Abstract
Hepatic artery pseudoaneurysm is a rare but potentially life-threatening condition that usually occurs after trauma. Early recognition and prompt management are essential for preventing catastrophic consequences, such as hemoperitoneum. We report a rare case of liver abscess caused by Klebsiella oxytoca resulting in hepatic artery pseudoaneurysm without iatrogenic injury. The unique feature of the present case is that the abscess cavity itself became a pseudoaneurysm as a result of fistula formation with the hepatic artery. Vascular complications should be considered in patients with unfavorable clinical course even in the absence of iatrogenic injury. Endovascular treatment is safe and effective.
Keywords: Klebsiella oxytoca; Aneurysm, False; Liver Abscess; Therapeutic Embolization; Catheters
Abstract
간동맥 거짓동맥류는 드문 질환이나 잠재적으로 생명을 위협할 수 있는 질환으로 외상 이후에 발생하는 경우가 많다. 때문에 빠른 진단과 적절한 치료가 혈복강과 같은 심각한 결과를 예방하기 위해 필수적이다. 우리는 의인적 손상 없이 Klebsiella oxytoca에 의한 간농양에 생긴 간동맥 거짓동맥류의 드문 증례를 보고하는 바이다. 이 증례의 독특한 특징은 농양 공간이 간동맥과 생긴 누공에 의해 거짓동맥류가 되었다는 점이다. 이 증례로부터 의인성 손상이 없이 환자의 임상 과정이 좋지 않은 경우 혈관 관련 합병증에 주의를 기울여야 함을 알 수 있으며 혈관 내 시술의 효과적이고 안전한 치료 방법이 될 수 있음을 알 수 있다
INTRODUCTION
Hepatic artery pseudoaneurysm (HAP) is potentially life-threatening condition, which occurs mostly after trauma. The association with liver abscess has rarely been described. Non-specific presentation frequently leads to delay in diagnosis. However, the immediate management is required to prevent irreversible consequences such as hemoperitoneum. Herein, we report a unique imaging feature of Klebsiella oxytoca liver abscess resulting in HAP without iatrogenic injury.
CASE REPORT
An 80-year-old man was admitted for fever and abdominal pain. The patient had diabetes and hypertension as an underlying disease. Laboratory results were as follows; white blood cell count 12380 cells/mm3, total bilirubin 5.2 mg/dL. Non-contrast abdominal CT and magnetic resonance cholangiopancreatography were performed on the day of visit and there were no specific findings except gallstones. Under suspicion of obstructive cholangitis, antibiotic therapy was initiated after blood culture. Common bile duct stone was removed endoscopically using a basket. None of the procedure were performed through peripheral bile duct.
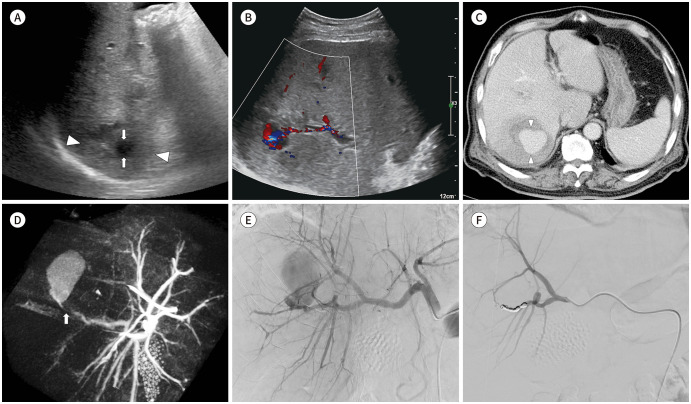
Blood culture grew Klebsiella oxytoca. On day 5, an abdominal ultrasound was performed because of persisted symptoms. Several poorly demarcated, hypoechoic lesions were identified over the right hepatic lobe suggesting abscess. The largest one was a 4.2 cm-sized lesion in segment VI of the liver with 3.2 cm of internal anechoic area (Fig. 1A). Color Doppler revealed a chaotic blood flow at internal anechoic area and a fistula formation from adjacent hepatic artery (Fig. 1B). Abdomen CT after 20 hours demonstrated that a 4.2 cm abscess cavity itself became pseudoaneurysm (Fig. 1C, D).
Fig. 1. Microcoil embolization of Klebsiella oxytoca liver abscess with hepatic artery pseudoaneurysm in an 80-year old man.
A. Ultrasonography demonstrates 4.2-cm hypoechoic lesion in segment VI with irregular margins (arrowheads) with a 3.2-cm internal anechoic area (arrows).
B. Color Doppler shows fistula formation between the anechoic space and the adjacent hepatic artery.
C. Contrast-enhanced liver dynamic computed tomography (portal phase) reveals rapid increase in the size of the pseudoaneurysm (arrowheads), which replaced the entire abscess pocket.
D. C-arm cone beam computed tomography during angiography shows a subtle connection (arrow) between the pseudoaneurysm and the hepatic artery branch supplying segment VI.
E. Digital subtraction angiography of the common hepatic artery reveals a large pseudoaneurysm from the hepatic artery branch supplying segment VI.
F. The final right hepatic angiography shows complete embolization of the culprit hepatic artery.
For angiographic evaluation, arterial access was obtained via the right common femoral artery using a 5-F vascular sheath (Radifocus Introducer II; Terumo, Tokyo, Japan). Common hepatic angiography and C-arm cone-beam CT using 5-F catheter (Rosch Hepatic; Cook, Bloomington, IN, USA), revealed a huge pseudoaneurysm arising from hepatic artery branch supplying segment VI. A 2.0-F microcatheter (Progreat; Terumo) was advanced into the distal circulation of pseudoaneurysm. Successful embolization was achieved with several detachable microcoils (Concerto, Medtronic, Sunnyvale, CA, USA) across the neck of pseudoaneurysm (Fig. 1E, F). Post-embolization hepatic angiography showed complete occlusion of culprit vascular branch. Seven days after the procedure, percutaneous catheter decompression was performed under ultrasound guidance for hematoma filled pocket with 8.5-F drainage catheter (Multipurpose Drainage Catheter Ultrathane; Cook). On day 12 of hospitalization, fever and hyperbilirubinemia subsided. Follow up CT taken at 12 days after the procedure confirmed complete resolution of hematoma and liver abscess.
DISCUSSION
Although HAP is the second most common splanchnic artery pseudoaneurysm that occurs mostly due to trauma, the association of HAP with liver abscess has rarely been described (1). Most cases of HAP with liver abscess has been rarely reported after iatrogenic injury including percutaneous aspiration or drainage catheter insertion. On the other hand, the development of HAP caused by liver abscess without iatrogenic event is extremely rare. There have been five case reports without iatrogenic event (Entamoeba histolytica in four cases and Enterococcus faecium in one case) (2,3,4,5,6). To the best of our knowledge, HAP with liver abscess caused by Klebsiella oxytoca has not been described previously in the English literature.
Klebsiella is known to cause opportunistic infections in patients with severe underlying disease or immunocompromised condition. Most of Klebsiella infection is caused by Klebsiella pneumoniae. Klebsiella oxytoca has been rarely identified in humans (7). In the literature, the overall incidence of Klebsiella oxytoca bacteremia was 2.6 per 10000 admissions per year. The biliary tract was the most common site of infection and liver abscess accounts for 5.6% of all infections (8). Ampicillin/sulbactam, second- and third-generation cephalosporins, aztreonam, imipenem, aminoglycosides and quinolones are potentially effective drugs. The combination of a b-lactam and a b-lactamase inhibitor has been suggested for Klebsiella oxytoca resistant to extended-spectrum b-lactam antibiotics (9).
The pathogenesis of HAP with liver abscess without iatrogenic injury is unclear. Several mechanisms have been suggested as possible causes of this rare comorbidity: 1) fibrinolytic proteins of bile from cavity can lyse the clot of unrecognized arterial injury, and 2) abscess directly related to endarteritis of hepatic artery (3). In this report, direct communication between abscess cavity and adjacent hepatic artery was identified in imaging study. The most plausible explanation is that abscess may spread into the surrounding tissue leading to erosion of adjacent hepatic artery and resulting in formation of fistula tract.
Depending on its location, HAP may rupture into hepatic vein, portal vein, biliary tree or abdominal cavity. The most catastrophic complication is hemoperitoneum from extrahepatic rupture, that can be life-threatening as large amounts of blood spill into the abdominal cavity. The mortality rate from HAP rupture ranges from 21% to 44% (10). In this report, the diagnosis could be made in time by early imaging follow up due to persistent symptoms.
The imaging appearance of HAP with liver abscess has been reported as characteristic turbulent blood flow in anechoic cystic structure on Doppler ultrasound (6). Contrast enhanced CT demonstrates rounded enhancing pseudoaneurysm which is located in or adjacent to the liver abscess (2,3,4,5,6). The unique feature of the present case was that abscess cavity itself became pseudoaneurysm as a result of fistula with adjacent hepatic artery. The vascular damage in the early stage of abscess before wall maturation may result in direct communication between abscess cavity and hepatic artery. Therefore, the size of pseudoaneurysm rapidly increased and early interventional management was required.
Treatment of HAP is not significantly different depending on etiologies. Endovascular embolization of the feeding artery is the current treatment of choice with success rate of approximately 90% (2). After successful thrombosis of pseudoaneurysm is achieved by endovascular treatment, subsequent percutaneous drainage could be performed safely as in the present case.
In summary, we discussed the diagnosis and treatment of HAP caused by liver abscess. Although it is rare, Klebsiella oxytoca may be a potential etiology of this rare comorbidity. Careful attention should be paid to vascular complication of liver abscess with unfavorable clinical course even without iatrogenic injury. Early management before rupture is imperative to avoid fatal outcome. Endovascular management is a safe and effective treatment option for HAP.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Writing—original draft, L.J.Y., L.H.N., L.W.H.
- writing—review & editing, all authors.
References
- 1.Baker KS, Tisnado J, Cho SR, Beachley MC. Splanchnic artery aneurysms and pseudoaneurysms: transcatheter embolization. Radiology. 1987;163:135–139. doi: 10.1148/radiology.163.1.3823426. [DOI] [PubMed] [Google Scholar]
- 2.Yadav AK, Gupta S, Hariprasad S, Kumar A, Ghuman SS, Gupta A. Amoebic liver abscess with hepatic artery pseudoaneurysm: successful treatment by interventional radiology. J Clin Exp Hepatol. 2015;5:86–88. doi: 10.1016/j.jceh.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gopanpallikar A, Rathi P, Sawant P, Gupta R, Dhadphale S, Deshmukh HL. Hepatic artery pseudoaneurysm associated with amebic liver abscess presenting as upper GI hemorrhage. Am J Gastroenterol. 1997;92:1391–1393. [PubMed] [Google Scholar]
- 4.Khan A, Pal KM, Khan HI. Hepatic artery pseudoaneurysm; a rare complication of amoebic liver abscess. J Pak Med Assoc. 2011;61:839–840. [PubMed] [Google Scholar]
- 5.Kim MD, Kim H, Kang SW, Jeong BG. Nontraumatic hepatic artery pseudoaneurysm associated with acute leukemia: a possible complication of pyogenic liver abscess. Abdom Imaging. 2002;27:458–460. doi: 10.1007/s00261-001-0078-8. [DOI] [PubMed] [Google Scholar]
- 6.Tacconi D, Lapini L, Giorni P, Corradini S, Caremani M. Pseudoaneurysm of the hepatic artery, a rare complication of an amebic liver abscess. J Ultrasound. 2009;12:49–52. doi: 10.1016/j.jus.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11:589–603. doi: 10.1128/cmr.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim BN, Ryu J, Kim YS, Woo JH. Retrospective analysis of clinical and microbiological aspects of Klebsiella oxytoca bacteremia over a 10-year period. Eur J Clin Microbiol Infect Dis. 2002;21:419–426. doi: 10.1007/s10096-002-0738-9. [DOI] [PubMed] [Google Scholar]
- 9.Wu SW, Dornbusch K, Norgren M, Kronvall G. Extended spectrum beta-lactamase from Klebsiella oxytoca, not belonging to the TEM or SHV family. J Antimicrob Chemother. 1992;30:3–16. doi: 10.1093/jac/30.1.3. [DOI] [PubMed] [Google Scholar]
- 10.Lu M, Weiss C, Fishman EK, Johnson PT, Verde F. Review of visceral aneurysms and pseudoaneurysms. J Comput Assist Tomogr. 2015;39:1–6. doi: 10.1097/RCT.0000000000000156. [DOI] [PubMed] [Google Scholar]