Abstract
Pseudomonas putida HS12, which is able to grow on nitrobenzene, was found to carry two plasmids, pNB1 and pNB2. The activity assay experiments of wild-type HS12(pNB1 and pNB2), a spontaneous mutant HS121(pNB2), and a cured derivative HS124(pNB1) demonstrated that the catabolic genes coding for the nitrobenzene-degrading enzymes, designated nbz, are located on two plasmids, pNB1 and pNB2. The genes nbzA, nbzC, nbzD, and nbzE, encoding nitrobenzene nitroreductase, 2-aminophenol 1,6-dioxygenase, 2-aminomuconic 6-semialdehyde dehydrogenase, and 2-aminomuconate deaminase, respectively, are located on pNB1 (59.1 kb). Meanwhile, the nbzB gene encoding hydroxylaminobenzene mutase, a second-step enzyme in the nitrobenzene catabolic pathway, was found in pNB2 (43.8 kb). Physical mapping, cloning, and functional analysis of the two plasmids and their subclones in Escherichia coli strains revealed in more detail the genetic organization of the catabolic plasmids pNB1 and pNB2. The genes nbzA and nbzB are located on the 1.1-kb SmaI-SnaBI fragment of pNB1 and the 1.0-kb SspI-SphI fragment of pNB2, respectively, and their expressions were not tightly regulated. On the other hand, the genes nbzC, nbzD, and nbzE, involved in the ring cleavage pathway of 2-aminophenol, are localized on the 6.6-kb SnaBI-SmaI fragment of pNB1 and clustered in the order nbzC-nbzD-nbzE as an operon. The nbzCDE genes, which are transcribed in the opposite direction of the nbzA gene, are coordinately regulated by both nitrobenzene and a positive transcriptional regulator that seems to be encoded on pNB2.
Release of massive amounts of nitroaromatic compounds into the environment has enabled a number of microorganisms to evolve the ability to mineralize these xenobiotic compounds. Nitrobenzene (NB), one of the recalcitrant nitroaromatic compounds, has been reported to be mineralized by microorganisms through oxidative (25) and partial reductive pathways (11, 24). In addition, construction of a hybrid pathway for the degradation of NB was attempted with Pseudomonas putida TB103 (13). Recently, Pseudomonas pseudoalcaligenes JS45 was reported to have a partial reductive pathway, and several enzymes involved in NB catabolism were purified and characterized (10, 12, 19, 37). We previously isolated P. putida HS12, which is able to utilize NB, and showed that P. putida HS12 possesses a partial reductive pathway (29).
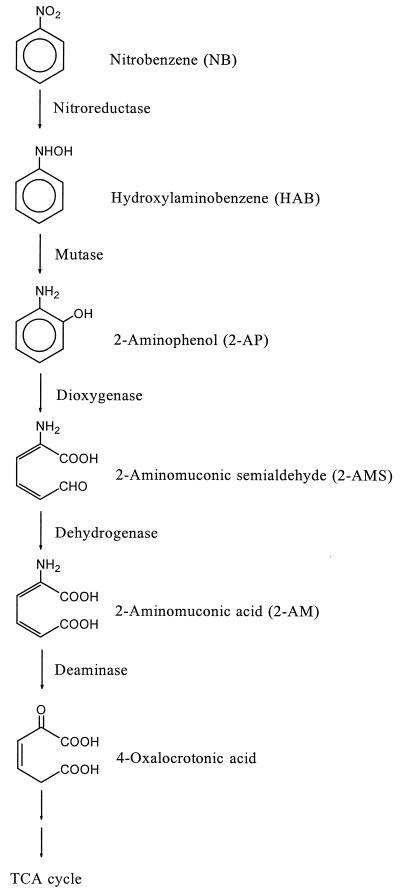
In the partial reductive pathway (Fig. 1), NB is first reduced to hydroxylaminobenzene (HAB) by the NB nitroreductase and then HAB is rearranged to 2-aminophenol (2-AP) by HAB mutase. 2-AP undergoes a meta ring cleavage to 2-aminomuconic 6-semialdehyde (2-AMS) by 2-AP 1,6-dioxygenase. 2-AMS dehydrogenase converts the resulting 2-AMS to 2-aminomuconate (2-AM), which is, by the action of 2-AM deaminase, finally deaminated to yield 4-oxalocrotonate, a well-known intermediate in the catechol meta cleavage pathway. Some of the NB catabolic enzymes, such as NB nitroreductase (37), 2-AP 1,6-dioxigenase (19), 2-AMS dehydrogenase (12), and 2-AM deaminase (10) in P. pseudoalcaligenes JS45, were purified and characterized, and DNA sequences of the genes coding for HAB mutase (GenBank no. AF028594), 2-AP 1,6-dioxygenase (GenBank accession no. U93363), and 2-AMS dehydrogenase (12) in P. pseudoalcaligenes JS45 were also released.
FIG. 1.
A partial reductive pathway for the degradation of nitrobenzene.
Many catabolic pathways involved in the degradation of aromatic compounds have been found to be encoded on catabolic plasmids, and biochemical and genetic studies of these catabolic plasmids have been extensively investigated. In the well-studied catabolic TOL plasmid, the genes encoding the enzymes for the oxidation of toluene via the meta cleavage of catechol are organized into two operons (7, 8, 41). The upper pathway operon is responsible for the conversion of toluene to benzoate, and the lower pathway operon is responsible for further conversion of benzoate to pyruvate and acetaldehyde. NAH7, another well-known catabolic plasmid, also carries two operons encoding catabolic enzymes for the oxidation of naphthalene via salicylate (5, 34, 42).
Although the NB catabolic pathway and the corresponding catabolic enzymes have been studied extensively, little is known about the molecular basis of NB catabolism, such as regulation and genetic organization of the catabolic genes. In this study, we demonstrate that the genes coding for the NB catabolic enzymes in P. putida HS12 are located on two plasmids, pNB1 and pNB2. We also report details on the genetic organization of the catabolic genes through cloning and functional analysis of these two plasmids and their subclones expressing the catabolic enzymes.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Induced cells of P. putida HS12 were grown in nitrogen- and carbon-free minimal salts medium (NCMSM) supplemented with 1 mM NB at 30°C, and additional NB was added after depletion (29). For the culture of uninduced cells of P. putida HS12, HS121, and HS124, 20 mM succinate and 10 mM NH4Cl instead of NB were used as sources of carbon and nitrogen. When necessary, 1 mM NB or 2 mM 2-AP was added to investigate the induction of catabolic enzymes. All of the E. coli strains were cultured in Luria-Bertani (LB) medium at 37°C. When necessary, ampicillin, chloramphenicol, and tetracycline were used at the final concentrations of 100, 50, and 20 μg/ml, respectively.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Descriptiona | Reference or source |
|---|---|---|
| Escherichia coli | ||
| JM109 | recA1 supE44 hsdR17 gyrA96 relA1 thi (lac-proAB) (F′ traD36 proAB+lacIqlac M15) | Promega |
| NFR502 | thr-1 ara-14 leuB6 DE(gpt-proA)62 lacY1 tsx-33 nfsB-2 qsr′-0 glnV44 galK2 LAM-nfsA1 Rac-0 hisG4 rfbD1 rpsL31(Smr) kdgK51 xylA5 mtl-1 argE3 thi-1 | 21; CGSC6838 |
| Pseudomonas putida | ||
| HS12 | NB+, wild-type, pNB1 and pNB2 carrier | |
| HS121 | NB−, a spontaneous mutant of HS12, pNB2 carrier | This study |
| HS124 | NB−, a cured mutant of HS12, pNB1 carrier | This study |
| Plasmids | ||
| pNB1 | 59.1-kb NB catabolic plasmid of P. putida HS12 | This study |
| pNB2 | 43.8-kb NB catabolic plasmid of P. putida HS12 | This study |
| pACYC184 | Cmr Tcr | New England Biolabs |
| pBR328 | Apr Cmr Tcr | New England Biolabs |
| pBluescript SK(+) | Apr | Stratagene |
| pBluescript KS(+) | Apr | Stratagene |
| pHS289 | pBluescript KS carrying a 1.1-kb SmaI-SnaBI fragment from pNB1 | This study |
| pHS291 | pBR328 carrying a 6.6-kb SnaBI-SmaI fragment from pNB1 | This study |
| pHS2910 | pBR328 carrying a 2.3-kb SnaBI-AsnI fragment from pNB1 | This study |
| pHS2911 | pBluescript SK carrying a 4.8-kb SnaBI-PstI fragment from pNB1 | This study |
| pHS2912 | pBluescript KS carrying a 2.4-kb EcoRI-PstI fragment from pNB1 | This study |
| pHS2916 | pBluescript SK carrying a 1.8-kb PstI fragment from pNB1 | This study |
| pHS35 | pACYC184 carrying a 9.0-kb EcoRI fragment with nbzB from pNB2 | This study |
| pHS3951 | pBR328 carrying a 1.1-kb SalI-SphI fragment from pNB2 | This study |
| pHS3952 | pBR328 carrying a 1.0-kb SspI-SphI fragment from pNB2 | This study |
| pHS941 | pBR328 carrying a 2.5-kb SspI-EcoRV fragment with nbzB from pNB2 | This study |
Abbreviations: NB+, growth on NB; NB−, no growth on NB; Apr, ampicillin resistance; Cmr, chloramphenicol resistance; Tcr, tetracycline resistance.
DNA manipulation.
DNA manipulations such as isolation, subcloning, digestion, ligation, and transformation were performed in accordance with the standard procedures of Sambrook et al. (33). Plasmids of P. putida strains were isolated by the method of O'Sullivan and Klaenhammer with a slight modification (28).
Cloning of the nbz genes from pNB1 and pNB2.
DNA fragments from pNB1 and pNB2 were cloned into pACYC184, pBR328, pBluescript SK, and pBluescript KS. Expression levels of the catabolic genes were confirmed by using the assay of the corresponding enzyme activity. For the screening of the nbzA gene encoding NB nitroreductase, DNA fragments of pNB1 were cloned into pHS941 (Table 1), pBluescript SK, and pBluescript KS and then transformed into E. coli NFR502 coexpressing the nbzB gene for HAB mutase. 2-AP produced from NB via HAB was determined by using high-performance liquid chromatography (HPLC) as described previously (29). Conversion of HAB to 2-AP by HAB mutase was monitored by using a similar procedure except that an acetonitrile-phosphate buffer (25 mM, pH 7.5) mixture (40:60, vol/vol) was used as the mobile phase.
Plasmid curing.
P. putida HS12 was grown on liquid LB medium at 37°C for 2 days, and the culture medium was diluted and plated onto an NB-supplemented minimal agar plate. Colonies showing poor growth on the selective plates were picked and examined for the loss of plasmids.
Enzyme assays.
Cultured cells were collected by centrifugation and washed with 50 mM potassium phosphate buffer (pH 7.2). The washed cells were suspended in the same buffer supplemented with 2 mM l-ascorbic acid, 1 mM dithiothreitol, and 0.1 mM phenylmethylsulfonyl fluoride and disrupted by sonication (29). The resulting suspension was centrifuged at 20,000 × g for 30 min at 4°C, and the pellet was discarded. The supernatant was used for enzyme assays. All of the enzyme assays were carried out at 30°C.
NB nitroreductase activity was determined by spectrophotometrically monitoring the decrease of NADPH at 340 nm according to a method described elsewhere (37). The reaction mixture contained 50 mM phosphate buffer (pH 7.2), 0.2 mM NB, and 0.5 mM NADPH. The enzyme reaction was initiated by adding a predetermined amount of crude extracts. When the reaction mixture was analyzed by HPLC, the amount of NB that disappeared was about half that of NADPH oxidized as reported before (37), and a quantitative analysis of HAB was not possible because it was highly reactive and decomposed rapidly under the reaction conditions. Since all the subclones carrying NB nitroreductase were constructed in E. coli NFR502 coexpressing HAB mutase, HAB produced from NB by NB nitroreductase was converted immediately into 2-AP instead of into aniline spontaneously. Thus, only NB nitroreductase was responsible for the consumption of NADPH under this condition. HAB mutase was assayed by analyzing the formation of 2-AP from HAB by HPLC. The reaction mixture was the same as that of NB nitroreductase except that only 0.2 mM HAB was added as a substrate. The reaction mixture was analyzed after the reaction was stopped by adding acetonitrile. For analysis of the 2-AP 1,6-dioxygenase activity, the disappearance of 2-AP was monitored spectrophotometrically as reported elsewhere (19). 2-AMS dehydrogenase was assayed by measuring the decrease in the concentration of 2-hydroxymuconic 6-semialdehyde (2-HMS) at 375 nm. 2-HMS was used as a substrate instead of 2-AMS, and the reaction mixture was composed of 50 mM phosphate buffer (pH 7.2), 0.02 mM 2-HMS, and 0.2 mM NAD+ (12). The activity of 2-AM deaminase was determined by tracing the decrease in absorbance at 326 nm due to the disappearance of 2-AM (10). The composition of the reaction mixture was the same as that described above except that 0.1 mM 2-AM was added as a substrate.
Protein concentrations were determined by the method of Bradford (1) with bovine serum albumin as the standard.
Chemicals.
Hydroxylaminobenzene (HAB) was chemically synthesized from NB through zinc dust reduction by the method of Patrick et al. (31). 2-HMS was prepared enzymatically from catechol by using catechol 2,3-dioxygenase. E. coli JM109 harboring pHS604, a derivative of pKK223-3 carrying a 2.1-kb SmaI-HindIII fragment with the todE gene from pDTG603 (44), was used for the conversion of catechol to 2-HMS. The 2-HMS produced was purified from the reaction mixture as described elsewhere (26). 2-AM was also synthesized enzymatically from 2-AP by using JM109(pHS2910) carrying the nbzC and nbzD genes and immediately used for the assay of 2-AM deaminase.
RESULTS
Identification of NB catabolic plasmids pNB1 and pNB2.
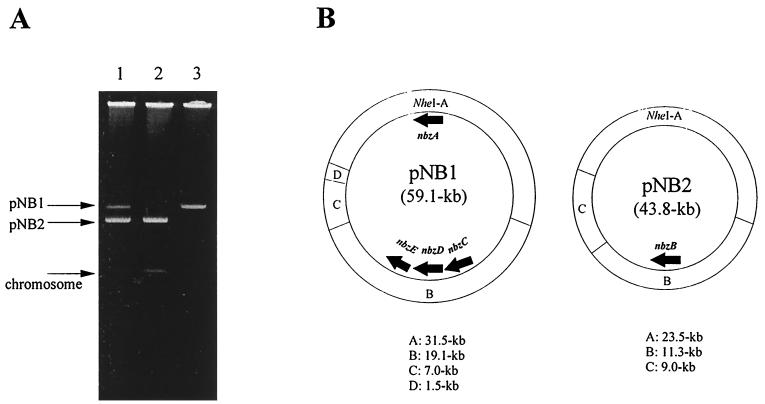
P. putida HS12 previously isolated from soil was able to grow on NB as the sole source of carbon, nitrogen, and energy (29). During serial transfer on nonselective agar plates (LB agar), however, spontaneous mutants which had lost the ability to grow on NB appeared. To determine whether the catabolism of NB was correlated with a plasmid(s), we examined the presence of a plasmid(s) in HS12. As shown in Fig. 2A, HS12 was found to harbor two plasmids. The larger one was designated pNB1, and the smaller one was designated pNB2. All of the spontaneous mutants were observed to lose the larger plasmid (pNB1) and carry only the smaller plasmid (pNB2). One of these mutants was selected and designated P. putida HS121. On the other hand, plasmid-cured P. putida strains were obtained by incubating the parent strain, P. putida HS12, at an elevated temperature. Of the cured strains, P. putida HS124 carrying only the larger plasmid pNB1 was selected, and this strain was also unable to grow on NB. These results indicate that at least some of the genes responsible for the degradation of NB are carried on both pNB1 and pNB2. The physical maps of pNB1 and pNB2 were constructed by using the restriction enzyme NheI (Fig. 2B). From the size of each NheI fragment cloned into pBR328, the sizes of pNB1 and pNB2 were estimated to be about 59.1 and 43.8 kb, respectively.
FIG. 2.
NB catabolic plasmids pNB1 and pNB2 in P. putida HS12 and its derivatives. (A) Plasmid profiles of HS12(pNB1 and pNB2) (lane 1), HS121(pNB2) (lane 2), and HS124(pNB1) (lane 3); (B) physical map and genetic organization of NB catabolic genes in pNB1 and pNB2.
NB catabolic genes in pNB1 and pNB2.
Since two plasmids, pNB1 and pNB2, were essential for the degradation of NB, the activities of the catabolic enzymes in HS12(pNB1 and pNB2), HS124(pNB1), and HS121(pNB2) grown under various conditions were determined (Table 2). While wild-type HS12 possessed all the catabolic enzymes for NB, HS124 was found to have the genes for NbzA (NB nitroreductase), NbzC (2-AP 1,6-dioxygenase), NbzD (2-AMS dehydrogenase), and NbzE (2-AM deaminase). HS121 was analyzed to have only the gene encoding NbzB (HAB mutase).
TABLE 2.
Enzyme activities in various strains
| Strain (plasmid) | Growth conditionsb | Enzyme sp act (nmol/min/mg of protein)a
|
||||
|---|---|---|---|---|---|---|
| NbzA (nitroreductase) | NbzB (mutase) | NbzC (dioxygenase) | NbzD (dehydrogenase) | NbzE (deaminase) | ||
| P. putida | ||||||
| HS12(pNB1,pNB2) | NB | 267 | 1,470 | 711 | 46 | 217 |
| HS12(pNB1,pNB2) | Suc | 204 | 1,530 | 12 | 2 | 8 |
| HS12(pNB1,pNB2) | Suc + AP | 211 | 1,490 | 10 | 4 | 21 |
| HS124(pNB1) | Suc | 193 | —c | 18 | 5 | 11 |
| HS124(pNB1) | Suc + NB | 215 | — | 13 | 2 | 19 |
| HS124(pNB1) | Suc + AP | 219 | — | 17 | 8 | 23 |
| HS121(pNB2) | Suc | — | 1,270 | — | — | — |
| HS121(pNB2) | Suc + NB | — | 1,302 | — | — | — |
| HS121(pNB2) | Suc + AP | — | 1,127 | — | — | — |
| E. colid | ||||||
| NFR502(pHS289) | 213 | — | — | — | — | |
| JM109(pHS3951) | — | 420 | — | — | — | |
| JM109(pHS3952) | — | 507 | — | — | — | |
| JM109(pHS291) | — | — | 175 | 74 | 164 | |
| JM109(pHS291R) | — | — | 42 | 14 | 35 | |
| JM109(pHS2910) | — | — | 154 | — | — | |
| JM109(pHS2911) | — | — | 333 | 208 | — | |
| JM109(pHS2912) | — | — | — | 362 | — | |
| JM109(pHS2916) | — | — | — | — | 725 | |
Enzyme assays were performed with cell extracts prepared as described in Materials and Methods, and each enzyme activity is a mean value of three independent measurements.
Cells were grown in NCMSM supplemented with 3 mM nitrobenzene for both carbon and nitrogen sources (NB) or 20 mM succinate and 10 mM NH4Cl for carbon and nitrogen sources (Suc), and 1 mM NB (Suc + NB) or 2 mM 2-AP (Suc + AP) were added additionally. See the text for more details.
—, not detected.
All E. coli plasmids were uninduced.
For some insight into the induction mechanism, P. putida HS12, HS121, and HS124 were incubated with NB and 2-AP to determine which one could serve as a growth substrate or an inducer. HAB was not suitable for a growth substrate and an inducer because of its toxic and mutagenic properties. Severe cell lysis was observed even in the presence of an additional carbon source (data not shown). In the case of NB, only P. putida HS12 could utilize it as a growth substrate and an inducer for the NB catabolic enzymes (Table 2). P. putida HS121 and HS124 were unable to grow on NB, and lysis of P. putida HS124 was observed, probably due to HAB accumulated from NB by NB nitroreductase. In contrast to NB, 2-AP was not utilized as a growth substrate for any of these strains. When P. putida HS12, HS121, and HS124 were incubated with 2 mM 2-AP in NCMSM containing 20 mM succinate and 10 mM NH4Cl, no significant increase in the activities of catabolic enzymes was found (Table 2), which suggests that 2-AP was not an inducer for the NB catabolic pathway. Therefore, it was concluded that only NB could serve as both a growth substrate and an inducer for the degradation of NB.
NbzA, the first catabolic enzyme for NB, was expressed in strains HS12 and HS124. When HS12 was induced by NB, the level of NbzA in crude extracts increased slightly, compared to that of uninduced HS12 (Table 2). Succinate-grown HS124 cells showed NbzA activity similar to those of the induced and uninduced wild-type HS12 cells. From the observation that there was no remarkable difference in the NbzA activities among induced HS12, uninduced HS12, and succinate-grown HS124, the nbzA gene was considered to be expressed constitutively. As in the case of NbzA, background levels of NbzB activities of HS12 and HS121 were relatively high regardless of NB induction, which suggests that the expression of NbzB is also constitutive. On the other hand, activities of NbzC, NbzD, and NbzE in wild-type HS12 increased from 23- to 60-fold by the addition of NB (Table 2). These results strongly imply that the genes encoding NbzC, NbzD, and NbzE might be organized as an operon. The activities of NbzC, NbzD, and NbzE were also found in HS124, but, unlike that of wild-type HS12, their expression was not induced by NB, showing a level comparable to that of uninduced HS12 (Table 2). Based on these results, it seems that plasmid pNB2 is essential for the induction of the genes nbzC, nbzD, and nbzE carried on pNB1 in HS12. The activities of 2-AMS dehydrogenase (NbzD) in P. putida strains were relatively low compared to those of other catabolic enzymes, and this might be due to the fact that the activity of NbzD was determined by using 2-HMS instead of the physiological substrate 2-AMS.
From the results described above, it was concluded that the genes coding for NbzA, NbzC, NbzD, and NbzE are located on pNB1 and the gene for NbzB is carried on pNB2. To investigate the genetic organization of the catabolic plasmids pNB1 and pNB2 in more detail, DNA fragments from each plasmid were cloned, and expression of the corresponding enzymes was examined.
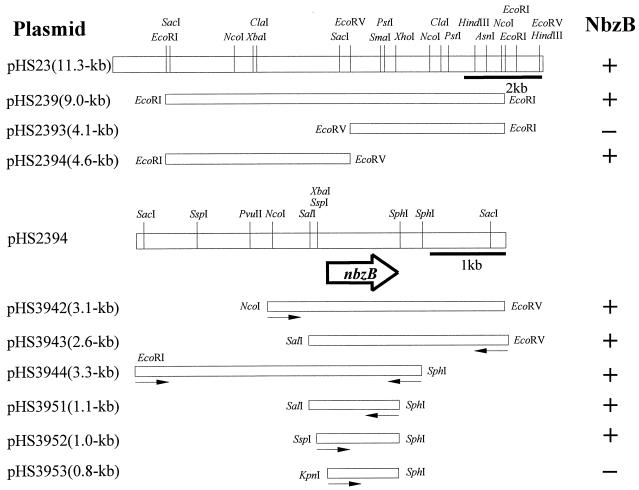
Cloning and location of the nbzB gene in pNB2.
For the identification of the region containing the gene for NbzB (HAB mutase), three NheI fragments of pNB2 isolated from HS121 were cloned. As mentioned before, a colony expressing the nbzB gene was screened by analyzing the production of 2-AP from HAB. E. coli JM109(pHS23) carrying a 11.3-kb NheI fragment of pNB2 was able to produce 2-AP from HAB (Fig. 3). The physical map of pHS23 was constructed by using restriction enzymes, and several subclones were generated by cloning into pBR328 to locate a more precise region of the nbzB gene. Of such clones, JM109(pHS2394) containing a 4.6-kb EcoRI-EcoRV fragment showed the activity of NbzB. The restriction map of pHS2394 was constructed, and additional subcloning was conducted. JM109(pHS3951) harboring a 1.1-kb SalI-SphI fragment was able to transform HAB to 2-AP. JM109(pHS3952) with a 1.0-kb SspI-SphI fragment, in which the insert was transcribed in the reverse direction of pHS3951, also exhibited a relatively high mutase activity similar to that of JM109 possessing pHS3951 (Table 2). This implies that the promoter of the nbzB gene originating from P. putida HS12 is expressed well in E. coli strains. JM109(pHS3953) with an 0.8-kb KpnI-SphI fragment exhibited no HAB mutase activity, and the direction of transcription of the nbzB gene by its own promoter was determined based on a partial sequence of the 1.0-kb SspI-SphI fragment (data not shown). These results indicate that the nbzB gene encoding the second-step enzyme in the NB catabolic pathway resides on the 1.0-kb SspI-SphI fragment of pNB2.
FIG. 3.
Cloning of DNA fragments of pNB2 in E. coli and localization of the nbzB gene. The ability of each clone to transform HAB to 2-AP is shown on the right. Arrows indicate the direction of transcription of the promoter on the E. coli vector.
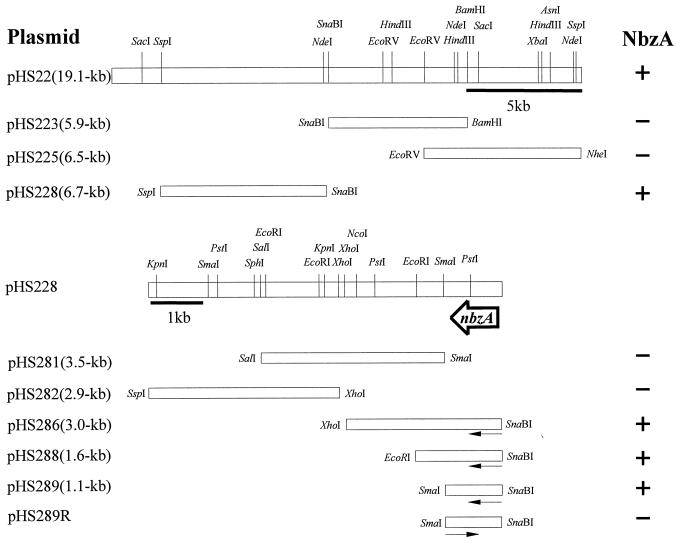
Cloning and location of the nbzA gene in pNB1.
pNB1 obtained from HS124 was digested with NheI and ligated into pHS941, a derivative of pBR328 containing the nbzB gene of pNB2 (Table 1). The resulting plasmids were transformed into E. coli NFR502, which is known as a nitroreductase-deficient mutant (21), and colonies expressing NB nitroreductase (NbzA) were screened by analyzing the production of 2-AP from NB via HAB. NFR502(pHS22) harboring a 19.1-kb NheI fragment exhibited a low level of NbzA activity (Fig. 4). Of several subclones from pHS22, NFR502(pHS228) containing a 6.7-kb SspI-SnaBI fragment was found to produce a significant amount of 2-AP from NB. Additional subclones were constructed by cloning specific fragments of pHS228 into pBluescript SK and pBluescript KS. To detect the activity of NbzA from subclones, NFR502 harboring pHS35, a derivative of pACYC184 carrying a 9.0-kb EcoRI fragment with the nbzB gene of pNB2, was used as a transforming host. The smallest fragment showing the activity of NbzA was found to be a 1.1-kb SmaI-SnaBI fragment in pHS289 (Fig. 4; Table 2), indicating that the nbzA gene is located on the 1.1-kb SmaI-SnaBI fragment of pNB1. pHS289R, a clone constructed in the opposite direction of pHS289, showed no nitroreductase activity, which suggests that, in contrast to the nbzB gene, the gene for NbzA is expressed not by its own promoter but by the vector promoter in E. coli strains.
FIG. 4.
Cloning of DNA fragments of pNB1 in E. coli and localization of the nbzA gene. The ability of each clone to transform NB to 2-AP via HAB is shown on the right. Arrows indicate the direction of transcription of the promoter on the E. coli vector.
Cloning and location of the genes nbzC, nbzD, and nbzE encoding aminophenol catabolic enzymes.
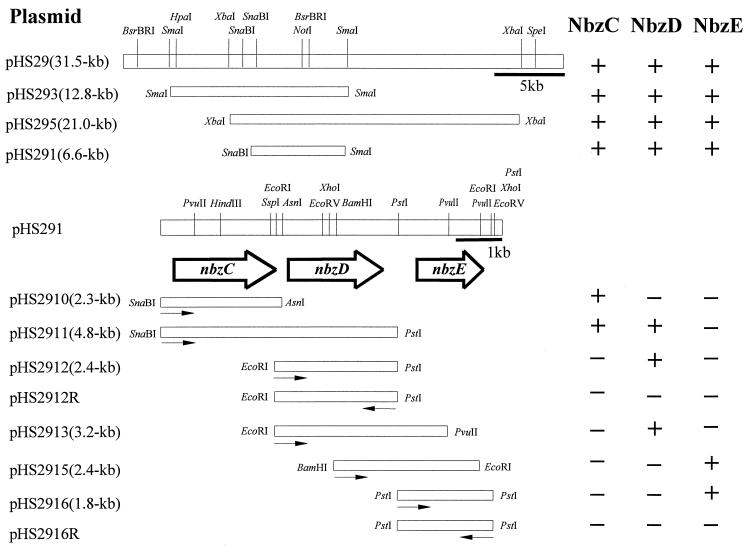
To locate the genes involved in the catabolism of 2-AP, NheI fragments of pNB1 isolated from HS124 were cloned into pBR328, and the disappearance of 2-AP was checked by HPLC. As shown in Fig. 5, plasmid pHS29 carrying a 31.5-kb fragment was found to express NbzC (2-AP 1,6-dioxygenase), NbzD (2-AMS dehydrogenase), and NbzE (2-AM deaminase). To locate the genes nbzC, nbzD, and nbzE more precisely, various fragments of pHS29 were subcloned, and each subclone was tested for whether it carried all the genes for 2-AP catabolism. As a result, E. coli JM109(pHS291) harboring a 6.6-kb SnaBI-SmaI fragment was found to express the genes nbzC, nbzD, and nbzE (Fig. 5; Table 2). JM109(pHS291R) containing the same fragment in reverse orientation also showed considerable activities of NbzC, NbzD, and NbzE, which suggests that the genes nbzC, nbzD, and nbzE are under the control of the promoter from P. putida HS12 (Table 2). For more precise information on the size and organization of the aminophenol catabolic genes, a 6.6-kb fragment of pHS291 was mapped and subcloned into pBR328, pBluescript SK, and pBluescript KS, and activities of the catabolic enzymes were determined as shown in Fig. 5. The activity of NbzC was detected in JM109 containing pHS2910 and pHS2911, and NbzD activity was observed in JM109 with pHS2911, pHS2912, and pHS2913. NbzE, the last catabolic enzyme examined, was expressed in JM109 harboring pHS2915 and pHS2916. The specific activities of NbzC, NbzD, and NbzE determined from the crude extracts of E. coli strains are shown in Table 2. From these results, it was determined that the genes nbzC, nbzD, and nbzE are located on the 2.3-kb SnaBI-AsnI, the 2.4-kb EcoRI-PstI, and the 1.8-kb PstI fragments, respectively. When the same fragments as those in pHS2912 and pHS2916 were cloned in the opposite direction (pHS2912R and pHS2916R), the activities of NbzD and NbzE were not detected. These results indicate that the aminophenol catabolic genes are organized in the order of nbzC, nbzD, and nbzE in pNB1.
FIG. 5.
Cloning of DNA fragments of pNB1 in E. coli and genetic organization of the genes nbzC, nbzD, and nbzD. The activities of NbzC, NbzD, and NbzE of each clone are shown on the right. Arrows indicate the direction of transcription of the promoter on the E. coli vector.
DISCUSSION
In this study, we have demonstrated the genetic organization of the NB catabolic pathway of P. putida HS12 by cloning and functional analysis of the catabolic genes. All the catabolic genes were found to be located on two plasmids, pNB1 and pNB2.
The smaller plasmid, pNB2, carried only the nbzB gene for HAB mutase involved in the conversion of HAB to 2-AP (Fig. 2B). Although the previously reported 3-hydroxylaminophenol mutase (36) with a similar catalytic property was rather large (62-kDa polypeptide), the size of the 1.0-kb SspI-SphI fragment showing NbzB activity was highly consistent with that of the HAB mutase gene from P. pseudoalcaligenes JS45. The level of NbzB activity was relatively high in NB-grown P. putida HS12 compared to that of other catabolic enzymes (Table 2). The high level of NbzB activity seems to be beneficial to HS12 in mineralization of NB when the high toxicity of HAB produced from NB is considered. From the sequence analysis of the region adjacent to the nbzB gene (data not shown), this gene was found to be flanked by resolvase and transposase genes having a sequence similar to those in Tn5501 reported by Lauf et al. (17). This might be a clue to why the nbzB gene is located on the smaller plasmid, pNB1, separate from other catabolic genes. Many catabolic transposons which carry some part or all of the genes for the catabolism of aromatic compounds have been reported thus far. Tn4371 in Alcaligenes eutrophus A5 (22), Tn4651 and Tn4653 in plasmid TOL (39), Tn4655 in plasmid NAH7 (40), Tn5271 in plasmid pBRC60 (23), and Tn5707 in pENH91 (27) have been revealed to carry the catabolic genes for chlorobiphenyls, toluene, naphthalene, 3-chlorobenzoate, and chlorocatechol, respectively. It is presumed that the nbzB gene was transposed from an unknown source into a region of plasmid pNB2 by a Tn5501-like transposon.
The NB catabolic genes except for nbzB were carried on the other plasmid, pNB1, but all the genes were not organized into a single operon. The nbzA gene for NB nitroreductase was found to be separated by 20.1 kb from the genes nbzC, nbzD, and nbzE encoding the aminophenol catabolic enzymes, and the transcription orientation of nbzA was opposite to that of the aminophenol catabolic genes (Fig. 2B). The expression of NbzA, the first enzyme for the degradation of NB, was not strongly induced by NB (Table 2). In other words, the expression level of NbzA remained unexpectedly high regardless of the presence of NB, suggesting that the nbzA gene is constitutively expressed in P. putida HS12.
As shown in Fig. 5, the genes nbzC, nbzD, and nbzE were found as a cluster and were transcribed in the same direction, and their expression was highly induced by NB as a whole in P. putida HS12 (Table 2). While subclones (JM109 with pHS291 and pHS291R) containing all the catabolic genes for 2-AP were highly expressed by its own promoter, subclones (JM109 with pHS2912R and pHS2916R) with a deletion of an upstream region including the nbzC gene led to no expression of the rest of the genes (the genes nbzD and nbzE) in the operon. These results provide strong evidence that the genes nbzC, nbzD, and nbzE are carried on a single coordinately regulated operon.
When uninduced P. putida HS12 was incubated with NB, the conversion rate of NB to 2-AP decreased slightly, but the degradation rate of 2-AP decreased significantly, as shown in our previous work (29). The increased expression levels of NbzCDE were observed only when HS12 was incubated with NB (Table 2). This strongly implies that pNB2 carries an unknown regulatory gene which is essential for the induction of the nbzCDE operon located on pNB1 in HS12. The low expression level of the nbzCDE genes in HS121 might be due to the absence of a regulatory gene of pNB2. Based on our experimental results, it is clear that the aminophenol catabolic operon is induced by NB and that pNB2 possesses a transcription-positive regulator which participates in the expression of the aminophenol catabolic operon, the nbzCDE genes. A number of transcription regulators involved in the catabolism of aromatic compounds, such as BenM (3), CatR (2), NahR (35), PcaQ (30), PhnS (18), TcbR (20), TdnR (6), TsaR (14), and TfdR (43), have been reported to belong to the LysR family. Further genetic analysis is needed to establish whether the positive transcriptional regulator of aminophenol catabolic operon is related to the LysR-type activator.
Most of the catabolic genes responsible for the degradation of xenobiotics have been reported to be organized into functional units (operons) in a single plasmid, such as the upper and lower catabolic operons in the TOL (7, 8) and NAH7 plasmids (5, 42). On the other hand, the NB catabolic genes were revealed to be carried on two different plasmids, pNB1 and pNB2, similar to the carbaryl catabolic plasmids (9), and they were organized into two separate genes (nbzA and nbzB) and one operon (nbzCDE) as depicted in Fig. 2B. The 2,4-d catabolic plasmid pJP4 was reported to have a similar genetic organization and carry three functional units, tfdA, tfdB, and tfdCDEF (4, 32, 38). However, the overall genetic regulation of the catabolic pathway is different. The tfdA gene and tfdCDEF operon were positively regulated by TfdR (15), and the tdfB gene was both positively and negatively regulated by TfdS (16) in Ralstonia eutropha JMP134. In the case of the NB catabolic pathway in P. putida HS12, only the nbzCDE operon was observed to be positively regulated.
From the results indicating that the catabolic genes for the degradation of NB are carried separately on two plasmids and that these functional units (the nbzA, nbzB, and nbzCDE genes) are regulated differently, it seems that P. putida HS12 has acquired these genes from different sources in the course of adaptation to the NB-contaminated environment through the mechanism of horizontal gene transfer, recombination, and transposition. A comparative study of the aminophenol catabolic operon with other well-known ring cleavage operons at the molecular level is expected to provide more information on the genetic adaptation and evolution process of microorganisms toward xenobiotics.
REFERENCES
- 1.Bradford M M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 2.Chugani S A, Parsek M R, Chakrabarty A M. Transcriptional repression mediated by LysR-type regulator CatR bound at multiple binding sites. J Bacteriol. 1998;180:2367–2372. doi: 10.1128/jb.180.9.2367-2372.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collier L S, Gaines G L, Neidle E L. Regulation of benzoate degradation in Acinetobacter sp. strain ADP1 by BenM, a LysR-type transcription activator. J Bacteriol. 1998;180:2493–2501. doi: 10.1128/jb.180.9.2493-2501.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Don R H, Pemberton J M. Genetic and physical map of the 2,4-dichlorophenoxyacetic acid-degradative plasmid pJP4. J Bacteriol. 1985;161:466–468. doi: 10.1128/jb.161.1.466-468.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eaton R W, Chapman P J. Bacterial metabolism of naphthalene: construction and use of recombinant bacteria to study ring cleavage of 1,2-dihydroxynaphthalene and subsequent reactions. J Bacteriol. 1992;174:7452–7554. doi: 10.1128/jb.174.23.7542-7554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukumori F, Saint C P. Nucleotide sequences and regulational analysis of genes involved in conversion of aniline to catechol in Pseudomonas putida UCC22(pDTN1) J Bacteriol. 1997;179:399–408. doi: 10.1128/jb.179.2.399-408.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harayama S, Rekik M. The meta cleavage operon of TOL degradative plasmid pWW0 comprises 13 genes. Mol Gen Genet. 1990;221:113–120. doi: 10.1007/BF00280375. [DOI] [PubMed] [Google Scholar]
- 8.Harayama S, Rekik M, Wubbolts M, Rose K, Leppik R A, Timmis K N. Characterization of five genes in the upper-pathway operon of TOL plasmid pWW0 from Pseudomonas putida and identification of the gene products. J Bacteriol. 1989;171:5048–5055. doi: 10.1128/jb.171.9.5048-5055.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayatsu M, Hirano M, Nagata T. Involvement of two plasmids in the degradation of carbaryl by Arthrobacter sp. strain RC100. Appl Environ Microbiol. 1999;65:1015–1019. doi: 10.1128/aem.65.3.1015-1019.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He Z, Spain J C. A novel 2-aminomuconate deaminase in the nitrobenzene degradation pathway of Pseudomonas pseudoalcaligenes JS45. J Bacteriol. 1998;180:2502–2506. doi: 10.1128/jb.180.9.2502-2506.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He Z, Spain J C. Studies of the catabolic pathway of degradation of nitrobenzene by Pseudomonas pseudoalcaligenes JS45: removal of the amino group from 2-aminomuconic semialdehyde. Appl Environ Microbiol. 1997;63:4839–4843. doi: 10.1128/aem.63.12.4839-4843.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He Z, Davis J K, Spain J C. Purification, characterization, and sequence analysis of 2-aminomuconic 6-semialdehyde dehydrogenase from Pseudomonas pseudoalcaligenes JS45. J Bacteriol. 1998;180:4591–4595. doi: 10.1128/jb.180.17.4591-4595.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung K-H, Lee J-Y, Kim H-S. Biodegradation of nitrobenzene through a hybrid pathway in Pseudomonas putida. Biotechnol Bioeng. 1995;48:625–630. doi: 10.1002/bit.260480610. [DOI] [PubMed] [Google Scholar]
- 14.Junker F, Kiewitz R, Cook A M. Characterization of the p-toluenesulfonate operon tsaMBCD and tsaR in Comamonas testosteroni T-2. J Bacteriol. 1997;179:919–927. doi: 10.1128/jb.179.3.919-927.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaphammer B, Kukor J J, Olsen R H. Regulation of tfdCDEF by tfdR of 2,4-dichlorophenoxyacetic acid degradation plasmid pJP4. J Bacteriol. 1990;172:2280–2286. doi: 10.1128/jb.172.5.2280-2286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaphammer B, Olsen R H. Cloning and characterization of tfdS, the repressor-activator gene of tfdB, from the 2,4-dichorophenoxyacetic acid catabolic plasmid pJP4. J Bacteriol. 1990;172:5856–5862. doi: 10.1128/jb.172.10.5856-5862.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lauf U, Muller C, Herrmann H. The transposable elements resident on the plasmids of Pseudomonas putida strain H, Tn5501 and Tn5502, are cryptic transposons of the Tn3 family. Mol Gen Genet. 1998;259:674–678. doi: 10.1007/s004380050862. [DOI] [PubMed] [Google Scholar]
- 18.Laurie A D, Lloyd-Jones G. The phn genes of Burkholderia sp. strain RP007 constitute a divergent gene cluster for polycyclic aromatic hydrocarbon catabolism. J Bacteriol. 1999;181:531–540. doi: 10.1128/jb.181.2.531-540.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lendenmann U, Spain J C. 2-Aminophenol 1,6-dioxygenase: a novel aromatic ring cleavage enzyme purified from Pseudomonas pseudoalcaligenes JS45. J Bacteriol. 1996;178:6227–6232. doi: 10.1128/jb.178.21.6227-6232.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leveau J H J, de Vos W M, van der Meer J R. Analysis of the binding site of the LysR-type transcriptional activator TcbR on the tcbR and tcbC divergent promoter sequences. J Bacteriol. 1994;176:1850–1856. doi: 10.1128/jb.176.7.1850-1856.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCalla D R, Kaiser C, Green M H L. Genetics of nitrofurazone resistance in Escherichia coli. J Bacteriol. 1978;133:10–16. doi: 10.1128/jb.133.1.10-16.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merlin C, Springael D, Mergeay M, Toussaint A. Organization of the bph gene cluster of transposon Tn4371, encoding enzymes for the degradation of biphenyl and 4-chlorobiphenyl compounds. Mol Gen Genet. 1997;253:499–506. doi: 10.1007/s004380050349. [DOI] [PubMed] [Google Scholar]
- 23.Nakatsu C H, Wyndham R C. Cloning and expression of the transposable chlorobenzoate-3,4-dioxygenase genes of Alcaligenes sp. strain BR60. Appl Environ Microbiol. 1993;59:3625–3633. doi: 10.1128/aem.59.11.3625-3633.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishino S F, Spain J C. Degradation of nitrobenzene by a Pseudomonas pseudoalcaligenes. Appl Environ Microbiol. 1993;59:2520–2525. doi: 10.1128/aem.59.8.2520-2525.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishino S F, Spain J C. Oxidative pathway for the biodegradation of nitrobenzene by Comamonas sp. strain JS765. Appl Environ Microbiol. 1995;61:2308–2313. doi: 10.1128/aem.61.6.2308-2313.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishizuka Y, Ichiyama A, Hayaishi O. Metabolism of the benzene ring of tryptophan (mammals) Methods Enzymol. 1970;17A:463–491. [Google Scholar]
- 27.Ogawa N, Miyashita K. The chlorocatechol-catabolic transposon Tn5707 of Alcaligenes eutrophus NH9, carrying a gene cluster highly homologous to that in the 1,2,4-trichlorobenzene-degrading bacterium Pseudomonas sp. strain P51, confers the ability to grow on 3-chlorobenzoate. Appl Environ Microbiol. 1999;65:724–731. doi: 10.1128/aem.65.2.724-731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Sullivan D, Klaenhammer T R. Rapid mini-prep isolation of high-quality plasmid DNA from Lactococcus and Lactobacillus spp. Appl Environ Microbiol. 1993;59:2730–2733. doi: 10.1128/aem.59.8.2730-2733.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park H-S, Lim S-J, Chang Y-K, Livinston A G, Kim H-S. Degradation of chloronitrobenzenes by a coculture of Pseudomonas putida and Rhodococcus sp. Appl Environ Microbiol. 1999;65:1083–1091. doi: 10.1128/aem.65.3.1083-1091.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parke D. Characterization of PcaQ, a LysR-type transcriptional activator required for catabolism of phenolic compounds, from Agrobacterium tumefaciens. J Bacteriol. 1996;178:266–272. doi: 10.1128/jb.178.1.266-272.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patrick T B, Schield J A, Kirchner D G. Synthesis of fluoroaromatic amines. J Org Chem. 1974;39:1758–1761. [Google Scholar]
- 32.Perkins E J, Gordon M P, Caceres O, Lurquin P F. Organization and sequence analysis of the 2,4-dichlorophenol hydroxylase and dichlorocatechol oxidative operons of plasmid pJP4. J Bacteriol. 1990;172:2351–2359. doi: 10.1128/jb.172.5.2351-2359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 34.Schell M A. Cloning and expression in Escherichia coli of the naphthalene degradation gene from plasmid NAH7. J Bacteriol. 1983;153:822–829. doi: 10.1128/jb.153.2.822-829.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schell M A, Poster E F. Demonstration, characterization, and mutational analysis of NahR protein binding in nah and sal promoters. J Bacteriol. 1989;171:837–846. doi: 10.1128/jb.171.2.837-846.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schenzle A, Lenke H, Spain J C, Knackmuss H-J. 3-Hydroxylaminophenol mutase from Ralstonia eutropha JMP134 catalyzes a Bamberger rearrangement. J Bacteriol. 1999;181:1444–1450. doi: 10.1128/jb.181.5.1444-1450.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Somerville C C, Nishino S F, Spain J C. Purification and characterization of nitrobenzene nitroreductase from Pseudomonas pseudoalcaligenes JS45. J Bacteriol. 1995;177:3837–3842. doi: 10.1128/jb.177.13.3837-3842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Streber W R, Timmis K N, Zenk M H. Analysis, cloning and high-level expression of 2,4-dichlorophenoxyacetate monooxygenase gene tfdA of Alcaligenes eutrophus JMP134. J Bacteriol. 1987;169:2950–2955. doi: 10.1128/jb.169.7.2950-2955.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsuda M, Minegishi K I, Iino T. Toluene transposons Tn4651 and Tn4653 are class II transposons. J Bacteriol. 1989;171:1386–1393. doi: 10.1128/jb.171.3.1386-1393.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsuda M, Iino T. Naphthalene degrading genes on plasmid NAH7 are on a defective transposon. Mol Gen Genet. 1990;223:33–39. doi: 10.1007/BF00315794. [DOI] [PubMed] [Google Scholar]
- 41.Worsey M J, Williams P A. Metabolism of toluene and xylenes by Pseudomonas putida (arvilla) mt-2: evidence for a new function of the TOL plasmid. J Bacteriol. 1975;124:7–13. doi: 10.1128/jb.124.1.7-13.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yen K-M, Gunsalus I C. Plasmid gene organization: naphthalene/salicylate oxidation. Proc Natl Acad Sci USA. 1982;79:874–878. doi: 10.1073/pnas.79.3.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.You I-S, Ghosal D. Genetic and molecular analysis of a regulatory region of the herbicide 2,4-dichlorophenoxyacetate catabolic plasmid pJP4. Mol Microbiol. 1995;16:321–331. doi: 10.1111/j.1365-2958.1995.tb02304.x. [DOI] [PubMed] [Google Scholar]
- 44.Zylstra G J, Gibson D T. Toluene degradation of Pseudomonas putida F1. Nucleotide sequence of the todC1C2BADE genes and their expression in Escherichia coli. J Biol Chem. 1989;264:14940–14946. [PubMed] [Google Scholar]