Abstract
Background
Limited data exist that compare clinical outcomes of 2-drug regimens (2DRs) and 3-drug regimens (3DRs) in people living with human immunodeficiency virus.
Methods
Antiretroviral treatment–experienced individuals in the International Cohort Consortium of Infectious Diseases (RESPOND) who switched to a new 2DR or 3DR from 1 January 2012–1 October 2018 were included. The incidence of clinical events (AIDS, non-AIDS cancer, cardiovascular disease, end-stage liver and renal disease, death) was compared between regimens using Poisson regression.
Results
Of 9791 individuals included, 1088 (11.1%) started 2DRs and 8703 (88.9%) started 3DRs. The most common 2DRs were dolutegravir plus lamivudine (22.8%) and raltegravir plus boosted darunavir (19.8%); the most common 3DR was dolutegravir plus 2 nucleoside reverse transcriptase inhibitors (46.9%). Individuals on 2DRs were older (median, 52.6 years [interquartile range, 46.7–59.0] vs 47.7 [39.7–54.3]), and a higher proportion had ≥1 comorbidity (81.6% vs 73.9%). There were 619 events during 27 159 person-years of follow-up (PYFU): 540 (incidence rate [IR] 22.5/1000 PYFU; 95% confidence interval [CI]: 20.7–24.5) on 3DRs and 79 (30.9/1000 PYFU; 95% CI: 24.8–38.5) on 2DRs. The most common events were death (7.5/1000 PYFU; 95% CI: 6.5–8.6) and non-AIDS cancer (5.8/1000 PYFU; 95% CI: 4.9–6.8). After adjustment for baseline demographic and clinical characteristics, there was a similar incidence of events on both regimen types (2DRs vs 3DRs IR ratio, 0.92; 95% CI: .72–1.19; P = .53).
Conclusions
This is the first large, international cohort to assess clinical outcomes on 2DRs. After accounting for baseline characteristics, there was a similar incidence of events on 2DRs and 3DRs. 2DRs appear to be a viable treatment option with regard to clinical outcomes. Further research on resistance barriers and long-term durability of 2DRs is needed.
Keywords: HIV, dual therapy, 2-drug regimens, antiretroviral treatment, clinical outcomes
In this analysis of 9791 antiretroviral-experienced individuals in the International Cohort Consortium of Infectious Diseases (RESPOND) (1088 on 2-drug regimens, 8703 on 3-drug regimens), there was a similar short-term incidence of severe clinical events after adjusting for baseline characteristics (incidence rate ratio, 0.92; 95% confidence interval, .72–1.19; P = .53).
Standard treatment of human immunodeficiency virus (HIV) involves combination antiretroviral therapy (ART), traditionally with 3 antiretroviral drugs (ARVs) [1]. Use of 3-drug regimens (3DRs) has been shown to be effective in maintaining viral suppression and increasing CD4 cell counts [2–4]. However, ART is a lifelong commitment, and there are concerns around long-term toxicities [5–8]. With an aging HIV population, the prevalence of non-AIDS comorbidities is increasing, and it is therefore increasingly important to reduce the potential risks associated with ART [8–10].
The emergence of new ARVs with a higher barrier against resistance and more potent antiretroviral activity has led to more interest in reducing ART to 2-drug regimens (2DRs). Several clinical trials and observational studies have shown good virologic and immunologic efficacy of 2DRs [11–19]. There are five 2DRs recommended in current treatment guidelines as switch strategies for individuals with a viral load (VL) below the limit of detection and without historical resistance or hepatitis B coinfection: dolutegravir (DTG) plus rilpivirine (RPV), DTG plus lamivudine (3TC), atazanavir (ATV/b) plus 3TC, boosted darunavir (DRV/b) plus 3TC [1, 20–22], and DRV/b plus RPV [1]. Additionally, DTG plus 3TC is widely recommended as an initial regimen for ART-naive individuals [1, 21, 22].
Despite increasing virologic and immunologic evidence to support 2DRs, there remains little research available on a large, international scale that has assessed clinical end points of 2DRs. Our aim was to compare clinical outcomes with use of 2DRs vs 3DRs.
METHODS
Study Design and Setting
The International Cohort Consortium of Infectious Diseases (RESPOND) is a prospective, multicohort collaboration that includes almost 30 000 people living with HIV-1 (PLWH) from 17 cohorts across Europe and Australia. Further details on RESPOND are published elsewhere [23]. Clinical and demographic data are collected on participants during routine clinical care at the time of enrollment and annually thereafter. Data are also retrospectively collected on the 5 years prior to enrollment and earlier if available. Data on clinical events including AIDS and non-AIDS–defining cancers (NADC), cardiovascular disease (CVD), end-stage liver and renal disease (ESLD and ESRD), and death are collected in real time. Events listed above, occurring from 12 months prior to the last cohort visit before RESPOND enrollment onward, are submitted using a case report form and validated by clinicians at the RESPOND coordinating center using prespecified algorithms [24]. Analyses in RESPOND are performed including validated and nonvalidated events; sensitivity analyses are performed including validated events only.
Participants
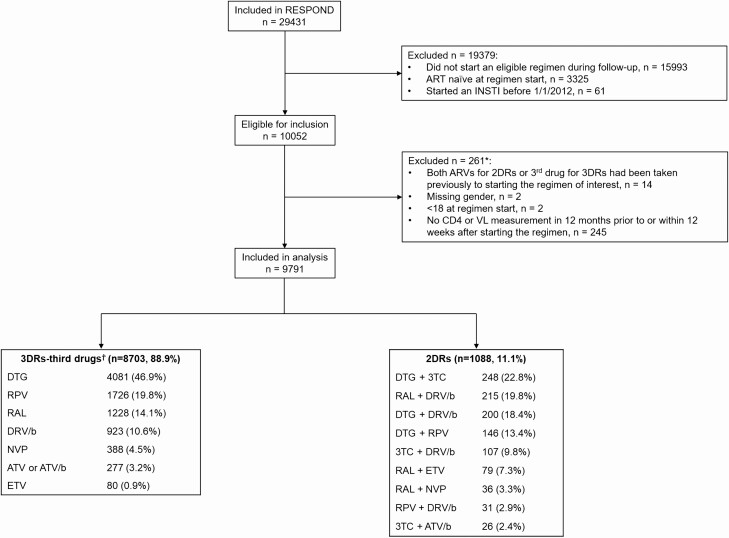
Inclusion criteria for RESPOND are detailed elsewhere [25]. For this analysis, ART-experienced individuals from RESPOND were included if they switched to an eligible 2DR or 3DR, with or without virologic suppression, after the latest of local cohort enrollment and 1 January 2012. ART-naive individuals were excluded as most 2DRs are currently recommended as switch strategies [1] and only 3% of those who started 2DRs in RESPOND were ART-naive. Eligible regimens, as listed in Figure 1, were chosen a priori by a working group to reflect 2DRs currently being prescribed in real-world settings, rather than limited only to those currently recommended. The third drug for 3DRs was chosen to include the same ARVs included in the 2DRs. Individuals were aged ≥18 years at regimen start (defined as baseline) and had a CD4 cell count and VL measurement 12 months prior to or within 12 weeks after starting the regimen of interest. Participants who started an eligible 2DR and 3DR during follow-up (FU) were included in the 2DR group. Participants who started eligible 3DRs were then identified from those not starting 2DRs.
Figure 1.
Study flow chart. *More than 1 reason can apply. †3DRs consisted of 2 nucleoside reverse transcriptase inhibitors plus the third drug listed. 3DRs were chosen so that the third drug included the same ARVs listed in the 2DRs. Abbreviations: 2DR, 2-drug regimen; 3DR, 3-drug regimen; 3TC, lamivudine; ART, antiretroviral therapy; ARV, antiretroviral; ATV, atazanavir; ATV/b, boosted ATV; DRV/b, boosted darunavir; DTG, dolutegravir; ETV, etravirine; INSTI, integrase inhibitor; NVP, nevirapine; RAL, raltegravir; RPV, rilpivirine; VL, viral load.
Outcome Definition
The primary outcome was a severe clinical event, defined as a composite outcome of AIDS (cancer and noncancer), NADC, CVD (defined as invasive cardiovascular procedures, myocardial infarction, or stroke), ESLD, ESRD, and death [24]. Individuals were followed until the first severe event of any type, last clinical visit, or 1 October 2018 (administrative censoring date), whichever occurred first.
Definitions of Potential Confounders
The following variables, defined prior to or at regimen start, were considered as potential confounders: year of starting the regimen of interest, age, gender, ethnicity, body mass index, smoking status, geographical region (categorized as in previous RESPOND analyses [26]), HIV risk category, nadir CD4 cell count, CD4 and CD8 cell counts at regimen start, VL at regimen start, number of ARVs and drug classes previously exposed to, and duration of total prior ART. Prior comorbidities considered included viral hepatitis B and C, hypertension, diabetes, AIDS, NADC, ESLD, ESRD, CVD, fracture, chronic kidney disease, chronic liver enzyme elevation, and dyslipidemia. Definitions of all variables are provided in the footnote of Table 1.
Table 1.
Characteristics of Participants at Regimen Start
| Overall | 3-Drug Regimens | 2-Drug Regimens | |||||
|---|---|---|---|---|---|---|---|
| Characteristic | N | (%) | n | (%) | n | (%) | |
| Total | 9791 | (100) | 8703 | (88.9) | 1088 | (11.1) | |
| Geographical region of Europea | Western | 4955 | (50.6) | 4537 | (52.1) | 418 | (38.4) |
| Southern | 2261 | (23.1) | 1763 | (20.3) | 498 | (45.8) | |
| Northern/Australia | 1679 | (17.1) | 1573 | (18.1) | 106 | (9.7) | |
| Eastern | 896 | (9.2) | 830 | (9.5) | 66 | (6.1) | |
| Gender | Male | 7048 | (72.0) | 6253 | (71.9) | 795 | (73.1) |
| Female | 2738 | (28.0) | 2445 | (28.1) | 293 | (26.9) | |
| Transgender | 3 | (0.0) | 3 | (0.0) | 0 | (0.0) | |
| Ethnicity | White | 6976 | (81.9) | 6147 | (81.5) | 829 | (84.9) |
| Black | 1125 | (13.2) | 1017 | (13.5) | 108 | (11.1) | |
| Other | 416 | (4.9) | 376 | (5.0) | 40 | (4.1) | |
| BMI/kg/m2 | <18.5 | 363 | (4.9) | 310 | (4.6) | 53 | (7.2) |
| 18.5 to <25 | 4182 | (56.0) | 3752 | (55.8) | 430 | (58.1) | |
| ≥25 | 2923 | (39.1) | 2666 | (39.6) | 257 | (34.7) | |
| Smoking status | Never | 2764 | (40.9) | 2505 | (40.8) | 259 | (41.5) |
| Current | 2836 | (41.9) | 2599 | (42.3) | 237 | (38.0) | |
| Previous | 1162 | (17.2) | 1034 | (16.8) | 128 | (20.5) | |
| HIV viral load, copies/mL | <200 | 8588 | (87.7) | 7648 | (87.9) | 940 | (86.4) |
| ≥200 | 1203 | (12.3) | 1055 | (12.1) | 148 | (13.6) | |
| HIV risk | Men who have sex with men | 4037 | (43.0) | 3631 | (43.5) | 406 | (39.6) |
| Intravenous drug user | 1536 | (16.4) | 1343 | (16.1) | 193 | (18.8) | |
| Heterosexual | 3469 | (37.0) | 3076 | (36.8) | 393 | (38.3) | |
| Other | 337 | (3.6) | 303 | (3.6) | 34 | (3.3) | |
| Prior AIDS, noncancer | 2021 | (22.1) | 1731 | (21.2) | 290 | (29.1) | |
| Prior ADC | 440 | (4.8) | 379 | (4.6) | 61 | (5.8) | |
| HCVb | 2568 | (28.0) | 2268 | (27.9) | 300 | (29.0) | |
| HBVd | 488 | (5.5) | 445 | (5.6) | 43 | (4.2) | |
| Hypertensione | 2620 | (33.1) | 2302 | (32.0) | 318 | (44.5) | |
| Diabetesf | 711 | (8.6) | 604 | (8.3) | 107 | (11.7) | |
| Prior non-ADC | 429 | (4.7) | 376 | (4.6) | 53 | (5.1) | |
| Prior ESLD | 87 | (1.2) | 72 | (1.1) | 15 | (1.8) | |
| Prior ESRD | 52 | (0.5) | 35 | (0.4) | 17 | (1.6) | |
| Prior CVDg | 362 | (4.1) | 285 | (3.6) | 77 | (8.0) | |
| Prior fracture | 508 | (6.6) | 468 | (6.7) | 40 | (6.2) | |
| Prior CKDh | 436 | (4.9) | 333 | (4.2) | 103 | (10.6) | |
| Prior CLEEi | 3628 | (38.7) | 3247 | (39.0) | 381 | (36.0) | |
| Dyslipidemiaj | 6587 | (75.7) | 5787 | (74.7) | 800 | (83.9) | |
| Any prior comorbidity | 7257 | (88.8) | 6373 | (87.9) | 884 | (95.6) | |
| Continuous variables, median (interquartile range) | |||||||
| Regimen start, month/year (mm/yy) | 07/15 | (04/14–08/16) | 07/15 | (03/14–07/16) | 12/15 | (11/14–01/17) | |
| Age, years | 48.3 | (40.3–54.9) | 47.7 | (39.7–54.3) | 52.6 | (46.7–59.0) | |
| CD4 cell count nadir, cells/mm3,k | 202.0 | (91.0–309.0) | 206.0 | (96.0–312.0) | 170.0 | (68.0–280.0) | |
| CD4 cell count at regimen start, cells/mm3,k | 608.0 | (423.0–810.0) | 605.0 | (424.0–809.0) | 622.0 | (408.6–814.1) | |
| CD8 cell count at regimen start, cells/mm3,k | 790.0 | (572.0–1087.0) | 786.0 | (571.0–1081.0) | 827.0 | (579.5–1119.5) | |
| Number of antiretrovirals previously exposed to | 6 | (4–9) | 6 | (4–8) | 8 | (5–11) | |
| Total previous treatment duration, months | 9.9 | (4.7–16.6) | 9.5 | (4.5–16.1) | 14.3 | (6.4–19.0) |
Baseline is defined as the date of starting a regimen of interest. P values for comparisons of 2-drug regimens and 3-drug regimens were all <0.05, except for gender (P = .59), prior ESLD (0.09), CD4 cell count at regimen start (0.55), and CD8 cell count at regimen start (0.08). Denominator for percentages is all participants with nonmissing data. Total unknown n (%): ethnicity, 1274 (13.0); BMI, 2323 (23.7); smoking status, 3029 (30.9); HIV risk, 412 (4.2); prior AIDS-non cancer, 636 (6.5); prior AIDS cancer, 570 (5.8); HCV, 623 (6.4); HBV, 896 (9.2); hypertension, 1880 (19.2); diabetes, 1565 (16.0); prior non-AIDS-defining cancer, 570 (5.8); prior ESLD, 2288 (23.4); prior ESRD, 721 (7.4); prior CVD, 866 (8.8); prior fracture, 2135 (21.8); prior CKD, 857 (8.8); prior CLEE, 405 (4.1); dyslipidemia, 1086 (11.1); prior comorbidity, 1616 (16.5).
Abbreviations: ADC, AIDS-defining cancer; BMI, body mass index; CKD, chronic kidney disease; CLEE, chronic liver enzyme elevation; CVD, cardiovascular disease; ESLD, end stage liver disease; ESRD, end stage renal disease; HBV, hepatitis B surface antigenemia; HCV, hepatitis C AB positive; HIV, human immunodeficiency virus.
aDue to small numbers, Australia was combined with Northern Europe, and Eastern Central Europe was combined with Eastern Europe.
bHCV was defined by use of anti-HCV medication, a positive HCV antibody test, a positive HCV RNA qualitative test, HCV RNA viral load (VL) >615 IU/mL, and/or a positive genotype test [26].
dHBV was defined by a positive HBV surface antigen and/or HBV-RNA VL >357 IU/mL.
eHypertension was confirmed by use of antihypertensives at any time before regimen start or if the most recent systolic or diastolic blood pressure measurement before regimen start was higher than 140 or 90 mm Hg, respectively.
fDiabetes was defined by a reported diagnosis, use of antidiabetic medication, glucose ≥11.1 mmol/L, and/or HbA1c ≥6.5% or ≥48 mmol/mol.
gCVD was defined using a composite diagnosis of myocardial infarction, stroke, or invasive cardiovascular procedure.
hCKD was confirmed if there were 2 consecutive measurements of estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 measured at least 3 months apart. eGFR was calculated using the CKD-epidemiology (EPI) creatinine equation [27].
iChronic liver enzyme elevation was confirmed if there were 2 consecutive measurements of ALT >50 IU/L for males or >35 IU/L for females, measured between 6 months and 2 years apart. One normal alanine aminotranferase (ALT) measurement was allowed between elevated measurements.
jDyslipidemia was defined as total cholesterol >239.4 mg/dL or high-density lipoprotein cholesterol <34.7 mg/dL or triglyceride >203.55 mg/dL or use of lipid-lowering treatments [28].
kCD4 and CD8 cell counts were taken as the most recent measurements in the 12 months prior to regimen start. If no measurements were taken prior to starting the regimen, the first measurement within 12 weeks after regimen start was used, and CD4 cell nadir was recorded the same as CD4 cell count at regimen start.
Statistical Methods
Reasons for discontinuing the previous regimen before starting the 2DR or 3DR were compared where the previous regimen was discontinued within 7 days prior to starting the new regimen.
Poisson regression was used to compare the incidence of any severe clinical event between regimen types, adjusted for baseline characteristics. Each characteristic was adjusted for separately in univariable models, and those with P value < .1 were simultaneously included in a multivariable model. Results of the multivariable model were compared according to the reason for discontinuing the previous regimen (toxicity vs other) before starting the 2DR or 3DR. Other prespecified subgroup analyses included age, gender, CD4 count, and VL at regimen start. All subgroup analyses were performed by fitting an interaction term between regimen type and the subgroup of interest.
In all models, an unknown category was used to account for missing data. Sensitivity analyses were performed using multiple imputation by chained equations with 10 imputations, including the same variables as those included in the primary analysis model. Results were combined using Rubin’s rules [29]. A complete case analysis was also performed excluding participants with missing data on any variables included in the model.
Other sensitivity analyses included restricting analyses to include centrally validated events only and comparing 2DRs that are currently recommended in treatment guidelines to matched 3DRs.
Finally, exploratory analyses were performed to compare the incidence of the most common individual events (AIDS [noncancer], NADC, CVD, death), adjusted for key baseline characteristics (age, CD4 cell count at regimen start, smoking status, and number of ARVs previously exposed to).
Analyses were performed using Stata/SE 15.0 (StataCorp LLC). All P values are 2-sided with a P value < .05 defined as statistically significant.
RESULTS
Among 10 052 eligible RESPOND participants, 9791 (97.4%) met the inclusion criteria and were included in the analysis. A larger proportion of those excluded were intravenous drug users compared with those included (26.3% excluded vs 16.4% included); other baseline characteristics were similar. Of those included, 1088 (11.1%) started 2DRs and 8703 (88.9%) started 3DRs. Figure 1 shows the reasons for exclusion of participants and the number included on each regimen. The most common 2DRs were DTG plus 3TC (22.8%), raltegravir (RAL) plus DRV/b (19.8%), and DTG plus DRV/b (18.4%). The most common 3DR was DTG plus 2 nucleoside reverse transcriptase inhibitors (NRTIs) (46.9%); the most common NRTI backbones were tenofovir disoproxil fumarate/emtricitabine (45.0%) and abacavir/3TC (40.5%).
Participant baseline characteristics are presented in Table 1. The median age at baseline was higher for those on 2DRs (52.6 years; interquartile range [IQR], 46.7–59.0 for 2DRs vs 47.7 years; IQR, 39.7–54.3 for 3DRs; P < .001). The median time between date of baseline VL measurement and regimen start was 21 days (6–55), and most participants on both regimen types had a suppressed VL (86.4% on 2DRs vs 87.9% on 3DRs, P = .16). CD4 cell count was also similar (622 cells/µL; IQR, 409–814 for 2DRs vs 605, IQR, 424–809 for 3DRs, P = .55). Approximately 89% of participants had at least 1 comorbidity, mainly driven by dyslipidemia. There was a higher proportion of most comorbidities in those on 2DRs, including prior CVD (8.0% vs 3.6%, P < .0001) and NADC (5.1 vs 4.6%, P = .007). Finally, participants on 2DRs had been exposed to more ARVs prior to starting the regimen of interest (8 ARVs [5–11] vs 6 [4–8], P < .001).
Of those who started a 2DR or 3DR, 1006 (92.5%) and 8071 (92.7%) discontinued their previous regimen within 7 days of starting the new regimen, respectively. The most common reason for discontinuation of the previous regimen was toxicity for both regimen types (30.9% among those on 2DRs vs 31.1% on 3DRs, P = .91). Among those who discontinued because of toxicity, the most common type of toxicity was related to the nervous system for those starting 3DRs (28.3%) and renal impairment for 2DRs (37.9%). Additionally, treatment simplification was reported for a larger proportion of discontinuations among participants starting a 3DR (9.3% 2DRs vs 15.2% 3DRs, P < .001).
Virologic and immunologic outcomes at 6 and 12 months FU were similar on 2DRs and 3DRs (Supplementary Material).
Severe Clinical Outcomes
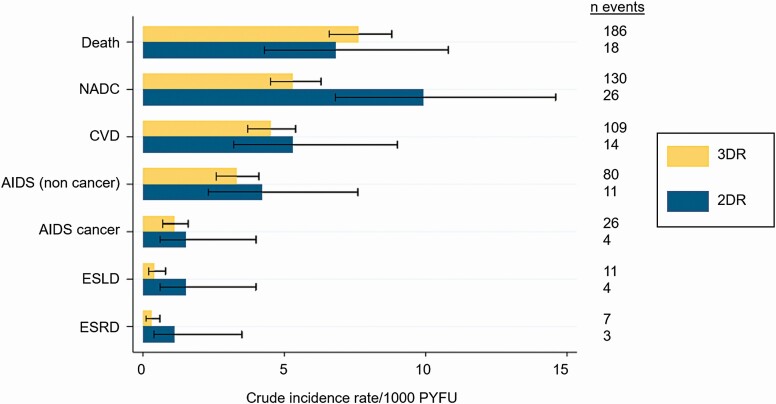
Median FU was 2.6 years (IQR, 1.4–3.8) and higher for those on 3DRs (2.7 years; IQR, 1.4–3.8) compared with 2DRs (2.2 years; IQR, 1.2–3.2). During a total FU of 27 159 years, there were 619 severe clinical events (incidence rate [IR] 23.3/1000 PYFU; 95% confidence interval [CI]: 21.6–25.2): 540 on 3DRs (22.5/1000 PYFU; 95% CI: 20.7–24.5) and 79 on 2DRs (30.9/1000 PYFU; 95% CI: 24.8–38.5). The most common events were death (IR 7.5/1000 PYFU; 95% CI: 6.5–8.6) and NADC (5.8/1000 PYFU; 95% CI: 4.9–6.8). Figure 2 shows the crude IRs of each event by regimen type. With the exception of death, the crude IR of each event was higher on 2DRs, although some of the event rates have wide CIs due to the small number of events.
Figure 2.
Crude incidence rate/1000 person-years of follow-up and 95% confidence interval for 2DRs vs 3DRs. Abbreviations: 2DR, 2-drug regimen; 3DR, 3-drug regimen; CVD, cardiovascular disease; ESLD, end stage liver disease; ESRD, end stage renal disease; NADC, non-AIDS–defining cancer; PYFU, person-years of follow-up.
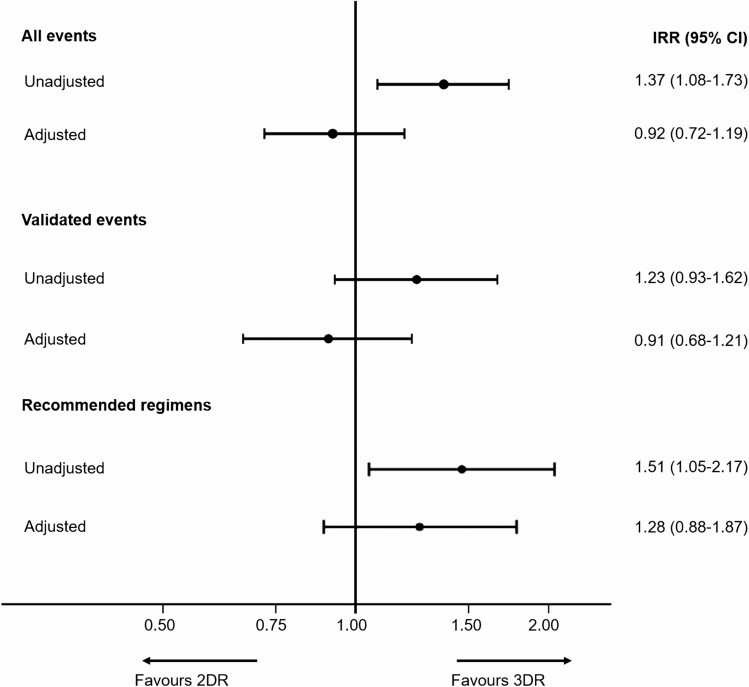
The unadjusted IR of any severe event was higher on 2DRs (IR ratio [IRR], 1.37; 95% CI: 1.08–1.73; P = .009), as shown in Figure 3. After adjustment for age, the difference was attenuated and no longer significant (IRR, 1.08; 95% CI: .85–1.37; P = .54); results were similar after adjustment for a wide range of baseline characteristics (IRR, 0.92; 95% CI: .72–1.19; P = .53). Of the 619 events, 462 were in the validation period, and 444 (96.1%) were validated, giving an IR of validated events of 28.1/1000 PYFU (95% CI: 25.4–31.0) for 3DRs, 34.6/1000 PYFU (95% CI: 26.7–44.7) for 2DRs, and a crude IRR comparing 2DRs to 3DRs of 1.23 (95% CI: 0.93–1.62; P = .14). Results after adjustment were similar to those from our main analysis (Figure 3).
Figure 3.
IRR comparing events on 2DRs vs 3DRs. All events and validated events—adjusted analyses adjusted for age, gender, ethnicity, body mass index, smoking status, human immunodeficiency virus (HIV) risk group, HIV viral load at regimen start, nadir CD4 count, CD4 cell count at regimen start, viral hepatitis C, viral hepatitis B, prior hypertension, prior diabetes, prior AIDS-defining event (excluding cancer), prior AIDS cancer, prior non-AIDS cancer, prior end stage liver disease, prior cardiovascular disease, prior fracture, prior chronic kidney disease, prior dyslipidemia, and number of drugs previously exposed to. Recommended regimens—adjusted analysis adjusted for age, gender, ethnicity, smoking status,CD4 cell count at regimen start, viral hepatitis C, prior AIDS-defining event (excluding cancer), prior non-AIDS cancer, prior cardiovascular disease, prior chronic kidney disease, number of drugs previously exposed to. Recommended regimens included 2DRs: dolutegravir (DTG) plus rilpivirine (RPV), DTG plus lamivudine (3TC), boosted atazanavir (ATV/b) plus 3TC, darunavir (DRV) plus 3TC, DRV plus RPV; 3DRs: DTG or RPV or ATV/b or DRV plus 2 nucleoside reverse transcriptase inhibitors. Abbreviations: 2DR, 2-drug regimen; 3DR, 3-drug regimen; CI, confidence interval; IRR, incidence rate ratio.
In a prespecified subgroup analysis, there was a significant interaction between regimen type and VL at regimen start (interaction P = .011); this showed there was no difference in the adjusted incidence of events between regimen types for those with a suppressed VL at regimen start (IRR, 1.12; 95% CI: .85–1.48); however, in those with uncontrolled viremia (VL ≥200 copies/mL), there was a lower incidence of events on 2DRs vs 3DRs (IRR, 0.51; 95% CI: .30–.89]). Similar results were seen when uncontrolled viremia was defined as VL ≥50 copies/mL (interaction P = .03). There was no interaction between the reason for discontinuing the previous regimen (toxicity vs other) and regimen type (interaction P = .35), indicating a similar incidence of severe events on 2DRs and 3DRs regardless of the reason for discontinuing the previous regimen. Other subgroup analyses were also nonsignificant.
Exploratory analyses that focused on individual events showed no significant differences between regimen types. However, after adjustment, there was a nonsignificant higher incidence of AIDS (IRR, 1.27; 95% CI: .67, 2.43; P = .47) and NADC (IRR, 1.35; 95% CI: .88, 2.09; P = .17) on 2DRs, and a lower incidence of CVD (IRR 0.80; 95% CI: .45–1.41; P = .44) and death (IRR, 0.69; 95% CI: .42–1.12; P = .13; Table 2). As the event rates were lower when looking at specific events and the analyses were adjusted for a limited number of potential confounders, these estimates have wide CIs, and the results should be interpreted with caution.
Table 2.
Comparison of the Incidence of Individual Severe Clinical Events Between 2-Drug Regimens and 3-Drug Regimens
| Univariable | Multivariablea | |||||||
|---|---|---|---|---|---|---|---|---|
| Event | Regimen type | N Events | IRR | (95% CI) | P Value | IRR | (95% CI) | P Value |
| Death | 3DR | 186 | 1 | 1 | ||||
| 2DR | 18 | 0.90 | (.55–1.46) | .66 | 0.69 | (.42–1.12) | .13 | |
| Non-AIDS–defining cancer | 3DR | 130 | 1 | 1 | ||||
| 2DR | 26 | 1.86 | (1.22–2.84) | .004 | 1.35 | (.88–2.09) | .17 | |
| Cardiovascular disease | 3DR | 109 | 1 | 1 | ||||
| 2DR | 14 | 1.19 | (.68–2.08) | .54 | 0.80 | (.45–1.41) | .44 | |
| AIDS, noncancer | 3DR | 80 | 1 | 1 | ||||
| 2DR | 11 | 1.28 | (.68–2.40) | .44 | 1.27 | (.67–2.43) | .47 |
Abbreviations: 2DR, 2-drug regimen; 3DR, 3-drug regimen; CI, confidence interval; IRR, incidence rate ratio.
aMultivariable model adjusted for age, CD4 cell count at regimen start, smoking status, and number of drugs previously exposed to.
Sensitivity Analyses
We restricted our analyses to include participants on recommended 2DRs compared with matched 3DRs, as listed in Figure 3 footnote. This included 558 PLWH (51.3%) on 2DRs and 7007 (80.5%) on 3DRs. Differences in baseline characteristics between 2DRs and 3DRs in this analysis were similar to those in the primary analysis, apart from a higher proportion on recommended 2DRs having suppressed VL compared with matched 3DRs (96.1% vs 88.0%, P < .0001). There were 363 events during 18 133 PYFU on 3DRs (IR 20.0/1000 PYFU; 95% CI: 18.1–22.2) and 32 events during 1059 PYFU on 2DRs (IR 30.2/1000 PYFU; 95% CI: 21.4–42.7). There was a similar distribution of events as in the main analysis. As in the primary analysis there was a higher crude incidence of events on 2DRs (IRR, 1.51; 95% CI: 1.05–2.17; P = .026; Figure 3); after adjustment there was no longer a significant difference between regimen types (IRR, 1.28; 95% CI: .88–1.87; P = .20). Using multiple imputation to account for missing data or performing a complete case analysis showed similar results.
We explored the role of the NRTI backbone for those on 3DRs to determine whether the incidence of events was driven by the backbone rather than the third drug. We compared the IRs on each 3DR before and after adjusting for the backbone but found similar results. We also repeated the main analysis adjusting for the Data Collection on Adverse events of Anti-HIV Drugs (D:A:D) CVD risk score, which accounts for previous exposure to ARVs [30], and found similar results. We repeated the main analysis using multiple imputation to account for missing data and performed a complete case analysis, both with similar results.
Discussion
In this study of almost 10 000 ART-experienced individuals (1088 on 2DRs) from across Europe and Australia, we found a similar incidence of severe clinical events on 2DRs vs 3DRs, after adjusting for baseline characteristics, primarily age. While several surrogate markers for clinical outcomes, such as inflammation, and immune activation biomarkers have been extensively compared between 2DRs and 3DRs with mixed results [11, 16, 31, 32], this is one of the first large studies to compare clinical outcomes. Baseline characteristics were notably different between groups, suggesting there is likely to be confounding by indication. However, our result was consistent across a wide range of sensitivity analyses, including restricting the analysis to centrally validated events and to individuals starting recommended regimens only.
Subgroup analyses showed consistent results among those with a suppressed VL at regimen start. Interestingly, there was a lower incidence of events on 2DRs vs 3DRs in those with uncontrolled viremia, although this group did include smaller numbers. This may be because the proportion of participants with comorbidities among those with uncontrolled viremia on 2DRs was lower than among those with a suppressed VL, which was not the case for those on 3DRs. However, research is needed to investigate this further.
For the primary analysis, we included all 2DRs shown to be noninferior to 3DRs, regardless of whether they are recommended in guidelines, to reflect current clinical practice across the regions included. Sensitivity analyses were performed including 2DRs recommended in international guidelines only, which showed a higher, although nonsignificant, incidence of clinical events on 2DRs. This analysis, however, included considerably smaller numbers and the results have wide CIs. It is expected that there may be a higher short-term incidence of events on 2DRs, as older individuals and those with comorbidities were more likely to be prescribed 2DRs in our analysis; therefore, further research of clinical outcomes with longer FU on 2DRs is needed.
Results from preplanned exploratory analyses comparing the incidence of individual events suggest that NADC and noncancer AIDS event rates may be higher for 2DR, but death and CVD rates may be lower. These analyses were limited by power and larger studies or studies focused on these end points alone are needed to investigate this further. Van Wyk et al [32] and Calza et al [33] showed a decrease in lipids with DTG plus 3TC and RAL plus etravirine, respectively, compared with 3DRs, suggesting the risk of CVD could be lower on 2DRs. However, other studies that compared lipids on 2DRs have shown mixed results [11, 12, 16]. Additionally, Serrano-Villar et al found increased long-term inflammation on 2DRs [33], the clinical implications of which warrant further investigation.
Switching from 3DRs to 2DRs has several potential advantages. Avoiding ARVs shown to be associated with an increased risk of toxicities, such as renal and bone toxicities, may further lead to fewer toxicities on 2DRs, although this requires further research with longer FU and comparison with newer 3DRs, such as those that include tenofovir alafenamide [11, 35–37]. Additionally, 2DRs provide a simpler regimen for those not currently on fixed combination pills, and some 2DRs have been shown to be more cost effective than many 3DRs [1, 8, 37, 38]. While most treatment guidelines recommend specific 2DRs as switch strategies, DTG plus 3TC is now recommended as a possible initial regimen for ART-naive individuals [1, 20, 22]. It is therefore important to compare the longer-term clinical outcomes of 2DRs vs 3DRs, data that will not be available from randomized clinical trials. While many studies have shown that 2DRs are noninferior to 3DRs for short-term virologic and immunologic end points, data that compare clinical end points remain scarce [11–19, 39, 40].
There are some limitations to our analysis. We prespecified the minimum number of participants on integrase inhibitors to be enrolled in RESPOND; therefore, participants are not randomly selected. As this is an observational study, confounding by indication may affect our results, and while we have adjusted for a wide range of baseline characteristics, residual confounding cannot be excluded. Additionally, there is a relatively high proportion of missing data, for example, for smoking status, and data completeness varies between cohorts. However, we performed several sensitivity analyses to handle missing data, all with similar results. Finally, the primary outcome of severe clinical outcome was analyzed as a composite end point due to the low incidence of specific events, and 2DRs and 3DRs were analyzed as groups. Specific regimens included in 2DRs and 3DRs were specified a priori and reflect real-world settings where individuals are treated with a range of regimens. The results may differ for specific events or for specific regimens.
There are, however, several important strengths to our analysis. To our knowledge, this is one of the first studies to assess clinical outcomes of 2DRs. RESPOND is a large and heterogeneous sample that provides results that are generalizable to PLWH in Europe and Australia. Further, due to the size of the study, we were able to include a variety of 2DRs and assess relatively uncommon clinical end points.
In conclusion, after accounting for demographic and clinical characteristics, there was a similar incidence of severe clinical events on 2DRs and 3DRs. 2DRs appear to be a viable treatment option with regard to clinical outcomes in the first 2–3 years of exposure, although further research on resistance barriers and long-term durability of 2DRs is needed.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by ViiV Healthcare LLC (grant 207709) and Gilead Sciences (grant CO-EU-311–4339). Additional support has been provided by the following participating cohorts contributing data in-kind: Austrian HIV Cohort Study, the Australian HIV Observational Database, Le Centre Hospitalier Universitaire Saint-Pierre, University Hospital Cologne, the EuroSIDA Cohort, Frankfurt HIV Cohort Study, Georgian National AIDS Health Information System, Modena HIV Cohort, San Raffaele Scientific Institute, Swiss HIV Cohort Study, AIDS Therapy Evaluation in the Netherlands Cohort, and the Royal Free HIV Cohort Study.
Potential conflicts of interest. G. W. reports grants paid to their institution from Gilead Sciences and grants from ViiV outside the submitted work. H. G. reports grants from the Swiss National Science Foundation and the RESPOND collaboration during the conduct of the study; grants from Swiss National Science Foundation, Swiss HIV Cohort Study, and the National Institutes of Health, and Yvonne Jacob Foundation; grants from unrestricted research grant from Gilead; and personal fees for being an advisor/consultant for Merck, Gilead Sciences, ViiV Healthcare, a member of a data safety monitoring boards outside the submitted work. C. M. reports grants from Gilead and personal fees from ViiV advisory board, Janssen for speaking at a conference, MSD (speaker bureau) outside the submitted work. C. Smith reports personal fees from Gilead Sciences outside the submitted work. S. D.W. reports grants from RESPOND during the conduct of the study. F. W. reports grants paid to their institution from the RESPOND Consortium during the conduct of the study and personal fees from ViiV Healthcare, Gilead, and MSD outside the submitted work. J. M. M. reports grants and personal fees (payments for lectures and academic and research grants) from AbbVie, Angelini, Contrafect, Cubist, Genentech, Gilead Sciences, Jansen, Medtronic, MSD, Novartis, Pfizer, and ViiV Healthcare outside the submitted work, and received a personal 80:20 research grant from Institut d’Investigacions Biomèdiques August Pi i Sunyer, Barcelona, Spain, during 2017–2021. M. L. reports grants from Gilead Sciences, Janssen Cilag, and ViiV Healthcare outside the submitted work. N. C. reports personal fees from Virology Education outside the submitted work. V. V. reports other financial support from ViiV Healthcare outside the submitted work and is a salaried employee of ViiV Healthcare and owner of GlaxoSmithKline stock. F. R. reports other financial support from Gilead Sciences outside the submitted work and is a salaried employee of Gilead Sciences. J. L. reports personal fees from ViiV Healthcare, Gilead Sciences, and Janssen Cilag outside the submitted work. C. D. reports grants and personal fees from Gilead Sciences and ViiV Healthcare, personal fees from Janssen Cilag, and grants from MSD outside the submitted work. J. Hoy reports advisory board fees paid to their institution from Gilead Sciences, ViiV Healthcare, and Merck, Sharp & Dohme outside the submitted work. M. B. reports grants and personal fees from Gilead Sciences (support to their institution for clinical research; support to them for travel to scientific meetings, lecturing, and medical advisory boards), ViiV Healthcare (support to their institution for clinical research; support to them for lecturing and medical advisory boards), and AbbVie (support to their institution for clinical research; support to them for lecturing and medical advisory boards); and grants from MSD (support to their institution for clinical research) outside the submitted work. H. B. has received support for travelling, consultancy fees, and honorarium from Gilead, BMS, Viiv Healthcare, and Roche outside the submitted work; serves as the president of the Association Contre le HIV etAautres Infections Transmissibles and in this function has received support for the Swiss HIV Cohort Study from ViiV Healthcare, Gilead, BMS, MSD, and Abbvie. A. C. reports unrestricted educational grants (to their institution) from Gilead, ViiV Healthcare, and MSD and financial support to the Institution for the Groupe LIPO and Metabolisme (Day Hospital) from Gilead, ViiV Healthcare, MSD, and AbbVie outside the submitted work. A. V. A. reports that he represents the European AIDS Treatment Group (EATF) in the RESPOND Steering Committee. The EATG received support funding from several drug companies (mainly, Viiv, Gilead, MSD, Janssen). A. P. M. reports personal fees from Gilead Sciences (one-off honorarium not related to the work presented here) outside the submitted work. A.M. reports grants from various sources (see funding acknowledgments) during the conduct of the study; personal fees for lectures, consultancy, travel support, and honoraria from ViiV, Gilead, and Eiland and Bonnin PC outside the submitted work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
RESPOND Study Group
AIDS Therapy Evaluation in the Netherlands Cohort: F Wit, P Reiss, M Hillebregt, Stichting HIV Monitoring, Amsterdam, Netherlands. The Australian HIV Observational Database: M Law, K Petoumenos, N Rose, UNSW, Sydney, Australia. Austrian HIV Cohort Study: R Zangerle, H Appoyer, Medizinische Universität Innsbruck, Innsbruch, Austria. Le Centre Hospitalier Universitaire (CHU) Saint-Pierre:S De Wit, M Delforge, Centre de Recherche en Maladies Infectieuses a.s.b.l., Brussels, Belgium. EuroSIDA Cohort: G Wandeler, Centre of Excellence for Health, Immunity and Infections (CHIP), Rigshospitalet, RegionH, Copenhagen, Denmark. Frankfurt HIV Cohort Study: C Stephan, M Bucht, Johann Wolfgang Goethe-University Hospital, Frankfurt, Germany. Infectious Diseases, AIDS and Clinical Immunology Research Center: N Chkhartishvili, O Chokoshvili, Infectious Diseases, AIDS and Clinical Immunology Research Center, Tbilisi, Georgia. Italian Cohort Naive Antiretrovirals: A d’Arminio Monforte, A Rodano, A Tavelli, L’Azienda Socio Sanitaria Territoriale (ASST) Santi Paolo e Carlo, Milan, Italy; I Fanti, Icona Foundation, Milan, Italy. Modena HIV Cohort: C Mussini, V Borghi, Università degli Studi di Modena, Modena, Italy. Nice HIV Cohort: C Pradier, E Fontas, K Dollet, C Caissotti, Université Côte d’Azur et Centre Hospitalier Universitaire, Nice, France. Projecte per la Informatització del Seguiment Clínic epidemiològic de la Infecció per VIH i Sida (PISCIS) Cohort Study: J Casabona, J M Miro, J M Llibre, A Riera, J Reyes- Urueña, Centre Estudis Epidemiologics de ITS i VIH de Catalunya, Badalona, Spain. Royal Free Hospital Cohort: C Smith, F Lampe, Royal Free Hospital, University College London, London, United Kingdom. San Raffaele Scientific Institute: A Castagna, A Lazzarin, A Poli, Università Vita-Salute San Raffaele, Milano, Italy. Swedish InfCare HIV Cohort: A Sönnerborg, K Falconer, V Svedhem, Karolinska University Hospital, Stockholm, Sweden. Swiss HIV Cohort Study: H Günthard, B Ledergerber, H Bucher, A Scherrer, University of Zurich, Zurich, Switzerland. University Hospital Bonn: J C Wasmuth, J. Rockstroh, Bonn, Germany. University Hospital Cologne: J J Vehreschild, G Fätkenheuer, Cologne, Germany.
RESPOND Executive Committee
A Mocroft, * J Rooney, F Rogatto, V Vannappagari, H Garges, G Wandeler, M Law, R Zangerle, C Smith, S De Wit, J Lundgren, H Günthard (*chair).
RESPOND Scientific Steering Committee
J Lundgren, * H Günthard, * J Kowalska, D Raben, L Ryom, A Mocroft, J Rockstroh, L Peters, A Volny Anne, N Dedes, E D Williams, N Chkhartishvili, R Zangerle, M Law, F Wit, C Necsoi, G Wandeler, C Stephan, C Pradier, A D’Arminio Monforte, C Mussini, A Bruguera, H Bucher, A Sönnerborg, J J Vehreschild, J C Wasmuth, C Smith, A Castagna, F Rogatto, R Haubrich, V Vannappagari, H Garges (*chairs).
RESPOND Outcomes Scientific Interest Group
L Ryom, A Mocroft, B Neesgaard, L Greenberg, L Bansi-Matharu, V Svedhem-Johansson, F Wit, K Grabmeier-Pfistershammer, R Zangerle, J Hoy, M Bloch, D Braun, A Calmy, G Schüttfort, M Youle, S De Wit, C Mussini, S Zona, A Castagna, A Antinori, N Chkhartishvili, N Bolokadze, E Fontas, K Dollet, C Pradier, J M Miro, J M Llibre, J J Vehreschild, C Schwarze-Zander, J -C Wasmuth, J Rockstroh, K Petoumenos, M Law, C Duvivier, G Dragovic, R Radoi, C Oprea, M Vasylyev, J Kowalska, R Matulionyte, V Mulabdic, G Marchetti, E Kuzovatova, N Coppola, J Begovac, I Aho, S Martini, H Bucher, A Harxhi, T Wæhre, A Pharris, A Vassilenko, G Fätkenheuer, J Bogner, A Maagaard, E Jablonowska, D Elbirt, G Marrone, C Leen, C Wyen, M Kundro, N Dedes, E Dixon Williams, J Gallant, D Thorpe, H Diaz Cuervo, V Vannappagari, H Garges.
Community Representatives
A Volny-Anne, N Dedes, L Mendao (European AIDS Treatment Group), E Dixon Williams. (United Kingdom).
RESPOND Staff
D Raben, L Peters, L Ryom, B Neesgaard, J F Larsen, M L Jakobsen, T Bruun, A Bojesen, E V Hansen, T W Elsing, D Kristensen, S Thomsen, T Weide, A Mocroft, L Greenberg.
Statistical Staff
A Mocroft, L Greenberg, L Bansi-Matharu, A Pelchen-Matthews, K Petoumenos, N Rose, D Byonanebye.
Contributor Information
Lauren Greenberg, Centre for Clinical Research, Epidemiology, Modelling and Evaluation (CREME), Institute for Global Health, University College London, London, United Kingdom.
Lene Ryom, Centre of Excellence for Health, Immunity and Infections (CHIP), Department of Infectious Diseases, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark.
Bastian Neesgaard, Centre of Excellence for Health, Immunity and Infections (CHIP), Department of Infectious Diseases, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark.
Gilles Wandeler, Department of Infectious Diseases, Bern University Hospital, University of Bern, Bern, Switzerland.
Therese Staub, Infectious Diseases, Centre Hospitalier Luxembourg (CHL), Luxembourg City, Luxembourg.
Martin Gisinger, Medizinische Universität Innsbruck , Innsbruck, Austria.
Michael Skoll, Wiener Medizinische Universität, Vienna, Austria.
Huldrych F Günthard, Department of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, Zurich, Switzerland; Institute of Medical Virology, University of Zurich, Zurich, Switzerland.
Alexandra Scherrer, Department of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, Zurich, Switzerland; Institute of Medical Virology, University of Zurich, Zurich, Switzerland.
Cristina Mussini, Modena HIV Cohort, Università degli Studi di Modena , Modena, Italy.
Colette Smith, Royal Free HIV Cohort Study, Royal Free Hospital, University College London, London, United Kingdom.
Margaret Johnson, Royal Free HIV Cohort Study, Royal Free Hospital, University College London, London, United Kingdom.
Stéphane De Wit, Saint Pierre University Hospital, Université Libre de Bruxelles , Brussels, Belgium.
Coca Necsoi, Saint Pierre University Hospital, Université Libre de Bruxelles , Brussels, Belgium.
Christian Pradier, Nice HIV Cohort, Université Côte d’Azur et Centre Hospitalier Universitaire, Nice, France.
Ferdinand Wit, AIDS Therapy Evaluation in the Netherlands Cohort (ATHENA), Stichting HIV Monitoring (SHM), Amsterdam, The Netherlands.
Clara Lehmann, University Hospital Cologne, Cologne, Germany.
Antonella d’Arminio Monforte, Italian Cohort Naive Antiretrovirals, (ICONA) L’Azienda Socio Sanitaria Territoriale (ASST) Santi Paolo e Carlo , Milano, Italy.
Jose M Miró, Hospital Clinic-August Pi i Sunyer Biomedical Research Institute (IDIBAPS), University of Barcelona, Barcelona, Spain.
Antonella Castagna, School of Medicine, Vita-Salute San Raffaele University, Milan, Italy.
Vincenzo Spagnuolo, School of Medicine, Vita-Salute San Raffaele University, Milan, Italy.
Anders Sönnerborg, Division of Infectious Diseases, Karolinska Institutet and Department of Infectious Diseases, Karolinska University Hospital, Stockholm, Sweden.
Matthew Law, Australian HIV Observational Database (AHOD), University of New South Wales (UNSW) , Sydney Australia.
Jolie Hutchinson, Australian HIV Observational Database (AHOD), University of New South Wales (UNSW) , Sydney Australia.
Nikoloz Chkhartishvili, Georgian National AIDS Health Information System (AIDS HIS), Infectious Diseases, AIDS and Clinical Immunology Research Center , Tbilisi, Georgia.
Natalia Bolokadze, Georgian National AIDS Health Information System (AIDS HIS), Infectious Diseases, AIDS and Clinical Immunology Research Center , Tbilisi, Georgia.
Jan-Christian Wasmuth, University Hospital Bonn, Bonn, Germany.
Christoph Stephan, Medical Department No. 2, Infectious Diseases Unit, Goethe-University Hospital Frankfurt, Frankfurt, Germany.
Vani Vannappagari, ViiV Healthcare, RTP, Research Triangle Park, North Carolina, USA.
Felipe Rogatto, Gilead science, Foster City, California, USA.
Josep M Llibre, Hospital Universitari Germans Trias i Pujol Department of Internal Medicine, HIV Unit, Barcelona, Spain.
Claudine Duvivier, Assistance Publique - Hôpitaux de Paris (APHP)-Hôpital Necker-Enfants Malades, Service de Maladies Infectieuses et Tropicales, Centre d’Infectiologie Necker-Pasteur, Institut hospitalo-universitaire (IHU) Imagine , Paris, France.
Jennifer Hoy, Department of Infectious Diseases, Alfred Hospital and Monash University, Melbourne, Victoria, Australia.
Mark Bloch, Australian HIV Observational Database (AHOD), University of New South Wales (UNSW) , Sydney Australia.
Heiner C Bucher, Department of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, Zurich, Switzerland.
Alexandra Calmy, HIV/AIDS Unit in Geneva University Hospital, Geneva, Switzerland.
Alain Volny Anne, European AIDS Treatment Group, Brussels, Belgium.
Annegret Pelchen-Matthews, Centre for Clinical Research, Epidemiology, Modelling and Evaluation (CREME), Institute for Global Health, University College London, London, United Kingdom.
Jens D Lundgren, Centre of Excellence for Health, Immunity and Infections (CHIP), Department of Infectious Diseases, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark.
Lars Peters, Centre of Excellence for Health, Immunity and Infections (CHIP), Department of Infectious Diseases, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark.
Loveleen Bansi-Matharu, Centre for Clinical Research, Epidemiology, Modelling and Evaluation (CREME), Institute for Global Health, University College London, London, United Kingdom.
Amanda Mocroft, Centre for Clinical Research, Epidemiology, Modelling and Evaluation (CREME), Institute for Global Health, University College London, London, United Kingdom.
for the RESPOND (International Cohort Consortium of Infectious Diseases) Study Group:
F Wit, P Reiss, M Hillebregt, M Law, K Petoumenos, N Rose, R Zangerle, H Appoyer, S De Wit, M Delforge, G Wandeler, C Stephan, M Bucht, N Chkhartishvili, O Chokoshvili, A d’Arminio Monforte, A Rodano, A Tavelli, I Fanti, C Mussini, V Borghi, C Pradier, E Fontas, K Dollet, C Caissotti, J Casabona, J M Miro, J M Llibre, A Riera, J Reyes- Urueña, C Smith, F Lampe, A Castagna, A Lazzarin, A Poli, A Sönnerborg, K Falconer, V Svedhem, H Günthard, B Ledergerber, H Bucher, A Scherrer, J C Wasmuth, J J Vehreschild, G Fätkenheuer, A Mocroft, J Rooney, F Rogatto, V Vannappagari, H Garges, G Wandeler, M Law, R Zangerle, C Smith, S De Wit, J Lundgren, H Günthard, J Lundgren, H Günthard, J Kowalska, D Raben, L Ryom, A Mocroft, J Rockstroh, L Peters, A Volny Anne, N Dedes, E D Williams, N Chkhartishvili, R Zangerle, M Law, F Wit, C Necsoi, G Wandeler, C Stephan, C Pradier, A D’Arminio Monforte, C Mussini, A Bruguera, H Bucher, A Sönnerborg, J J Vehreschild, J C Wasmuth, C Smith, A Castagna, F Rogatto, R Haubrich, V Vannappagari, H Garges, L Ryom, A Mocroft, B Neesgaard, L Greenberg, L Bansi-Matharu, V Svedhem-Johansson, F Wit, K Grabmeier-Pfistershammer, R Zangerle, J Hoy, M Bloch, D Braun, A Calmy, G Schüttfort, M Youle, S De Wit, C Mussini, S Zona, A Castagna, A Antinori, N Chkhartishvili, N Bolokadze, E Fontas, K Dollet, C Pradier, J M Miro, J M Llibre, J J Vehreschild, C Schwarze-Zander, J -C Wasmuth, J Rockstroh, K Petoumenos, M Law, C Duvivier, G Dragovic, R Radoi, C Oprea, M Vasylyev, J Kowalska, R Matulionyte, V Mulabdic, G Marchetti, E Kuzovatova, N Coppola, J Begovac, I Aho, S Martini, H Bucher, A Harxhi, T Wæhre, A Pharris, A Vassilenko, G Fätkenheuer, J Bogner, A Maagaard, E Jablonowska, D Elbirt, G Marrone, C Leen, C Wyen, M Kundro, N Dedes, E Dixon Williams, J Gallant, D Thorpe, H Diaz Cuervo, V Vannappagari, H Garges, A Volny-Anne, N Dedes, L Mendao, E Dixon Williams, D Raben, L Peters, L Ryom, B Neesgaard, J F Larsen, M L Jakobsen, T Bruun, A Bojesen, E V Hansen, T W Elsing, D Kristensen, S Thomsen, T Weide, A Mocroft, L Greenberg, A Mocroft, L Greenberg, L Bansi-Matharu, A Pelchen-Matthews, K Petoumenos, N Rose, and D Byonanebye
References
- 1. EACS . EACS guidelines version 10.1. EACS (European AIDS Clinical Society), 2020. Available at: https://www.eacsociety.org/files/guidelines-10.1_finaljan2021_1.pdf. [Google Scholar]
- 2. Tseng A, Seet J, Phillips EJ. The evolution of three decades of antiretroviral therapy: challenges, triumphs and the promise of the future. Br J Clin Pharmacol 2015; 79:182–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Palella FJ Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998; 338:853–60. [DOI] [PubMed] [Google Scholar]
- 4. Trickey A, May MT, Vehreschild JJ, et al. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV 2017; 4:e349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fernandez-Montero JV, Eugenia E, Barreiro P, Labarga P, Soriano V. Antiretroviral drug-related toxicities— clinical spectrum, prevention, and management. Expert Opin Drug Saf 2013; 12:697–707. [DOI] [PubMed] [Google Scholar]
- 6. Margolis AM, Heverling H, Pham PA, Stolbach A. A review of the toxicity of HIV medications. J Med Toxicol 2014; 10:26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Troya J, Bascuñana J. Safety and tolerability: current challenges to antiretroviral therapy for the long-term management of HIV infection. AIDS Rev 2016; 18:127–37. [PubMed] [Google Scholar]
- 8. Holtzman C, Armon C, Tedaldi E, et al. ; and the HOPS Investigators . Polypharmacy and risk of antiretroviral drug interactions among the aging HIV-infected population. J Gen Intern Med 2013; 28:1302–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011; 53:1120–6. [DOI] [PubMed] [Google Scholar]
- 10. Pelchen-Matthews A, Ryom L, Borges ÁH, et al. ; EuroSIDA Study . Aging and the evolution of comorbidities among HIV-positive individuals in a European cohort. AIDS 2018; 32:2405–16. [DOI] [PubMed] [Google Scholar]
- 11. Llibre JM, Hung CC, Brinson C, et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet 2018; 391:839–49. [DOI] [PubMed] [Google Scholar]
- 12. Pulido F, Ribera E, Lagarde M, et al. ; DUAL-GESIDA-8014-RIS-EST45 Study Group . Dual therapy with darunavir and ritonavir plus lamivudine vs triple therapy with darunavir and ritonavir plus tenofovir disoproxil fumarate and emtricitabine or abacavir and lamivudine for maintenance of human immunodeficiency virus type 1 viral suppression: randomized, open-label, noninferiority DUAL-GESIDA 8014-RIS-EST45 trial. Clin Infect Dis 2017; 65:2112–8. [DOI] [PubMed] [Google Scholar]
- 13. Taiwo B, Zheng L, Gallien S, et al. ; ACTG A5262 Team . Efficacy of a nucleoside-sparing regimen of darunavir/ritonavir plus raltegravir in treatment-naive HIV-1-infected patients (ACTG A5262). AIDS 2011; 25:2113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perez-Molina JA, Rubio R, Rivero A, et al. ; GeSIDA 7011 Study Group . Simplification to dual therapy (atazanavir/ritonavir + lamivudine) versus standard triple therapy [atazanavir/ritonavir + two nucleos(t)ides] in virologically stable patients on antiretroviral therapy: 96 week results from an open-label, non-inferiority, randomized clinical trial (SALT Study). J Antimicrob Chemother 2017; 72:246–53. [DOI] [PubMed] [Google Scholar]
- 15. Arribas JR, Girard PM, Landman R, et al. ; OLE/RIS-EST13 Study Group . Dual treatment with lopinavir-ritonavir plus lamivudine versus triple treatment with lopinavir-ritonavir plus lamivudine or emtricitabine and a second nucleos(t)ide reverse transcriptase inhibitor for maintenance of HIV-1 viral suppression (OLE): a randomised, open-label, non-inferiority trial. Lancet Infect Dis 2015; 15:785–92. [DOI] [PubMed] [Google Scholar]
- 16. Cahn P, Madero JS, Arribas JR, et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferior. Lancet 2018; 6736:1–13. [DOI] [PubMed] [Google Scholar]
- 17. Gantner P, Cuzin L, Allavena C, et al. ; Dat’AIDS Study Group . Efficacy and safety of dolutegravir and rilpivirine dual therapy as a simplification strategy: a cohort study. HIV Med 2017; 18:704–8. [DOI] [PubMed] [Google Scholar]
- 18. Neesgaard B, Pelchen-Matthews A, Ryom L, et al. ; EuroSIDA Study . Uptake and effectiveness of two-drug compared with three-drug antiretroviral regimens among HIV-positive individuals in Europe. AIDS 2019; 33:2013–24. [DOI] [PubMed] [Google Scholar]
- 19. Reynes J, Trinh R, Pulido F, et al. Lopinavir/ritonavir combined with raltegravir or tenofovir/emtricitabine in antiretroviral-naive subjects: 96-week results of the PROGRESS study. AIDS Res Hum Retroviruses 2013; 29:256–65. [DOI] [PubMed] [Google Scholar]
- 20. Australasian Society for HIV VH and SHM . Antiretroviral Guidelines. US DHHS Guidelines with Australian commentary. Available at: https://arv.ashm.org.au/. Accessed 15 May 2019.
- 21. Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Department of Health and Human Services. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed 15 May 2019.
- 22. Saag MS, Gandhi RT, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the International Antiviral Society–USA panel. JAMA 2020; 324:1651–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. RESPOND Study Group . How to RESPOND to modern challenges for people living with HIV (PLWHIV): a new Cohort collaboration. Microorganisms 2020; 8:1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. RESPOND Coordinating Centre . RESPOND manual of operations (MOOP) for clinical events v1.6 [Internet].2019. Available at: https://chip.dk/Portals/0/files/RESPOND/RESPONDManualofOperationsMOOP__Version1.6.pdf?ver=2019-11-05-124535-643. Accessed 10 September 2020.
- 25. RESPOND Coordinating Centre . RESPOND International Cohort Consortium of Infectious Diseases Version 1.0 May 2019 [Internet].2019. Available at: https://www.chip.dk/Portals/0/files/RESPOND/RESPOND_Consortium-description_V1.0_2019MAY29.pdf?ver=2019-10-02-144627-317. Accessed 10 September 2020.
- 26. Greenberg L, Ryom L, Wandeler G, et al. ; RESPOND Study Group . Uptake and discontinuation of integrase inhibitors (INSTIs) in a large cohort setting. J Acquir Immune Defic Syndr 2020; 83:240–50. [DOI] [PubMed] [Google Scholar]
- 27. Disease Epidemiology Collaboration . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Friis-Møller N, Reiss P, Sabin C, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007; 356:1723–35. [DOI] [PubMed] [Google Scholar]
- 29. Rubin DB. Multiple imputation for nonresponse in surveys. New York: John Wiley and Sons, 1987. [Google Scholar]
- 30. Friis-Møller N, Thiébaut R, Reiss P, et al. ; DAD Study Group . Predicting the risk of cardiovascular disease in HIV-infected patients: the Data Collection on Adverse Effects of Anti-HIV Drugs Study. Eur J Cardiovasc Prev Rehabil 2010; 17:491–501. [DOI] [PubMed] [Google Scholar]
- 31. Gutierrez MDM, Mateo MG, Vidal F, Domingo P. Does choice of antiretroviral drugs matter for inflammation? Expert Rev Clin Pharmacol 2019; 12:389–96. [DOI] [PubMed] [Google Scholar]
- 32. van Wyk J, Ajana F, Bisshop F, et al. Efficacy and safety of switching to dolutegravir/lamivudine fixed-dose 2-drug regimen vs continuing a tenofovir alafenamide–based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with human immunodeficiency virus type 1: phase 3, randomized, noninferiority TANGO Study. Clin Infect Dis 2020; 71:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Calza L, Magistrelli E, Colangeli V, et al. Dual raltegravir-etravirine combination as maintenance regimen in virologically suppressed HIV-1-infected patients. AIDS Res Hum Retroviruses 2017; 33:632–8. [DOI] [PubMed] [Google Scholar]
- 34. Serrano-Villar S. Reducing ART to less than 3-ARV regimen linked to increased systemic inflammation. Presented at the 23rd AIDS Conference, 6–10 July. Available at: https://cattendee.abstractsonline.com/meeting/9289/Presentation/801. Accessed 10 September 2020.
- 35. Maggiolo F, Di Filippo E, Valenti D, Serna Ortega PA, Callegaro A. NRTI sparing therapy in virologically controlled HIV-1 infected subjects: results of a controlled, randomized trial (probe). J Acquir Immune Defic Syndr 2016; 72:46–51. [DOI] [PubMed] [Google Scholar]
- 36. Rossetti B, Montagnani F, De Luca A. Current and emerging two-drug approaches for HIV-1 therapy in ART-naïve and ART-experienced, virologically suppressed patients. Expert Opin Pharmacother 2018; 19:713–38. [DOI] [PubMed] [Google Scholar]
- 37. Boswell R, Foisy MM, Hughes CA. Dolutegravir dual therapy as maintenance treatment in HIV-infected patients: a review. Ann Pharmacother 2018; 52:681–9. [DOI] [PubMed] [Google Scholar]
- 38. Baril JG, Angel JB, Gill MJ, et al. Dual therapy treatment strategies for the management of patients infected with HIV: a systematic review of current evidence in ARV-naive or ARV-experienced, virologically suppressed patients. PLoS One 2016; 11:e0148231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nyaku AN, Zheng L, Gulick RM, et al. ; ACTG A5353 Study Team . Dolutegravir plus lamivudine for initial treatment of HIV-1-infected participants with HIV-1 RNA <500 000 copies/mL: week 48 outcomes from ACTG 5353. J Antimicrob Chemother 2019; 74:1376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Taiwo BO, Marconi VC, Berzins B, et al. Dolutegravir plus lamivudine maintains human immunodeficiency virus-1 suppression through week 48 in a pilot randomized trial. Clin Infect Dis 2018; 66:1794–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.