from 19% to 5% (Fig. Fig. 2, t test, P < 0.0001). The average number of drinks per any drinking day decreased from 5.7 to 3.5 drinks (t test, P = 0.009). The average percentage of total drinking days did not change significantly (49–38%, t test, P = NS). Finally, the average number of drinks per month decreased from 80 to 35 (t test, P = 0.002). Subjectively, several participants reported a decreased desire to initiate and continue drinking. Subjects also reported decreased feelings of craving alcohol, increased ability to moderate and control drinking, and decreased rate of drinking. This is reflected in the average PACS score dropping from 12.2 pre-treatment to 4.5 post-treatment (t test, P < 0.01). One subject also reported a changed taste of alcohol. After the trial, three subjects continued to stay on bupropion and naltrexone, while three continued on bupropion alone. These subjects continued to do well on medication as subjectively reported to JCG during clinical follow-up visits.
Fig. 1 .
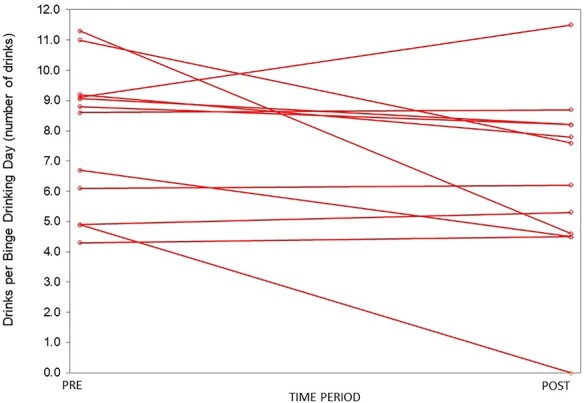
A comparison of drinks per binge drinking day pre- and post-treatment. The number of drinks per binge drinking day over the 3 months prior to treatment (PRE) was calculated for each subject and graphed above. The subjects then received a combined bupropion and naltrexone therapy. The number of drinks per binge drinking day over 3-month treatment period (POST) was calculated for each subject and graphed above. There was no significant difference in drinks per binge drinking day pre- and post-treatment.
Fig. 2 .
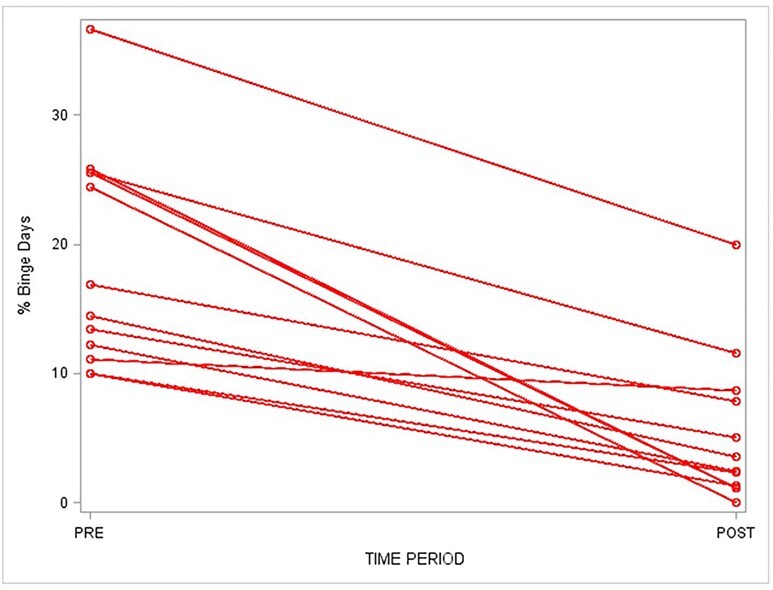
A comparison of percent binge drinking days pre- and post-treatment. The percent binge drinking days over the 3 months prior to treatment (PRE) was calculated for each subject and graphed above. The subjects then received a combined bupropion and naltrexone therapy. The percent binge drinking days over the 3-month treatment period (POST) was calculated for each subject and graphed above. There was a significant decrease (P < 0.0001, t-test) in percent binge drinking days post-treatment compared to pre-treatment.
DISCUSSION
In this open-label, feasibility and tolerability study, binge-drinking individuals receiving bupropion and naltrexone reported an average decrease in drinks per binge drinking day of 7.8–6.4 drinks and a decrease in percent binge drinking days of 19–5%. Reductions in drinks per any drinking day (5.7–3.5 drinks), percent total drinking days (49–38%) and drinks per month (80–35 drinks) were also noted. While the decrease in drinks per binge drinking day was modest, the decrease in percentage of binge drinking days was noteworthy and, in many cases, was quite profound. Substantive reductions in overall drinking were also present.
While difficult to interpret fully, combined naltrexone and bupropion appear to reduce overall alcohol intake and potentially blunt the likelihood of a drinking day progressing to a binge drinking day. It is not clear what the underlying mechanism is, though it is known that pharmacological treatments can impact the initiation of alcohol-seeking behavior, as well as the amount of alcohol consumption, depending on the drug used (Czachowski, 2005; Czachowski et al., 2006). It is noteworthy that subjects reported increased ability to moderate and control drinking, decreased rate of drinking and decreased feelings of craving alcohol. It is of interest that, recently, naltrexone was reported to enhance neural connections that may increase executive control over drinking (Elton et al., 2019), which could underlie some of the reported increased sense of control over drinking. Interestingly, studies examining the effects of bupropion and naltrexone on weight loss noted decreased frequency and intensity of food cravings, as well as increased control over cravings, similar to the effects our patients reported with alcohol (Greenway et al., 2010). Overall, these data do suggest that bupropion and naltrexone may be useful for decreasing binge drinking. It is noteworthy that half of the subjects chose to stay on bupropion alone or bupropion and naltrexone after the trial. This ‘voting with their feet’ is another positive indicator that bupropion and naltrexone could have value in helping individuals with binge drinking.
The combination of bupropion and naltrexone was moderately tolerated. The most common side effects were insomnia/restlessness, headache, and nausea/diarrhea. Other side effects included dry mouth, dizziness, constipation and tremor. These side effects are typical of those seen with bupropion and naltrexone (FDA, 2009; FDA, 2010; Greenway et al., 2010). Side effects were typically mild to moderate in severity and often decreased with time. Three subjects did stop naltrexone because of side effects, though one went back on the medication. One subject also stopped bupropion. On average, blood pressure was not impacted by treatment and HR was moderately elevated. Finally, treatment with combined naltrexone and bupropion did not impact basic laboratory studies, including CBC with differential, serum electrolytes, BUN, creatinine or glucose, LFTs and urinalysis. Overall, we would consider tolerability as generally acceptable, suggesting that the combination of naltrexone and bupropion therapy merits further study in larger clinical trials. It should be noted individuals with physical dependence on alcohol or a history of seizures were excluded from the trial given the evidence that bupropion can lower seizure thresholds (Skowron and Stimmel, 1992).
It is interesting that more women than men volunteered for the study. Historically, clinical trials in AUDs uniformly enroll more men than women, often in a 2:1 male: female ratio or higher, e.g. Garbutt et al., 2016. This is partially explained by the fact that AUDs have been a male dominant disease. Therefore, it was surprising in the current trial to recruit a 1:4 male:female ratio. One possible explanation for this is that women are more likely than men to take action for their problematic alcohol consumption before more serious problems emerge and at a younger age (Grosso et al., 2013).
While the current trial does not prove efficacy, it does provide evidence to support further exploration of melanocortin agonists with/without opioid antagonists in treating binge drinking. Naltrexone monotherapy has been reported to reduce binge drinking in young adults (O’Malley et al., 2015). However, the current trial grew out of the preclinical evidence that melanocortin activation on its own and when coupled with opioid blockade could produce a more robust reduction in binge drinking (Navarro et al., 2015). The clinical relevance of this for reducing appetite and producing weight loss was demonstrated in obese individuals where the combination of naltrexone with the melanocortin activator, bupropion, was found to be effective in suppressing food cravings with subsequent weight loss (Greenway et al., 2010). Furthermore, the combination of naltrexone and bupropion was superior to either monotherapy for weight loss (Greenway et al., 2010). Accordingly, the continued investigation of MCR agonists with/without opioid antagonism for binge drinking appears to be a valuable direction for study.
There were several limitations to this study. First, without a placebo control, it is unclear what the effect of study participation on drinking outcomes is. Indeed, placebo effects are known to be important in helping individuals reduce drinking (Weiss et al., 2008). Therefore, the direct contribution of naltrexone and bupropion to drinking reductions is unknown. An additional limitation includes the small number of participants. With a small sample size, it is unclear how generalizable these results are to the larger population. Finally, this study enrolled a very small number of men that does not represent the proportion of men seen in the binge drinking and AUD population.
In summary, this exploratory, open-label, single-arm, 12-week prospective trial examined the use of naltrexone and bupropion therapy for the treatment of binge drinking. Twelve individuals with a history of 5 (men) or 3 (women) binge drinking episodes per month over the past 3 months were recruited and treated with naltrexone and bupropion therapy for 3 months. The combination was moderately tolerated and half of the subjects elected to stay on medication after the trial. Treatment was associated with decreased drinks per binge drinking day and decreased percent binge drinking days, as well as reductions in other drinking measures. Subjectively, participants reported decreased feelings of craving alcohol, increased ability to moderate and control drinking and decreased rate of drinking. These results suggest naltrexone and bupropion may be useful for treating binge drinking and should be further studied.
Acknowledgments
The authors would like to acknowledge Melissa Stansbury for her help with the study. We also acknowledge the assistance of the North Carolina Translational and Clinical Sciences (NC TraCS) Institute, which is supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR002489.
Note: Corresponding author is relocating and does not currently have a permanent address, telephone, or fax.
Contributor Information
T Jordan Walter, Pharmacology Department, University of North Carolina, 120 Mason Farm Road, 4010 Genetic Medicine Building, Campus Box 7365, Chapel Hill, NC 27514, USA.
Montserrat Navarro, Psychology & Neuroscience Department, University of North Carolina, 235 E Cameron Ave, Chapel Hill, NC 27599, USA; Bowles Center for Alcohol Studies, 104 Manning Drive, CB #7178, Chapel Hill, NC 27599, USA.
Todd E Thiele, Psychology & Neuroscience Department, University of North Carolina, 235 E Cameron Ave, Chapel Hill, NC 27599, USA; Bowles Center for Alcohol Studies, 104 Manning Drive, CB #7178, Chapel Hill, NC 27599, USA.
Cort Pedersen, Psychiatry Department, University of North Carolina, 101 Manning Drive, Ste 7160, Chapel Hill, NC 27514, USA.
Alexey Kampov-Polevoy, Psychiatry Department, University of North Carolina, 101 Manning Drive, Ste 7160, Chapel Hill, NC 27514, USA.
J C Garbutt, Bowles Center for Alcohol Studies, 104 Manning Drive, CB #7178, Chapel Hill, NC 27599, USA; Psychiatry Department, University of North Carolina, 101 Manning Drive, Ste 7160, Chapel Hill, NC 27514, USA.
Funding
This work was funded by the North Carolina Translational and Clinical Sciences (NC TraCS) Institute, which is supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR002489.
CONFLICT OF INTEREST
None of the authors have any conflicts of interest to report.
STATEMENT OF ETHICAL STUDY OF HUMAN SUBJECTS
This study was approved by the Institutional Review Board at the University of North Carolina at Chapel Hill. Subjects gave written informed consent for inclusion in the trial.
References
- Addolorato G, Vassallo GA, Antonelli G, et al. (2018) Binge drinking among adolescents is related to the development of alcohol use disorders: results from a cross-sectional study. Sci Rep 8:12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billes SK, Sinnayah P, Cowley MA. (2014) Naltrexone/bupropion for obesity: an investigational combination pharmacotherapy for weight loss. Pharmacol Res 84:1–11. [DOI] [PubMed] [Google Scholar]
- Czachowski CL. (2005) Manipulations of serotonin function in the nucleus accumbens core produce differential effects on ethanol and sucrose seeking and intake. Alcohol Clin Exp Res 29:1146–55. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Legg BH, Stansfield KH. (2006) Ethanol and sucrose seeking and consumption following repeated administration of the GABAB agonist baclofen in rats. Alcohol Clin Exp Res 30:812–8. [DOI] [PubMed] [Google Scholar]
- Elton A, Dove S, Spencer C, et al. (2019) Naltrexone acutely enhances connectivity between the ventromedial prefrontal cortex and a left frontoparietal network. Alcohol Clin Exp Res 43:965–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery BA, Volpicelli JR, Pettinati HM. (1999) Psychometric properties of the Penn alcohol craving scale. Alcohol Clin Exp Res 23:1289–95. [PubMed] [Google Scholar]
- Food and Drug Administration . 2009. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/018644s039s040.pdf (4 July 2019, date last accessed).
- Food and Drug Administration . 2010. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021897s015lbl.pdf 4 July 2019, date last accessed).
- Garbutt JC. (2009) The state of pharmacotherapy for the treatment of alcohol dependence. J Subst Abuse Treat 36:S15. [PubMed] [Google Scholar]
- Garbutt JC, Kampov-Polevoy AB, Kalka-Juhl LS, et al. (2016) Association of the sweet-liking phenotype and craving for alcohol with the response to naltrexone treatment in alcohol dependence: a randomized clinical trial. JAMA Psychiatry 73:1056–63. [DOI] [PubMed] [Google Scholar]
- Greenway FL, Fujioka K, Plodkowski RA, et al. (2010) Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 376:595–605. [DOI] [PubMed] [Google Scholar]
- Grosso JA, Epstein EE, McCrady BS, et al. (2013) Women's motivators for seeking treatment for alcohol use disorders. Addict Behav 38:2236–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR. (2006) Age at drinking onset and alcohol dependence: Age at onset, duration, and severity. Arch Pediatr Adolesc Med 160:739–46. [DOI] [PubMed] [Google Scholar]
- Leeman RF, Palmer RS, Corbin WR, et al. (2008) A pilot study of naltrexone and BASICS for heavy drinking young adults. Addict Behav 33:1048–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JW, Naimi TS, Brewer RD, et al. (2007) Binge drinking and associated health risk behaviors among high school students. Pediatrics 119:76–85. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, et al. (2008) Effect of naltrexone and ondansetron on alcohol cue–induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry 65:466–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism . (2004) NIAAA council approves definition of binge drinking. NIAAA Newsletter 3:3. [Google Scholar]
- Navarro M, Carvajal F, Lerma-Cabrera JM, et al. (2015) Evidence that melanocortin receptor agonist melanotan-II synergistically augments the ability of naltrexone to blunt binge-like ethanol intake in male C57BL/6J mice. Alcohol Clin Exp Res 39:1425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Luhn KL, Kampov-Polevoy AB, et al. (2019) Bupropion, alone and in combination with naltrexone, blunts binge-like ethanol drinking and intake following chronic intermittent access to ethanol in male C57 BL/6J mice. Alcohol Clin Exp Res 43:783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley SS, Corbin WR, Leeman RF, et al. (2015) Reduction of alcohol drinking in young adults by naltrexone: a double-blind, placebo-controlled, randomized clinical trial of efficacy and safety. J Clin Psychiatry 76:e207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinati HM, Weiss RD, Dundon W, et al. (2005) A structured approach to medical management: a psychosocial intervention to support pharmacotherapy in the treatment of alcohol dependence. J Stud Alcohol Suppl 15:170–8. [DOI] [PubMed] [Google Scholar]
- Puddey IB, Rakic V, Dimmitt SB, et al. (1999) Influence of pattern of drinking on cardiovascular disease and cardiovascular risk factors—a review. Addiction. 94:649–63. [DOI] [PubMed] [Google Scholar]
- Rinker JA, Marshall SA, Mazzone CM, et al. (2017) Extended amygdala to ventral tegmental area corticotropin-releasing factor circuit controls binge ethanol intake. Biol Psychiatry 81:930–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, et al. (2015) 2010 National and state costs of excessive alcohol consumption. Am J Prevent Med 49:e73–9. [DOI] [PubMed] [Google Scholar]
- Skowron DM, Stimmel GL. (1992) Antidepressants and the risk of seizures. Pharmacotherapy 12:18–22. [PubMed] [Google Scholar]
- Sobell LC, Maisto SA, Sobell MB, et al. (1979) Reliability of alcohol abusers’ self-reports of drinking behavior. Behav Res Ther 17:157–60. [DOI] [PubMed] [Google Scholar]
- Sprow GM, Thiele TE. (2012) The neurobiology of binge-like ethanol drinking: evidence from rodent models. Physiol Behav 106:325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprow GM, Rinker JA, Lowery-Gointa EG, et al. (2016) Lateral hypothalamic melanocortin receptor signaling modulates binge-like ethanol drinking in C 57 BL/6 J mice. Addiction biology. 21:835–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) . 2015. 2015 National Survey on Drug Use and Health (NSDUH). Table 2.46B—Alcohol Use, Binge Alcohol Use, and Heavy Alcohol Use in Past Month among Persons Aged 12 or Older, by Demographic Characteristics: Percentages, 2014 and 2015.https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.htm#tab2-46b.
- Weiss RD, O'Malley SS, Hosking JD, et al. (2008) Do patients with alcohol dependence respond to placebo? Results from the COMBINE study. J Stud Alcohol Drugs 69:878–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright FL, Rodgers RJ. (2013) Acute behavioural effects of bupropion and naltrexone, alone and in combination, in non-deprived male rats presented with palatable mash. Psychopharmacology 228:291–307. [DOI] [PubMed] [Google Scholar]


