Abstract
Purpose
To analyze the altered brain regions and intrinsic brain activity patterns in trauma-exposed firefighters without posttraumatic stress disorder (PTSD).
Materials and Methods
Resting-state functional MRI (rsfMRI) was performed for all subjects. Thirty-one firefighters over 40 years of age without PTSD (31 men; mean age, 49.8 ± 4.7 years) were included. Twenty-six non-traumatized healthy controls (HCs) (26 men; mean age, 65.3 ± 7.84 years) were also included. Voxel-based morphometry was performed to investigate focal differences in the brain anatomy. Seed-based functional connectivity analysis was performed to investigate differences in spontaneous brain characteristics.
Results
The mean z-scores of the Seoul Verbal Learning Test for immediate and delayed recall, Controlled Oral Word Association Test (COWAT) score for animals, and COWAT phonemic fluency were significantly lower in the firefighter group than in the HCs, indicating decreased neurocognitive function. Compared to HCs, firefighters showed reduced gray matter volume in the left superior parietal gyrus and left inferior temporal gyrus. Further, in contrast to HCs, firefighters showed alterations in rsfMRI values in multiple regions, including the fusiform gyrus and cerebellum.
Conclusion
Structural and resting-state functional abnormalities in the brain may be useful imaging biomarkers for identifying alterations in trauma-exposed firefighters without PTSD.
Keywords: Brain; Firefighters; Magnetic Resonance Imaging; Stress Disorder, Post-Traumatic
Abstract
목적
외상 후 스트레스 장애(posttraumatic stress disorder; 이하 PTSD)가 없는 외상에 노출된 소방관들에서 뇌 구조의 변화와 휴식기 뇌기능 변화를 연구하고자 한다.
대상과 방법
모든 피험자는 휴식기 기능 뇌자기공명영상(resting-state functional MRI; 이하 rsfMRI) 검사를 시행하였다. PTSD가 없는 40세 이상의 31명의 소방관(31명, 평균 연령, 49.8 ± 4.7세)이 포함되었다. 26명의 외상을 받지 않은 건강한 대조군(26명, 평균 연령, 65.3 ± 7.84세)도 포함되었다. Voxel-based morphometry 분석을 시행하여 뇌 해부학상의 국소적 차이를 조사하였으며, 휴식기 뇌기능의 차이를 조사하기 위해 seed-based functional connectivity analysis 분석을 시행하였다.
결과
서울언어학습결과(Seoul Verbal Learning Test)의 평균 z 값을 비교했을때 소방관은 건강한 대조군에 비해 즉각회상(immediate recall), 지연 회상(delayed recall), 통제단어연상검사(Controlled Oral Word Association Test; 이하 COWAT)의 동물(animal)과 음소(phonemic) 항목에서 점수가 유의하게 낮았으며, 신경인지 기능이 감소한 것으로 나타났다. 소방관은 좌위마루이랑(left superior parietal gyrus)과 좌하관자이랑(left inferior temporal gyrus)의 회색질 부피가 건강한 대조군에 비해 감소되어 있었다. 소방관은 방추향이랑(fusiform gyrus)과 소뇌(cerebellum) 등을 포함한 여러 부위에서 rsfMRI 값의 변화를 보였다.
결론
구조적 뇌 및 휴식 상태 기능 이상은 외상에 노출된 소방관의 변화를 확인하는 데 유용한 이미징 바이오 마커일 수 있다.
INTRODUCTION
The extremely stressful (psychologically traumatic) events in the firefighting duty can increase the risk for posttraumatic stress disorder (PTSD) symptoms in firefighters (1,2). According to previous literature (2,3,4), the prevalence rate of PTSD is two times greater in firefighters (18–37%) than general population (7–9%). Previous neuroimaging studies showed various abnormalities in brain structure (5,6,7) and function (8,9) in patients with PTSD. However, the firefighters that fail to satisfy the criteria of PTSD that frequently experience extremely stressful (traumatic) experiences have been understudied, although this population may provide a unique opportunity to examine the neural system related to work-related traumatic stressors. Emerging evidence suggests that traumatic stress itself may have a substantial impact on brain structure and function even in the absence of PTSD symptoms (10,11). Also, disturbances in brain function have already been suggested to contribute to cognitive impairments in patients with psychiatric disorders including PTSD (12,13). However, the consequences of trauma on neurocognitive function in firefighters without PTSD remain unclear.
Previous studies have shown that human brain structure and function can be explored in vivo by neuroimaging techniques, such as voxel-based morphometry (VBM) and resting-state functional MRI (rsfMRI). VBM allows a whole-brain regional specific assessment of deep gray matter (GM) and the brain cortex, which consists of neuronal cell bodies generating and processing nerve signals underlying brain function (14). On the other hand, rsfMRI examines spontaneous fluctuations in blood oxygen level-dependent signals in the absence of a stimulus or task and enables the investigation of regional neural activity and functional connectivity (15). Hypothesis-driven seed-based resting state functional connectivity (RSFC) analysis performs temporal cross-correlation and intuitive interpretation. Study on trauma-exposed firefighters without PTSD using these methods can inform the field's understanding of the neural correlates of stress exposure.
The purpose of this study was to analyze the altered brain regions and intrinsic brain activity patterns in trauma-exposed firefighters without PTSD.
MATERIALS AND METHODS
PARTICIPANTS
This study was approved by the Yonsei Heath System Institutional Review Board, and all participants provided signed informed consent forms after receiving a complete description of the study (IRB no. 4-2016-0187). A total of 35 retired firefighters and incumbents over 40 years old were prospectively included in this study. Participants underwent comprehensive medical and psychiatric interviews. According to the Structural Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders-IV Axis I Disorders, none of the participants were diagnosed with PTSD. The psychometric qualities of the Korean version of the Post-traumatic Diagnosis Scale (PDS) (16) was also performed. The PDS is a 17-item self-report instrument that can provide both a diagnosis of PTSD and measures of overall and subscale symptom severity (17). Respondents rate each item on a 4-point scale (0 = not at all to 3 = very much) over a period of the past month. A symptom is counted as present if a score of 1 or higher is selected. These frequency scores are summed to produce a severity score. The Seoul Neuropsychological Screening Battery (SNSB) was performed to determine the cognitive status by trained clinical neuropsychologists (18). The Seoul Verbal Learning Test recall test (memory), the Seoul Verbal Learning Test delayed recall test (memory), Rey Complex Figure delayed recall test (memory), Korean-Boston Naming Test (language), Rey Complex Figure copy test (visuospatial), Controlled Oral Word Association Test (COWAT) animal (frontal/executive), COWAT phonemic (frontal/executive), Stroop Test (frontal/executive), Korean-Trail Making Test-Elderly's version (frontal/executive), and Digit Symbol Coding correct (frontal/executive) was performed. The Beck anxiety inventory (BAI) (19), Center for Epidemiologic Studies Depression scale (CES-D) (20), Alcohol Use Disorders Identification Test (AUDIT) (21) and the Pittsburgh Sleep Quality Index (PSQI) (22) test were also performed. The exclusion criteria are as follows: 1) history of traumatic brain injury, 2) presence of comorbid medical conditions, 3) history of psychiatric disorder (e.g., psychotic or mood disorders), 4) history of substance abuse, and 5) absence of imaging sequences. A total of 31 firefighters (31 men, 49.8 ± 4.7 years) were enrolled. We also recruited 26 healthy controls (HCs) (26 men, 65.3 ± 7.8 years) who were sex-matched, serving as a general non-traumatized group. In the HC group, only the SNSB test was performed.
IMAGE ACQUISITION
MRI scanning was conducted on a 3T scanner (Achieva; Philips Healthcare, Best, the Netherlands or Ingenia CX; Philips Healthcare) with a 32-channel head coil. T2*-weighted functional neuroimaging data were collected with a single shot echo-planar imaging sequence, allowing for full-brain coverage collected axially [repetition time (TR) = 2000 ms, echo time (TE) = 40 ms, field of view (FOV) = 220 × 220 mm2, number of slices = 31 (interleaved), number of axial volumes = 165, voxel size = 2.75 × 2.75 × 4.5 mm3, flip angle (FA) = 90°, and total acquisition time = 5 min 38 sec]. Participants were instructed to rest and keep their eyes closed, lie still, and think of nothing in particular. For anatomical imaging, a 3 dimensional-T1-turbo field echo sequence was used with following parameters: TR = 9.9 ms, TE = 4.6 ms, FOV = 220 × 220 mm2, section gap = 0 mm, voxel size = 0.859 × 0.859 × 1.0 mm3, FA = 15°, total acquisition time = 5 min 29 sec. Head motion was minimized with restraining foam pads provided by the manufacturer.
IMAGE ANALYSIS
ANATOMICAL DATA PREPROCESSING AND VBM
All images were processed and analyzed using the CAT12 toolbox (C. Gaser, Structural Brain Mapping Group, Jena University Hospital, Jena, Germany; http://dbm.neuro.uni-jena.de/cat/) implemented in SPM12 (Wellcome Trust Centre for Neuroimaging; http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). CAT12 served as the platform for all the analyses, as it offers processing pipelines for VBM allowing us to perform all analysis with this software package. For processing- and analysis-steps, pre-set parameters in accordance with standard protocol (http://www.neuro.uni-jena.de/cat12/CAT12-Manual.pdf) were used, applying default settings unless indicated otherwise. Preprocessing included correction for bias-field inhomogeneities, normalization using the DARTEL-algorithm (23) and segmentation into GM, white matter (WM) and cerebrospinal fluid (CSF) (24). The segmentation was followed by accounting for partial volume effects (25). Data were smoothed with a 8 mm full-width at half-maximum (FWHM) Gaussian kernel. Processing also included a two-step quality assurance: first, all images were visually inspected for artefacts (prior to preprocessing); secondly, all underwent a statistical quality control for inter-subject homogeneity and overall image quality as included in the CAT12 toolbox (“check homogeneity” function) after segmentation. This second step again included a visual inspection procedure for potential newly introduced artefacts.
FUNCTIONAL DATA PREPROCESSING
Standard image preprocessing methods were conducted employing the SPM12 software (http://www.fil.ion.ucl.ac.uk/spm/) with the CONN toolbox (http://www.nitrc.org/projects/conn) for functional connectivity analysis in MATLAB R2017b (MathWorks Inc., Natick, Massachusetts, US) environment. The functional images were corrected for slice time and motion, co-registered with a high-resolution anatomical scan, normalized into the Montreal Neurological Institute space, resampled at 3 mm3 and smoothed with a Gaussian kernel of 8 mm3 FWHM. In addition, the ARtifact detection Tools (ART: http://www.nitrc.org/projects/artifact_detect) were used to measure motion artefacts in all individuals in both groups. We controlled for motion artefacts using 32 parameters: 12 realignment parameters with 1st order temporal derivatives, ten WM-related artefacts with 1st order temporal derivatives, and ten CSF-related artefacts with 1st order temporal derivatives by realignment parameters detected with ART.
ROIs DEFINITION
We identified the brain regions which showed significant [false discovery rate (FDR)-corrected p < 0.05] gray-matter volume difference in the VBM analysis and used the automated anatomical labeling to define the regions-of-interests (ROIs). As a result, the following two brain regions were selected: the left superior parietal gyrus and left inferior temporal gyrus.
RSFC ANALYSIS
Following the preprocessing steps outlined above, the blood oxygenated level-dependent (BOLD) signal data were passed through a band pass filter (0.009–0.08 Hz) within the CONN toolbox in SPM12 for further data correction. The mean BOLD signal time course was then extracted from each of the predefined ROIs. The time course for each ROI was then correlated with the time course of the whole-brain voxels, allowing for the calculation of a correlation coefficient for each ROI by Pearson's product–moment calculation.
STATISTICAL ANALYSIS
To analyze demographic data and neuropsychological test scores, assumptions of normal distribution were tested with the Kolmogorov-Smirnov test. For neuropsychological performance, z-scores according to age- and education-specific norms were compared between groups. Student's t-test and Mann-Whitney test were performed according to normality. Results were considered significant with p < 0.05.
For VBM analysis, we performed comparison of GM volumes in the two groups. In addition, we correlated VBM results with our neuropsychological scores, using a multiple regression design, with age as covariates and separate design matrices for each executive test. To allow more exploratory examination, the threshold for statistical analysis was first set to an uncorrected p < 0.001, and among the regions found from the uncorrected threshold, a voxellevel FDR-corrected p < 0.05 and a cluster-level uncorrected p < 0.05 were applied to reduce the potential Type I error. For RSFC analysis, the threshold for statistical analysis was first set to an uncorrected p < 0.001, and among the regions found from the uncorrected threshold, a cluster-level uncorrected p < 0.05 were applied. To exclude possible confounding factors, we covariated out age in the statistical test after normalization across 2 groups interest by using the z score function in Matlab in both VBM and RSFC analyses.
RESULTS
There were 31 subjects identified as the trauma-exposed firefighter group without PTSD (all male, mean age 49.8 ± 4.7 years), and 26 HC subjects (all male, mean age 65.3 ± 7.8 years). The demographic and clinical characteristics of each group are provided in Table 1. There were no differences in the years of educations (p = 0.22). The group was sex-matched, but the age was significantly different between 2 groups (p < 0.001). The firefighters showed BAI, CES-D, AUDIT, PDS, and PSQI scores of 5.10 ± 5.58, 7.61 ± 3.72, 8.03 ± 5.5, 4.35 ± 5.5, and 6.55 ± 3.06, respectively.
Table 1. Demographic and Clinical Characteristics.
| Trauma-Exposed Firefighters (n = 31) | Healthy Controls (n = 26) | p-Value* | |
|---|---|---|---|
| Age, years | 49.77 ± 4.70 | 65.31 ± 7.84 | < 0.001 |
| Gender, male | 31 (100) | 26 (100) | N/A |
| Education duration, years | 13.84 ± 2.35 | 12.65 ± 4.60 | 0.22 |
Data are mean ± standard deviation or n (%) values.
*Calculated from student t test for continuous variables and chi-square test for categorical variables, to compare the patient characteristics of firefighters and the healthy controls.
N/A = not available
NEUROPSYCHOLOGICAL TEST RESULTS
The results of neuropsychological tests presented as group mean z-scores based on age, education, and gender specific information in the firefighters and HC groups are summarized in Table 2. The Seoul Verbal Learning Test on immediate recall, delayed recall, COWAT animal, COWAT phonemic were significantly lower in the firefighters group than the HCs (p = 0.002, < 0.001, < 0.001, and < 0.001, respectively). The Rey Complex Figure copy test were significantly higher in the firefighters group than the controls (p = 0.001). There was no significant difference in the Rey Complex Figure copy test (p = 0.329), Korean-Boston Naming Test (p = 0.153), or Stroop color-word test (p = 0.22).
Table 2. Neuropsychological Test Results Presented as Mean Z-Scores Based on the Age, Educational Level, and Gender-Specific Normative Information.
| Trauma-Exposed Firefighters (n = 31) | Healthy Controls (n = 26) | p-Value* | |
|---|---|---|---|
| SVLT recall test | −0.53 ± 0.90 | 0.19 ± 0.85 | 0.002 |
| SVLT delayed recall test | −0.51 ± 0.92 | 0.36 ± 0.69 | < 0.001 |
| RCF delayed recall test | 0.59 ± 1.12 | 0.74 ± 0.92 | 0.329 |
| Naming K-BNT | 0.54 ± 0.83 | 0.41 ± 0.66 | 0.153 |
| RCF copy test | 0.76 ± 0.60 | 0.13 ± 0.68 | 0.001 |
| COWAT animal | −0.62 ± 0.95 | 0.42 ±1.00 | < 0.001 |
| COWAT phonemic | −0.20 ± 0.89 | 0.18 ± 0.67 | < 0.001 |
| Stroop Test-color reading correct | 0.08 ± 0.71 | 0.33 ± 0.83 | 0.22 |
*Calculated from Student t test for continuous variables to compare the patient characteristics of firefighters and the healthy controls.
COWAT = Controlled Oral Word Association Test, K-BNT = Korean-Boston Naming Test, RCF = Rey Complex Figure, SVLT = Seoul Verbal Learning Test
VBM RESULTS
GROUP COMPARISON OF GM VOLUMES
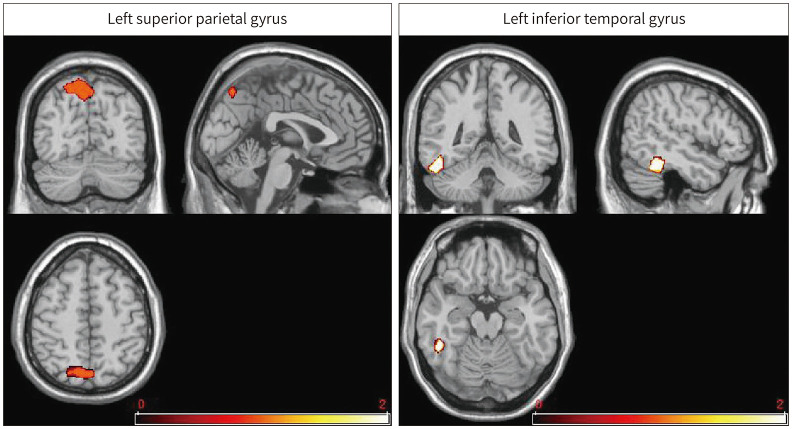
Compared with the HCs, the firefighters showed lower GM volumes at the left superior parietal (p < 0.05, FDR-corrected) and left inferior temporal gyrus (p < 0.05, FDR-corrected) relative to the HCs (Fig. 1, Table 3). No GM increase was found in firefighters compared with HCs. These significantly different areas on VBM were used as seeds to perform seed-based functional connectivity analyses.
Fig. 1. Brain regions showing less gray-matter volume in trauma-exposed firefighters than in healthy controls (p < 0.05, false discovery rate-corrected).
Table 3. Decreased Gray-Matter Volume in Trauma-Exposed Firefighters Compared to Healthy Controls.
| Brain Regions | Peak MNI Coordinates | Voxels (n) | Peak t Value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Left superior parietal gyrus | −12 | −69 | 54 | 1104 | 5.76 |
| Left inferior temporal gyrus | −49.5 | −45 | −19.5 | 450 | 4.47 |
The regions that survived a whole-brain false discovery rate-corrected threshold of p < 0.05, cluster size > 20.
MNI = Montreal Neurological Institute
CORRELATIONS BETWEEN NEUROPSYCHOLOGICAL TEST AND VBM DATA
No significant correlation was found for the neuropsychological variables and VBM data.
rsfMRI RESULTS
SEED-BASED RSFC RESULTS
Group comparison of RSFC by using the left superior parietal gyrus as seed:
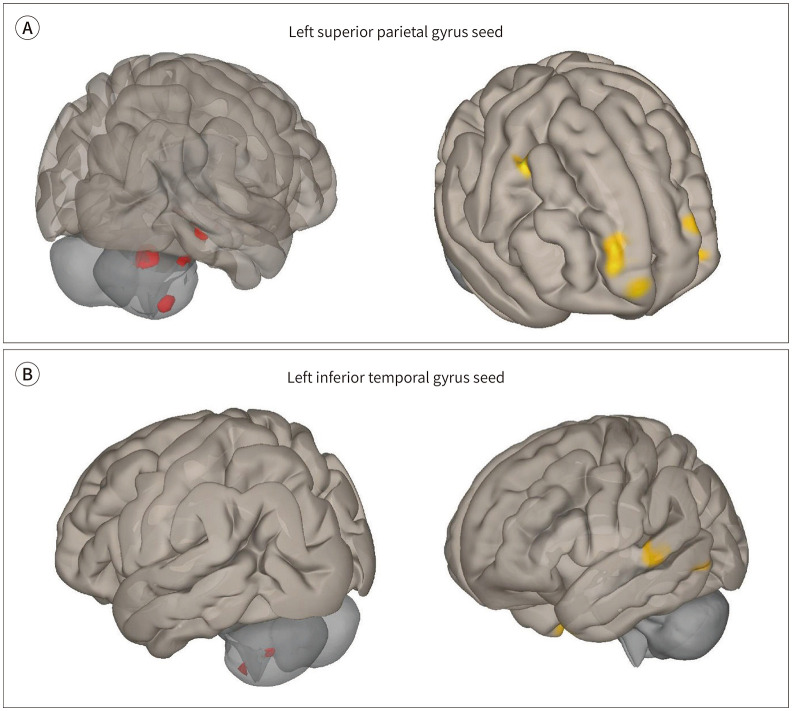
Compared with the HC, the firefighters showed decreased RSFC in the right superior frontal gyrus, right precentral gyrus, right fusiform gyrus, left superior frontal gyrus, left inferior orbital gyrus, and left superior frontal gyrus (uncorrected voxel-wise p < 0.001, uncorrected cluster-wise p < 0.05). The firefighters showed increased RSFC in the right cerebellum lobule VIII, left parahipppocampal gyrus, right fusiform gyrus, and right cerebellum lobules IV and V (uncorrected voxel-wise p < 0.001, uncorrected cluster-wise p < 0.05).
Group comparison of RSFC by using the left inferior temporal gyrus as seed:
Compared with the HC, the firefighters showed decreased RSFC in the left superior temporal gyrus, right precentral gyrus, and left middle temporal gyrus (uncorrected voxel-wise p < 0.001, uncorrected cluster-wise p < 0.05). The firefighters showed increased RSFC in the right cerebellum lobules VI and IX (uncorrected voxel-wise p < 0.001, uncorrected cluster-wise p < 0.05). The results of RSFC using the left superior parietal gyrus and left inferior temporal gyrus is summarized in Fig. 2, Table 4.
Fig. 2. Seed-based functional connectivity analysis in firefighters using the left superior parietal gyrus and left inferior temporal gyrus as seeds. Red color indicates increased connectivity, and yellow color indicates decreased connectivity in the firefighter group compared to the healthy controls (uncorrected voxel-wise p < 0.001, uncorrected cluster-wise p < 0.05).
A. Results of tests using the left superior parietal gyrus as a seed.
B. Results of tests using the left inferior temporal gyrus as a seed.
Table 4. Regions Showing Significant Differences in Functional Connectivity when Using the Left Superior Parietal Gyrus and Left Inferior Temporal Gyrus as Seeds.
| Brain Regions | Peak MNI Coordinates | Voxels (n) | Peak t Value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Seed: left superior parietal gyrus | |||||
| HC > firefighter | |||||
| Right superior frontal | 24 | 68 | 12 | 383 | 5.41 |
| Right precentral gyrus | 42 | 2 | 48 | 108 | 5.02 |
| Right fusiform gyrus | 32 | 12 | −44 | 100 | 4.97 |
| Left superior frontal gyrus | −24 | 60 | 22 | 53 | 4.37 |
| Left inferior orbital gyrus | −24 | 28 | −22 | 50 | 4.34 |
| Left superior frontal gyrus | −30 | 54 | 0 | 42 | 4.12 |
| Firefighter > HC | |||||
| Right lobule VIII of cerebellar hemisphere | 26 | −50 | −54 | 150 | 7.85 |
| Left parahippocampal gyrus | −12 | −2 | −20 | 95 | 4.66 |
| Right fusiform gyrus | 36 | −38 | −16 | 66 | 4.37 |
| Right lobule IV, V of cerebellar hemisphere | 30 | −38 | −28 | 76 | 3.97 |
| Seed: left inferior temporal gyrus | |||||
| HC > firefighter | |||||
| Left superior temporal gyrus | −60 | −24 | 2 | 74 | 4.31 |
| Right precentral gyrus | 52 | 0 | 34 | 104 | 4.20 |
| Left middle temporal gyrus | −48 | −58 | −4 | 53 | 4.16 |
| Firefighter > HC | |||||
| Left lobule IX of cerebellar hemisphere | −2 | −25 | −46 | 64 | 4.51 |
| Right lobule VI of cerebellar hemisphere | 30 | −52 | −32 | 53 | 4.17 |
The regions that survived a threshold of uncorrected voxel-wise p < 0.001, uncorrected cluster-wise p < 0.05.
HC = healthy control, MNI = Montreal Neurological Institute
DISCUSSION
Our study evaluated structural brain and resting state functional activity alterations to determine difference between trauma-exposed firefighters and HCs. The firefighters showed lower mean z-scores on neurocognitive abilities compared to the HCs. The VBM results revealed trauma-exposed structural abnormalities in left superior frontal gyrus and left inferior temporal gyrus. Also, trauma-exposed alterations in rsfMRI values were noted in multiple regions, including the fusiform gyrus and cerebellum.
Disturbances in intrinsic brain function have already been suggested to contribute to neurocognitive impairments in patients with psychiatric disorders, including PTSD (26,27). Previous studies on childhood trauma has shown that traumatized patients show poor performance on measures of executive function, processing speed, and working memory (28,29). However, the neurocognitive function and the relationships between neurocognitive function and resting state functional activity alterations has not been clear in trauma-exposed firefighters. Our study results suggest that exposure to traumatic stress events in firefighters might adversely impact the cognitive systems that support executive functioning, even in firefighters without PTSD.
In our study, the VBM results showed trauma-exposed structural abnormalities in left superior parietal gyrus and left inferior temporal gyrus. The left superior parietal gyrus is known to contribute to higher cognitive functions and working memory processing. The decreased volume of the left superior parietal gyrus and left inferior temporal gyrus in firefighters may partially account for the fact of decreased neurocognitive test results; the superior parietal gyrus is related to spatial orientation and attention-related activation (30), whereas the inferior temporal gyrus is critical for working and recognition memory (31). These results may reflect neural damage due to release of neurotoxic agents induced by traumatic stress (32). Previous studies have shown that different types of trauma exposure leads to heterogeneous VBM results (33), and further studies are indicated to assess the generalizability of these results. As our study compared trauma-exposed firefighters from non-trauma-exposed HCs, our findings may be affected by the stressor but not a PTSD effect.
Although there are ample studies focusing either on the brain structural or functional differences of PTSD compared to either a non-trauma or a trauma-exposed control groups, there is limited literature focusing in the differences between a trauma-exposed group to HCs. Also, multiple previous studies have demonstrated impaired fusiform activity and connectivity in PTSD (34,35); alteration in fusiform gyrus activity was also seen in firefighters from our studies, although it did not reach statistical significance. These impairments in connectivity may underline symptoms of trauma-exposed subjects. There is a growing body of evidence indicating that disrupted cerebellar activity may contribute to psychiatric illness; a previous metaanalysis of rsfMRI in PTSD showed altered activities in the cerebellar hemisphere (cerebellar pyramis) in PTSD patients (36), which may be related to our results (36). A recent study focusing on trauma-exposed firefighters with partial PTSD reported that partial PTSD individuals exhibited altered global network properties as well as altered local properties in the temporal and parietal cortices (37), which are similar to our results Another recent study demonstrated that the right anterior insula could be a core region of the network undergoing changes after experiencing a traumatic or painful event, but may not be specifically involved in the development of PTSD, but this finding was not noted in this study (38).
The study has several limitations. First, this study was performed with a relatively small sample size. Our results should be considered preliminary until confirmed in larger samples. Second, the age was not matched between the firefighters and HCs. Although we performed regression analysis to compensate for the age difference, limitation remains and there may have been more widespread differences in the results if the age of HCs was matched. Third, because none of the firefighters were diagnosed with PTSD, no PTSD cases were included. Fourth, the results from the seed-based RSFC did not reach statistical significance with FDRcorrected p-value; large studies are indicated for further validation. Fourth, correlation analyses were not performed between neuropsychological test and rsfMRI results.
In conclusion, structural brain and resting-state functional abnormalities may be useful imaging biomarkers for identifying alterations in trauma-exposed firefighters without PTSD.
Acknowledgments
This research was supported by the Fire Fighting Safety & 119 Rescue Technology Research and Development Program funded by National Fire Agency (“MPSS-Firesafety-2015-80”).
Footnotes
- Conceptualization, P.Y.W., K.C., L.S.
- data curation, P.Y.W., N.J., C.S.J.
- formal analysis, J.S.
- investigation, N.J., K.C.
- methodology, J.S.
- project administration, L.P.H., K.C., L.S.
- resources, K.C.
- software, J.S.
- supervision, L.P.H., K.C., L.S.
- validation, K.C.
- writing—original draft, P.Y.W.
- writing—review & editing, P.Y.W., J.S., L.S.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Beaton RD, Murphy SA. Sources of occupational stress among firefighter/EMTs and firefighter/paramedics and correlations with job-related outcomes. Prehosp Disaster Med. 1993;8:140–150. doi: 10.1017/s1049023x00040218. [DOI] [PubMed] [Google Scholar]
- 2.Corneil W, Beaton R, Murphy S, Johnson C, Pike K. Exposure to traumatic incidents and prevalence of post-traumatic stress symptomatology in urban firefighters in two countries. J Occup Health Psychol. 1999;4:131–141. doi: 10.1037//1076-8998.4.2.131. [DOI] [PubMed] [Google Scholar]
- 3.Bryant RA, Harvey AG. Posttraumatic stress in volunteer firefighters. Predictors of distress. J Nerv Ment Dis. 1995;183:267–271. doi: 10.1097/00005053-199504000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Wagner D, Heinrichs M, Ehlert U. Prevalence of symptoms of posttraumatic stress disorder in German professional firefighters. Am J Psychiatry. 1998;155:1727–1732. doi: 10.1176/ajp.155.12.1727. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Wu M, Liao Y, Ouyang L, Du M, Lei D, et al. Grey matter reduction associated with posttraumatic stress disorder and traumatic stress. Neurosci Biobehav Rev. 2014;43:163–172. doi: 10.1016/j.neubiorev.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Meng Y, Qiu C, Zhu H, Lama S, Lui S, Gong Q, et al. Anatomical deficits in adult posttraumatic stress disorder: a meta-analysis of voxel-based morphometry studies. Behav Brain Res. 2014;270:307–315. doi: 10.1016/j.bbr.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 7.O'Doherty DC, Chitty KM, Saddiqui S, Bennett MR, Lagopoulos J. A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry Res. 2015;232:1–33. doi: 10.1016/j.pscychresns.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes KC, Shin LM. Functional neuroimaging studies of post-traumatic stress disorder. Expert Rev Neurother. 2011;11:275–285. doi: 10.1586/ern.10.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stark EA, Parsons CE, Van Hartevelt TJ, Charquero-Ballester M, McManners H, Ehlers A, et al. Post-traumatic stress influences the brain even in the absence of symptoms: a systematic, quantitative meta-analysis of neuroimaging studies. Neurosci Biobehav Rev. 2015;56:207–221. doi: 10.1016/j.neubiorev.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 2012;71:286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Brandes D, Ben-Schachar G, Gilboa A, Bonne O, Freedman S, Shalev AY. PTSD symptoms and cognitive performance in recent trauma survivors. Psychiatry Res. 2002;110:231–238. doi: 10.1016/s0165-1781(02)00125-7. [DOI] [PubMed] [Google Scholar]
- 13.Lee RS, Hermens DF, Porter MA, Redoblado-Hodge MA. A meta-analysis of cognitive deficits in first-episode major depressive disorder. J Affect Disord. 2012;140:113–124. doi: 10.1016/j.jad.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 14.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 15.Lv H, Wang Z, Tong E, Williams LM, Zaharchuk G, Zeineh M, et al. Resting-state functional MRI: everything that nonexperts have always wanted to know. AJNR Am J Neuroradiol. 2018;39:1390–1399. doi: 10.3174/ajnr.A5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nam BR, Kwon HI, Kwon JH. Psychometric qualities of the Korean version of the Post-traumatic Diagnosis Scale (PDS-K) Korean J Clin Psychol. 2010;29:147–167. [Google Scholar]
- 17.Foa EB, Cashman L, Jaycox L, Perry K. The validation of a self-report measure of posttraumatic stress disorder: The Posttraumatic Diagnostic Scale. Psychol Assess. 1997;9:445. [Google Scholar]
- 18.Kang Y, Na DL, Hahn S. Seoul neuropsychological screening battery. Incheon: Human Brain Research & Consulting Co; 2003. [Google Scholar]
- 19.Ulusoy M, Sahin NH, Erkmen H. The Beck anxiety inventory: psychometric properties. J Cogn Psychother. 1998;12:163–172. [Google Scholar]
- 20.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 21.Saunders JB, Aasland OG, Babor TF, De la, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption--II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 22.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 23.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 25.Tohka J, Zijdenbos A, Evans A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage. 2004;23:84–97. doi: 10.1016/j.neuroimage.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Parslow RA, Jorm AF. Pretrauma and posttrauma neurocognitive functioning and PTSD symptoms in a community sample of young adults. Am J Psychiatry. 2007;164:509–515. doi: 10.1176/ajp.2007.164.3.509. [DOI] [PubMed] [Google Scholar]
- 27.Huang M, Lu S, Yu L, Li L, Zhang P, Hu J, et al. Altered fractional amplitude of low frequency fluctuation associated with cognitive dysfunction in first-episode drug-naïve major depressive disorder patients. BMC Psychiatry. 2017;17:11. doi: 10.1186/s12888-016-1190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saleh A, Potter GG, McQuoid DR, Boyd B, Turner R, MacFall JR, et al. Effects of early life stress on depression, cognitive performance and brain morphology. Psychol Med. 2017;47:171–181. doi: 10.1017/S0033291716002403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu S, Pan F, Gao W, Wei Z, Wang D, Hu S, et al. Neural correlates of childhood trauma with executive function in young healthy adults. Oncotarget. 2017;8:79843–79853. doi: 10.18632/oncotarget.20051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rizzolatti G, Fogassi L, Gallese V. Parietal cortex: from sight to action. Curr Opin Neurobiol. 1997;7:562–567. doi: 10.1016/s0959-4388(97)80037-2. [DOI] [PubMed] [Google Scholar]
- 31.Miller EK, Li L, Desimone R. A neural mechanism for working and recognition memory in inferior temporal cortex. Science. 1991;254:1377–1379. doi: 10.1126/science.1962197. [DOI] [PubMed] [Google Scholar]
- 32.Jatzko A, Rothenhöfer S, Schmitt A, Gaser C, Demirakca T, Weber-Fahr W, et al. Hippocampal volume in chronic posttraumatic stress disorder (PTSD): MRI study using two different evaluation methods. J Affect Disord. 2006;94:121–126. doi: 10.1016/j.jad.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, Tan Q, Yin H, Zhang X, Huan Y, Tang L, et al. Decreased gray matter volume in the left hippocampus and bilateral calcarine cortex in coal mine flood disaster survivors with recent onset PTSD. Psychiatry Res. 2011;192:84–90. doi: 10.1016/j.pscychresns.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Wu RZ, Zhang JR, Qiu CJ, Meng YJ, Zhu HR, Gong QY, et al. Study on resting-state default mode network in patients with posttraumatic stress disorder after the earthquake. Sichuan Da Xue Xue Bao Yi Xue Ban. 2011;42:397–400. [PubMed] [Google Scholar]
- 35.Yin Y, Jin C, Eyler LT, Jin H, Hu X, Duan L, et al. Altered regional homogeneity in post-traumatic stress disorder: a resting-state functional magnetic resonance imaging study. Neurosci Bull. 2012;28:541–549. doi: 10.1007/s12264-012-1261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koch SB, Van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M. Aberrant resting-state brain activity in posttraumatic stress disorder: a meta-analysis and systematic review. Depress Anxiety. 2016;33:592–605. doi: 10.1002/da.22478. [DOI] [PubMed] [Google Scholar]
- 37.Jung WH, Chang KJ, Kim NH. Disrupted topological organization in the whole-brain functional network of trauma-exposed firefighters: a preliminary study. Psychiatry Res Neuroimaging. 2016;250:15–23. doi: 10.1016/j.pscychresns.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Preis MA, Schmidt-Samoa C, Dechent P, Kroener-Herwig B. The effects of prior pain experience on neural correlates of empathy for pain: an fMRI study. Pain. 2013;154:411–418. doi: 10.1016/j.pain.2012.11.014. [DOI] [PubMed] [Google Scholar]