Abstract
Objective:
Otitis media and associated otorrhea are frequent complications following tympanostomy tube insertion; the most common otologic procedure performed in children in the United States. Current treatments include the administration of antibiotic or antibiotic/anti-inflammatory combination drops to the affected ear. Several studies have demonstrated that using an antibiotic/anti-inflammatory combination product is more effective than the use of antibiotics alone. However, administration of any drops through the tympanostomy tube is very difficult in children, and patient compliance can be an issue. Our group has developed a novel combination drug/hydrogel formulation for the treatment of otitis media/otorrhea that releases both ciprofloxacin and dexamethasone over a 2–3 week period. This has the potential to offer significant advantages over current treatments in use in the clinic.
Methods:
The release of drugs from the combination hydrogel was validated in vitro over the desired time frame and the activity of the released drugs was monitored via assays to confirm retention of full activity throughout the dissolution period. The safety of the ciprofloxacin/dexamethasone hydrogel and its inactive excipients was evaluated through in vivo otic toxicity studies in guinea pigs, including hearing tests, gross microscopy, and cytocochleogram analysis.
Results:
Extended release of both drugs was demonstrated in vitro and antibiotic/anti-inflammatory activities were retained. The hydrogel components and its excipients did not cause adverse reactions in animals, demonstrating safety of the hydrogel combination in vivo.
Conclusion:
The studies presented lay the groundwork for extended release middle ear hydrogel formulations that are capable of safely releasing combinations of active pharmaceutical agents over a desired period of time. This would be more advantageous than therapeutics that are currently used in the clinic for the treatment of otitis media/otorrhea associated with tympanostomy tube insertion.
Keywords: Otitis media, otorrhea, ciprofloxacin, dexamethasone, hydrogel, tympanostomy tube
1. Introduction
1.1. Tympanostomy tube associated otitis media
Myringotomy with tympanostomy tube (TT) insertion is the most frequently performed otologic procedure in children in the United States. Approximately 2 million TT insertion procedures are performed each year for the treatment of otitis media [1]. TT insertion is a surgical procedure involving the insertion of a pressure-equalizing tube into the tympanic membrane. This surgical procedure helps eliminate fluid in the middle ear space that can cause otitis media and/or hearing loss. Insertion of a TT can ventilate the middle ear space and prevent further retraction of the eardrum under negative pressure. TT surgery is typically required for children with recurrent otitis media and/or otitis media with effusion that persist for more than 3 months despite medical therapy. [2].
Tympanostomy tube otorrhea (TTO) is one of the most common post-operative complications following TT insertion. Studies vary in reported incidence, ranging from 17% of TT ears [3] to up to 52% of children experiencing one or more episodes of TTO [4]. Early or persistent otorrhea is characterized by drainage from the middle ear space through the tube into the outer ear canal due to otitis media [5]. The most common pathogens associated with TTO are also associated with otitis media. These include Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, Staphylococcus aureus, and Pseudomonas aeruginosa, all of which can induce an inflammatory response in the middle ear. Children under 7 years of age are especially vulnerable to the increased risk of OM because of their undeveloped immune systems and limited performance of their Eustachian tubes. For children, otorrhea can cause foul odor, fever, and pain, which can have a negative impact on social development and quality of life. Therefore, the current treatment for post-operative TTO focuses on eradication of bacterial infection and reduction of the duration and severity of symptoms [2].
1.2. Limitations with Current Treatment Methods of TTO
Multiple antibiotics are used in the clinic to treat otorrhea. Ciprofloxacin (0.3%), has been shown to reduce TTO in trials when compared to no treatment [6]. The addition of anti-inflammatories in combination with antibiotics has been shown to have an even greater success in treatment in TTO. In one report, 90% of children reported being otorrhea-free at 7 days when receiving the combination of antibiotics and anti-inflammatories as compared to only 78% of patients who only received only topical antibiotic post-operatively [7]. Furthermore, several studies have documented more rapid resolution of chronic suppurative otitis media in a primate model [8] and in animals with tympanostomy tubes suffering from acute otitis media when using a combined antibiotic/anti-inflammatory therapy compared to antibiotics alone [9].
Although the treatment is ultimately effective, issues with patient compliance and unreliable drug delivery to the middle ear exist. The administration of topical drops is difficult and frequently traumatic for children. Parents are required to administer the drops daily. The drops must be pushed through the ear canal to drive the drops through the TT into the middle ear space. In addition, it is nearly impossible for a parent to ensure that the dose has reached the targeted area. Even when performed correctly, only a small fraction of the dose enters the middle ear space through the TT [7, 10]. To combat issues with patient compliance and unreliable drug delivery, extended release medications have been developed. An extended release ciprofloxacin poloxamer gel has been commercialized [11] to mitigate these issues. This gel is injected into the middle ear space at the time of TT placement and is designed to release the antibiotic for a 14 day period. Treatment with this ciprofloxacin poloxamer gel lead to a decrease in ottorhea and the decreased use of additional antibiotics over 15 days when compared to TT alone [11]. Despite the prolonged presence of the gel in the middle ear, the treatment did not impair hearing function, middle ear function, or tube patency over 29 days. However, the lack of steroids in its formulation make it less effective than ciprofloxacin/steroid drops and adoption of this treatment in the clinical setting has been slow.
1.3. Antibiotic/anti-inflammatory hydrogel combination (OR-404IT)
The study presented here aims to demonstrate the safety and efficacy of a novel extended release ciprofloxacin/dexamethasone combination hydrogel preparation (OR-404IT) for the prevention of otorrhea associated with tympanostomy tube insertion. OR-404IT is designed to release both ciprofloxacin and dexamethasone over a 2–3 week period. The release and activity of the drugs were monitored in vitro and in vivo safety studies were performed in a guinea pig model. The successful development of an extended release ciprofloxacin/dexamethasone hydrogel preparation for injection into the middle ear at the time of surgery would not only decrease infection and associated hearing loss, but also allow for increased patient compliance and efficacy as compared to currently available treatments.
2. Materials and Methods:
2.1. Manufacture of particles:
USP ciprofloxacin and dexamethasone were purchased from Xi’an Shunyi Bio-Chemical Technology Co., LTD. Both drugs were crystallized using standard crystallization procedures. After crystallization, ciprofloxacin and dexamethasone were separately milled and sieved into desired size ranges to produce drug particles. Ciprofloxacin particles were coated with a semi-porous layer of parylene C via vapor deposition. Dexamethasone particles were also coated with a semi-porous layer of parylene C. Coated drug particles were sieved to specific size ranges.
2.2. Hydrogel suspension:
A 1% hyaluronic acid (HA) solution was used as the hydrogel carrier to produce a suspension of coated drug particles.
2.3. In vitro release studies:
The release profiles of drugs from either extended release dexamethasone or ciprofloxacin formulations were tested as follows: 3 mg of coated ciprofloxacin or coated dexamethasone particles were incubated with shaking in water at 37°C. At specific time points, a 200 μl sample was removed and used to measure the absorbance of the solution at 272 nm (ciprofloxacin) or 242 nm (dexamethasone) using a UV-3100PC, UV/VIS Scanning Spectrophotometer (VWR, Radnor, PA) and then replaced back into the dissolution chamber. For the hydrogel, the release of individual drugs was monitored from the hydrogel suspension. Briefly, 0.6 g of extended release ciprofloxacin or 0.6 g of extended release dexamethasone was mixed with 900 μl of 1% HA. The suspension was then mixed by gentle pipetting. Following production of a stable suspension, 300 μl of suspension was added onto the membranes of three separate wells of a 6-well Snapwell plate. 4 ml of distilled water was added to bottom wells and the plates were incubated with shaking at 37°C. At specific time points, a 200 μL sample was removed and used to measure the absorbance of the solution at 272 nm (ciprofloxacin) or 242 nm (dexamethasone) and then replaced back into the dissolution well. For the combination hydrogel, the release of both drugs was monitored from the same hydrogel suspension. Dissolution preparation and sampling conditions were identical to the individual drug hydrogel suspensions. The absorbance of samples was monitored at both 272 nm (ciprofloxacin) and 242 nm (dexamethasone) and then replaced back into the dissolution well.
2.4. Ciprofloxacin Eluate Assay Activity:
Day 14 ciprofloxacin eluate from the in vitro dissolution studies was tested for anti-microbial activity using the Broth Microdilution Method following National Committee for Clinical Laboratory Standards (NCCLS) guidelines. Decreasing concentrations of the drug were added to Haemophilus Test Medium (HTM) and inoculated with H. influenzae. One positive growth control of HTM broth was used and one sterile control of HTM broth was not inoculated to ensure sterility of the H. influenzae broth. The multi-well plate was incubated at 37°C for 20–24 hours. Wells that displayed visible cloudiness were marked as being positive for bacterial growth.
2.5. Dexamethasone Eluate Assay Activity:
Day 14 dexamethasone eluate from the in vitro dissolution studies was added to RAW 264.7 murine macrophage cells prior to addition of 10 ng/ml of lipopolysaccharide (LPS). LPS activates inflammatory pathways in the RAW 264.7 cells, including increasing expression of interleukin-6 (IL-6). Active dexamethasone prevents the increase in IL-6 expression. β-actin expression was used as a load control in order to normalize expression levels of IL-6. Following addition of dexamethasone, cells were incubated with LPS for 4 hours. Quantitative PCR was performed on cDNA using a BIORAD CFX96 Real Time System with IL-6 and β-actin primer/probe combinations.
2.6. Animal studies:
Animal ototoxicity studies were conducted by the Contract Research Organization (CRO) MPI Research. The safety study was conducted in accordance with the U.S. Department of Agriculture’s Animal Welfare Act, the Public Health Service Policy on Humane Care and Use of Laboratory Animals, and the Guide for the Care and Use of Laboratory Animals. Animals were fasted on the day of surgery prior to administration of anesthesia. Prior to any procedure, the animals received a combination of buprenorphine (0.05 mg/kg) and dexmedetomidine (0.25 mg/kg). General anesthesia was maintained using isoflurane. While under general anesthesia, a single slow bolus intratympanic injection of 75 μL of test sample was administered into each ear in male Crl:HA (Albino Hartley) guinea pigs. Under visualization with an operating microscope, the injection was performed using a 27 g x 1 ½ Exel hypodermic disposable needle that pierced the intratympanic membrane to allow for injection of the gel into the middle ear. The same test sample was used in each animal. Terminal studies were performed after a 21-day recovery period. Two different dosages of OR-404IT were used: a High Dose (1.5 mg ciprofloxacin + 0.6 mg dexamethasone) and a Low Dose (0.5 mg ciprofloxacin + 0.2 mg dexamethasone), both designed to release the drugs over a two to three week period. Additionally, OR-404IT vehicle (excipients only, no active drugs) was tested. Lastly, a saline solution was used as a negative control and a 10% Neomycin injection was used as a positive control for ototoxicity. Details of the study design are listed in Table 1. Five groups of Crl:HA (Albino Hartley) guinea pigs (N=35 total) were used. Bilateral auditory brainstem response (ABR) values were evaluated on animals at pretest for baseline value at frequencies of 4, 10, and 20 kHz and ABR threshold shifts were measured by BioSigRZ v5.7. All animals were anesthetized and placed in a sound-attenuated electrically shielded closure. An MF-1 open field speaker was placed approximately 4 cm away from the tested ear while the opposite ear was plugged.
Table 1: Study Design.
Animal were injected bilaterally via the transtympanic route by slow bolus injection as a fixed dose once on Day 1 (75 μL each ear).
| GROUP | Treatment | Dose Level (mg/kg) | NUMBER OF ANIMALS | |||
|---|---|---|---|---|---|---|
| Number | ABR | Cytocochleogram | Otic Histopathology | |||
| M | M | M | M | |||
| 1 | Vehicle | 0 | 7 | 7 | 7 | 7 |
| 2 | Saline | - | 5 | 5 | 5 | 5 |
| 3 | OR-404IT | 0.5 mg ciprofloxacin + 0.2 mg dexamethasone | 8 | 8 | 8 | 8 |
| 4 | OR-404IT | 1.5 mg ciprofloxacin + 0.6 mg dexamethasone | 10 | 10 | 10 | 10 |
| 5 | OR-IT Control | 10% Neomycin | 5 | 5 | 5 | 5 |
Toxicity assessment was based on mortality, body weight, clinical observations, ABR evaluations, otoscopic examinations, anatomic pathology, and cytocochleogram analysis.
Following euthanasia, the cochleae of the animals were collected and processed for cytocochleogram analysis (left ears) and histopathological analysis (right ears). For cytocochleograms, cochleae were fixed in 4% paraformaldehyde and decalcified using 10% EDTA. The tissues were stained with phalloidin and mounted on slides for analysis. For histological analysis, tissues were fixed in 0.5% paraformaldehyde and decalcified in 10% EDTA. Tissues were processed into paraffin blocks, sectioned, and stained with hematoxylin and eosin.
3. Results
3.1. In Vitro dissolutions:
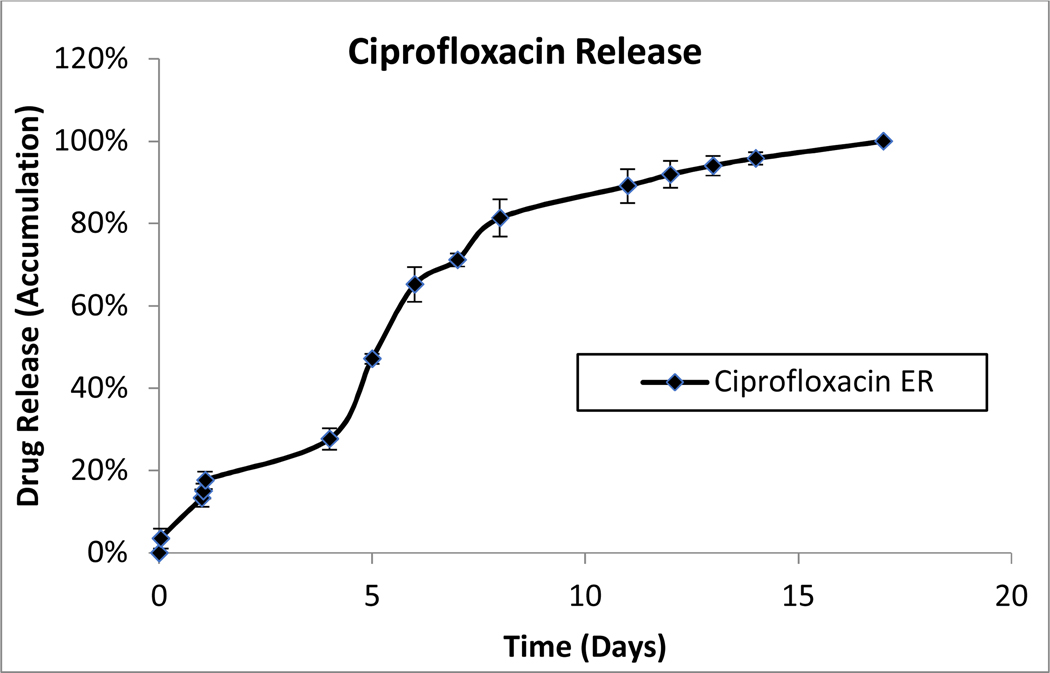
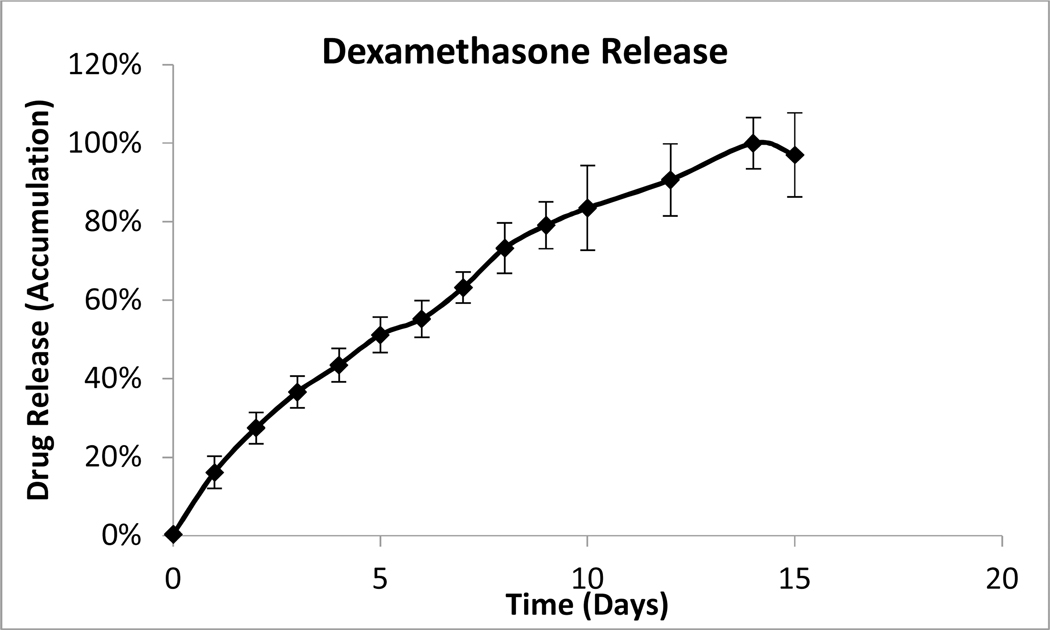
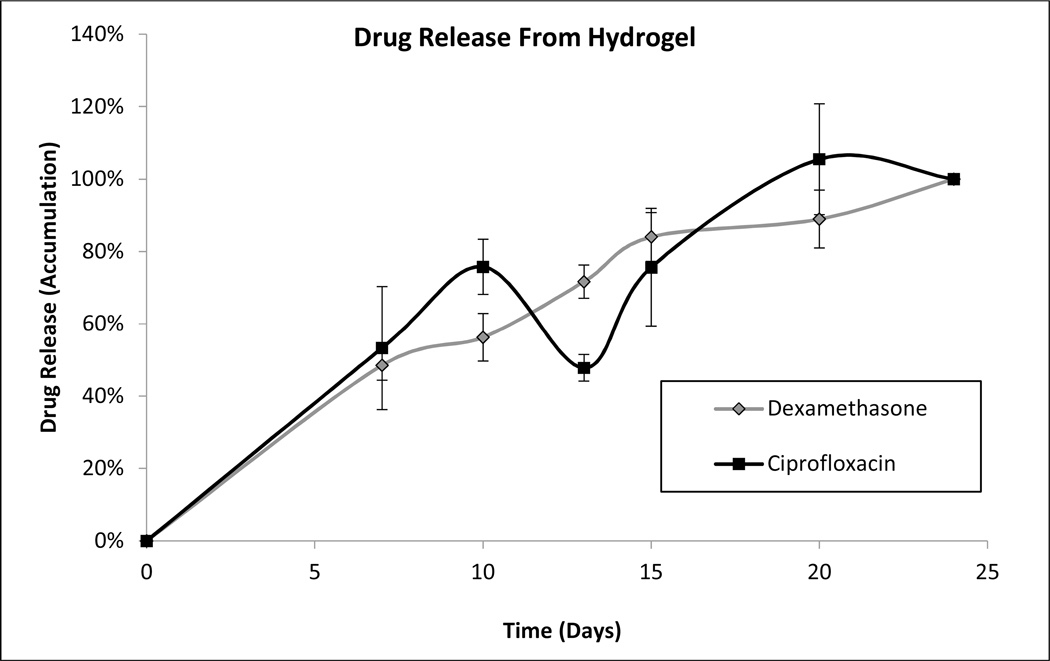
Individual formulations of coated dexamethasone or ciprofloxacin were monitored for drug release as described above. Near constant release was observed from both coated ciprofloxacin and dexamethasone particles over a two-week period (Figure 1). The in vitro release from a combination hydrogel suspension of coated ciprofloxacin and dexamethasone (OR-404IT) was also monitored. The rate of dissolution of each drug decreased by approximately 50%, giving a three-week release for both dexamethasone and ciprofloxacin from the combination hydrogel (Figure 1). However, both drugs maintained their near-constant release profiles over the duration of release. Hydrogel suspensions of either dexamethasone or ciprofloxacin were also tested to confirm that the release rate of each drug was independently controlled. The rate of drug release was the same whether a single drug or a combination of drugs was present in the hydrogel suspension (data not shown). Released drugs eluted from individual drug suspension formulations were used in activity assays below.
Figure 1: Drug Dissolution Studies.
A) Parylene-coated ciprofloxacin particles (3 mg) were stirred in water at 37°C. At the indicated time points, samples were removed and their absorbance at 272 nm was measured. Data is presented as Mean ± Standard Deviation.
B) Parylene-coated dexamethasone particles (3 mg) were stirred in water at 37°C. At the indicated time points, samples were removed and their absorbance at 242 nm was measured. Data is presented as Mean ± Standard Deviation.
C) Drug release from combination hydrogel: Dissolutions were performed as described in Methods. In vitro release of both dexamethasone and ciprofloxacin was assayed from a single combination hydrogel. Data is presented as Mean ± Standard Deviation.
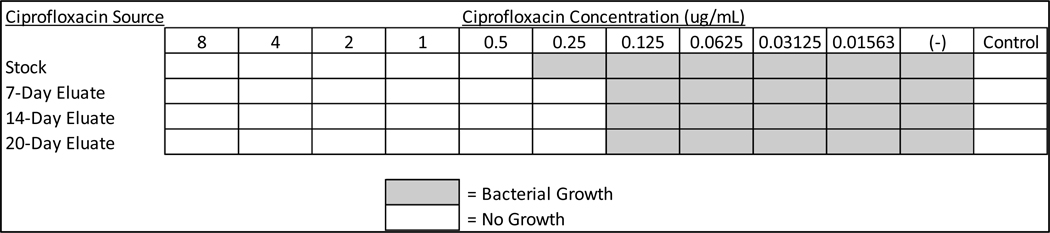
3.2. Anti-microbial activity by eluted ciprofloxacin:
The activity of eluted ciprofloxacin was monitored to confirm that the drug maintains activity throughout manufacture and elution. Released drug was collected after two weeks of dissolution in vitro as described in Materials and Methods. The concentration of eluted drug was calculated via UV-VIS spectroscopy and its activity was compared to freshly prepared ciprofloxacin solutions using the Broth Microdilution Method following NCCLS guidelines. Ciprofloxacin eluted from the extended release formulation was shown to inhibit H. influenzae growth up to a concentration of 0.125 μg/ml. Freshly prepared ciprofloxacin was shown to inhibit growth up to a concentration of 0.25 μg/ml. (Figure 2) This study demonstrated that ciprofloxacin eluate remained as active as freshly-made ciprofloxacin stock throughout the release period.
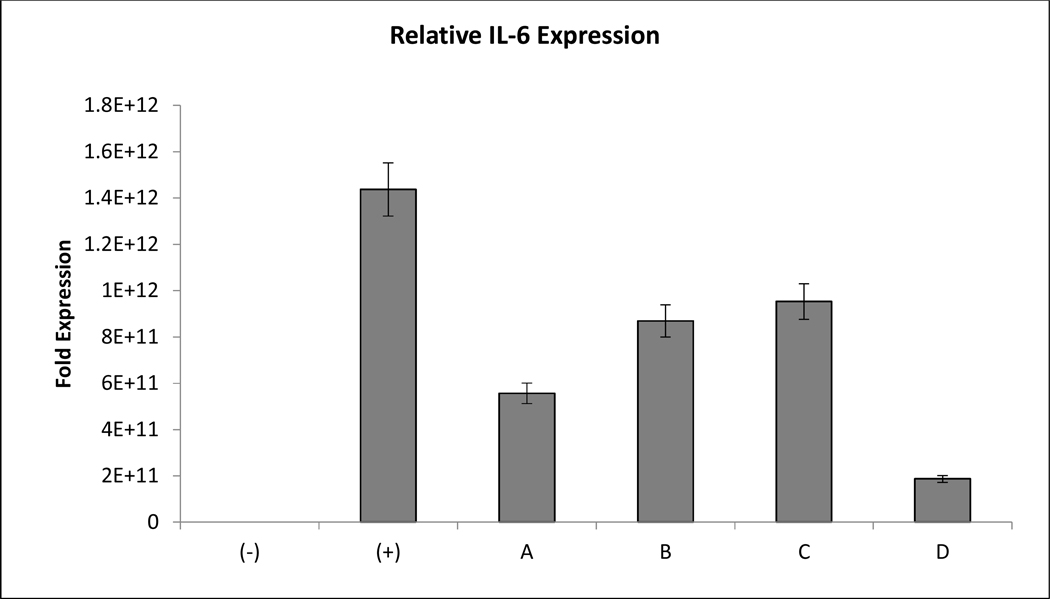
Figure 2. Dexamethasone Eluate Retains Anti-Inflammatory Activity Over 14 Days.
Freshly prepared dexamethasone stocks were compared to two-week dexamethasone in vitro eluate. Drug was assayed using real time polymerase chain reaction. Expression of interleukin-6 (IL-6) was used to assess anti-inflammatory activity. Actin expression was used as a load control. The columns are as follows: (−): no LPS, (+): + LPS, A: LPS + 0.1 μM fresh DEX, B: LPS + 1.0 μM fresh DEX, C: LPS + 0.1 μM eluted DEX, D: LPS + 1.0 μM eluted DEX. Data is presented as Mean ± Standard Deviation.
3.3. Inflammation suppression by eluted dexamethasone:
The activity of eluted dexamethasone was also monitored to confirm that the drug maintains activity throughout manufacture and elution. Released drug was collected after two weeks of dissolution. The concentration of eluted drug was calculated via UV-VIS spectroscopy and its activity was compared to freshly prepared dexamethasone solutions using Real Time Polymerase Chain Reaction (RT-PCR) to monitor the suppression of lipopolysaccharide (LPS) dependent inflammation in RAW 264.7 murine macrophage cells. Freshly prepared dexamethasone suppressed IL-6 expression at 0.1 and 1 μM. Interestingly, it did not suppress IL-6 expression in a dose-dependent manner. Dexamethasone eluate from the sustained release formulation suppressed IL-6 expression in a manner comparable to freshly prepared dexamethasone and exhibited dose-dependent suppression. (Figure 3) The results of the assay demonstrate that dexamethasone activity is maintained throughout particle/hydrogel manufacture and release.
Figure 3: Ciprofloxacin Eluate Retains Anti-Microbial Activity Over 14 Days.
Freshly prepared ciprofloxacin stocks were compared to ciprofloxacin in vitro eluate. Drug was assayed via the Broth Microdilution Method testing antimicrobial susceptibility of Haemophilus influenza to freshly prepared or eluted ciprofloxacin.
3.4. Ototoxicity assessment of combination hydrogel materials in Guinea Pigs:
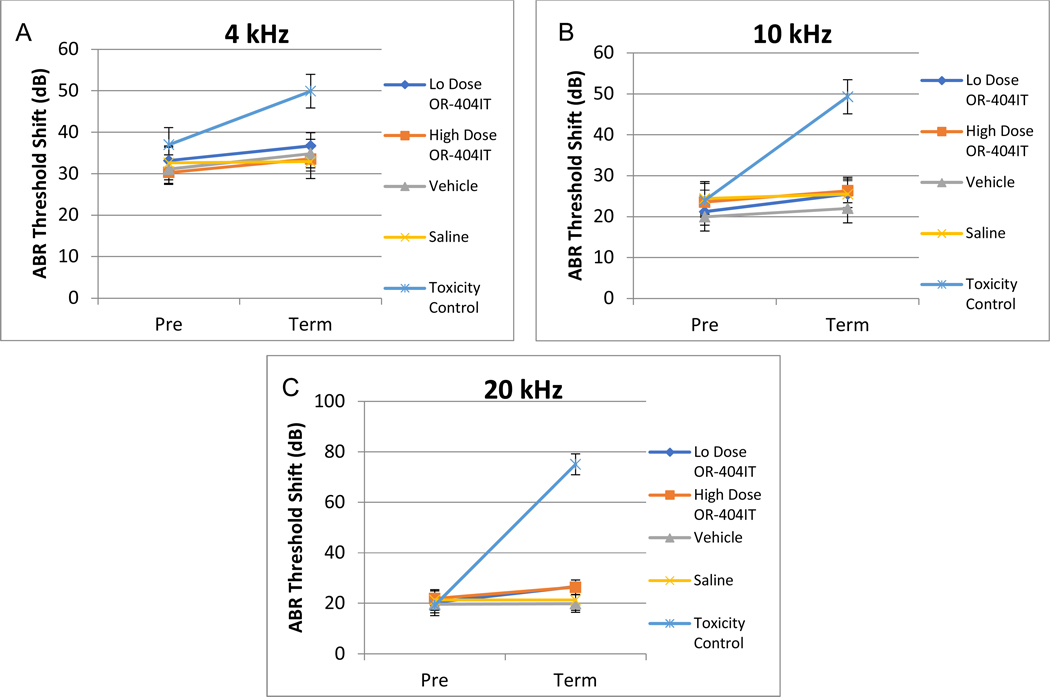
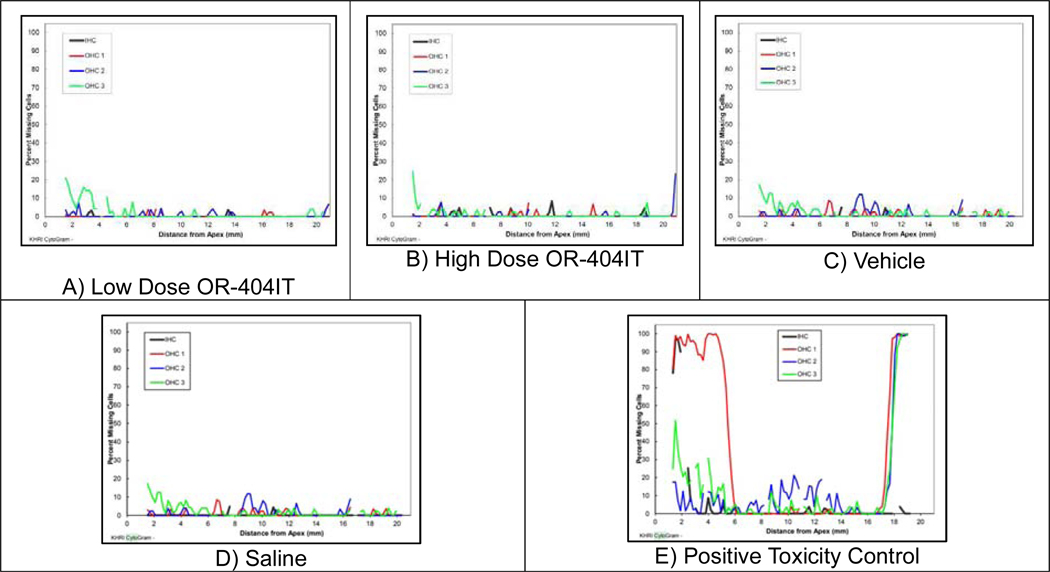
A safety study was performed to assess any potential ototoxicity of the drug and excipient components of OR-4040IT administered via transtympanic administration, followed by a 21 day recovery. At both doses tested, OR-404IT did not cause mortality and exhibited no effect on body weights, pathology findings, otoscopic examinations, ABR thresholds (Figure 4), or cytocochleograms (Figure 5). Excipients (parylene and HA) used in OR-404IT and a negative control (Saline) were also tested and exhibited no toxic effect in animals.
Figure 4: Auditory Brainstem Responses for OR-404IT.
The Mean ABR threshold shifts for Low Dose OR-404IT (0.5 mg ciprofloxacin + 0.2 mg dexamethasone), High Dose OR-404IT (1.5 mg ciprofloxacin + 0.6 mg dexamethasone), Vehicle (2.1 mg inactive excipients), Saline (negative control), and Positive Toxicity Control (10% neomycin). Animals were injected with similar treatment bilaterally into the middle ear space. No statistically significant difference was observed between ears in individual animals. ABR data for both ears was combined. No significant difference was noted with the exception of the toxic control. The threshold shifts were measured at 4 kHz (A), 10 kHz (B) and 20 kHz (C). Pre represents the ABR threshold of animals before implantation and Post represents the ABR threshold prior to euthanasia.
Figure 5: Cytocochleogram Hair Cell Analysis.
The representative cytocochleograms presented correlate with the histological findings. A) Low Dose OR-404IT (0.5 mg ciprofloxacin + 0.2 mg dexamethasone), B) High Dose OR-404IT (1.5 mg ciprofloxacin + 0.6 mg dexamethasone), C) Vehicle (2.1 mg inactive excipients), and D) Saline (negative control), via the transtympanic route did not cause mortality and did not affect cytocochleograms. As expected, E) Positive Toxicity Control (10% neomycin) displayed significant loss of outer and inner hair cells.
Microscopic fragments of foreign material were present in the middle ear of animals at 0.5/0.2 (Low Dose) and 1.5/0.6 mg (High Dose) OR-404IT and in the tympanic membrane of animals at 0.5/0.2 mg OR-404IT. Similar material was present in vehicle control (excipients) animals and was most likely a component of the hyaluronic acid hydrogel or polymers used in the formulation. A 10% Neomycin positive control (Positive Toxicity Control) was used to validate the assay and exhibited the expected ototoxicity in control animals (Figure 4 and 5E). The Positive Toxicity Control related microscopic findings were present in the inner ear, spiral ganglion, and auditory nerve. Minimal to mild hair cell loss was present in the organ of corti and minimal necrosis was present in the spiral ganglion. Myelin degeneration was also present in the auditory nerve. These microscopic findings are typical of the toxicity from Neomycin and from drugs in the same class of compound. No such toxicity was present in animals tested with any dose of OR-404IT or formulation vehicle (excipients).
Two control animals (one Saline control and one Positive Toxicity Control) died during the animal study. The Saline control death was considered secondary to the anesthesia procedure as the microscopic findings in the ears of this animal were similar to those of other animals from the Saline control group that survived. Microscopic findings in the Positive Toxicity Control animal were also found to be similar to the other animals in the same group that survived. It is unlikely that the administration of Saline or of the OR-IT Control were related to their deaths.
4. Discussion
This study demonstrated the effectiveness of OR-404IT to release both dexamethasone and ciprofloxacin over a 2–3 week period and to retain antibiotic/anti-inflammatory activity following release as a potential treatment of otorrhea/otitis media. In guinea pigs, OR-404IT did not cause any adverse reactions throughout a three week period, thus demonstrating its safety in vivo.
4.1. Activity assays
The activity studies of ciprofloxacin and dexamethasone eluates aimed to assess if the eluted drugs had comparable antibiotic and anti-inflammatory activities as their freshly-prepared counterparts. The eluted drugs were shown to retain activity following release in vitro. Although the addition of freshly prepared dexamethasone as a control suppressed IL-6 expression at 0.1 and 1 μM, it did not suppress IL-6 expression in the same dose-dependent manner as the dexamethasone eluate. As stock solutions were prepared in ethanol, this effect may have been due to the drug precipitating out of the solution when working samples were made. Regardless, these results still verify that the activity of dexamethasone is retained throughout hydrogel manufacture and release. Ciprofloxacin eluate was shown to inhibit H. influenzae growth as effectively as freshly prepared ciprofloxacin. These results demonstrate that the activity of eluted ciprofloxacin is retained throughout hydrogel manufacture and release. Therefore, the polymer coating and hydrogel manufacture processes appear to have no effect on the abilities of ciprofloxacin and dexamethasone to prevent infection and inflammation.
4.2. Animal studies
Vehicle (OR-404IT excipients), saline, and OR-404IT doses up to 1.5 mg ciprofloxacin/0.6 mg dexamethasone did not cause any apparent changes in average ABR thresholds, body weights, pathology findings, cytocochleograms, or otoscopic examinations. The 10% Neomycin control (Positive Toxicity Control) produced the expected increase in ABR thresholds across all frequencies tested and exhibited associated hair cell loss, thus validating the methods used to assess middle and inner ear ototoxicity. Collectively, these results demonstrate the excellent in vivo safety of OR-404IT and its components when injected into the middle ear of guinea pigs.
4.3. Otitis Media, Otorrhea, and Current Treatments
Otitis media (OM) causes recurrent, or persistent otorrhea and can lead to middle ear mucosal thickening. According to the World Health Organization, the risk factors for otitis media include young age and poor socioeconomic conditions. Diagnosis is difficult in children aged 0 to 30 months and is often uncertain, as draining ears are not always indicative of OM [12]. Hearing loss and otorrhea associated with OM during the first two years of life can also cause learning disabilities and poor scholastic performance [13], thus OM/otorrhea usually has the largest impact on children. The standard treatment for OM/otorrhea currently involves the systemic administration of the antibiotic, ciprofloxacin, which helps eradicate the gram-negative organisms often observed with OM. However, systemic quinolones such as ciprofloxacin can cause a number of adverse effects [14]. Therefore, a number of topical therapeutics for the treatment of OM have been developed. One advantage of topical administration is in the ability to achieve higher concentrations of drug at the treatment site compared to systemic administration, therefore improving clinical outcome. A 2006 review in the Cochrane Database assessed the effectiveness of topical quinolone antibiotics (such as ciprofloxacin) versus systemic antibiotics for the treatment of chronic suppurative OM [15]. The authors concluded that topical quinolone antibiotics are more effective at clearing aural discharge than systemic antibiotics. A second advantage of local administration of antibiotics is the reduction of adverse systemic side effects. Thus, an effective, local extended release formulation has the dual advantages of being safer and more effective than systemic treatment with antibiotics.
4.4. Advantages of antibiotic/corticosteroid combination therapy
Although topical antibiotics have been shown to be more effective than systemic antibiotics, the combination of local antibiotics with corticosteroids has been shown to reduce otorrhea with better efficacy than antibiotics alone [16]. A randomized, observer-masked, parallel-group, multicenter trial with 80 children with TTO compared topical ciprofloxacin/dexamethasone to oral amoxicillin/clavulanic acid (antibiotic combination) and demonstrated a more rapid median cessation time of otorrhea with the topical ciprofloxacin/dexamethasone group compared to the oral antibiotic combination group (4.0 days vs 7.0 days). In addition, fewer systemic adverse events were observed in the group receiving the topical ciprofloxacin/dexamethasone suspension [17]. In a randomized, multicenter trial comparing topical ciprofloxacin/dexamethasone combination to topical ciprofloxacin alone in 201 children with acute OM and visible otorrhea, the mean time for resolution of otorrhea was significantly shorter with the topical ciprofloxacin/dexamethasone combination (4.22 versus 5.31 days) [9]. In two randomized, controlled trials on adults, the combination of topical antibiotics with corticosteroids significantly reduced otorrhea compared with placebo. One of the trials involved 123 adults with OM. Significantly fewer people had OM (52%) after treatment with an antibiotic/corticosteroid combination as compared to placebo (75%) when compliance to the medication protocol was greater than 70%. A second, smaller trial demonstrated similar results in 31 adults at the end of 4 weeks of treatment with a combined antibiotic/corticosteroid treatment (35% with treatment and 79% with placebo) [18]. In these early trials on the combined drug therapy, issues with administration compliance strongly suggested the need for a local extended release treatment. A commercialized, extended release ciprofloxacin hydrogel has demonstrated clinical success in the treatment of otorrhea [11]. However, its lack of antiinflammatory may make it a less effective treatment than combination antibiotic/antiinflammatory drops.
4.5. OR-404IT may be more effective than current treatments
The present study highlights the proof of concept of a novel extended release combination antibiotic/anti-inflammatory hydrogel (ciprofloxacin/dexamethasone hydrogel, OR-404IT) for the treatment of otorrhea associated with tympanostomy tube placement. The successful development of OR-404IT could lead to better clinical outcomes for these pediatric patients and its use could potentially be expanded for the treatment of other middle and outer ear infections. The studies herein demonstrate the in vitro efficacy and in vivo safety of OR-404IT. The use of a localized, extended release combination antibiotic/anti-inflammatory drug formulation injected into the middle ear at the time of surgery would negate the need for daily drug administration while achieving accurate dosage and reduce the incidence of adverse effects [11].
5. Conclusion
A ciprofloxacin/dexamethasone hydrogel formulation (OR-404IT) has been developed by our group for the treatment/prevention of otorrhea/OM associated with tympanostomy tube insertion. Both ciprofloxacin and dexamethasone remained active throughout a period of 2–3 weeks after drug and hydrogel manufacture. The in vivo ototoxicity animal studies demonstrated the safety of the active and inactive components in guinea pigs, laying the groundwork for extended release middle ear hydrogel formulations that can efficaciously and safely deliver drugs over a desired period of time, making more effective treatments as compared to those currently used in the clinic.
Acknowledgements
Grant Support from the National Institute on Deafness and Other Communication Disorders #R44DC014416
Abbreviations:
- TT
tympanostomy tube
- ABR
auditory brainstem response
- TTO
tympanostomy tube otorrhea
Footnotes
Declaration of Interests
E.P. and W.S. own stock in O-Ray Pharma.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Isaacson G, Rosenfeld RM, Care of the child with tympanostomy tubes. Ped. Otolaryngol 43 (6) (1996) 1183–1193. [DOI] [PubMed] [Google Scholar]
- 2.Rosenfeld RM, Schwartz SR, Pynnonen MA, Tunkel DE, Hussey HM, Fichera JS, Grimes AM, Hackell JM, Harrison MF, Haskell H, Haynes DS, Kim TW, Lafreniere DC, LeBlanc K, Mackey WL, Netterville JL, Pipan ME, Raol NP, Schellhase KG, Clinical practice guideline: Tympanostomy tubes in children. Otolaryngol. Head Neck Surg. 149 (1 Suppl) (2013) S1–35. [DOI] [PubMed] [Google Scholar]
- 3.Schmelzle J, Birtwhistle RV, Tan AKW, Acute otitis media in children with tympanostomy tubes. Can. Fam. Physician 54 (8) (2008) 1123–1127. [PMC free article] [PubMed] [Google Scholar]
- 4.van Dongen TM, van der Heijden GJ, Freling HG, Venekamp RP, Schilder AG, Parent-reported otorrhea in children with tympanostomy tubes: incidence and predictors. PLoS One 8 (7) (2013) e69062pmid:23874870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strother CG, Sadow K, Evaluation of otorrhea (ear discharge) in children. [Google Scholar]
- 6.Heslop A, Lildholdt T, Gammelgaard N, Ovesen T, Topical ciprofloxacin is superior to topical saline and systemic antibiotics in the treatment of tympanostomy tube Otorrhea in children: the results of a randomized clinical trial. Laryngoscope 120 (12) (2010) 2516–2520. [DOI] [PubMed] [Google Scholar]
- 7.Roland PS, Kreisler LS, Reese B, Anon JB, Lanier B, Conroy PJ, Wall GM, Dupre SJ, Potts S, Hogg G, Stroman DW, McLean C, Topical ciprofloxacin/dexamethasone otic suspension is superior to ofloxacin otic solution in the treatment of children with acute otitis media with otorrhea through tympanostomy tubes. Pediatrics 113 (1) (2004) e40–46. [DOI] [PubMed] [Google Scholar]
- 8.Alper CM, Dohar JE, Gulhan M, Treatment of chronic suppurative otitis media with topical tobramycin and dexamethasone. Arch. Otolaryngol. Head Neck Surg 126 (2) (2000) 165–173. [DOI] [PubMed] [Google Scholar]
- 9.Roland PS, Anon JB, Moe RD, Conroy PJ, Wall GM, Dupre SJ, Krueger KA, Potts S, Hogg G, Stroman DW, Topical ciprofloxacin/dexamethasone is superior to ciprofloxacin alone in pediatric patients with acute otitis media and otorrhea through tympanostomy tubes. Laryngoscope 113 (12) (2003) 2116–2122. [DOI] [PubMed] [Google Scholar]
- 10.UPMC Children’s Hospital of Pittsburgh. Bilateral Myringotomy and Tubes (BM-T). Retrieved from http://www.chp.edu/our-services/ent/patient-procedures/bilateral-myringotomy
- 11.Edmunds AL, Otiprio: An FDA-Approved Ciprofloxacin Suspension Gel for Pediatric Otitis Media With Effusion. P. T 42 (5) (2017) 307–311. [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Prevention of hearing impairment from chronic otitis media: report of a WHO/CIBA Foundation workshop. Paper presented at: Prevention of hearing impairment from chronic otitis media: report of a WHO/CIBA Foundation Workshop; November 19–21, 1996; London, UK. [Google Scholar]
- 13.Teele DW, Klein JO, Chase C, Menyuk P, Rosner BA, Otitis media in infancy and intellectual ability, school achievement, speech, and language at age 7 years. Greater Boston Otitis Media Study Group. J. Infect. Dis 162 (3) (1990) 685–94. [DOI] [PubMed] [Google Scholar]
- 14.Goodman LS Goodman and Gilman’s the Pharmacological Basis of Therapeutics. Vol. 1157 New York, NY: Pergamon Press; 1990. [Google Scholar]
- 15.Macfadyen CA, Acuin JM, Gamble C Systemic antibiotics versus topical treatments for chronically discharging ears with underlying eardrum perforations. Cochrane Database Syst Rev, 2006. (1):CD005608. [DOI] [PubMed] [Google Scholar]
- 16.Jr Kutz JW, Roland PS, Lee KH, Ciprofloxacin 0.3% + dexamethasone 0.1% for the treatment for otitis media. Expert Opin. Pharmacother 14 (17) (2013) 2399–405. [DOI] [PubMed] [Google Scholar]
- 17.Dohar J, Giles W, Roland P, Bikhazi N, Carroll S, Moe R, Reese B, Dupre S, Wall M, Stroman D, McLean C, Crenshaw K, Topical ciprofloxacin/dexamethasone superior to oral amoxicillin/clavulanic acid in acute otitis media with otorrhea through tympanostomy tubes. Pediatrics. 118 (3) (2006) e561–9. [DOI] [PubMed] [Google Scholar]
- 18.Browning GG, Gatehouse S, Calder IT, Medical management of active chronic otitis media: a controlled study. J. Laryngol. Otol 102 (6) (1988) 491–5. [DOI] [PubMed] [Google Scholar]