Abstract
Exopolysaccharide production by Sinorhizobium meliloti is required for invasion of root nodules on alfalfa and successful establishment of a nitrogen-fixing symbiosis between the two partners. S. meliloti wild-type strain Rm1021 requires production of either succinoglycan, a polymer of repeating octasaccharide subunits, or EPS II, an exopolysaccharide of repeating dimer subunits. The reason for the production of two functional exopolysaccharides is not clear. Earlier reports suggested that low-phosphate conditions stimulate the production of EPS II in Rm1021. We found that phosphate concentrations determine which exopolysaccharide is produced by S. meliloti. The low-phosphate conditions normally found in the soil (1 to 10 μM) stimulate EPS II production, while the high-phosphate conditions inside the nodule (20 to 100 mM) block EPS II synthesis and induce the production of succinoglycan. Interestingly, the EPS II produced by S. meliloti in low-phosphate conditions does not allow the invasion of alfalfa nodules. We propose that this invasion phenotype is due to the lack of the active molecular weight fraction of EPS II required for nodule invasion. An analysis of the function of PhoB in this differential exopolysaccharide production is presented.
The soil bacterium Sinorhizobium meliloti (Rhizobium meliloti) fixes nitrogen in symbiotic association with the leguminous plant Medicago sativa (alfalfa). Molecular analyses have revealed that early steps in the establishment of this symbiosis, including attraction of the bacteria to the plant and formation of the plant nodule, depend upon an exchange of small signaling molecules between the two partners (12, 27, 28). Bacterial invasion of root nodules requires exopolysaccharide production by S. meliloti (25), although the function of these exopolysaccharides in invasion is not yet clear.
S. meliloti wild-type strain Rm1021 produces succinoglycan (26), a well-characterized exopolysaccharide polymer of repeating octasaccharide subunits. Each subunit consists of one galactose and seven glucose residues and is decorated with a succinyl, an acetyl, and a pyruvyl modification (2, 36, 37). Mutants of Rm1021 that fail to synthesize succinoglycan (exo mutants) form empty nodules that are devoid of bacteria and are unable to fix nitrogen (24, 25). A 25-kb cluster of exo genes has been identified on the second symbiotic megaplasmid (8, 9, 16, 17, 24, 35) that is required for the production of succinoglycan (14, 22, 25), and biosynthetic roles have been assigned to most of the gene products (38). A tetramer fraction of succinoglycan was originally assigned as the active species in nodule invasion (7, 43), but recent evidence suggests that the active fraction is a trimer of the octasaccharide subunit (19).
Rm1021 also has the capacity to make a second distinct exopolysaccharide, designated EPS II (15), which is a polymer of repeating disaccharide subunits. Subunits of EPS II consist of an acetylated glucose connected to a pyruvylated galactose residue (15, 21). A 32-kb cluster of exp genes (distinct from the exo genes) on the second symbiotic megaplasmid (15) is responsible for the synthesis of EPS II. EPS II production by the wild-type strain Rm1021 has been previously characterized as cryptic (15). EPS II is produced at extremely low levels by this strain, but production is greatly increased in derivatives carrying the expR101 mutation, which results in overexpression of the exp genes. The expR101-mediated synthesis of EPS II was shown to suppress the symbiotic defects of succinoglycan-deficient strains on alfalfa (15). Further analysis revealed that a low-molecular-weight fraction, consisting of 15 to 20 disaccharide subunits of EPS II, efficiently rescued nodule invasion in an exopolysaccharide-deficient strain (18), suggesting that low-molecular-weight EPS II may act as a symbiotic signal during infection.
The reason that S. meliloti is capable of producing two functional exopolysaccharides and how their production is regulated are not yet clear. It has been shown that EPS II production in wild-type Rm1021 increases in conditions of phosphate limitation (44). This observation provides some interesting insight when the two environments that Sinorhizobium may encounter (the soil and inside the nodule) are considered. The concentration of available phosphate in the soil is very low (typically 1 to 10 μM) (11). The phosphate concentrations inside plant tissues are very high (10 to 20 mM) (11). In addition, legumes that obtain nitrogen from symbiosis require higher levels of phosphate for optimal growth than plants that receive nitrogen from fertilizers (23). It has also been suggested that the concentration of phosphate inside the nodule is much higher than any other part of the plant (up to 100 mM) (23).
This report shows how phosphate concentration determines which exopolysaccharides are produced by S. meliloti. Low phosphate concentrations stimulate EPS II production, while high phosphate concentrations dramatically increase succinoglycan production. We show that the EPS II produced by Rm1021 in low-phosphate conditions does not allow nodule invasion to occur. We also observe that the phoB gene is required for the production of EPS II in low-phosphate conditions. We propose that phosphate concentration serves as a signal to regulate which exopolysaccharide is produced according to the requirements of its environment.
MATERIALS AND METHODS
Bacterial strains, media, and genetic techniques.
The strains used in this study are listed in Table 1. The construction of strains by general transduction has been previously described (13). Overnight cultures were grown in Luria-Bertani broth supplemented with 2.5 mM CaCl2 and 2.5 mM MgSO4 (LB-MC) and inoculated (1:200 dilution) into MOPS-MGS media modified for phosphate concentration. Modified MOPS-MGS minimal medium (44) consisted of 50 mM MOPS (morpholine propane sulfonic acid; pH 7.4), 55 mM mannitol, 1 mM MgSO4, 0.25 mM CaCl2, 19 mM glutamic acid, and 0.004 mM biotin. Either 0 or 0.1 mM (low-phosphate) or 20, 100, 150, or 200 mM (high-phosphate) K2HPO4 was added. Due to osmolarity considerations, 50 mM MOPS was omitted from the 150 and 200 mM K2HPO4 liquid media. To provide buffering capacity and the correct pH, these media were adjusted to the correct phosphate concentration by adding equal molar amounts of K2HPO4 and KH2PO4. MOPS-MGS plates were solidified with 1.5% Difco Bacto Agar. Cultures were grown with 500 μg of streptomycin, 200 μg of neomycin, or 40 μg gentamicin per ml where appropriate and incubated for 96 h at 30°C.
TABLE 1.
Bacterial strains used in this work
| Strain | Relevant characteristics | Reference or Source |
|---|---|---|
| Rm1021 | SU47 str-21 | 25 |
| Rm8501 | Rm1021 lac mutant | 15 |
| Rm7210 | Rm1021, exoY210::Tn5-233 | 25 |
| Rm10002 | Rm1021, expA3::Tn5 | 15 |
| Rm11003 | Rm1021, exoY210::Tn5-233, expA3::Tn5 | This work |
| Rm8530 | Rm1021, expR101 | G. C. Walker |
| Rm9020 | Rm1021, expR101, exoY210::Tn5-132 | G. C. Walker |
| Rm11002 | Rm1021, expR101, expA3::Tn5 | This work |
| Rm9001 | Rm1021, mucR::Tn5-233, exoY210::Tn5 | 18 |
| RmH615 | Rm1021, lac mutant, ΩphoB3::Tn5 | 4 |
| Rm11000 | RmH615, exoY-GY-Tn3hohokm | This work |
| Rm11001 | RmH615, expA404-Tn3hohokm | This work |
| RmAR9007 | Rm1021, exoY-GY-Tn3hohokm | G. C. Walker |
| RmAR1001 | Rm1021, expA404-Tn3hohokm | G. C. Walker |
Carbohydrate composition analyses.
To characterize the sugar composition of the exopolysaccharides produced by S. meliloti, a culture was grown on a 0.1 or 100 mM MOPS-buffered minimal medium plate over a nylon filter disk, which was used to minimize carbohydrate transfer from the medium. The culture was suspended in 1 ml of distilled H2O (dH2O) and centrifuged at 20,800 × g for 3 min to pellet the cells. The supernatant was dialyzed against dH2O for 2 days by using a 1000 MWCO membrane (Spectrum). The exopolysaccharides in the supernatant were hydrolyzed in 10 volumes of 2 M trifluoroacetic acid at 100°C for 18 h in a sealed glass vial. The hydrolyzed exopolysaccharides were dried in a Brinkmann SpeedVac centrifuge and resuspended in a small volume of water for analysis by high-pressure anion-exchange chromatography (HPAEC). HPAEC was performed on a Dionex DX 500 with a CarboPac MA1 (anion-exchange) column (4 by 250 mm) using a pulsed amperometric detector with a gold working electrode (Dionex). The waveform used for the analysis was as follows: 0.1 V for 0.4 s, 0.7 V for 0.2 s, and −0.1 V for 0.4 s. The samples were eluted isocratically by using 500 mM NaOH. The carbohydrate composition of the exopolysaccharides was calculated as a glucose/galactose ratio based on the elution times of known glucose and galactose standards. Because there was no free glucose or galactose before hydrolysis, all calculated Glc/Gal ratios represent hydrolyzed exopolysaccharide.
Quantitation of carbohydrate production.
Quantitation of exopolysaccharide production by the various S. meliloti derivatives was performed with Dreywood's anthrone reagent (31). Cultures (2 ml) of MOPS-MGS medium with the appropriate phosphate concentrations were inoculated with the strains and grown for 72 h at 30°C. Cell density measurements were taken at an optical density of 600 nm (OD600), and the cultures were centrifuged at 20,800 × g for 3 min to pellet the cells. The supernatants were tested for total carbohydrate content by the anthrone assay. Anthrone unit/OD600 ratios reflect exopolysaccharide production per cell density unit. Due to signal detection differences between glucose (1 U) and galactose (0.54 U) in the assay and the structures of the subunits of succinoglycan (7 Glc + 1 Gal = 7.54 U) and EPS II (1 Glc + 1 Gal = 1.54 U), a correction factor of 4.9 (7.54/1.54) was implemented for samples that only contain EPS II (exoY in 0 mM K2HPO4). Likewise, a correction was made for Rm1021 in 0 mM K2HPO4 because this strain synthesizes approximately equimolar amounts of succinoglycan and EPS II in low phosphate. Background anthrone-positive material in the cultures was determined by using an exoY expA strain, a derivative that produces neither exopolysaccharide.
Analysis of the molecular weight of EPS II.
A 5-ml culture of a strain unable to produce succinoglycan (exoY) was grown in LB-MC for 48 h at 30°C. The culture was spun at 20,800 × g for 3 min to pellet the cells, washed twice with sterile dH2O, and inoculated into 1 liter of MOPS-buffered minimal medium with 0 mM phosphate. The culture was incubated for 7 days in a rotary shaker and EPS II was isolated and assayed for molecular weight by HPAEC (18, 39). HPAEC was performed by using a P10 column matrix (Dionex), and samples were eluted in a gradient with 100 mM NaOH (eluent A) and 1 M sodium acetate in 100 mM NaOH (18, 39). The gradient was a linear variation of the proportion of eluent B, as follows: 0 min and 10% B, 3 min and 10% B, 10 min and 20% B, 25 min and 100% B, 50 min and 100% B, 55 min and 10% B, and 60 min and 10% B. The flow rate was 1 ml/min. The waveform used for the analysis was as follows: 0.05 V for 0.6 s, 0.75 V for 0.2 s, and −0.15 V for 0.4 s. EPS II purified from expR101 exoY (low- and high-molecular-weight EPS II) and mucR exoY (high-molecular-weight EPS II only) strains was used as a standard. These strains were grown and the EPS II was prepared and analyzed as previously described (18).
β-Galactosidase assay of gene expression.
All cultures tested for β-galactosidase activity were grown to log phase in MOPS-MGS medium with different phosphate concentrations (0, 20, 100, 150, and 200 mM K2HPO4) at 30°C and assayed as previously described (30) to determine Miller units of activity.
Plant inoculation experiments.
All indicated strains were incubated with M. sativa as previously described on standard (25) and modified Jensen's media. Modifications included omission of K2HPO4, exchange of CaHPO4 with an equivalent amount of CaCO3, and supplementation with 0.1 mM (low-phosphate) or 25 mM (high-phosphate) KH2PO4-K2HPO4 equimolar buffer as the sole phosphate source. Bacterial cultures were grown in 0 mM K2HPO4 MOPS-MGS (low), LB-MC (standard), or 150 mM K2HPO4 MGS (high) liquid medium for 72 h. Plants were scored for pink and white nodules after 30 days of growth at 25°C, 60% relative humidity, and a 16-h light cycle.
RESULTS
Low-phosphate conditions stimulate the synthesis of EPS II in S. meliloti.
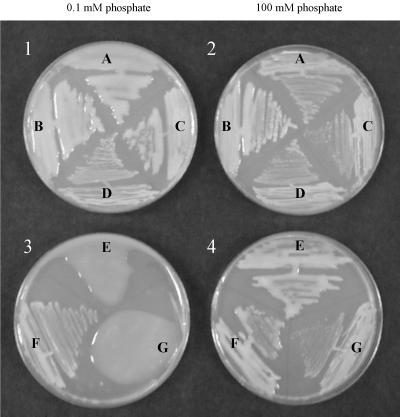
In order to stimulate the limiting phosphate concentrations that Sinorhizobium may encounter in its natural soil environment, MOPS-buffered minimal medium plates with 0.1 mM K2HPO4 (low phosphate) were prepared (44). Rm1021 (wild type) is very mucoid in these low-phosphate conditions (Fig. 1-1A). To determine which exopolysaccharides are produced by the wild-type strain Rm1021, strains with a mutation in a gene responsible for the production of each of the two known S. meliloti exopolysaccharides were tested in the same conditions. A strain with an expA mutation is unable to synthesize EPS II (15) but is mucoid under these conditions (Fig. 1-1B), suggesting that succinoglycan is a component of the exopolysaccharide produced by Rm1021. An exoY mutant cannot produce succinoglycan (25), but it also develops mucoid colonies (Fig. 1-1C), indicating that EPS II also comprises part of the exopolysaccharide production in low phosphate. An exoY expA double mutant was constructed to establish the correlation between colony mucoidy and exopolysaccharide production. This strain is nonmucoid at all phosphate concentrations (Fig. 1-1D and 1-2D).
FIG. 1.
Exopolysaccharide production by S. meliloti on plates with different phosphate concentrations. Plates with 0.1 mM (1 and 3) or 100 mM (2 and 4) K2HPO4 were prepared as indicated in Materials and Methods. Plates 1 and 2: A, Rm1021; B, expA; C, exoY; and D, exoY expA. Plates 3 and 4: E, expR101; F, expR101 expA; and G, expR101 exoY.
We also analyzed expR101, a Rm1021 derivative with a mutation that increases EPS II production (15), to determine if this strain behaved like Rm1021 in these different phosphate conditions. The expR101 mutant and derivatives with either an exoY or expA mutation were plated on low-phosphate medium (Fig. 1–3). Because most of the mucoidy of the expR101 strain is eliminated with the introduction of an expA mutation, it is apparent that the main component of the exopolysaccharides produced in low phosphate by the expR101 mutant is EPS II. The expR101 expA derivative retained a low level of mucoidy, revealing that there is also some succinoglycan production at this low phosphate level.
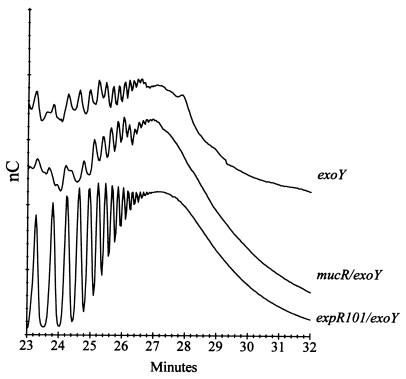
FIG. 3.
Chromatographic analysis of the molecular weight of EPS II produced by an exoY strain in low phosphate. The graph is a vertical overlay of chromatographic profiles of three independent samples. Separation by HPAEC of EPS II was performed as previously described (18). The carbohydrate electrochemical response is expressed in nanocoulombs. Purified EPS II from mucR exoY and expR101 exoY strains was used as a standard (18). The biologically active fraction of EPS II elutes with a retention time of 22 to 26 min.
To confirm biochemically which exopolysaccharides were produced by the various strains, we sampled the cultures grown on low-phosphate plates for carbohydrate composition analyses (Table 2). We took advantage of the very different glucose/galactose ratios of succinoglycan (7:1) (2) and EPS II (1:1) (21) to differentiate the two exopolysaccharides. Analysis of the exopolysaccharide production by the exoY derivative (1:1 Glc/Gal ratio) verifies that only EPS II is produced by this strain in low-phosphate conditions. Exopolysaccharide analysis of the expA derivative yields a ratio of 7.2:1, confirming that the exopolysaccharide produced by this strain in low phosphate is succinoglycan. The exoY expA double mutant yielded only background levels of glucose that we attribute to cyclic glucan production. Rm1021 produces exopolysaccharides with a Glc/Gal ratio of 3.4:1, suggesting that there is a mixture of succinoglycan and EPS II produced by wild-type Sinorhizobium in low phosphate concentrations. The results of the carbohydrate composition analyses for expR101 (1.3:1 ratio) and expR101 exoY (1:1 ratio) strains support the observation that the exopolysaccharide produced by expR101 in low phosphate is primarily EPS II. The Glc/Gal ratio of the expR101 expA strain (7.6:1) confirms that expR101 derivatives retain the ability to produce succinoglycan in low phosphate levels.
TABLE 2.
Carbohydrate composition analyses of exopolysaccharide production by S. meliloti derivatives
| Strain | Glc/Gal ratioa at:
|
|
|---|---|---|
| 0.1 mM phosphate | 100 mM phosphate | |
| Rm1021 | 3.4:1 | 6.8:1 |
| exoY | 1.0:1 | None |
| expA | 7.2:1 | 6.2:1 |
| exoY expA | Noneb | None |
| expR101 | 1.3:1 | 6.2:1 |
| expR101 exoY | 1.0:1 | None |
| expR101 expA | 7.6:1 | 6.4:1 |
| phoBc | 7.6:1 | 6.4:1 |
| phoB exoYc | None | None |
| phoB expAc | 7.6:1 | 6.2:1 |
The ratios of glucose and galactose were calculated by total peak area. Glucose and galactose were assigned based on the chromatographic profiles of known carbohydrate standards.
None, background levels of glucose due to cyclic glucan production.
RmH615 is a derivative of Rm1021 with a phoB mutation.
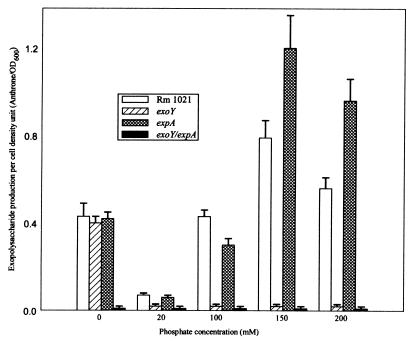
A wild-type S. meliloti strain produces significant amounts of exopolysaccharides when grown at low levels of phosphate (0 mM), produces very little at intermediate levels of phosphate (20 mM), and achieves maximal production of exopolysaccharides in high-phosphate (150 mM) concentrations (Fig. 2). An exoY derivative of Rm1021 (which lacks the ability to synthesize succinoglycan) shows some exopolysaccharide production at low levels of phosphate concentration but produces negligible amounts of exopolysaccharide at higher concentrations of phosphate, confirming that most of the exopolysaccharide produced at high phosphate is indeed succinoglycan. On the other hand, an expA mutant (that is unable to synthesize EPS II) shows a pattern of exopolysaccharide production very similar to wild type throughout the entire phosphate concentration spectrum. Both Rm1021 and the expA mutant produce high levels of exopolysaccharides at higher levels of phosphate, although the absolute quantities of exopolysaccharides produced varied between the two strains. Interestingly, this mutant only produces succinoglycan, even at low concentrations of phosphate, suggesting that in an exp mutant background, succinoglycan production is derepressed at low phosphate concentrations. Based on this analysis, exopolysaccharide production under most laboratory conditions (LB-MC, MOPS-MGS, and Jensen's media) may be at minimal levels.
FIG. 2.
Exopolysaccharide production by S. meliloti in different phosphate concentrations. Supernatant exopolysaccharides were purified as described in Materials and Methods, and the total carbohydrate content was determined by anthrone assay (31). The OD600 of each culture was determined, and the total carbohydrate per cell density unit was plotted against the concentration of phosphate in the growth medium.
High-phosphate conditions block synthesis of EPS II and induce synthesis of succinoglycan.
In order to observe the exopolysaccharide production of S. meliloti in the high-phosphate conditions present inside a plant nodule, MOPS-buffered minimal medium plates with 100 mM K2HPO4 (high phosphate) were prepared. Upon plating the strains on this medium, two dramatic effects were observed. First, no EPS II production could be detected in either Rm1021 or an expR101 mutant in high-phosphate concentrations (Fig. 1–2C and 1-4G). An increase in phosphate concentration to 2 mM K2HPO4 was sufficient to block EPS II production in Rm1021 (reference 44 and data not shown), but an increase to 100 mM phosphate was required to block EPS II production in an expR101 mutant (Fig. 1-4G). The expR101 exoY strain can only produce EPS II and is nonmucoid at 100 mM phosphate, suggesting that the EPS II production in an expR101 background at this high phosphate concentration is very low or absent altogether.
We also observed that there was a large increase in the amount of mucoidy in succinoglycan-producing strains at high phosphate levels compared to the same strains grown on media with intermediate levels (2 to 20 mM K2HPO4) of phosphate (data not shown). Both Rm1021 and expA strains, which are capable of producing succinoglycan, became very mucoid at this level, while the exoY strain was nonmucoid (Fig. 1–2). Also, the mucoidy of expR101 strain at this high phosphate concentration was eliminated with an exoY but not with an expA mutation (Fig. 1-4E, F, and G). This observation demonstrates that an expR101 strain produces succinoglycan, not EPS II, at this high phosphate level.
We performed carbohydrate composition analyses to confirm which exopolysaccharides were produced at high phosphate levels. The Glc/Gal ratios of both wild-type Rm1021 (6.8:1) and expA (6.2:1) strains suggest that succinoglycan is the exopolysaccharide being produced under these high-phosphate conditions (Table 2). No EPS II production could be detected in either the exoY or the expR101 exoY strain in high phosphate. Analyses of expR101 and expR101 expA strains yielded Glc/Gal ratios of 6.2:1 and 6.4:1, respectively. These ratios confirm that expR101 strains, like Rm1021, produce succinoglycan at this high phosphate concentration. The dramatic change in the glucose/galactose ratio when an expR101 mutant is grown in low (1.3:1 ratio)- and high (6.3:1 ratio)-phosphate conditions demonstrates how exopolysaccharide synthesis in this strain shifts from EPS II in low phosphate concentrations to succinoglycan in high-phosphate concentrations.
Exopolysaccharide gene expression is subject to phosphate-dependent regulation.
We attempted to determine if there was a correlation between phosphate-dependent exopolysaccharide production and changes in exopolysaccharide gene expression. Chromosomal (single-copy) exoY-lacZ and expA-lacZ fusions were used to measure changes in expression from the genes responsible for the production of succinoglycan (exo) and EPS II (exp). In Rm8501 (a lac mutant of Rm1021), there is an eightfold decrease in transcription from the exp genes when the cells are grown in 200 mM (high) phosphate versus 0 mM (low) phosphate conditions (Table 3). In addition, most of this decrease occurs between 0 and 20 mM phosphate, suggesting that EPS II production in Rm1021 is only seen at very low levels of phosphate. This observation is consistent with the observed increase in EPS II synthesis by Rm1021 in low-phosphate conditions and is in agreement with the sevenfold increase in exp gene expression in low phosphate with an exp-lacZ fusion seen previously (40, 44). Conversely, expression from the exo genes increases 15-fold when the phosphate concentration in the medium is increased from 0 to 200 mM K2HPO4. This is consistent with the increase in exopolysaccharide production seen in succinoglycan-producing strains at high levels of phosphate. Thus, transcription from the exp genes decreases and transcription from the exo genes increases as phosphate levels rise. From these data, we suggest that one possible source of the differences in exopolysaccharide production at different levels of phosphate is the differential expression of the genes responsible for the production of succinoglycan and EPS II.
TABLE 3.
exo and exp gene expression in different phosphate conditions in S. melilotia
| Strain and fusion | Mean Miller units ± SD of β-galactosidase activity at:
|
||||
|---|---|---|---|---|---|
| 0 mM phosphate | 20 mM phosphate | 100 mM phosphate | 150 mM phosphate | 200 mM phosphate | |
| Rm8501 | |||||
| exoY-lacZ | 8.1 ± 1.0 | 11 ± 0.7 | 37 ± 3.0 | 55 ± 4.0 | 120 ± 13 |
| expA-lacZ | 10 ± 1.5 | 1.9 ± 0.1 | 1.4 ± 0.2 | 1.7 ± 0.1 | 1.3 ± 0.5 |
| RmH615 | |||||
| exoY-lacZ | 4.9 ± 1.0 | 8.5 ± 1.7 | 19 ± 2.0 | 98 ± 4.0 | 119 ± 13 |
| expA-lacZ | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.7 ± 0.2 | 0.9 ± 0.2 | 0.7 ± 0.2 |
Rm8501 is a lac mutant of Rm1021. RmH615 is a lac mutant and has a phoB mutation. All data are calculated in Miller units of β-galactosidase activity.
The EPS II produced in low-phosphate conditions by Rm1021 does not allow nodule invasion.
It was previously shown that an active low-molecular-weight fraction of EPS II synthesized in strains with an expR101 mutation could function in lieu of succinoglycan in nodule invasion (15). We investigated how exopolysaccharide production in different phosphate concentrations, especially EPS II production by Rm1021, might affect the symbiosis between Sinorhizobium and M. sativa. Using modified Jensen's agar, the exopolysaccharide mutants were incubated with M. sativa on high-, standard-, and low-phosphate plant media. Standard Jensen's medium (25) has a total phosphate concentration of 8.5 mM, an intermediate concentration between the low (0.1 mM)- and high (25 mM)-phosphate Jensen's media. Attempts to create Jensen's agar with higher phosphate concentrations (50 or 100 mM) resulted in wilting and death of the plants due to high salt concentrations. The Rm1021, expA, and expR101 strains are capable of producing succinoglycan and effectively invaded nodules at all three levels of phosphate. Interestingly, the invasion efficiency of the expR101 exoY strain was poor on high-phosphate medium (Table 4). This is consistent with the observation that EPS II production is very low at this level, even in a strain with an expR101 mutation. We also observed that the exoY mutant did not invade nodules in any phosphate conditions, even though there is increased EPS II production in this strain at low phosphate levels. The expR101 exoY strain, however, did effectively invade nodules in the same low-phosphate conditions, even though it exclusively produced EPS II as well. These results suggested that there could be a difference in the EPS II being produced by the exoY and expR101 exoY strains.
TABLE 4.
Nodule invasion phenotype of S. meliloti exopolysaccharide mutants on modified Jensen's media
| Strain | No. of plants with pink nodulesa at:
|
||
|---|---|---|---|
| 0.1 mM phosphate | 8.5 mM phosphate | 25 mM phosphate | |
| Rm1021 | 30 | 30 | 30 |
| exoY | 0 | 0 | 0 |
| expA | 30 | 30 | 30 |
| exoY expA | 0 | 0 | 0 |
| expR101 | 30 | 30 | 30 |
| expR101 exoY | 30 | 30 | 10b |
Plants without pink nodules developed white nodules characteristic of inoculation of M. sativa with an exo mutant.
The plants had an average of 1.6 pink nodules per plant versus 9.1 pink nodules per plant for those inoculated with expR101 in high-phosphate media.
The EPS II induced in low-phosphate conditions lacks the low-molecular-weight fraction crucial for efficient nodule invasion.
Because the EPS II produced by the exoY derivative in low phosphate did not allow nodule invasion, we analyzed whether the EPS II contained the molecular weight fraction that is symbiotically active in nodule invasion (18). Two strains with mutations that increase EPS II production in Rm1021 were used as controls. The MucR protein is a repressor of EPS II production in S. meliloti (34), but the EPS II produced by a mucR derivative does not allow nodule invasion (33). Analyses have revealed that the EPS II produced by mucR derivatives is composed primarily of high-molecular-weight chains and lacks the active fraction (15 to 20 dimer subunits) found in strains with the expR101 mutation (18). To determine how the molecular weight of EPS II from an exoY derivative compared to that produced by expR101 exoY and mucR exoY strains, EPS II from a culture of each strain was purified and analyzed by HPAEC (Fig. 3). The expR101 exoY mutant produces a large amount of the biologically active fraction of low-molecular-weight EPS II that elutes with a retention time of 22 to 26 min and high-molecular-weight EPS II that elutes between 26 and 30 min (18). The EPS II from a mucR exoY mutant, however, is mostly high molecular weight. The EPS II from an exoY mutant grown in low-phosphate medium also lacks the low-molecular-weight EPS II fraction that is active in nodule invasion. The EPS II isolated from both exoY and mucR exoY is composed of primarily high-molecular-weight polymers.
A functional phoB gene is required for production of EPS II at low phosphate concentrations.
We were interested in determining if this pattern of phosphate-dependent exopolysaccharide production in S. meliloti could be subject to PhoB-dependent regulation. It has been shown that some genes that are not directly involved in the acquisition and assimilation of phosphate are regulated by the PhoB protein in S. meliloti (42). The PhoB protein is a transcriptional regulator that binds to characteristic sequences (called pho boxes) upstream from phosphate-regulated genes during phosphate starvation (29). Binding of PhoB to these sequences can increase (4) or decrease (6, 41) transcription of these genes, depending on the location of the pho boxes in the promoter region. Previous work by Zhan et al. (44) demonstrated that Rm8002, a derivative of Rm1021 with an uncharacterized mutation that affected alkaline phosphatase activity, failed to induce EPS II production in low-phosphate conditions. Later, Summers et al. found the expC gene (a gene in the exp operon responsible for EPS II production) during a search for genes that were expressed differently in low phosphate (42). They demonstrated that this difference was eliminated with a phoB mutation. RmH615 is a derivative of Rm1021 that has a phoB mutation (4). In high-phosphate conditions, RmH615 behaves much like Rm1021. The mucoidy of RmH615 colonies is eliminated with the introduction of an exoY mutation, and carbohydrate analyses of the exopolysaccharides produced by RmH615 (a 6.3:1 Glc/Gal ratio) confirm that succinoglycan is the exopolysaccharide produced at high phosphate (Table 2). When RmH615 (phoB) is grown in low-phosphate conditions, however, the mucoidy that results is eliminated by the introduction of an exoY mutation into the strain but is not affected by an expA mutation. Unlike Rm1021 or the expR101 mutant, there appears to be no EPS II production in low phosphate in RmH615. Carbohydrate composition analyses confirm that succinoglycan is the only exopolysaccharide produced in low phosphate (a 7.6:1 Glc/Gal ratio), and only background levels of glucose were detected in the RmH615 exoY double mutant (Table 2). Analysis of the RmH615 expA double mutant revealed a Glc/Gal ratio that was identical (i.e., 7.6:1) to that for RmH615, indicating that an expA mutation does not affect exopolysaccharide production in this strain. While β-galactosidase assays in RmH615 resulted in a pattern of exo gene expression that was similar to Rm1021, only background levels of exp expression were detected at all phosphate levels (Table 3).
DISCUSSION
In this report we have analyzed how phosphate concentration has a regulatory effect on exopolysaccharide production. In Luria broth, Rm1021 synthesizes low levels of succinoglycan and very little (if any) EPS II (15, 34). Jensen's agar, the medium used to determine nodulation efficiency, contains 8.5 mM total phosphate, which is between 103- and 104-fold higher than the natural soil phosphate concentration. The large difference in phosphate concentrations between the soil and the nodule is an example of the dissimilar conditions that Sinorhizobium may encounter during its lifetime, and certain physiological adjustments may occur to satisfy the requirements of these environments. Exopolysaccharides may play a role in the transition between the soil and the plant environments. In both Rm1021 and the expR101 strain, increasing the phosphate concentration decreases EPS II production to very low levels. Merely increasing the osmolarity of the medium has been previously shown (10) to have no significant effect on either the amount of succinoglycan produced or the expression of the exo genes. EPS II production cannot be detected in Rm1021 when the phosphate level is raised to 2 mM and in the expR101 background when the phosphate level is raised to 100 mM. Because EPS II production is greatest at low phosphate levels and the nodule is a high-phosphate environment, we assume that EPS II is produced primarily while Sinorhizobium is in the soil and not inside the nodule. This low level of EPS II production in high-phosphate conditions is evident since the expR101 exoY strain is very inefficient in invading alfalfa nodules on high-phosphate Jensen's media. The expR101 exoY mutant is invasion proficient on low- and standard-phosphate Jensen's media, but most of the plants inoculated with this strain on the high-phosphate medium failed to develop any pink nodules. In this example, the invasion phenotype of a strain depends on the phosphate concentration of the medium.
In general, S. meliloti strains that exclusively produce EPS II invade M. sativa with a much lower efficiency than do succinoglycan-producing strains (15) (Table 4). While EPS II produced in an expR101 background is functional in nodule invasion, the lack of pink nodules on plants inoculated with an exoY mutant in low-phosphate conditions suggests that the EPS II produced by Rm1021 in low-phosphate conditions does not allow invasion of nodules. The molecular weight of exopolysaccharides in Sinorhizobium is critical in allowing efficient infection of nodules (7, 18–20, 43). Both succinoglycan and EPS II are known to have low-molecular-weight fractions that are essential for nodule invasion. Addition of succinoglycan trimer in trans rescues the nodule invasion defects of an exo mutant (7, 43). A fraction of 15 to 20 dimer subunits of EPS II in concentrations that are as low as 7 pmol also allows invasion of nodules by exo derivatives (18). We show that the EPS II produced by exoY lacks the low-molecular-weight fraction shown to be symbiotically active in nodule invasion. The EPS II from the exoY derivative closely resembles the high-molecular-weight EPS II produced by a mucR exoY derivative, which does not invade nodules despite overproducing EPS II (18) (Fig. 3).
The observation that EPS II from the exoY strain does not allow nodule invasion leads to the question of what role EPS II may play in Sinorhizobium-alfalfa symbiosis. Our data suggest that the lack of EPS II production by expA does not affect the efficiency of nodule invasion in the conditions we use to assay invasion. Because production of EPS II in the amounts we have observed at low phosphate is energetically expensive, it probably has a role important to the bacteria. Perhaps EPS II is important to S. meliloti as a free-living organism. For example, EPS II could mitigate the effects of desiccation or neutralize potentially harmful compounds in the soil. On the other hand, EPS II production in low phosphate could increase the host specificity of S. meliloti for M. sativa. It has been previously shown that EPS II does not substitute for succinoglycan in nodule invasion on several hosts that can be effectively nodulated by Rm1021 derivatives that produce succinoglycan (15).
High-phosphate conditions, such as those probably found inside the plant nodule, inhibit EPS II production and stimulate succinoglycan production. Analyses of Rm1021 and expR101 in high-phosphate conditions (Fig. 1 and 2) suggest that succinoglycan appears to be the primary exopolysaccharide produced at these levels. The fact that expR101 produces large amounts of succinoglycan in high-phosphate conditions is novel, since expR101 strains have been observed to produce mostly EPS II and suppress succinoglycan production in S. meliloti under most experimental conditions. The function of high levels of succinoglycan production in high-phosphate conditions is not yet clear. β-Galactosidase assays suggest a 15-fold increase in transcription from the exo genes in high-phosphate conditions (Table 2). In Rm1021, succinoglycan production is essential for nodule invasion (exoY derivatives do not invade nodules in any phosphate conditions), but succinoglycan may also have a function inside the nodule. Succinoglycan production in the nodule could protect Sinorhizobium from plant defense mechanisms or other adverse conditions inside the nodule.
The details of how exopolysaccharide production is regulated by phosphate remain to be elucidated. We show that no EPS II production occurs in a phoB null strain in low-phosphate conditions. It has been demonstrated in Escherichia coli that the PhoB protein binds to its target DNA sequence in conditions of phosphate starvation (29). Upon binding, PhoB can either increase or decrease transcription from the genes that it regulates, depending on the position of the pho box with respect to the RNA polymerase binding site. In high-phosphate conditions, the PhoB protein becomes phosphorylated and is released from the pho box (29). Unlike Rm1021, there is no EPS II production induced at low phosphate in a phoB mutant. Carbohydrate composition analysis confirms that succinoglycan (a 7.6:1 Glc/Gal ratio) is the sole exopolysaccharide produced by a phoB mutant, and β-galactosidase assays show no stimulation in transcription from the exp genes in low phosphate (40, 42). This observation is in agreement with the differential expression of the expC gene in low phosphate observed by Summers et al. (42). These observations suggest that PhoB positively regulates the expression of the exp genes, but whether this control is direct or indirect has yet to be resolved.
A possibility of indirect regulation of the exp genes by PhoB is through regulation of phosphate transport. The phosphate transport systems of S. meliloti have been elegantly characterized by others (1, 4, 5, 32). The phoCDET operon encodes a phosphate transport system that is induced in limiting phosphate conditions but repressed under high-phosphate conditions (4). The orfA-pit operon encodes a phosphate transport system that is activated when S. meliloti is grown in excess phosphate but repressed in limiting phosphate conditions (6). The common regulator for these two systems is the PhoB protein. The effect of a phoB mutant on phosphate transport is dual. Transcription from the phoCDET phosphate transport system is suppressed and transcription from the orfA-pit operon is stimulated (6). Perhaps phoCDET transcription, which is suppressed in a phoB background, is used as a signal of low-phosphate conditions and is required for EPS II production in low phosphate. We are currently investigating how the products of the phoCDET and orfA-pit operons affect exopolysaccharide production in S. meliloti.
Previous work has suggested that the exp operon may have sites that are responsible for the induction of EPS II production in low-phosphate conditions. Mutation of the mucS locus of the exp operon eliminates the induction of EPS II synthesis in low phosphate (3). Due to the position of the mucS locus in the intergenic region between the two oppositely transcribed arms of the exp operon, this site could act as a regulatory region for the operon. A study by Summers et al. has suggested a region of limited homology to a pho box within the mucS locus (42), and others have suggested regions of weak homology throughout the exp operon (40). We have noticed a very strong putative pho box about 150 bp upstream of this locus, but the significance of this region is still unknown.
We also noticed the presence of two putative PhoB-binding elements upstream from the promoter region of exoY. Due to its position between the two oppositely transcribed arms of the exo operon, the exoX-exoY intergenic region has been suggested to be a logical site for transcriptional regulation of the exo operon. Recent work has demonstrated that the exoX-exoY intergenic region may have sites for binding of the MucR protein, a positive regulator of succinoglycan production (10). The first of the two putative pho boxes resides upstream (−63 to −46) of a putative start site for exoY and is very similar in placement to a putative pho box upstream (−62 to −45) of the phosphate-regulated phoCDET operon in S. meliloti (4). The second box, however, has an unusual placement (−27 to −10 versus −40 to −23 in phoCDET) that could block the ribosome-binding site when PhoB binds in low-phosphate conditions. We do not know what role, if any, these putative PhoB-binding regions may have on the production of succinoglycan and/or expression of the exo genes. Site-directed mutagenesis of these putative PhoB-binding elements could elucidate what role, if any, PhoB could play in succinoglycan production. The environment that Sinorhizobium encounters as a free-living organism in the soil is very different from that found inside the nodule. The radical change in phosphate concentration between the soil and the nodule could provide a strong signal of the changing environmental conditions. Many other environmental signals may play a role in facilitating the adjustment of the bacteria between the soil and the nodule. It would be interesting to see how other factors important to symbiosis may be affected by changes in phosphate concentration and what other signals may play a role in controlling exopolysaccharide production in Sinorhizobium.
ACKNOWLEDGMENTS
We thank Graham C. Walker and Turlough Finan for providing strains. We also thank Lawrence Reitzer and the members of our laboratory for their helpful discussions and insights on this project.
This work was supported by National Science Foundation grant MCB-9733532 to J.E.G.
REFERENCES
- 1.al-Niemi T S, Summers M L, Elkins J G, Kahn M L, McDermott T R. Regulation of the phosphate stress response in Rhizobium meliloti by PhoB. Appl Environ Microbiol. 1997;63:4978–4981. doi: 10.1128/aem.63.12.4978-4981.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aman P, McNeil M, Franzen L-E, Darvill A G, Albersheim P. Structural elucidation, using HPLC-MS and GLC-MS, of the acidic exopolysaccharide secreted by Rhizobium meliloti strain Rm1021. Carbohydr Res. 1981;95:263–282. [Google Scholar]
- 3.Astete S G, Leigh J A. mucS, a gene involved in activation of galactoglucan (EPS II) synthesis gene expression in Rhizobium meliloti. Mol Plant-Microbe Interact. 1996;9:395–400. doi: 10.1094/mpmi-9-0395. [DOI] [PubMed] [Google Scholar]
- 4.Bardin S, Dan S, Osteras M, Finan T M. A phosphate transport system is required for symbiotic nitrogen fixation by Rhizobium meliloti. J Bacteriol. 1996;178:4540–4547. doi: 10.1128/jb.178.15.4540-4547.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bardin S D, Finan T M. Regulation of phosphate assimilation in Rhizobium (Sinorhizobium) meliloti. Genetics. 1998;148:1689–1700. doi: 10.1093/genetics/148.4.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bardin S D, Voegele R T, Finan T M. Phosphate assimilation in Rhizobium (Sinorhizobium) meliloti: identification of a pit-like gene. J Bacteriol. 1998;180:4219–4226. doi: 10.1128/jb.180.16.4219-4226.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Battisti L, Lara J C, Leigh J A. Specific oligosaccharide form of the Rhizobium meliloti exopolysaccharide promotes nodule invasion in alfalfa. Proc Natl Acad Sci USA. 1992;89:5625–5629. doi: 10.1073/pnas.89.12.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker A, Kleickmann A, Arnold W, Pühler A. Analysis of the Rhizobium meliloti exoH, exoK, exoL fragment: ExoK shows homology to excreted endo-β-1,3-1,4 glucanases and ExoH resembles membrane proteins. Mol Gen Genet. 1993;238:145–154. doi: 10.1007/BF00279541. [DOI] [PubMed] [Google Scholar]
- 9.Becker A, Kleickmann A, Keller M, Arnold W, Pühler A. Identification and analysis of the Rhizobium meliloti exoAMNOP genes involved in exopolysaccharide biosynthesis and mapping of promoters located on the exoHKLAMONP fragment. Mol Gen Genet. 1993;241:367–379. doi: 10.1007/BF00284690. [DOI] [PubMed] [Google Scholar]
- 10.Bertram-Drogatz P A, Ruberg S, Becker A, Pühler A. The regulatory protein MucR binds to a short DNA region located upstream of the mucR coding region in Rhizobium meliloti. Mol Gen Genet. 1997;254:529–538. doi: 10.1007/s004380050448. [DOI] [PubMed] [Google Scholar]
- 11.Bieleski R. Phosphate pools, phosphate transport, and phosphate availability. Annu Rev Plant Physiol. 1973;24:225–252. [Google Scholar]
- 12.Denarie J, Cullimore J. Lipo-oligosaccharide nodulation factors: a new class of signaling molecules mediating recognition and morphogenesis. Cell. 1993;74:951–954. doi: 10.1016/0092-8674(93)90717-5. [DOI] [PubMed] [Google Scholar]
- 13.Finan T M, Hartweig E, LeMieux K, Bergman K, Walker G C, Signer E R. General transduction in Rhizobium meliloti. J Bacteriol. 1984;159:120–124. doi: 10.1128/jb.159.1.120-124.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finan T M, Kunkel B, De Vos G F, Signer E R. Second symbiotic megaplasmid in Rhizobium meliloti carrying exopolysaccharide and thiamine synthesis genes. J Bacteriol. 1986;167:66–72. doi: 10.1128/jb.167.1.66-72.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glazebrook J, Walker G C. A novel exopolysaccharide can function in place of the Calcofluor-binding exopolysaccharide in nodulation of alfalfa by Rhizobium meliloti. Cell. 1989;56:661–672. doi: 10.1016/0092-8674(89)90588-6. [DOI] [PubMed] [Google Scholar]
- 16.Glucksmann M A, Reuber T L, Walker G C. Family of glycosyl transferases needed for the synthesis of succinoglycan by Rhizobium meliloti. J Bacteriol. 1993;175:7033–7044. doi: 10.1128/jb.175.21.7033-7044.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glucksmann M A, Reuber T L, Walker G C. Genes needed for the modification, polymerization, export, and processing of succinoglycan by Rhizobium meliloti: a model for succinoglycan biosynthesis. J Bacteriol. 1993;175:7045–7055. doi: 10.1128/jb.175.21.7045-7055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.González J E, Reuhs B L, Walker G C. Low molecular weight EPS II of Rhizobium meliloti allows nodule invasion in Medicago sativa. Proc Natl Acad Sci USA. 1996;93:8636–8641. doi: 10.1073/pnas.93.16.8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.González J E, Semino C E, Wang L X, Castellano-Torres L E, Walker G C. Biosynthetic control of molecular weight in the polymerization of the octasaccharide subunits of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Proc Natl Acad Sci USA. 1998;95:13477–13482. doi: 10.1073/pnas.95.23.13477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.González J E, York G M, Walker G C. Rhizobium meliloti exopolysaccharides: synthesis and symbiotic function. Gene. 1996;179:141–146. doi: 10.1016/s0378-1119(96)00322-8. [DOI] [PubMed] [Google Scholar]
- 21.Her G-R, Glazebrook J, Walker G C, Reinhold V N. Structural studies of a novel exopolysaccharide produced by a mutant of Rhizobium meliloti strain Rm1021. Carbohydr Res. 1990;198:305–312. doi: 10.1016/0008-6215(90)84300-j. [DOI] [PubMed] [Google Scholar]
- 22.Hynes M F, Simon R, Müller P, Niehaus K, Labes M, Pühler A. The two megaplasmids of Rhizobium meliloti are involved in the effective nodulation of alfalfa. Mol Gen Genet. 1986;202:356–362. [Google Scholar]
- 23.Israel D. Investigation of the role of phosphorus in symbiotic dinitrogen fixation. Plant Physiol. 1987;84:835–840. doi: 10.1104/pp.84.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keller M, Müller P, Simon R, Pühler A. Rhizobium meliloti genes for exopolysaccharide synthesis and nodule infection located on megaplasmid 2 are actively transcribed during symbiosis. Mol Plant-Microbe Interact. 1988;1:267–274. [Google Scholar]
- 25.Leigh J A, Signer E R, Walker G C. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc Natl Acad Sci USA. 1985;82:6231–6235. doi: 10.1073/pnas.82.18.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leigh J A, Walker G C. Exopolysaccharides of Rhizobium: synthesis, regulation and symbiotic function. Trends Genet. 1994;10:63–67. doi: 10.1016/0168-9525(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 27.Long S R, Staskawicz B J. Prokaryotic plant parasites. Cell. 1993;73:921–935. doi: 10.1016/0092-8674(93)90271-q. [DOI] [PubMed] [Google Scholar]
- 28.Losick R, Kaiser D. Why and how bacteria communicate. Sci Am. 1997;276:68–73. doi: 10.1038/scientificamerican0297-68. [DOI] [PubMed] [Google Scholar]
- 29.Makino K, Amemura M, Kim S K, Nakata A, Shinawaga H. Mechanism of transcriptional activation of the phosphate regulon in Escherichia coli. In: Torriani-Gorini A, Yagil E, Silver S, editors. Phosphate in microorganisms: cellular and molecular biology. Washington, D.C.: ASM Press; 1994. pp. 5–12. [Google Scholar]
- 30.Miller J. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 31.Morris D. Quantitative determination of carbohydrates with Dreywood's anthrone reagent. Science. 1948;108:254–255. doi: 10.1126/science.107.2775.254. [DOI] [PubMed] [Google Scholar]
- 32.Oresnik I J, Charles T C, Finan T M. Second site mutations specifically suppress the Fix− phenotype of Rhizobium meliloti ndvF mutations on alfalfa: identification of a conditional ndvF-dependent mucoid colony phenotype. Genetics. 1994;136:1233–1243. doi: 10.1093/genetics/136.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pühler A, Arnold W, Buendia-Claveria A, Kapp D, Keller M, Niehaus K, Quandt J, Roxlau A, Weng W M. The role of the Rhizobium meliloti exopolysaccharides EPS I and EPS II in the infection process of alfalfa nodules. In: Hennecke H, Verma D P S, editors. Advances in molecular genetics of plant-microbe interactions. Vol. 1. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1991. pp. 189–194. [Google Scholar]
- 34.Pühler A M, Arnold W, Becker A, Roxlau A, Keller M, Kapp D, Lagares A, Lorenzen J, Niehaus K. The role of Rhizobium meliloti surface polysaccharides in nodule development. In: Palacios R, Mora J, Newton W E, editors. New horizons in nitrogen fixation. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1993. pp. 207–212. [Google Scholar]
- 35.Reed J W, Capage M, Walker G C. Rhizobium meliloti exoG and exoJ mutations affect the ExoX-ExoY system for modulation of exopolysaccharide production. J Bacteriol. 1991;173:3776–3788. doi: 10.1128/jb.173.12.3776-3788.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reinhold B B, Chan S Y, Reuber T L, Marra A, Walker G C, Reinhold V N. Detailed structural characterization of succinoglycan, the major symbiotically important exopolysaccharide of Rhizobium meliloti Rm1021. J Bacteriol. 1994;176:1997–2002. doi: 10.1128/jb.176.7.1997-2002.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reuber T L, Walker G C. The acetyl substituent of succinoglycan is not necessary for alfalfa nodule invasion by Rhizobium meliloti Rm1021. J Bacteriol. 1993;175:3653–3655. doi: 10.1128/jb.175.11.3653-3655.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reuber T L, Walker G C. Biosynthesis of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Cell. 1993;74:269–280. doi: 10.1016/0092-8674(93)90418-p. [DOI] [PubMed] [Google Scholar]
- 39.Reuhs B L, Carlson R W, Kim J S. Rhizobium fredii and Rhizobium meliloti produce 3-deoxy-d-manno-2-octulosonic acid-containing polysaccharides that are structurally analogous to group II K antigens (capsular polysaccharides) found in Escherichia coli. J Bacteriol. 1993;175:3570–3580. doi: 10.1128/jb.175.11.3570-3580.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruberg S, Pühler A, Becker A. Biosynthesis of the exopolysaccharide galactoglucan in Sinorhizobium meliloti is subject to a complex control by the phosphate-dependent regulator PhoB and the proteins ExpG and MucR. Microbiology. 1999;145:603–611. doi: 10.1099/13500872-145-3-603. [DOI] [PubMed] [Google Scholar]
- 41.Smith M W, Payne J W. Expression of periplasmic binding proteins for peptide transport is subject to negative regulation by phosphate limitation in Escherichia coli. FEMS Microbiol Lett. 1992;100:183–190. doi: 10.1111/j.1574-6968.1992.tb14038.x. [DOI] [PubMed] [Google Scholar]
- 42.Summers M L, Elkins J G, Elliott B A, McDermott T R. Expression and regulation of phosphate stress inducible genes in Sinorhizobium meliloti. Mol Plant-Microbe Interact. 1998;11:1094–1101. doi: 10.1094/MPMI.1998.11.11.1094. [DOI] [PubMed] [Google Scholar]
- 43.Urzainqui A, Walker G C. Exogenous suppression of the symbiotic deficiencies of Rhizobium meliloti exo mutants. J Bacteriol. 1992;174:3403–3406. doi: 10.1128/jb.174.10.3403-3406.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhan H J, Lee C C, Leigh J A. Induction of the second exopolysaccharide (EPSb) in Rhizobium meliloti SU47 by low phosphate concentrations. J Bacteriol. 1991;173:7391–7394. doi: 10.1128/jb.173.22.7391-7394.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]