Abstract
Nose and lungs are considered as anatomic and functional unit, as they share morphological, pathophysiological and immunological basis. Allergic rhinitis and asthma often coexist in the same individual, and the treatment of one also improves the symptoms of the other (one airway, one disease). The aim of this review is to discuss the interaction between upper and lower airways, based on recent scientific evidence and, critically, analyze the important implications the new findings have for the future therapy and prevention of these common diseases. (www.actabiomedica.it)
Keywords: united airway disease, rhinitis, asthma, children, allergy, IgE, aeroallergen, COVID-19
Introduction
The concept of United Airway Disease (UAD) was introduced in the first 2000s to indicate the mor-pho-functional connection existing between the lower and upper airways (1).
The nasopharynx and the lungs, in addition to being connected from an anatomical point of view, share a common pathophysiological and immunological basis that supports the concept of “one airway, one dis-ease”(2).
UAD was classically born to define the link between allergic rhinitis (AR) and asthma by considering them as manifestations of one syndrome in different parts of the respiratory tract and that the more severe the rhinitis, the more severe the asthma. Currently it has taken on a more heterogeneous and complex meaning, including other diseases of the upper and lower airways such as rhinitis with and without nasal polyposis and crhonic obstructive pulmonary disease (COPD) (3). In childhood UAD has been described mainly through the association between allergic rhinitis and asthma (4-6).
An association between upper and lower airways has also been observed in COVID-19 infection. Children were found to have significantly lower expression of COVID-19 receptors (angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2)) in the nasal and bronchial mucosa, than adults (7) resulting in a less severe infection, mostly, limited to the upper airways (8).
Epidemiological and clinical evidence of united airway disease
Allergic rhinitis is one of the most common diseases in children, though it is often underdiagnosed or undertreated. According to Asher et al (9), the prevalence of AR varies between 0.8 to 14.9% in 6-7-years old and 1.4 to 39.7% in 13-14-years old children worldwide. AR occurs in association with several other diseases, including asthma, allergic conjunctivitis, at-opic dermatitis and sinusitis.
AR is a risk factor for asthma both in adults (10,11) and children (12). Approximately 30% of patients with rhinitis develops asthma (13) and up to 80% of patients with perennial asthma have rhinitis (14). In a German cohort of 1314 healthy newborns recruited in 1990, Cough et al. found that, at age 20, asthma occurred more frequently with coexisting allergic rhinitis and/or eczema than as a single entity from pre-puberty to adulthood (15).
Severity of rhinitis was positively correlated with a score of asthma severity (16) and inversely correlated to an index of quality of life (17). On the other hand, patients with severe uncontrolled asthma commonly have severe nasal disease (often chronic rhinosinusitis 18).
Burgess et al. demonstrated that allergic rhinitis increases the likelihood of new-onset asthma after childhood and the likelihood of having persisting asthma from childhood into middle age (19). Some studies have shown an increase of nonspecific bronchial hyperreactivity (BHR) in patients with allergic and non-allergic rhinitis, especially during the exacerbation stage (20,21). Ciprandi et al. found an increase of BHR and spirometric impairment both in patients with perennial and seasonal AR (22,23).
In COpenhagen Prospective Studies on Asthma in Childhood (COPSAC) birth cohort, Chawes et al. observed the association between upper and lower airway diseases also in non-atopic individuals (24), finding that both children with allergic and non-allergic forms of rhinitis have a similar risk of developing asthma (25).
According to Allergic Rhinitis and its Impact on Asthma (ARIA) 2019 guidelines for the management of AR and asthma comorbidities, the presence of asthma should always be excluded in patients with allergic rhinitis, especially in those with moderate to severe persistent rhinitis (26).
The link between upper and lower airways is also reflected in therapeutic practice. It has been shown that a careful AR management is associated with better asthma control and, likewise, the improvement of asthma was associated with a resolution of allergic rhinitis symptoms (27,28). Inhaled corticosteroids used for the treatment of rhinitis are associated with a better control of asthma symptoms and viceversa (29) and im-munotherapy (not widely used in clinical management of rhinitis) was found to prevent the development of the asthma (30,31). Novembre et al. found that three years of short-term co-seasonal sublingual immuno-therapy improves seasonal allergic rhinitis symptoms and reduces the development of seasonal asthma in children with hay fever (32).
UAD underlying mechanisms
The interaction between lower and upper airways is supported by anatomical, pathophysiological and immunological mechanisms. First of all, the upper airways are the first filter for the entry of pathogens and allergens in the respiratory tract and have the function of humidifying and heating the air. In rhinitis the inflammation and edema of the nasal mucosa, together with the increase of oral breathing, promote the entry of inhalants into the lungs, resulting in inflammation and bronchial hyperreactivity (33.) From an anatomical and histological point of view, upper and lower airways share common elements, including ciliary epithelium, basement membrane, lamina propria, mucous glands. Unlike the upper airways, the bronchi have a fibrocar-tilage structure and smooth muscle cells. In a comparative study between asthmatic and control subjects, Chanez et al. found a thickening of the lamina reticularis of the nasal and bronchial mucosa in control subjects and in subjects with perennial rhinitis and asthma. In both, the thickening of the nasal lamina reticularis correlated with that of the bronchial tissue (34).
Allergic rhinitis and asthma share common pathophysiological mechanisms including Th2-medi-ated response, IgE-mediated hypersensitivity and increased eosinophilic counts (figure 1).
Figure 1. The connection between upper and lower airways: possible mechanisms and results.
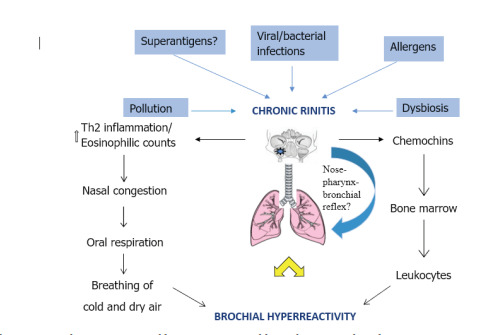
Ballardini et al. observed that Ige-mediated sensi-tization to common epitopes is associated with an increased risk of developing both asthma and allergic rhinitis in childhood (35). In a 2010 cross-sectional study, it was found that sputum eosinophilia was associated with 52 times increase in odds of nasal eosinophilia and, therefore, monitoring of nasal eosinophilia by cytology may be a useful substitute for sputum cytology in the composite assessment of airway inflammation (36).
The spread of inflammation from upper to lower airways has been subject of many studies (37,38,39).
It has been suggested that local airway inflammation may result in systemic inflammation through the activation of bone marrow with chemotaxis of white blood cells both in upper and lower airways (40). In a recent meta-analysis (41), researchers have studied the role of Staphylococcus aureus superantigens in the persistence and severity of asthma and allergic rhinitis. If superantigens are confirmed to modify, if not cause, allergic airways diseases then therapeutic interventions included antibiotics, anti-IgE when SE (staphylococ-cal enterotoxin) specific IgE are found in serum or respiratory tissues, antibodies against Th2-orientated cytokines and, ultimately, vaccination therapy to prevent Staphylococcal infections, should be considered.
In a 2018 study (42), it was noted, in a cohort of 73 6-year-old children with moderate/severe AR, that those who developed asthma had a significant difference in respiratory resistance (Rrs) and reactance (Xrs) before and after bronchodilation (BD), but only at AR exacerbations. This study reveals that small-airway dysfunction precedes the development of asthma in children with allergic rhinitis and changes in respiratory impedance at AR exacerbation may assist in identifying those at risk to progress to asthma. In children with asthma, higher exhaled NO levels were found in atopic than in non-atopic subjects (43).
Another connection between nose and lungs was suggested by the existence of the nose-pharynx-bronchial reflex, that can occur after stimulation by pathogens or allergens (40,44) (figure 2).
Figure 2. The representation of nose-pharynx-bronchial reflex. BV: blood vessel. CNS: central nervous system.
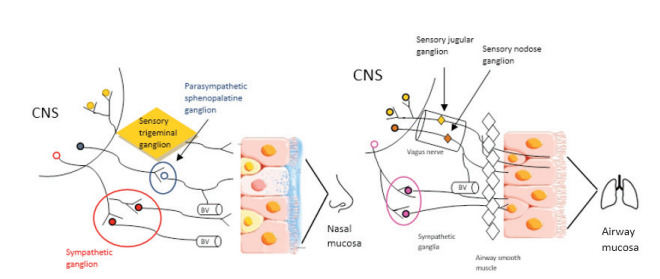
Nervous signals transmitted through afferent fibers of the trigeminal nerve are elaborated in the brain-stem, from which efferent fibers, that control bronchial muscle tone and lower airways constriction, branch off. Evidence supporting the hypothesis is the application of pepper, silicon particles, or cold air to the nasal mucosa can produce immediate effects on FEV1 or respiratory resistance and trigeminal resection prevents bronchoconstriction induced by the application of silicon particles to the nasal mucosa (37). However, the most recent studies do not seem to confirm the existence of a nasal bronchial reflex, focusing on the study of inflammatory and immunological mechanisms underlying the relationship between upper and lower airways (45,46).
In recent years, the role of the microbiome in the pathophysiology of chronic rhinosinusitis (47) and bronchial hyperresponsiveness has been studied (48): dysbiosis can have an impact on the health of the mu-cosa and on the severity of the disease and can affect the development of other disorders along the respiratory tract.
Conclusion
Several studies confirm the reciprocal interaction between upper and lower airways, although many mechanisms still remain unknown. A better comprehension of sinus-nasal microbiome in healthy patients and in chronic rhinosinusitis and the link with bronchial hyperreactivity can help in developing new prognostics, diagnostics, and therapeutics strategies. The use of probiotics, widely used in childhood, can restore the native sinus ecology with significant therapeutic and preventive implications. Also the study of Staphylococcus Aureus superantigens can help find new treatment and prevention approaches both in chronic rhinitis and asthma.
Moreover, in children, the nose-lung interaction is part of a broader scenario, called ‘atopic march’, where, classically, atopic dermatitis in followed by food allergy, asthma and AR. In fact, in childhood, AR and asthma often coexist, following different temporal and development trajectories. Investigating the role of risk factors (onset, severity, skin sensibilization, family history, genetics) could help recognize patients at risk and break the progression of the allergic march.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- Passalacqua G, Ciprandi G, Canonica GW. The nose-lung interaction in allergic rhinitis and asthma: united airways disease. CurrOpin Allergy Clin Immunol. 2001;1:7–13. doi: 10.1097/01.all.0000010978.62527.4e. [DOI] [PubMed] [Google Scholar]
- Grossman J. One airway, one disease. Chest. 1997;11:11S–6S. doi: 10.1378/chest.111.2_supplement.11s. [DOI] [PubMed] [Google Scholar]
- Yii ACA, Tay TR, Choo XN, Koh MSY, Tee AKH, Wang DY. Precision medicine in united airways disease: A “treatable traits” approach. Allergy. 2018;73:1964–1978. doi: 10.1111/all.13496. [DOI] [PubMed] [Google Scholar]
- Ciprandi G, Caimmi D, Miraglia Del Giudice M, La Rosa M, Salpietro C, Marseglia GL. Recent developments in United airways disease. Allergy Asthma Immunol Res. 2012;4:171–7. doi: 10.4168/aair.2012.4.4.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrorilli C, Posa D, Cipriani F, Caffarelli C. Asthma and allergic rhinitis in childhood: what’s new. Pediatr Allergy Immunol. 2016;27:795–803. doi: 10.1111/pai.12681. [DOI] [PubMed] [Google Scholar]
- Caimmi D, Marseglia A, Pieri G, Benzo S, Bosa L. Cai- mmi S. Nose and lungs: one way, one disease. Ital J Pediatr. 2012;38:60. doi: 10.1186/1824-7288-38-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saheb Sharif-Askari N, Saheb Sharif-Askari F, Alabed M, et al. Airways expression of SARS-CoV-2 receptor, ACE2, and TMPRSS2 is lower in children than adults and increases with smoking and COPD. Mol Ther Methods Clin Dev. 2020;18:1–6. doi: 10.1016/j.omtm.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi GF, Brindisi G, Indolfi C, et al. Upper airway involvement in pediatric COVID-19. Pediatr Allergy Immunol. 2020;26(31 Suppl):85–88. doi: 10.1111/pai.13356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher MI, Keil U, Anderson HR, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8:483–91. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- Shaaban R, Zureik M, Soussan D, et al. Allergic rhinitis and onset of bronchial hyperresponsiveness: a population-based study. Am J Respir Crit Care Med. 2007;176:659–66. doi: 10.1164/rccm.200703-427OC. [DOI] [PubMed] [Google Scholar]
- Tohidinik HR, Mallah N, Takkouche B. History of allergic rhinitis and risk of asthma; a systematic review and metaanalysis. World Allergy Organ J. 2019;12:100069. doi: 10.1016/j.waojou.2019.100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochat MK, Illi S, Ege MJ, et al. Allergic rhinitis as a predictor for wheezing onset in school-aged children. J Allergy Clin Immunol. 2010;126:1170–5.e2. doi: 10.1016/j.jaci.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Cruz AA, Popov T, Pawankar R, et al. Common characteristics of upper and lower airways in rhinitis and asthma: ARIA update, in collaboration with GA (2) LEN. Allergy. 2007;62(suppl):1–41. doi: 10.1111/j.1398-9995.2007.01551.x. [DOI] [PubMed] [Google Scholar]
- Brozek JL, Bousquet J, Baena-Cagnani CE, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466–476. doi: 10.1016/j.jaci.2010.06.047. [DOI] [PubMed] [Google Scholar]
- Gough H, Grabenhenrich L, Reich A, et al. Allergic multimorbidity of asthma, rhinitis and eczema over 20 years in the German birth cohort MAS. Pediatr Allergy Immunol. 2015;26:431–7. doi: 10.1111/pai.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponte EV, Franco R, Nascimento HF, et al. Lack of control of severe asthma is associated with co-existence of moder- ate-to-severe rhinitis. Allergy. 2008;63:564–9. doi: 10.1111/j.1398-9995.2007.01624.x. [DOI] [PubMed] [Google Scholar]
- Benazzo M, Leonardi S, Corsico A, et al. Cetirizine modifies quality of life and symptoms in children with seasonal allergic rhinitis: a pilot study. Acta Biomed. 2020;92:e2021003. doi: 10.23750/abm.v92i1.9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresciani M, Paradis L, Des Roches A, et al. Rhinosinusitis in severe asthma. J Allergy Clin Immunol. 2001;107:73–80. doi: 10.1067/mai.2001.111593. [DOI] [PubMed] [Google Scholar]
- Burgess JA, Walters EH, Byrnes GB, et al. Childhood allergic rhinitis predicts asthma incidence and persistence to middle age: a longitudinal study. J Allergy Clin Immunol. 2007;120:863–9. doi: 10.1016/j.jaci.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Gonzalez Hernandez J, Gomez Vera J, Orea Solano M, Flores Sandoval G, Rfos Nava R, de la Torre F. Hiperrespuesta de las viasaereasen los pacientes con rinitisalergica y no ale- rgica [Airway hyperreactivity in patients with allergic and non-allergic rhinitis] Rev Alerg Mex. 2003;50:86–90. [PubMed] [Google Scholar]
- Ciprandi G, Tosca MA, Cirillo I, Capasso M. Impact of allergic rhinitis on asthma in children: effects on bronchial hyperreactivity. Allergy. 2010;65:1199–201. doi: 10.1111/j.1398-9995.2009.02321.x. [DOI] [PubMed] [Google Scholar]
- Ciprandi G, Cirillo I, Tosca MA, Vizzaccaro A. Bronchial hyperreactivity and spirometric impairment in patients with perennial allergic rhinitis. Int Arch Allergy Immunol. 2004;133:14–8. doi: 10.1159/000075249. [DOI] [PubMed] [Google Scholar]
- Ciprandi G, Cirillo I, Tosca MA, Vizzaccaro A. Bronchial hyperreactivity and spirometric impairment in patients with seasonal allergic rhinitis. Respir Med. 2004;98:826–31. doi: 10.1016/j.rmed.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Chawes BL. Upper and lower airway pathology in young children with allergic- and non-allergic rhinitis. Dan Med Bull. 2011;58:B4278. [PubMed] [Google Scholar]
- Chawes BL. B0nnelykke K, Kreiner-M0ller E, Bisgaard H. Children with allergic and nonallergic rhinitis have a similar risk of asthma. J Allergy Clin Immunol. 2010;126:567–73. doi: 10.1016/j.jaci.2010.06.026. [DOI] [PubMed] [Google Scholar]
- Bousquet J, Schunemann HJ, Togias A, et al. Next-generation Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines for allergic rhinitis based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) and real-world evidence. J Allergy Clin Immunol. 2020;145:70–80. doi: 10.1016/j.jaci.2019.06.049. [DOI] [PubMed] [Google Scholar]
- Camargos P, Ibiapina C, Lasmar L, Cruz AA. Obtaining concomitant control of allergic rhinitis and asthma with a nasally inhaled corticosteroid. Allergy. 2007;62:310–6. doi: 10.1111/j.1398-9995.2007.01241.x. [DOI] [PubMed] [Google Scholar]
- Greisner WA, Settipane RJ, Settipane GA. The course of asthma parallels that of allergic rhinitis: a 23-year follow-up study of college students. Allergy Asthma Proc. 2000;21:371–5. doi: 10.2500/108854100778249123. [DOI] [PubMed] [Google Scholar]
- De Vittori V, Pacilio A, Indinnimeo L, et al. When asthma and rhinitis coexist, could rhinitis reduce asthma control in children? Allergy Asthma Proc. 2019;40:e8–e13. doi: 10.2500/aap.2019.40.4219. [DOI] [PubMed] [Google Scholar]
- Caffarelli C, Mastrorilli C, Procaccianti M, Santoro A. Use of sublingual immunotherapy for aeroallergens in children with asthma. J Clin Med. 2020;9:3381. doi: 10.3390/jcm9103381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morjaria JB, Caruso M, Emma R, Russo C, Polosa R. Treatment of allergic rhinitis as a strategy for preventing asthma. Curr Allergy Asthma Rep. 2018;18:23. doi: 10.1007/s11882-018-0781-y. [DOI] [PubMed] [Google Scholar]
- Novembre E, Galli E, Landi F, et al. Coseasonal sublingual immunotherapy reduces the development of asthma in children with allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2004;114:851–7. doi: 10.1016/j.jaci.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Valdesoiro L, Bosque M, Marco MT, Asensio O, Anton J, Larramona H. Rinitisalergica e hiperreactividadbronquial Allergic rhinitis and bronchial hyperreactivity. Aller- golImmunopathol (Madr) 2004;32:340–3. doi: 10.1016/s0301-0546(04)79266-6. [DOI] [PubMed] [Google Scholar]
- Chanez P, Vignola AM, Vic P, et al. Comparison between nasal and bronchial inflammation in asthmatic and control subjects. Am J Respir Crit Care Med. 1999;159:588–95. doi: 10.1164/ajrccm.159.2.9801022. [DOI] [PubMed] [Google Scholar]
- Ballardini N, Bergstrom A, Wahlgren CF, et al. IgE antibodies in relation to prevalence and multimorbidity of eczema, asthma, and rhinitis from birth to adolescence. Allergy. 2016;71:342–9. doi: 10.1111/all.12798. [DOI] [PubMed] [Google Scholar]
- Amorim MM, Araruna A, Caetano LB, Cruz AC, Santoro LL, Fernandes AL. Nasal eosinophilia: an indicator of eosinophilic inflammation in asthma. Clin Exp Allergy. 2010;40:867–74. doi: 10.1111/j.1365-2222.2009.03439.x. [DOI] [PubMed] [Google Scholar]
- Togias A. Rhinitis and asthma: evidence for respiratory system integration. J Allergy Clin Immunol. 2003;111:1171–83. doi: 10.1067/mai.2003.1592. [DOI] [PubMed] [Google Scholar]
- Braunstahl GJ. United airways concept: what does it teach us about systemic inflammation in airways disease? Proc Am Thorac Soc. 2009;6:652–4. doi: 10.1513/pats.200906-052DP. [DOI] [PubMed] [Google Scholar]
- Kanda A, Kobayashi Y, Asako M, Tomoda K, Kawauchi H, Iwai H. Regulation of interaction between the upper and lower airways in united airway disease. Med Sci (Basel) 2019;7:27. doi: 10.3390/medsci7020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togias A. Mechanisms of nose-lung interaction. Allergy. 1999;57(54 Suppl):94–105. doi: 10.1111/j.1398-9995.1999.tb04410.x. [DOI] [PubMed] [Google Scholar]
- Pastacaldi C, Lewis P, Howarth P. Staphylococci and staphylococcal superantigens in asthma and rhinitis: a systematic review and meta-analysis. Allergy. 2011;66:549–55. doi: 10.1111/j.1398-9995.2010.02502.x. [DOI] [PubMed] [Google Scholar]
- Skylogianni E, Triga M, Douros K, et al. Small-airway dysfunction precedes the development of asthma in children with allergic rhinitis. Allergol Immunopathol (Madr) 2018;46:313–321. doi: 10.1016/j.aller.2017.09.025. [DOI] [PubMed] [Google Scholar]
- Miraglia del Giudice M, Capasso M, Maiello N, et al. Exhaled nitric oxide and atopy in children. J Allergy Clin Immunol. 2003;111:193. doi: 10.1067/mai.2003.13. [DOI] [PubMed] [Google Scholar]
- Poddighe D, Brambilla I, Licari A, Marseglia GL. Pediatric rhinosinusitis and asthma. Respir Med. 2018;141:94–99. doi: 10.1016/j.rmed.2018.06.016. [DOI] [PubMed] [Google Scholar]
- Giavina-Bianchi P, Aun MV, Takejima P, Kalil J, Agondi RC. United airway disease: current perspectives. J Asthma Allergy. 2016;9:93–100. doi: 10.2147/JAA.S81541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda A, Kobayashi Y, Asako M, Tomoda K, Kawauchi H, Iwai H. Regulation of interaction between the upper and lower airways in united airway disease. Med Sci (Basel) 2019;7:27. doi: 10.3390/medsci7020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miraglia Del Giudice M, Parisi GF, Indolfi C, et al. Nasal microbiome in chronic rhinosinusitis. Minerva Pediatr Online ahead of print. 2020 doi: 10.23736/S2724-5276.20.05850-8. [DOI] [PubMed] [Google Scholar]
- Huang YJ, Nelson CE, Brodie EL, et al. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127:372–381.el-3. doi: 10.1016/j.jaci.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]


