Abstract
Purpose
The purpose of this study was to describe the clinical features and chest computed tomography (CT) findings of coronavirus disease 2019 (COVID-19) pneumonia.
Materials and Methods
An Institutional Review Board-approved retrospective review was performed for 51 laboratory-confirmed COVID-19 pneumonia patients. Patients were divided into two groups depending on their clinical status: mild and severe. Clinical characteristics and chest CT findings were compared between the two groups.
Results
Among the 51 patients (22 men, 29 women; mean age, 56.5 ± 16 years; range, 22–88 years), 37 (72.5%) were in the mild group and 14 (27.5%) were in the severe group. The patients in the severe group (68.7 ± 12.5 years) were older than the patients in the mild group (51.8 ± 14.9 years, p < 0.001). Premorbid conditions and decreased lymphocyte counts were more often observed in the severe group than in the mild group (71% vs. 41%, p = 0.049 and 86% vs. 32%, p = 0.001, respectively). On chest CT, most patients exhibited a mixed ground-glass opacification (GGO) with consolidation (76%) or a GGO (22%) pattern. The majority of lesions were predominantly bilateral in the lower lung with a posterior, peripheral distribution. The patients in the severe group had higher severity scores than those in the mild group.
Conclusion
Patients with laboratory-confirmed COVID-19 pneumonia have typical chest CT findings that provide important information regarding expected disease severity.
Keywords: Coronavirus Disease 2019; COVID-19; Pneumonia; Computed Tomography, X-Ray; Republic of Korea
Abstract
목적
본 연구는 Coronavirus disease 2019 (이하 COVID-19) 폐렴 환자의 임상 양상과 흉부전산화단층촬영(이하 CT) 소견을 분석하고자 하였다.
대상과 방법
IRB의 승인을 받은 연구로, 51명의 COVID-19 확진 환자들을 후향적으로 분석하였다. 환자들을 임상 양상에 따라 경증과 중증으로 나누어 두 그룹 간에 임상 양상과 흉부 CT 소견을 비교하였다.
결과
총 51명의 환자(남자 22명, 여자 29명, 평균 56.5 ± 16세, 범위 22~88세) 중 37명(72.5%)은 경증, 14명(27.5%)은 중증이었다. 중증 환자들의 평균 연령(68.7 ± 12.5세)은 경증 환자들(51.8 ± 14.9세)보다 많았다(p < 0.001). 중증 환자가 기저질환을 가지고 있는 경우가 많았으며(71% vs. 41%, p = 0.049), 혈액검사에서 림프구 백분율 감소를 보이는 경우가 많았다(86% vs. 32%, p = 0.001). 흉부 CT 소견은 대부분의 환자들에서 간유리음영과 폐경화가 혼합된 양상이거나(76%) 간유리음영으로(22%) 나타났고, 양측 폐 하부, 후방, 변연부에 나타나는 경우가 많았다. 중증 환자에서 병변이 더 많은 수의 폐엽을 침범했고, CT 위중도 점수도 높았다.
결론
COVID-19 폐렴 확진 환자의 특징적인 흉부 CT 소견을 숙지하는 것이 빠른 진단과 적절한 치료에 도움이 될 것이다.
INTRODUCTION
On December 31, 2019, an outbreak of pneumonia of unknown etiology was confirmed in Wuhan City, Hubei Province of China and informed to the World Health Organization (WHO) China Country Office (1). On January 7, 2020, the Chinese Center for Disease Control and Prevention identified a new type of coronavirus, which was isolated from patient lower respiratory tract samples (1). On February 11, 2020, the International Committee on Taxonomy of Viruses named this novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (2), and the WHO named the infection caused by this novel corona virus identified in 2019, coronavirus disease 2019 (COVID-19) (3). Despite considerable efforts to reduce transmission, the infection spread rapidly via human-to-human transmission through respiratory droplets and close contacts (4), and in January 2020, patients in Thailand, Japan, and South Korea were reported (1). On January 30, 2020, the WHO declared a public health emergency (5), and less than 3 months since the outbreak occurred in Wuhan City, the infection had spread globally. On March 11, 2020, the WHO officially declared the COVID-19 outbreak a pandemic (6). As of April 7, 2020, the SARS-CoV-2 infection has been confirmed in 1279722 patients including 72614 deaths globally (7). In South Korea, the first confirmed case of COVID-19 was reported on January 20, 2020 (8), and additional clusters of the infection emerged in Daegu City, the epicenter of the local outbreak in South Korea, on February 18, 2020. This local outbreak in Daegu City was found to be due to rapid spread of the virus, because many people had gathered to worship at a religious facility (“Shincheonji”) in Daegu City. Since then, the number of confirmed cases of COVID-19 in South Korea has increased rapidly. As of April 7, 2020, South Korea has reported a total of 10331 laboratory-confirmed cases (6794 in Daegu City), including 192 deaths (7,9).
Real-time reverse transcription polymerase chain reaction (RT-PCR) of viral nucleic acid is considered a reference standard for the diagnosis of COVID-19; however, several recently published studies have reported the importance of chest computed tomography (CT) findings in patients with false-negative RT-PCR (10,11,12). Chest CT plays an important role in the diagnosis and management of COVID-19 pneumonia. CT is a useful modality for early detection of lung abnormalities, assessment of the severity of the disease, and monitoring during treatment (13,14,15). The purpose of this study was to describe the chest CT findings of COVID-19 pneumonia and investigate the relationship between patient clinical features and chest CT findings.
MATERIALS AND METHODS
This retrospective study was approved by the Institutional Review Board; the requirement of informed consent was waived (IRB No. 2020-03-019).
PATIENTS
Between February 24, 2020 and March 8, 2020, 75 consecutive patients who were admitted to our hospital with laboratory-confirmed SARS-CoV-2 infection using RT-PCR were screened. Of these, 56 patients who underwent chest CT after admission were included; thereafter, we excluded patients who had normal findings on chest CT. The clinical data analyzed were as follows: age, sex, premorbid conditions, symptoms, date of onset of symptoms, and laboratory findings. As there were various treatment regimens depending on patient clinical status, we grouped patients into two groups—mild and severe; the patients in the mild group presented with mild symptoms (e.g., fever, mild respiratory symptoms) and needed general hospitalization (supportive care without mechanical ventilation therapy). The patients in the severe group presented with severe clinical symptoms (e.g., respiratory distress with respiratory rate ≥ 30 times/min, oxygen saturation < 93% at rest, arterial oxygen partial pressure/fractional inspired oxygen ≤ 300 mm Hg, or respiratory failure needing mechanical ventilation, shock, or a combination with extrapulmonary organ failure) and needed intensive care unit management.
IMAGE ACQUISITION
All chest CT scans were obtained using a multi-detector CT scanner (Somatom Definition AS+, Siemens Healthineers, Erlangen, Germany), and the scanning protocol included both non-enhanced (37 patients) and contrast-enhanced (14 patients) scans. The scanning parameters were as follows: detector collimation, 128 × 0.6 mm; beam pitch, 1.2; gantry speed, 0.5 s/rotation; tube voltage, 100–120 kVp; tube current with automatic exposure control, non-enhanced scan (reference 50 mAs, ranging between 32 and 82 mAs) and contrast-enhanced scan (reference 134 mAs, ranging between 120 and 250 mAs); slice thickness, 3.0 mm; reconstruction interval, 3.0 mm; and a sharp reconstruction kernel. Contrast medium (Iomeprol, Iomeron 350®; BRACCO, Milan, Italy) was administered using a mechanical injector (Stellant, Medrad®, Bayer, Leverkusan, Germany). All CT scans were obtained with the patient in a supine position at full inspiration.
IMAGE ANALYSIS
Two radiologists (with 7 and 10 years of chest imaging experience, respectively) reviewed the chest CT images independently on a picture archiving and communication system (PACS, INFINITT Healthcare Co., Ltd., Seoul, Korea), and final decisions were reached by consensus. The readers evaluated the chest CT images on both lungs (width 1500 HU; level, −600 HU) and mediastinal (width, 400 HU; level, 40 HU) window settings; images were analyzed with a focus on the 1) pattern of the lesions [e.g., ground glass opacification (GGO), mixed pattern (combined consolidation and GGO), and consolidation without GGO], 2) laterality, and 3) distribution in both axial (peripheral, central, mixed) and anteroposterior locations (anterior, posterior, mixed), 4) number of involved lobes, 5) degree of disease involvement for each lobe measured using a “total severity score,” as detailed below, and 6) other findings [e.g., interlobular septal thickening, air bronchogram, adjacent pleural thickening, pleural effusion, lymph node enlargement (≥ 10 mm in the short-axis dimension), reversed halo sign, and pulmonary embolism]. All descriptive terms for chest CT imaging followed the Fleischner Society glossary of terms for thoracic imaging (16). A lesion involving the outer one-third of the lung was defined as peripheral, and a lesion involving the inner two-thirds of the lung was defined as central. Lesions without the predilection of peripheral or central regions were defined as mixed. The anteroposterior location was determined based on the horizontal line dividing the lung into anterior and posterior halves.
Using the “total lung severity score,” the degree of involvement of each lobe was estimated, and the severity of the lesion was evaluated. The degree of involvement of each of the five lobes was scored as follows: 0, no involvement; 1, 1–25% involvement; 2, 26–50% involvement; 3, 51–75% involvement; or 4, 76–100% involvement. The “total lung severity score” of CT was the sum of the individual scores from each lobe and ranged from 0 to 20.
DATA AND STATISTICAL ANALYSIS
All statistical analyses were performed using SPSS statistics version 25 for Windows (IBM Corp., Armonk, NY, USA), and p < 0.05 was considered significantly different. Continuous variables were presented as the mean ± standard deviation and tested using Student's t-test. Categorical variables were presented as numbers and percentages and were compared using the chi square test or Fisher's exact test.
RESULTS
CLINICAL CHARACTERISTICS
Five patients (1 man and 4 women; mean age, 25.6 ± 7.5 years; range, 21–39 years) were excluded based on normal CT findings. The clinical characteristics and laboratory findings of the 51 included patients are described in Table 1. Of these patients with COVID-19 pneumonia and chest CT scans enrolled in this study, 37 (72.5%) were in the mild group and 14 (27.5%) were in the severe group. These included 22 (43%) men and 29 (57%) women with an age range of 22–88 years (mean, 56.5 ± 16 years). For patients in the mild group, the mean age and age range were 51.8 ± 14.9 years and 22–88 years, respectively, while for patients in the severe group, the mean age and age range were 68.7 ± 12.5 years and 38–84 years, respectively. Patients in the severe group were significantly older than those in the mild group (p < 0.001).
Table 1. Clinical Characteristics and Laboratory Findings of the 51 Patients with COVID-19 Pneumonia.
| Characteristics | Total (n = 51) | Mild (n = 37) | Severe (n = 14) | p-Value§ |
|---|---|---|---|---|
| Age (years)‡ | < 0.001 | |||
| Median age (range) | 58 (22–88) | 54 (22–88) | 71 (38–84) | |
| Mean age (SD) | 56.5 (16) | 51.8 (14.9) | 68.7 (12.5) | |
| Sex | 0.543 | |||
| Men | 22 (43) | 15 (41) | 7 (50) | |
| Women | 29 (57) | 22 (59) | 7 (50) | |
| Premorbid condition | ||||
| Any* | 25 (49) | 15 (41) | 10 (71) | 0.049 |
| Hypertension | 14 (28) | 11 (30) | 3 (21) | 0.730 |
| Diabetes | 8 (16) | 4 (11) | 4 (29) | 0.192 |
| Cardiovascular disease | 7 (14) | 4 (11) | 3 (21) | 0.376 |
| Malignancy | 4 (8) | 1 (3) | 3 (21) | 0.058 |
| COPD | 2 (4) | 1 (3) | 1 (7) | 0.478 |
| Asthma | 2 (4) | 1 (3) | 1 (7) | 0.478 |
| Chronic renal failure | 1 (2) | 0 (0) | 1 (7) | 1.000 |
| Symptoms | ||||
| Fever | 38 (75) | 26 (70) | 12 (86) | 0.472 |
| Maximum temperature, ℃ | 0.502 | |||
| < 37.3 | 13 (26) | 11 (30) | 2 (14) | |
| 37.3–38 | 19 (37) | 14 (38) | 5 (36) | |
| 38.1–39 | 15 (29) | 10 (27) | 5 (36) | |
| > 39 | 4 (8) | 2 (5) | 2 (14) | |
| Cough | 38 (75) | 29 (78) | 9 (64) | 0.309 |
| Dyspnea | 26 (51) | 17 (46) | 9 (64) | 0.242 |
| Sputum | 24 (47) | 17 (46) | 7 (50) | 0.796 |
| Headache | 16 (31) | 14 (38) | 2 (14) | 0.176 |
| Myalgia | 15 (29) | 12 (32) | 3 (21) | 0.513 |
| Sore throat | 9 (18) | 7 (19) | 2 (14) | 1.000 |
| Fatigue | 7 (14) | 6 (12) | 1 (7) | 0.657 |
| Diarrhea | 7 (14) | 6 (16) | 1 (7) | 0.657 |
| Nausea/vomiting | 5 (10) | 4 (11) | 1 (7) | 1.000 |
| Chest pain/tightness | 5 (10) | 4 (11) | 1 (7) | 1.000 |
| Anorexia | 5 (10) | 4 (11) | 1 (7) | 1.000 |
| Rhinorrhea | 2 (4) | 2 (5) | 0 (0) | 1.000 |
| Laboratory findings | ||||
| Normal or decreased leukocyte count | 45 (88) | 34 (92) | 11 (79) | 0.198 |
| Decreased lymphocyte percentile† | 24 (47) | 12 (32) | 12 (86) | 0.001 |
| Increased C-reactive protein level | 47 (92) | 34 (92) | 13 (93) | 1.000 |
Data are presented as mean (SD) or n (%).
*p < 0.05.
†p < 0.01.
‡p < 0.001.
§Difference between mild and severe group.
COPD = chronic obstructive pulmonary disease, COVID-19 = coronavirus disease 2019, SD = standard deviation
Among the 51 patients, 25 (49%) had a premorbid condition, such as hypertension, diabetes, cardiovascular disease, malignancy, chronic obstructive pulmonary disease, or chronic renal failure. Premorbid conditions were observed more frequently in patients in the severe group than in patients in the mild group (p = 0.049).
Symptoms of COVID-19 pneumonia included fever, cough, sputum, sore throat, rhinorrhea, dyspnea, chest pain, headache, myalgia, fatigue, diarrhea, nausea/vomiting, and anorexia. The most common symptoms were cough (75%), dyspnea (51%), and sputum (47%). Forty-five (88%) patients had normal or slightly decreased leukocyte counts, 24 (47%) had a decreased lymphocyte percentile, and 47 (92%) had increased C-reactive protein (CRP) levels. Patients in the severe group were more likely to have decreased lymphocyte percentiles than those in the mild group (p = 0.001).
CHEST CT IMAGING FINDINGS
The chest CT findings of the 51 patients with COVID-19 pneumonia are listed in Tables 2 and 3 and shown in Figs. 1, 2, 3, and 4. The mean time from development of symptoms to completion of the chest CT scan was 7.1 ± 4 days; there was no significant difference between the mild group and the severe group in this respect. Forty of the 51 (78%) patients had bilateral lung lesions, and four or five lobes were involved in 32 (63%) patients. A higher number of lobes was involved in patients in the severe group than those in mild group (p = 0.003). Bilateral lower lobes had higher lung severity scores in both groups. The total lung severity score was significantly higher in the severe group (13.4 ± 5) than that in mild group (6.2 ± 4, p < 0.001). Moreover, lung severity scores for each involved lobe were significantly higher in the severe group than in the mild group. The bilateral lower lobes were most commonly involved in both groups. Posterior (53%) and peripheral (71%) distributions were dominant overall, but severe patients more commonly exhibited mixed (anterior and posterior, central and peripheral) distributions.
Table 2. Distribution of the Lesions and CT Severity Scores in 51 Patients with COVID-19 Pneumonia.
| Characteristics | All (n = 51) | Mild (n = 37) | Severe (n = 14) | p-Value§ |
|---|---|---|---|---|
| Time from symptom development to chest CT (days) (mean, SD) | 7.1 (4) | 7.4 (4) | 6.4 (5) | 0.414 |
| Laterality | ||||
| Unilateral | 11 (22) | 10 (27) | 1 (7) | 0.251 |
| Bilateral | 40 (78) | 27 (73) | 13 (93) | |
| Number of involved lobes† | 0.003 | |||
| 1 | 9 (18) | 9 (24) | 0 (0) | |
| 2 | 4 (8) | 3 (8) | 1 (7) | |
| 3 | 6 (12) | 5 (14) | 1 (7) | |
| 4 | 2 (4) | 2 (5) | 0 (0) | |
| 5 | 30 (59) | 18 (49) | 12 (86) | |
| Lung severity score (mean, SD) | ||||
| Right upper lobe‡ | 1.3 (1) | 0.8 (1) | 2.5 (1) | < 0.001 |
| Right middle lobe‡ | 1.3 (1) | 0.8 (1) | 2.5 (1) | < 0.001 |
| Right lower lobe† | 2.1 (1) | 1.8 (1) | 2.9 (1) | 0.006 |
| Left upper lobe‡ | 1.5 (1) | 1.1 (1) | 2.6 (1) | < 0.001 |
| Left lower lobe† | 2.0 (1) | 1.7 (1) | 2.8 (1) | 0.005 |
| Total‡ | 2.0 (1) | 1.7 (1) | 2.8 (1) | 0.005 |
| Involved lobe distribution | ||||
| Right upper lobe* | 31 (60) | 19 (51) | 12 (86) | 0.025 |
| Right middle lobe† | 36 (71) | 22 (60) | 14 (100) | 0.005 |
| Right lower lobe | 43 (84) | 30 (81) | 13 (93) | 0.419 |
| Left upper lobe | 38 (75) | 25 (68) | 13 (93) | 0.082 |
| Left lower lobe | 45 (88) | 32 (86) | 13 (93) | 1.000 |
| Anteroposterior distribution† | 0.001 | |||
| Anterior | 1 (2) | 1 (3) | 0 (0) | |
| Posterior | 27 (53) | 25 (68) | 2 (14) | |
| Mixed | 23 (45) | 11 (29) | 12 (86) | |
| Central to peripheral distribution* | 0.019 | |||
| Central | 2 (4) | 1 (3) | 1 (7) | |
| Peripheral | 36 (71) | 30 (81) | 6 (43) | |
| Mixed | 13 (26) | 6 (16) | 7 (50) |
Data are presented as mean (SD) or n (%).
*p < 0.05.
†p < 0.01.
‡p < 0.001.
§Difference between mild and severe group.
COVID-19 = coronavirus disease 2019, CT = computed tomography, SD = standard deviation
Table 3. Chest CT Findings in 51 Patients with COVID-19 Pneumonia.
| CT Findings | All (n = 51) | Mild (n = 37) | Severe (n = 14) | p-Value‡ |
|---|---|---|---|---|
| Pattern of lesion | 0.229 | |||
| Pure GGO | 11 (22) | 10 (27) | 1 (7) | |
| Mixed GGO | 39 (76) | 26 (70) | 13 (93) | |
| Pure consolidation | 1 (2) | 1 (3) | 0 (0) | |
| Other findings | ||||
| Interlobular septal thickening† | 21 (41) | 11 (20) | 10 (71) | 0.007 |
| Air bronchogram* | 14 (28) | 7 (19) | 7 (50) | 0.026 |
| Adjacent pleural thickening | 5 (10) | 3 (8) | 2 (14) | 0.606 |
| Pleural effusion | 3 (6) | 1 (3) | 2 (14) | 0.179 |
| Reversed halo sign | 1 (2) | 1 (3) | 0 (0) | 1.000 |
| Lymphadenopathy | 0 (0) | 0 (0) | 0 (0) |
Data are represented as n (%).
*p < 0.05.
†p < 0.01.
‡Difference between mild and severe group.
COVID-19 = coronavirus disease 2019, CT = computed tomography, GGO = ground glass opacification
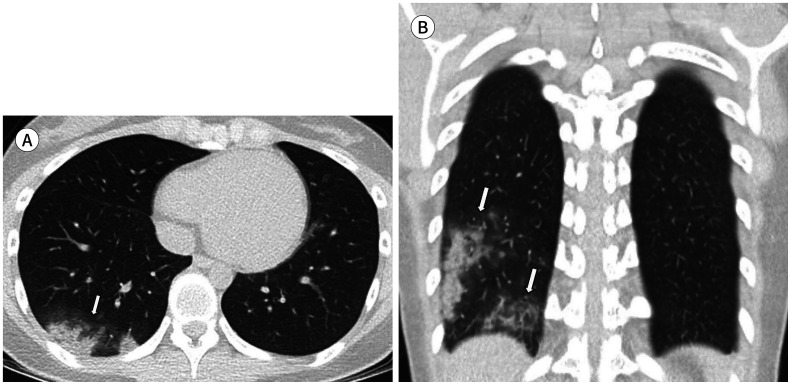
Fig. 1. A 22-year-old woman with coronavirus disease 2019 pneumonia in the mild group.
She had a headache, cough, and sputum 7 days before CT. Axial (A) and coronal (B) non-contrast chest CT show mixed ground glass opacification and consolidation in the posterior basal and lateral basal segments of the right lower lobe (arrow, A and arrows, B). The lesions are mainly present in a posterior, peripheral distribution. The total lung severity score was 1.
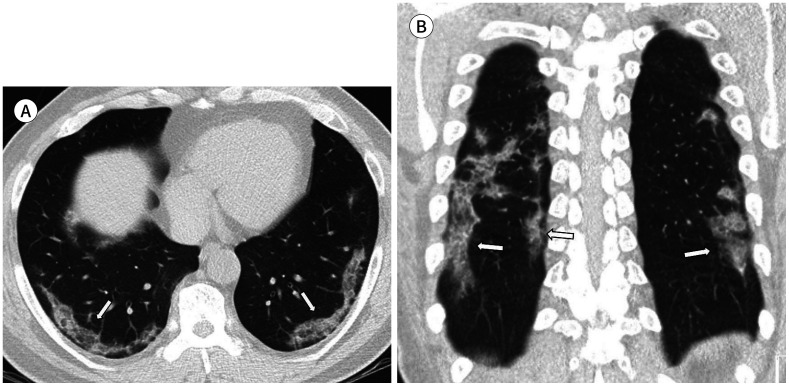
Fig. 2. A 53-year-old man with coronavirus disease 2019 pneumonia in the mild group.
He had a fever, cough, sputum, sore throat, and myalgia 12 days before CT. His premorbid condition was hypertension. Axial (A) and coronal (B) non-contrast chest CT show mixed ground glass opacification and consolidation in peripheral bilateral lower lobes (arrows). The total lung severity score was 6.
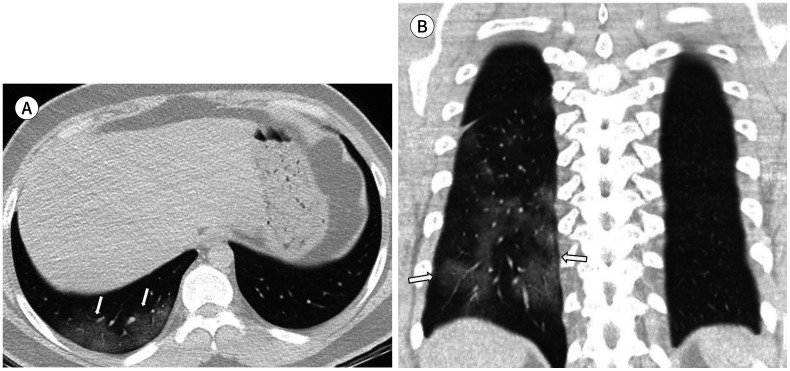
Fig. 3. A 23-year-old man with coronavirus disease 2019 pneumonia in the mild group.
He had a fever, headache, cough, and sputum 10 days before CT. Axial (A) and coronal (B) non-contrast chest CT show pure ground glass opacification in the right lower lobe with a posterior, peripheral distribution (arrows). The total lung severity score was 2.
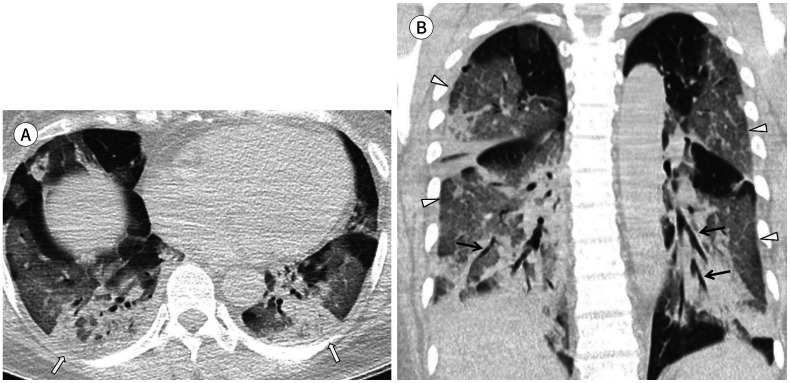
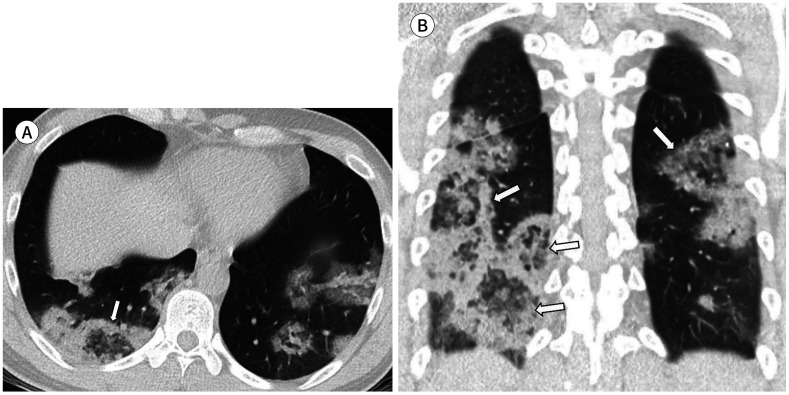
Fig. 4. A 50-year-old woman with coronavirus disease 2019 pneumonia in the severe group.
She had a fever, cough, and sputum 10 days before CT. Her premorbid condition was hypertension. Axial (A) and coronal (B) non-contrast chest CT scans show extensive involvement of mixed ground glass opacification and consolidation (white arrows). Multifocal interlobular septal thickening (arrowheads) and air bronchogram (black arrows) are also observed. The total lung severity score was 16.
Of the 51 patients, 39 (76%) had mixed GGO and consolidation, 11 (22%) had GGO, and 1 (2%) had pure consolidation. There was no significant difference between mild and severe groups regarding the pattern of lesions. Interlobular septal thickening was observed in 21 (41%) of 51 patients, including 11 of 37 (20%) in the mild group and 10 of 14 (71%) in the severe group. Air bronchogram was observed in 14 (28%) of 51 patients, including 7 (19%) mild and 7 (50%) severe patients. Interlobular septal thickening and air bronchogram were more often observed in the severe group than in the mild group (p = 0.007 and p = 0.026, respectively). Five of 51 (10%) patients had lesions with adjacent pleural thickening and 3 (6%) patients had pleural effusion. No patients had lymphadenopathy. Pulmonary embolism was not observed in patients with contrast-enhanced scans.
DISCUSSION
Before the novel SARS—CoV-2 virus, there have been six coronavirus strains known to be pathogenic in humans. Four of these are less pathogenic and cause mild respiratory infections; the other two have caused two outbreaks in the 21st century—SARS, caused by the SARS-CoV in 2003, and Middle East respiratory syndrome (MERS), caused by the MERS-associated coronavirus (MERS-CoV) in 2012 and 2015 (17). The chest CT findings of COVID-19 pneumonia show similarities with those of SARS and MERS pneumonia, that is, bilateral GGO and consolidative lesions with a peripheral distribution (18,19,20,21).
In this study, we analyzed clinical and chest CT findings of 51 patients with laboratory-confirmed COVID-19 pneumonia. Most (72.5%) of the patients were in the mild group. The patients in the severe group were older and had more premorbid conditions than patients in the mild group. The clinical symptoms of COVID-19 pneumonia were fever, myalgia, headache, and respiratory and gastrointestinal symptoms. The types of symptoms did not differ significantly between the mild and severe groups. Most patients had a normal or decreased leukocyte count (88%) and increased CRP (92%) levels, regardless of severity. Decreased lymphocyte percentiles were more often observed in patients in the severe group (86%) than in the mild group (32%). In most previous studies, leukocyte counts were normal or decreased, but CRP and lymphocyte counts varied (14,22,23,24,25).
Analysis of the chest CT findings of 51 patients with COVID-19 pneumonia revealed that most patients manifested with mixed GGO and consolidation (76%) or a pure GGO (22%) pattern. The lesions patterns were not different between the mild and severe groups, but the extent of the lesions and severity scores were higher in the severe group than those in the mild group. This is similar to the results of a previous study by Zhao et al. (13). In this study, overall, the lesions tended to involve both lower lungs with a posterior, peripheral distribution, but they appeared to be more extensive in the severe group—there was a wider invasion of the entire lung rather than a dominant distribution. However, the severity score of chest CT did not always correlate with clinical status for individual cases in each group. Some patients in the mild group showed high severity CT scores, and some patients in the severe group showed low severity CT scores. Considering the results of previous studies, this may be related to the duration of the disease (25).
Interestingly, interlobular septal thickening and air bronchogram were more often observed in the severe group than in the mild group. Zhou et al. (22) also reported that GGO with a reticular pattern (a reticular shadow on the background of GGO and interlobular septal thickening) and air bronchogram is more often observed in advanced-phase (8–14 days after the onset of symptoms) rather than early-phase (≤ 7 days after the onset of symptoms) disease. However, they divided the patients into early-phase and advanced-phase groups based on the time elapsed after symptom onset, while in this study, the time elapsed between symptom development and chest CT was not significantly different between groups. As the initial symptoms might be vague and the contact history in Daegu City, where regional infections were spreading, was unclear, there was a limit to knowing exactly when the disease began in the patients included in this study.
The reversed halo sign was observed in one patient (Fig. 5) in our study. The definition of a reversed halo sign is “a focal rounded area of GGO due to a more or less complete ring of consolidation” (16). The term was initially reported to be specific for cryptogenic organizing pneumonia (26,27), but has been subsequently used to describe other diseases, including pulmonary paracoccidioidomycosis (28). There have been several cases with the reversed halo sign in recently published reports of COVID-19 pneumonia (14,29,30,31,32), and it might reflect an organizing pneumonia pattern.
Fig. 5. A 39-year-old man with coronavirus disease 2019 pneumonia in the mild group with a multifocal reversed halo sign on chest CT.
He had a fever, headache and cough 6 days before CT. Axial (A) and coronal (B) non-contrast chest CT scans show a multifocal mixed ground glass opacification and consolidation pattern with a multifocal reversed halo sign (arrow, A and arrows, B). The total lung severity score was 9.
Grillet et al. (33) reported that 23% of patients with severe clinical features of COVID-19 pneumonia have acute pulmonary embolus on pulmonary CT angiography. In this study, pulmonary embolus was not observed in the 14 patients (8 in the mild group and 6 in the severe group) with contrast-enhanced scans.
This study had some limitations. First, we focused on initial chest CT findings at the time of diagnosis, and analysis of follow-up CT was not performed because most patients are still hospitalized and have not yet undergone follow-up CT scans. Moreover, the time of chest CT scan after symptom onset was variable, so it was difficult to evaluate CT findings that reflected the whole course of COVID-19 pneumonia. However, COVID-19 pneumonia is a new, highly contagious viral pneumonia that has caused a public health emergency; therefore, we considered urgent reporting necessary to establish appropriate management guidelines for the management of this virus. Second, the number of enrolled patients in our study was relatively small, and the comparison of CT findings may be deviated. Third, we do not know exactly when and where many of our patients were exposed to the virus. It is possible that most of our cases were imported, while some were secondary cases. It is also important to investigate whether viral mutations arise in the process of infection and whether they show different manifestations on chest CT. Finally, there was no histopathological data from deceased patients.
In summary, the most common chest CT findings in patients with COVID-19 pneumonia were the mixed GGO and consolidation or pure GGO patterns, predominantly involving the bilateral lower lungs with a posterior, peripheral distribution. In patients in the severe group, interlobular septal thickening and air bronchogram were more often observed, and the pneumonia appeared to be more extensive and have a wider invasion of the entire lung than a dominant distribution. The patients in the severe group were older and more likely to have premorbid conditions and a decreased lymphocyte percentile than patients in the mild group. In conclusion, patients with laboratory-confirmed COVID-19 pneumonia have typical chest CT findings that provide important information regarding expected disease severity. Thus, chest CT findings may help in the optimal management of COVID-19 pneumonia.
Footnotes
- Conceptualization, K.Y.S.
- data curation, all authors.
- formal analysis, K.Y.S.
- investigation, all authors.
- methodology, all authors.
- project administration, all authors.
- resources, all authors.
- supervision, K.Y.S.
- visualization, L.S.E.
- writing—original draft, all authors.
- writing—review & editing, all authors.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.World Health Organization. Coronavirus disease 2019 (COVID-19) situation report-1. 2020. Jan 21, [Accessed Apr 7, 2020]. Available at. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf?sfvrsn=20a99c10_4 .
- 2.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO director-general's remarks at the media briefing on 2019-nCoV. 2020. Feb 11, [Accessed Apr 7, 2020]. Available at. https://www.who.int/dg/speeches/detail/research-and-innovation-forum-on-novel-coronavirus-2019 .
- 4.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. WHO director-general's remarks at the media briefing on 2019-nCoV. 2020. Jan 30, [Accessed Apr 7, 2020]. Available at. https://www.who.int/dg/speeches/detail/who-director-general-s-statement-on-ihr-emergency-committee-on-novel-coronavirus-(2019-ncov)
- 6.World Health Organization. WHO director-general's opening remarks at the media briefing on COVID-19. 2020. Mar 11, [Accessed Apr 7, 2020]. Available at. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 .
- 7.World Health Organization. Coronavirus disease 2019 (COVID-19) situation report-78. 2020. Apr 07, [Accessed Apr 7, 2020]. Available at. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200407-sitrep-78-covid-19.pdf?sfvrsn=bc43e1b_2 .
- 8.Kim JY, Choe PG, Oh Y, Oh KJ, Kim J, Park SJ, et al. The first case of 2019 novel coronavirus pneumonia imported into Korea from Wuhan, China: implication for infection prevention and control measures. J Korean Med Sci. 2020;35:e61. doi: 10.3346/jkms.2020.35.e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korea Centers for Disease Control & Prevention. The uptates on COVID-19 in Korea as of 7 April 2000. 2020. Apr 07, [Accessed Apr 7, 2020]. Available at. https://www.cdc.go.kr/board/board.es?mid=a30402000000&bid=0030 .
- 10.Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020 doi: 10.1148/radiol.2020200343. [In press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang P, Liu T, Huang L, Liu H, Lei M, Xu W, et al. Use of chest CT in combination with negative RT-PCR assay for the 2019 novel coronavirus but high clinical suspicion. Radiology. 2020;295:22–23. doi: 10.1148/radiol.2020200330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang Y, Zhang H, Xie J, Lin M, Ying L, Pang P, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020 doi: 10.1148/radiol.2020200432. [In press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR Am J Roentgenol. 2020;214:1072–1077. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]
- 14.Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee KS. Pneumonia associated with 2019 novel coronavirus: can computed tomographic findings help predict the prognosis of the disease? Korean J Radiol. 2020;21:257–258. doi: 10.3348/kjr.2020.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 17.Guan CS, Lv ZB, Yan S, Du YN, Chen H, Wei LG, et al. Imaging features of coronavirus disease 2019 (COVID-19): evaluation on thin-section CT. Acad Radiol. 2020;27:609–613. doi: 10.1016/j.acra.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ajlan AM, Ahyad RA, Jamjoom LG, Alharthy A, Madani TA. Middle East respiratory syndrome coronavirus (MERS-CoV) infection: chest CT findings. AJR Am J Roentgenol. 2014;203:782–787. doi: 10.2214/AJR.14.13021. [DOI] [PubMed] [Google Scholar]
- 19.Das KM, Lee EY, Enani MA, AlJawder SE, Singh R, Bashir S, et al. CT correlation with outcomes in 15 patients with acute Middle East respiratory syndrome coronavirus. AJR Am J Roentgenol. 2015;204:736–742. doi: 10.2214/AJR.14.13671. [DOI] [PubMed] [Google Scholar]
- 20.Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong KT, Antonio GE, Hui DS, Lee N, Yuen EH, Wu A, et al. Thin-section CT of severe acute respiratory syndrome: evaluation of 73 patients exposed to or with the disease. Radiology. 2003;228:395–400. doi: 10.1148/radiol.2283030541. [DOI] [PubMed] [Google Scholar]
- 22.Zhou S, Wang Y, Zhu T, Xia L. CT features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR Am J Roentgenol. 2020;214:1287–1294. doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]
- 23.Xu X, Yu C, Qu J, Zhang L, Jiang S, Huang D, et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020;47:1275–1280. doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu YH, Dong JH, An WM, Lv XY, Yin XP, Zhang JZ, et al. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. J Infect. 2020;80:394–400. doi: 10.1016/j.jinf.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Z, Guo D, Li C, Fang Z, Chen L, Yang R, et al. Coronavirus disease 2019: initial chest CT findings. Eur Radiol. 2020 doi: 10.1007/s00330-020-06816-7. [In press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zompatori M, Poletti V, Battista G, Diegoli M. Bronchiolitis obliterans with organizing pneumonia (BOOP), presenting as a ring-shaped opacity at HRCT (the atoll sign). A case report. Radiol Med. 1999;97:308–331. [PubMed] [Google Scholar]
- 27.Kim SJ, Lee KS, Ryu YH, Yoon YC, Choe KO, Kim TS, et al. Reversed halo sign on high-resolution CT of cryptogenic organizing pneumonia: diagnostic implications. AJR Am J Roentgenol. 2003;180:1251–1254. doi: 10.2214/ajr.180.5.1801251. [DOI] [PubMed] [Google Scholar]
- 28.Gasparetto EL, Escuissato DL, Davaus T, De Cerqueira EM, Souza AS, Jr, Marchiori E, et al. Reversed halo sign in pulmonary paracoccidioidomycosis. AJR Am J Roentgenol. 2005;184:1932–1934. doi: 10.2214/ajr.184.6.01841932. [DOI] [PubMed] [Google Scholar]
- 29.Yoon SH, Lee KH, Kim JY, Lee YK, Ko H, Kim KH, et al. Chest radiographic and CT findings of the 2019 novel coronavirus disease (COVID-19): analysis of nine patients treated in Korea. Korean J Radiol. 2020;21:494–500. doi: 10.3348/kjr.2020.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang Y, Zhang H, Xu Y, Xie J, Pang P, Ji W. CT manifestations of two cases of 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295:208–209. doi: 10.1148/radiol.2020200280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020 doi: 10.1148/radiol.2020200463. [In press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye Z, Zhang Y, Wang Y, Huang Z, Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. 2020 doi: 10.1007/s00330-020-06801-0. [In press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grillet F, Behr J, Calame P, Aubry S, Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. 2020 doi: 10.1148/radiol.2020201544. [In press] [DOI] [PMC free article] [PubMed] [Google Scholar]