Abstract
Objectives
To review the diagnostic criteria for encephalitis and encephalopathy of presumed infectious etiology, as well as the diagnostic workup for viral encephalitis and its treatment approaches. The authors also intended to summarize relevant information on specific viruses frequently found in Brazil.
Source of data
Literature search on Pubmed/MEDLINE using the following keywords: “viral”, “encephalitis”, “child”, or “adolescents”, filtering for articles on humans and in English.
Summary of data
Viral encephalitis is the most common cause of encephalitis and is responsible for high rates of morbidity, permanent neurologic sequelae, and according to the virus, may have high mortality rates. The most common etiologies are herpesviruses 1 and 2 (HSV-1 and HSV-2), non-polio enterovirus, and arboviruses (in Brazil, dengue, Zika, and chikungunya). Other relevant etiologies are seasonal influenza, cytomegalovirus (CMV), Epstein–Barr virus (EBV), human herpesvirus 6 (HHV-6), and the re-emergent measles.
Conclusion
Clinical data, laboratory results, and neuroimaging findings support the diagnosis of encephalitis and the specific viral etiology. To increase the likelihood of etiologic confirmation, it is important to know the best approach to collecting samples and to choose the best identification technique for each virus. The differential diagnosis of viral encephalitis includes other infections and immune-mediated inflammatory central nervous system disorders.
Keywords: Viral encephalitis, Diagnosis, Treatment
Resumo
Objetivos
Revisar os critérios diagnósticos para encefalite e encefalopatia de etiologia infecciosa presumida, assim como a investigação diagnóstica para encefalite viral e suas abordagens terapêuticas. Além disso, pretendemos resumir tópicos relevantes sobre os vírus específicos frequentemente encontrados no Brasil.
Fonte de dados
Pesquisa bibliográfica feita nos bancos de dados Pubmed/Medline utilizando as seguintes palavras-chave: “viral”, “encephalitis”, “child” ou “adolescents”, limitando os artigos a estudos em humanos e escritos em inglês.
Resumo dos dados
A encefalite viral é a causa mais comum de encefalite e é responsável por altas taxas de morbidade, sequelas neurológicas permanentes e, de acordo com o vírus, altas taxas de mortalidade. As etiologias mais comuns são herpes vírus 1 e 2 (HSV-1 e HSV-2), enterovírus não pólio e arbovírus (no Brasil, Dengue, Zika e Chikungunya). Outras etiologias relevantes são a influenza sazonal, o citomegalovírus (CMV), o vírus Epstein-Barr (EBV), o herpes vírus humano 6 (HHV-6) e o sarampo reemergente.
Conclusão
Dados clínicos, resultados laboratoriais e de neuroimagem apoiam o diagnóstico de encefalite e a etiologia viral específica. Para aumentar a probabilidade de confirmação etiológica, é importante conhecer a melhor abordagem para coletar amostras e escolher a melhor técnica de identificação para cada vírus. O diagnóstico diferencial de encefalite viral inclui outras infecções e distúrbios inflamatórios do sistema nervoso central imunomediados.
PALAVRAS-CHAVE: Encefalite viral, Diagnóstico, Tratamento
Introduction
The reported annual incidence of encephalitis in children is around 16/100,000 child-years during the second year of life, remaining high until the age of 10, and it is about 1/100,000 child-years at the age of 15.1 The most common etiology is viral2 and the frequency of specific agents varies according to geographic location, season, patient immunological status, and viral genetic mutations over time. Viral encephalitides usually occur after hematogenic viral dissemination into the central nervous system (CNS). However, herpesviruses and Lyssavirus (rabies) can cause neurologic disease through nerve-rout dissemination.3 They are responsible for high rates of morbidity, can cause neurologic permanent sequelae and, according to the virus, may have high mortality rates. Therefore, all efforts should be made to guarantee prevention through vaccination and provide antiviral treatment when available.
The most common viral encephalitis agents are herpesviruses 1 and 2 (HSV-1 and HSV-2), non-polio enterovirus, and arbovirus.1 Other relevant etiologies are seasonal influenza, cytomegalovirus (CMV), Epstein-Barr virus (EBV), and human herpesvirus 6 (HHV-6). The present study reviews the clinical aspects of the more common viral encephalitides listed above, with a special focus on Brazilian epidemiology and the re-emergent measles. This review does not cover viruses that cause more frequently chronic progressive encephalopathies such as human immunodeficiency virus (HIV) or John Cunningham virus (JCV), nor arboviruses unreported in Brazil.
Methodology
The authors performed a literature search on Pubmed/MEDLINE using the following keywords: “viral,” “encephalitis,” “child,” or “adolescents,” filtering for articles on humans and in English. Relevant articles for this review were selected and reviewed in detail. The authors also looked for relevant manuscripts in the references of the selected articles and included additional work on specific topics from the authors’ personal files.
Definition of encephalitis
Encephalitis is defined by inflammation of the brain parenchyma with resulting neurologic dysfunction that can be caused by infection or autoimmunity.4 It is confirmed by identification of the inflammation in brain tissue specimens. However, this is rarely indicated, thus indirect evidence of inflammation in clinical presentation and ancillary non-invasive tests such as neuroimaging and cerebrospinal fluid (CSF) analysis are used.
Several other neurologic conditions may cause encephalopathy without any evidence of brain inflammation. Besides encephalitis per se, this review includes encephalopathy of presumed viral etiology (systemic symptoms and signs or laboratory confirmation of systemic viral infection associated with neurologic dysfunction).
In general, encephalitis should be suspected when symptoms or signs of neurologic dysfunction (headache, decreased level of consciousness, seizures, focal deficits, papilledema, behavioral changes) present acutely (24–72 h) along with systemic manifestations such as fever, lymphadenopathy, rash, arthralgia, myalgia, respiratory symptoms, or gastrointestinal symptoms, or with history of exposure to known risk factors (traveling to endemic areas, animal bites, exposure to insects or ticks).5, 6 Then, brain imaging – usually non-contrasted computed tomography (CT) of the brain – is recommended before lumbar puncture for CSF analysis when elevated intracranial pressure is suspected.6 Magnetic resonance imaging (MRI) may provide better characterization of brain inflammation, demonstrate focal lesions, and help in the differential diagnosis of idiopathic inflammatory CNS disorders.7 Electroencephalography (EEG) should be ideally performed in all patients6 to detect focal or generalized epileptiform activity. In selected cases, EEG may also be helpful to determine the origin of behavioral issues as being primarily psychiatric or caused by underlying encephalopathy.6
Diagnostic criteria of encephalitis and encephalopathy of presumed infectious etiology
In 2013, the International Encephalitis Consortium published recommendations for case definitions of encephalitis and encephalopathy of presumed infectious etiology, as well as the diagnostic workup on those cases.
The following criteria were recommended:7
Major criterion (required)
Patients presenting to medical attention with altered mental status (defined as decreased or altered level of consciousness, lethargy, or personality change) lasting ≥24 h with no alternative cause identified.
Minor criteria (two required for possible encephalitis; three or more required for probable or confirmed encephalitis)
-
•
Documented fever ≥38 °C within the 72 h before or after presentation.
-
•
Generalized or partial seizures not fully attributable to a pre-existing seizure disorder.
-
•
New onset of focal neurologic findings.
-
•
CSF leukocyte count ≥5 mm3
-
•
Abnormality of brain parenchyma on neuroimaging suggestive of encephalitis that is either new from prior studies or appears acute in onset.
-
•
Abnormality on electroencephalography consistent with encephalitis and not attributable to another cause.
Initial workup of viral encephalitis
After identifying a possible or probable case of encephalitis/encephalopathy of presumed infectious etiology, it is desirable to identify the specific etiology (Table 1). However, even with comprehensive testing, more than 50% of the encephalitides will remain without determination of the specific causative agent.2 The International Encephalitis Consortium7 has suggested an algorithm for etiologic workup. Here the relevant aspects of the investigations for viral etiologies are listed:
-
1)
When performing lumbar puncture, collect at least 5 mL of fluid and freeze unused fluid for additional testing.
-
2)
Identify CSF leukocyte count with differential, proteins, lactate, and glucose.
-
3)
Test for HSV-1/2 (polymerase chain reaction [PCR]) in CSF (if test available, also consider HSV CSF IgG and IgM).
-
4)
Test for enterovirus (PCR) (more sensitive in throat swab and stool than in CSF)
-
5)
Test EBV serology (VCA IgG and IgM and EBNA IgG).
-
6)
Hold acute serum and collect convalescent serum 10–14 days later for paired antibody testing.
-
7)
When clinical features of extra-CNS involvement are present, additional testing is recommended (e.g., biopsy of skin lesions, bronchoalveolar lavage and/or endobronchial biopsy in those with pneumonia/pulmonary lesions, throat swab PCR/culture in those with upper respiratory illness; stool culture in those with diarrhea).
Table 1.
Preferred diagnostic test according to suspected etiology.
| Virus | Preferred diagnostic test |
|---|---|
| HSV-1/HSV-2 | CSF PCR Consider repeating within 2-7 days of disease onset if negative with high clinical suspiciona |
| VZV | CSF specific IgG |
| Enterovirus | Stool and throat PCR are preferred over CSF PCR |
| EBV | Serum EBV capsid antigen IgG and IgM (VCA) and EBV nuclear antigen IgG (EBNA) |
| HHV-6 | CSF PCR paired with serum PCRb |
| Influenza | Culture, antigen test, PCR of respiratory secretions |
| Dengue/Zika/Chikungunya | CSF PCR or CSF-specific IgM |
| Measles | CS- specific IgG |
| CMV | CSF PCR or CSF-specific IgM |
CSF, cerebal spinal fluid; PCR, polymerase chain reaction; HSV, herpes virus; VZV, varicella zoster virus; EBV, Epstein-Barr virus; HHV-6, human herpes virus 6; CMV, cytomegalovirus.
If not tested for CSF PCR, consider testing CSF for HSV-IgG after 10–14 days of disease onset.
To exclude viral integration to the host DNA (causing false positive results).
Patients with viral encephalitis might have increased opening pressure on lumbar puncture. They usually present lymphocytic pleocytosis, mildly elevated protein, and normal glucose.6, 8, 9 In many cases brain MRI is normal, but it is likely to have specific findings, especially in case of HSV encephalitis.10 EEG is abnormal in more than 80% of patients with viral encephalitis, showing diffuse high amplitude slow waves and/or focal epileptiform activity.8 Continuous EEG monitoring may be necessary to identify non-convulsive status.11
Empiric treatment
When viral encephalitis is suspected, the first measures include supportive treatment and correction of any electrolyte disturbance, autonomic dysregulation, and renal and hepatic dysfunction.12 Also, it is important to treat seizures and non-convulsive status epilepticus.
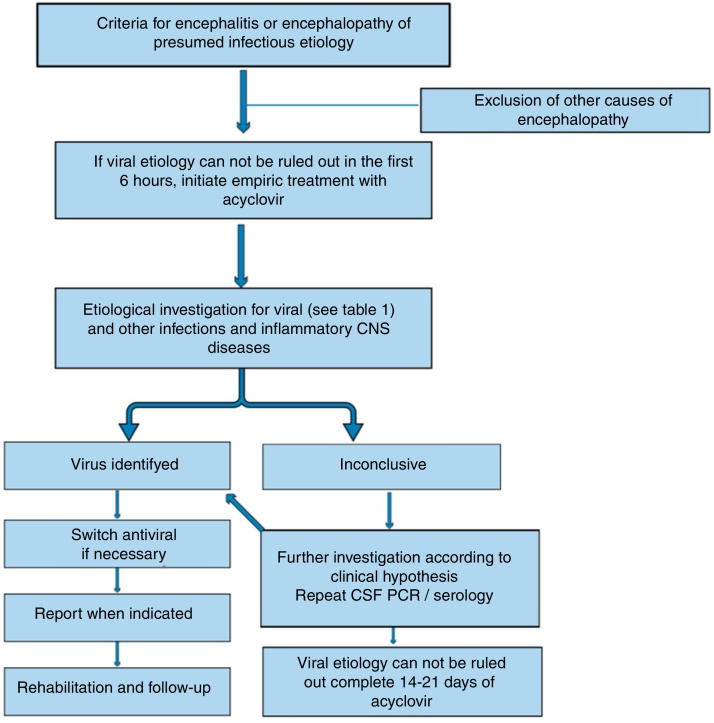
If viral encephalitis cannot be ruled out in the first six hours of admission, it is recommended to start empirical treatment with acyclovir.4 (Fig. 1) Acyclovir has antiviral activity against HSV and related viruses6 that are usually the most common causes of viral encephalitis. The dosing should be 500 mg/m2 every eight hours in children with ages ranging from 3 months to 18 years, and 10 mg/kg/dose every eight hours in children older than 12 years. Dosing should be adjusted if there is previous renal impairment.6
Figure 1.
Algorithm for management of encephalitis/encephalopathy of presumed viral etiology.
Up to 20% of patients treated with acyclovir may develop crystal-induced nephropathy; this usually manifests after four days of treatment.13 The nephropathy is reversible, but renal function should be monitored and the patients should be maintained adequately hydrated. Oral acyclovir does not achieve therapeutic CNS levels and should not be used to treat viral encephalitis.6
Confirmed cases of HSV encephalitis in children or adolescents should receive acyclovir treatment for 14–21 days. Some authors recommend CSF PCR re-evaluation after this period, and if still positive, they advise to maintain treatment and repeat CSF analysis every week until negative before stopping acyclovir.14, 15 In addition, even in cases in which the confirmation of HSV encephalitis is not possible, the treatment should be continued unless CSF is negative for HSV after 72 h of disease onset and there is normal MRI and CSF leukocyte count, and a normal level of consciousness is observed or an alternative diagnosis is made.6
Specific considerations
Each viral agent has clinical and laboratorial particularities (Table 1) that should be remembered to increase etiological identification.
HSV encephalitis
HSV-1 is the main cause of viral encephalitis in pediatric patients in most reports. HSV-2 more commonly causes meningitis in adults. Besides the positive PCR for HSV in CSF, there are some clues to increase the suspicion of HSV encephalitis.
Brain CT may show specific abnormalities (reduced attenuation in one or both temporal lobes or areas of hyperintensity9) suggestive of HSV in up to 80% of patients at presentation.16 Brain MRI may demonstrate hyperintense areas on the T2-weighted image (T2WI), fluid-attenuated inversion recovery (FLAIR), or diffusion-weighted images, and T1-weighted image (T1WI) with gadolinium enhancement on temporal lobes, cingulate gyrus, orbitofrontal, and insular cortex.10
CSF may be hemorrhagic in HSV encephalitis in up to 50% of the patients.1 CSF PCR on days two through ten of illness has more than 95% sensitivity and specificity for HSV.14 If there is high clinical suspicion and the first result is negative, it is recommended to take another sample in two to seven days. In this situation, PCR is usually positive even after acyclovir.17 If the CSF has not been tested for HSV by PCR, a CSF sample should be tested for IgG after ten to 14 days of disease onset.6
The EEG may demonstrate non-specific slowing or periodic lateralizing epileptiform discharges (PLEDS).9
VZV encephalitis
In children, VZV encephalitis usually occurs concurrently with chickenpox. However, it might occur as reactivation of a previous infection without skin lesions.18 Brain MRI may demonstrate grey and white matter lesions and their transition. Most lesions are ischemic, but hemorrhagic lesions might also occur along with areas of stenosis in small and large vessels.19 Detection of CSF antibodies to VZV appears to be more sensitive than the identification of viral DNA, especially if there is evidence of vasculopathy.19 Furthermore, the presence of cutaneous vesicular lesions might help to establish the diagnosis.
The treatment of VZV encephalitis is intravenous acyclovir 500 mg/m2 (3 months–12 years) or 10–15 mg/kg/dose every eight hours if older than 12 years of age. If there is evidence of vasculopathy, corticosteroids can be used (60–80 mg of prednisolone).6 No specific antiviral treatment is recommended for VZV cerebellitis, due to the lack of benefit in those cases.6
Enterovirus encephalitis
Enterovirus is the second most common cause of viral encephalitis after the Herpesviridae family. There are several reports over time of enterovirus encephalitis outbreaks in summer.20, 21 Patients usually present with diarrhea, vomiting, and depending on the virus serotype, they may present with hand-foot-and-mouth disease, herpangina, or other types of exanthema.
Brain MRI may demonstrate hyperintense T2WI and FLAIR lesions in the midbrain, pons, and medulla, especially in enterovirus type 71 (E71) CNS infection.4 The lesions are usually severe and characterized by necrosis and demyelination. It is advised to collect CSF, stool, and throat samples for PCR because it has been observed that the isolated CSF analysis may yield false-negative results.22
There is no specific treatment for immunocompetent children with enteroviral encephalitis.
EBV encephalitis
EBV is an important cause of encephalitis in adolescents. As PCR is associated with false-positive and false-negative results in EBV, serology is recommended.12 Doja et al.23 reported that patients with EBV encephalitis are more likely to present unspecific prodromes rather than classic mononucleosis syndrome. Then, clinically it may be difficult to distinguish EBV encephalitis from other viral encephalitides. Interestingly, focal abnormalities may be seen in the basal ganglia on the brain MRI and might help in the differential diagnosis.12
Some groups reported the use of acyclovir and ganciclovir to treat EBV encephalitis, but there is no strong evidence in the literature to support this approach.
HHV-6
Usually, in HHV-6 encephalitis, patients are below the age of 2 or immunocompromised, and develop gastrointestinal symptoms along with ataxia and prolonged convulsions.6 T2WI mesial temporal lobe hyperintensity might be observed on the brain MRI. CSF-positive PCR results should be confirmed through negative serum PCR due to the possibility of viral DNA integration to the host DNA.24 There are no clinical trials regarding treatment duration and dosing, but usually ganciclovir and foscarnet isolated or in combination are indicated to treat HHV-6 encephalitis.25
Influenza
Influenza encephalitis has variable severity phenotypes. It may present with mild encephalopathy, malignant brain edema, or acute necrotizing encephalopathy. Neurological symptoms (seizure, altered or loss of consciousness, decreased cognitive processing speed, motor paralysis or sensory loss, abnormal behavior) develop approximately two days after systemic symptoms (fever, myalgia, respiratory symptoms).26 There is evidence that the H1N1 strain of influenza A may cause more neurological manifestations than seasonal flu.6
Brain MRI may help in the differential diagnosis, showing bilateral thalamic necrosis27 in severe cases or reversible lesions on the splenium of the corpus callosum.28 Other neuroimaging findings can be used to define severity and prognosis, such as multifocal, symmetrically distributed brain lesions of the thalamus, cerebral white matter, brainstem, cerebellum, and parenchyma.29 The definite diagnosis is made by positive viral culture, viral antigen test, or viral RNA PCR of respiratory secretions, or by significant increases in the titer of the hemagglutination inhibition test.30
As it has been reported that the identification of influenza in the CSF is infrequent, it is not clear if the encephalitis occurs due to direct CNS infection or by cytokine release.27 Usually, treatment with amantadine and/or oseltamivir is indicated.
Arbovirus encephalitis
Arbovirus infections associated with encephalitis are transmitted to humans by arthropods. In Brazil, the most relevant arboviruses causing encephalitis are flaviviruses (dengue and Zika virus) and alphavirus (chikungunya virus).
It is difficult to differentiate dengue encephalitis from encephalopathy caused by systemic metabolic complications secondary to the infection. Encephalitis is defined by the identification of the virus in the CNS through PCR, IgM CSF positivity, or NS1 antigen in the CNS along with CSF pleocytosis.31 However, Soares et al.32 reported that most patients with CNS infection confirmed by PCR did not have CSF pleocytosis. In their prospective cohort dengue was the most common cause of encephalitis in adults and adolescents.
For Zika virus encephalitis, there are only few case reports of encephalitis associated to acute viral infection. Those reports have described brain MRI abnormalities characterized by cortical edema33 and hyperintense T2WI lesions in the cortical and subcortical white matter.34
Chikungunya encephalitis is more common in infants under 1 year of age, with a case-fatality rate of 16.6%.35 No specific MRI finding has been identified yet. In clinical studies, chikungunya viral RNA or positive IgM in the CSF have been used to define chikungunya-associated encephalitis.35
Measles encephalitis
Even though measles was declared eliminated from the Americas in 2016, recently another outbreak of the disease has begun. Thus, it is important to recognize possible neurological complications of the measles infection. Measles encephalitis may be characterized by mild symptoms, with brain MRI showing reversible lesions on the splenium of the corpus callosum. However, these MRI findings may also be found in influenza encephalitis, as mentioned above.36
However, one severe fatal complication of measles is the subacute sclerosing panencephalitis. This is especially important in the differential diagnosis of autoimmune encephalitis. It usually occurs in immunocompetent individuals who acquired the infection before vaccination, causing widespread CNS demyelination and neuronal loss.37, 38 The neurologic symptoms (intellectual deterioration, personality changes, and behavioral abnormalities, weakness, rigidity, myoclonus, and autonomic failure) occur six to 11 years after the primary infection, and they are associated with the persistence of the virus in the brain. The diagnosis is confirmed by the presence of measles antibodies in the CSF.38 No therapy is considered curative for this condition and the only way to prevent it is through vaccination.
CMV encephalitis
Although CMV encephalitis is more frequent in immunocompromised patients, it should be considered in patients under 6 months of age with new-onset seizures, fever, poor-feeding, and CSF protein elevation.39 To confirm the diagnosis, either CSF PCR or IgM can be used; however, PCR appears to be more sensitive. The indicated treatment is ganciclovir for four weeks.39
Differential diagnosis
Viral encephalitis can be suspected by clinical history, physical examination findings, and epidemiological information. Travel history may also give clues to the viral or alternative etiology (e.g., subacute and chronic clinical evolution for mycobacteria and fungi, animal contact for fungi and bacteria, suspected food for bacteria and parasites, geographic location for endemic infections). During the diagnosis workup, they may be differentiated from other infectious encephalitides by general CSF and blood laboratory findings (e.g., neutrophilic CSF predominance in bacterial, positive blood cultures), as well as specific detection of viral particles by PCR or antibodies.
Nevertheless, the most challenging differential diagnosis in the pediatric age group is between viral encephalitis and immune-mediated inflammatory CNS diseases such as acute disseminated encephalomyelitis (ADEM) and autoimmune encephalitis. Both viral and ADEM/autoimmune encephalitis can present subacutely, with CSF lymphocytic predominance, and systemic prodromal syndromes (mild respiratory symptoms, fever, myalgia).
ADEM patients have encephalopathy with or without focal symptoms, usually associated with previous history of vaccination or infection in the previous two months. The brain MRI may reveal diffuse, bilateral, poorly defined T2WI hyperintense lesions with involvement of white matter and deep grey matter.40 Lesions are usually formed at the same time, so all may (or may not) show contrast enhancement. Most patients have a monophasic disease, but a few patients with ADEM-like presentations may have recurrent disease.
The increasing recognition of immune-mediated encephalitis is also important in the differential diagnosis of infectious encephalitis. Autoimmune encephalitis has specific clinical phenotypes according to the specific autoantibody. The most relevant autoimmune encephalitis in the pediatric age group is anti-N-methyl-D-aspartate receptor (anti-NMDAr) encephalitis, which might present as worsening of neurologic symptoms after HSV encephalitis. In these cases, the autoimmunity is probably due to the immune response to neuronal surface antigens released during infection of the CNS.41 Another relevant autoimmune encephalitis in the pediatric age group is the GABAa encephalitis, which usually presents with seizures and has specific brain MRI findings.41 A better understanding of these disorders will help clinicians to understand the interfaces between infections and immune responses that may drive neuroinflammation by over-activation of the immune system.
Conclusions
Viral encephalitis is the most common cause of encephalitis in children and adolescents. All possible efforts should be made to establish the specific diagnosis using epidemiological information, clinical presentation, and ancillary tests. The differential diagnosis is not restricted among different viruses, but also includes other infections and immune-mediated inflammatory CNS disorders like ADEM and autoimmune encephalitis.
In order to increase the likelihood of positive results for a specific virus, it is important to know the best approach to collecting samples and to choose the best identification technique for each virus. Further studies are required to investigate defective mechanisms of defense against pathogens that might be genetically determined.
Treatment for specific viral etiologies may be initiated as soon as possible, and sometimes, when there is a high level of suspicion, empirical antiviral treatment is reasonable until etiologic confirmation is possible. Further research is also required to develop new therapies for unmet medical needs like arboviral and enterovirus infections.
Conflicts of interest
Bruna Klein da Costa received scholarship from CNPq Brazil; received scholarship from the Brazilian Committee for Research and Treatment of Multiple Sclerosis; received speaking honoraria from Libbs; received travel support from Merck and Roche; research support from CNPq/Brasil (425331/2016-4), FAPERGS/MS/CNPq/SESRS (17/2551-0001391-3) PPSUS/Brazil, TEVA (research grant for EMOCEMP Investigator Initiated Study). Douglas Kazutoshi Sato Received a scholarship from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan; a Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (KAKENHI 15K19472); research support from CNPq/Brasil (425331/2016-4), FAPERGS/MS/CNPq/SESRS (17/2551-0001391-3) PPSUS/Brazil, TEVA (research grant for EMOCEMP Investigator Initiated Study), and Euroimmun AG (Neuroimmunological Complications associated with Arboviruses); and speaker honoraria from Biogen, Novartis, Genzyme, TEVA, Merck-Serono, Roche, and Bayer and has participated in advisory boards for Shire, Roche, TEVA, Merck- Serono and Quest/Athena Diagnostics.
Acknowledgements
The authors would like to acknowledge the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Finance Code 001 – Brazilian Federal Agency for Support and Evaluation of Graduate Education (PROEX Program).
Footnotes
Please cite this article as: Costa BK, Sato DK. Viral encephalitis: a practical review on diagnostic approach and treatment. J Pediatr (Rio J). 2020;96(S1):12–9.
References
- 1.Koskiniemi M., Rautonen J., Lehtokoski-Lehtiniemi E., Vaheri A. Epidemiology of encephalitis in children: a 20-year survey. Ann Neurol. 1991;29:492–497. doi: 10.1002/ana.410290508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glaser C.A., Honarmand S., Anderson L.J., Schnurr D.P., Forghani B., Cossen C.K., et al. Beyond viruses: clinical profiles and etiologies associated with encephalitis. Clin Infect Dis. 2006;43:1565–1577. doi: 10.1086/509330. [DOI] [PubMed] [Google Scholar]
- 3.Johnson R.T. Viral infections of the nervous system. Viral Infect Nerv Syst. 1998 https://www.cabdirect.org/cabdirect/abstract/20002006583 Ed. 2. [cited 2019 Jun 30]. Available from: [Google Scholar]
- 4.Tunkel A.R., Glaser C.A., Bloch K.C., Sejvar J.J., Marra C.M., Roos K.L., et al. The Management of encephalitis: clinical practice guidelines by the infectious diseases society of america. Clin Infect Dis. 2008;47:303–327. doi: 10.1086/589747. [DOI] [PubMed] [Google Scholar]
- 5.Wang I.-J., Lee P.-I., Huang L.-M., Chen C.-J., Chen C.-L., Lee W.-T. The correlation between neurological evaluations and neurological outcome in acute encephalitis: a hospital-based study. Eur J Paediatr Neurol. 2007;11:63–69. doi: 10.1016/j.ejpn.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Kneen R., Michael B.D., Menson E., Mehta B., Easton A., Hemingway C., et al. Management of suspected viral encephalitis in children - Association of British Neurologists and British Paediatric Allergy, Immunology and Infection Group National Guidelines. J Infect. 2012;64:449–477. doi: 10.1016/j.jinf.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Venkatesan A., Tunkel A.R., Bloch K.C., Lauring A.S., Sejvar J., Bitnun A., et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. 2013;57:1114–1128. doi: 10.1093/cid/cit458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mcgrath N., Anderson N.E., Croxson M.C., Powell K.F., Anderson N. Herpes simplex encephalitis treated with acyclovir: diagnosis and long term outcome. J Neurol Neurosurg Psychiatry. 1997;63:321–326. doi: 10.1136/jnnp.63.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kennedy P.G. Viral encephalitis: causes, differential diagnosis, and management. J Neurol Neurosurg Psychiatry. 2004;75:i10–5. doi: 10.1136/jnnp.2003.034280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitley R.J., Kimberlin D.W. Herpes simplex encephalitis: children and adolescents. Semin Pediatr Infect Dis. 2005;16:17–23. doi: 10.1053/j.spid.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Gold J.J., Crawford J.R., Glaser C., Sheriff H., Wang S., Nespeca M. The role of continuous electroencephalography in childhood encephalitis. Pediatr Neurol. 2014;50:318–323. doi: 10.1016/j.pediatrneurol.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Bale J.F. Virus and immune-mediated encephalitides: epidemiology, diagnosis, treatment, and prevention. Pediatr Neurol. 2015;53:3–12. doi: 10.1016/j.pediatrneurol.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Pacheco L.R., Tavares H.M., Moysés Neto M., Dantas M., Rocha L.S.D., Ribeiro K.M., et al. Acute renal failure related to intravenous acyclovir. Rev Assoc Med Bras. 2005;51:275–278. doi: 10.1590/s0104-42302005000500019. [DOI] [PubMed] [Google Scholar]
- 14.Cinque P., Cleator G.M., Weber T., Monteyne P., Sindic C.J., Van Loon A.M. The role of laboratory investigation in the diagnosis and management of patients with suspected herpes simplex encephalitis: a consensus report. J Neurol Neurosurg Psychiatry. 1996;61:339–345. doi: 10.1136/jnnp.61.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donoso Mantke O., Vaheri A., Ambrose H., Koopmans M., de Ory F., Zeller H., et al. Analysis of the surveillance situation for viral encephalitis and meningitis in Europe. Euro Surveill. 2008;13:8017–8345. doi: 10.2807/ese.13.03.08017-en. [DOI] [PubMed] [Google Scholar]
- 16.Raschilas F., Wolff M., Delatour F., Chaffaut C., De Broucker T., Chevret S., et al. Outcome of and prognostic factors for herpes simplex encephalitis in adult patients: results of a multicenter study. Clin Infect Dis. 2002;35:254–260. doi: 10.1086/341405. [DOI] [PubMed] [Google Scholar]
- 17.Weil A.A., Glaser C.A., Amad Z., Forghani B. Patients with suspected herpes simplex encephalitis: rethinking an initial negative polymerase chain reaction result. Clin Infect Dis. 2002;34:1154–1157. doi: 10.1086/339550. [DOI] [PubMed] [Google Scholar]
- 18.De Broucker T., Mailles A., Chabrier S., Morand P., Stahl J.-P., steering committee and investigators group Acute varicella zoster encephalitis without evidence of primary vasculopathy in a case-series of 20 patients. Clin Microbiol Infect. 2012;18:808–819. doi: 10.1111/j.1469-0691.2011.03705.x. [DOI] [PubMed] [Google Scholar]
- 19.Gilden D., Cohrs R.J., Mahalingam R., Nagel M.A. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol. 2009;8:731–740. doi: 10.1016/S1474-4422(09)70134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akhmadishina L.V., Govorukhina M.V., Kovalev E.V., Nenadskaya S.A., Ivanova O.E., Lukashev A.N. Enterovirus A71 meningoencephalitis outbreak, Rostov-on-Don, Russia, 2013. Emerg Infect Dis. 2015;21:1440–1443. doi: 10.3201/eid2108.141084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casas-Alba D., de Sevilla M.F., Valero-Rello A., Fortuny C., García-García J.-J., Ortez C., et al. Outbreak of brainstem encephalitis associated with enterovirus- A71 in Catalonia, Spain (2016): a clinical observational study in a children’s reference centre in Catalonia. Clin Microbiol Infect. 2017;23:874–881. doi: 10.1016/j.cmi.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Velez C.M., Anderson M.S., Robinson C.C., McFarland E.J., Nix W.A., Pallansch M.A., et al. Outbreak of neurologic enterovirus type 71 disease: a diagnostic challenge. Clin Infect Dis. 2007;45:950–957. doi: 10.1086/521895. [DOI] [PubMed] [Google Scholar]
- 23.Doja A., Bitnun A., Ford Jones E.L., Richardson S., Tellier R., Petric M., et al. Pediatric Epstein–Barr virus—associated encephalitis: 10-year review. J Child Neurol. 2006;21:384–391. doi: 10.1177/08830738060210051101. [DOI] [PubMed] [Google Scholar]
- 24.Ward K.N., Leong H.N., Thiruchelvam A.D., Atkinson C.E., Clark D.A. Human herpesvirus 6 DNA levels in cerebrospinal fluid due to primary infection differ from those due to chromosomal viral integration and have implications for diagnosis of encephalitis. J Clin Microbiol. 2007;45:1298–1304. doi: 10.1128/JCM.02115-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agut H., Bonnafous P., Gautheret-Dejean A. Laboratory and clinical aspects of human herpesvirus 6 infections. Clin Microbiol Rev. 2015;28:313–335. doi: 10.1128/CMR.00122-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang G.F., Li W., Li K. Acute encephalopathy and encephalitis caused by influenza virus infection. Curr Opin Neurol. 2010;23:305–311. doi: 10.1097/wco.0b013e328338f6c9. [DOI] [PubMed] [Google Scholar]
- 27.Sugaya N. Influenza-associated encephalopathy in Japan: pathogenesis and treatment. Pediatr Int. 2000;42:215–218. doi: 10.1046/j.1442-200x.2000.01200.x. [DOI] [PubMed] [Google Scholar]
- 28.Notebaert A., Willems J., Coucke L., Van Coster R., Verhelst H. Expanding the spectrum of MERS type 2 lesions, a particular form of encephalitis. Pediatr Neurol. 2013;48:135–138. doi: 10.1016/j.pediatrneurol.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Mizuguchi M., Abe J., Mikkaichi K., Noma S., Yoshida K., Yamanaka T., et al. Acute necrotising encephalopathy of childhood: a new syndrome presenting with multifocal, symmetric brain lesions. J Neurol Neurosurg Psychiatry. 1995;58:555–561. doi: 10.1136/jnnp.58.5.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morishima T., Togashi T., Yokota S., Okuno Y., Miyazaki C., Tashiro M., et al. Encephalitis and encephalopathy associated with an influenza epidemic in Japan. Clin Infect Dis. 2002;35:512–517. doi: 10.1086/341407. [DOI] [PubMed] [Google Scholar]
- 31.Carod-Artal F.J., Wichmann O., Farrar J., Gascón J. Neurological complications of dengue virus infection. Lancet Neurol. 2013;12:906–919. doi: 10.1016/S1474-4422(13)70150-9. [DOI] [PubMed] [Google Scholar]
- 32.Soares C.N., Cabral-Castro M.J., Peralta J.M., De Freitas M.R., Zalis M., Puccioni- Sohler M. Review of the etiologies of viral meningitis and encephalitis in a dengue endemic region. J Neurol Sci. 2011;303:75–79. doi: 10.1016/j.jns.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Pradhan F., Burns J.D., Agameya A., Patel A., Alfaqih M., Small J.E., et al. Case report: Zika Virus meningoencephalitis and myelitis and associated magnetic resonance imaging findings. Am J Trop Med Hyg. 2017;97:340–343. doi: 10.4269/ajtmh.16-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carteaux G., Maquart M., Bedet A., Contou D., Brugières P., Fourati S., et al. Zika virus associated with meningoencephalitis. N Engl J Med. 2016;374:1595–1596. doi: 10.1056/NEJMc1602964. [DOI] [PubMed] [Google Scholar]
- 35.Gérardin P., Couderc T., Bintner M., Tournebize P., Renouil M., Lémant J., et al. Chikungunya virus–associated encephalitis. Neurology. 2016;86:94–102. doi: 10.1212/WNL.0000000000002234. [DOI] [PubMed] [Google Scholar]
- 36.Melenotte C., Craighero F., Girard N., Brouqui P., Botelho-Nevers E. Measles encephalitis the return: mild encephalitis with reversible splenial lesion. Int J Infect Dis. 2013;17:e72–3. doi: 10.1016/j.ijid.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Gutierrez J., Issacson R.S., Koppel B.S. Subacute sclerosing panencephalitis: an update. Dev Med Child Neurol. 2010;52:901–907. doi: 10.1111/j.1469-8749.2010.03717.x. [DOI] [PubMed] [Google Scholar]
- 38.Holt R.L., Kann D., Rassbach C.E., Schwenk H.T., Ritter J.M., Rota P.A., et al. Subacute sclerosing panencephalitis: the foothold in undervaccination. J Pediatr. 2016;179:259–262. doi: 10.1016/j.jpeds.2016.08.051. [DOI] [PubMed] [Google Scholar]
- 39.Guo Y., Jiang L. Cytomegalovirus encephalitis in immunocompetent infants: a 15-year retrospective study at a single center. Int J Infect Dis. 2019;82:106–110. doi: 10.1016/j.ijid.2019.02.045. [DOI] [PubMed] [Google Scholar]
- 40.Tenembaum S.N. Pediatric multiple sclerosis. Neuroimaging Clin N Am. 2017;27:229–250. doi: 10.1016/j.nic.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Dalmau J., Geis C., Graus F. Autoantibodies to synaptic receptors and neuronal cell surface proteins in autoimmune diseases of the central nervous system. Physiol Rev. 2017;97:839–887. doi: 10.1152/physrev.00010.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]