Abstract
Objective
To compare endoscopic and histologic features of pediatric patients with eosinophilic esophagitis (EoE) responding to proton pump inhibitor (PPI) to those not responding to PPI.
Methods
Endoscopic reports and photographs of patients with symptoms of esophageal dysfunction and ≥15 eosinophils per high-powered field (eos/hpf) in esophageal biopsies prior to PPI trial were reviewed. Patients were classified as responsive to PPI (PPIREoE) or non-responsive to PPI (PPINREoE) according to response totreatment (<15 eos/hpf) at second endoscopy after 8 weeks.
Results
Of the 231 patients (72.3% male), 64 (27.7%) were responsive to the proton pump inhibitors. Edema (77.3% vs. 62.5%, p = 0.031) and vertical lines (69.5% vs. 51.6%, p = 0.014) were more frequent in PPINREoE patients. An eosinophil count in the mid-esophagus ≥ 35 eos/HPF (25.1% vs. 12.5%) was more frequent in these patients (p = 0.001). Those with eosinophil count < 15 eos/HPF in the mid-esophagus at the first endoscopy were more likely to respond to treatment with proton pump inhibitors compared to patients with 15-34 eos/HPF (p = 0.004, OR: 3.26, 95% CI: 1.46-7.24) and to patients with ≥ 35 eos/HPF (p = 0.006, OR: 3.20, 95% CI: 1.39-7.41).
Conclusion
Edema and vertical lines at the endoscopy and a higher eosinophil count in the mid-esophagus were more frequent in patients who were non-responsive to proton pump inhibitors. As there were no significant differences in the other findings between the groups, it cannot be affirmed that these characteristics are sufficient to differentiate between PPINREoE and PPIREoE patients.
Keywords: Eosinophilic esophagitis, Endoscopy, Histology, Proton pump inhibitors
Resumo
Objetivo
Comparar características endoscópicas e histológicas entre pacientes com esofagite eosinofílica responsiva (EoERIBP) e não responsiva (EoENRIBP) ao tratamento com inibidores de bomba de prótons.
Métodos
Avaliados laudos e imagens endoscópicas de pacientes com sintomas de disfunção esofágica associados a contagem ≥15 eosinófilos por campo de grande aumento (eos/CGA) em biópsia do esôfago. Os pacientes foram classificados em responsivos (EoERIBP) ou não responsivos (EoENRIBP) aos inibidores de bomba de prótons conforme resposta ao tratamento na segunda endoscopia (<15 eos/CGA) após 8 semanas.
Resultados
Dos 231 pacientes (72,3% masculino), 64 (27,7%) foram responsivos aos inibidores de bomba de prótons. Edema (77,3% vs. 62,5%, p = 0,031) e linhas verticais (69,5% vs. 51,6%, p = 0,014) foram mais frequentes nos EoENRIBP. A contagem de eosinófilos em esôfago médio ≥35 eos/CGA (25,1% vs. 12,5%) foi mais frequente nesses pacientes (p = 0,001). Os que apresentaram contagem de eosinófilos <15 eos/CGA no esôfago médio à primeira endoscopia apresentaram maior chance de responder ao tratamento com inibidores de bomba de prótons em comparação aos pacientes com 15-34 eos/CGA (p = 0,004; OR: 3,26; IC95%: 1,46 - 7,24) e aos pacientes com ≥35 eos/CGA (p = 0,006; OR: 3,20; IC95%: 1,39 - 7,41).
Conclusão
Edema e linhas verticais à endoscopia e maior contagem de eosinófilos em esôfago médio foram mais frequentes nos pacientes não responsivos aos inibidores de bomba de prótons. Uma vez que não houve diferenças significativas nos outros achados entre os grupos, não se pode afirmar que essas características sejam suficientes para distinguir pacientes com EoENRIBP dos pacientes com EoERIBP.
Palavras-chave: Esofagite eosinofílica, Endoscopia, Histologia, Inibidores de bomba de prótons
Introduction
Eosinophilic esophagitis (EoE) is characterized by the presence of eosinophilic infiltrate in the esophagus, without affecting other segments of the gastrointestinal tract and has been recognized with increasing frequency in the last 20 years.1, 2, 3 It is not clear whether this represents an increase in diagnostic perception, or if it is a true increase in the onset of new cases.
The symptoms of EoE vary according to age. Infants and young children may present with feeding difficulties, vomiting, and regurgitation, while older children, adolescents, and adults may also experience dysphagia and a feeling that food is obstructing the esophagus (food impaction).4, 5, 6
Upper gastrointestinal endoscopy with biopsy is essential for the diagnosis. Endoscopic findings include edema, furrows or vertical lines, concentric rings, and white exudates, whereas the macroscopic appearance may be normal.5, 7
Hirano et al. developed a classification system for endoscopic findings of EoE, which may help the diagnosis and can be used to monitor the disease, since the endoscopic score tends to decrease, especially in patients who show histological improvement after treatment.7
The diagnosis of EoE is clinical, endoscopic, and histologic, that is. There must be clinical manifestations of esophageal dysfunction, associated with mucosal changes at endoscopy and/or eosinophilic infiltrate in esophageal biopsies, with eosinophil counts ≥15 eosinophils per high-power field (eos/HPF) in the area of higher eosinophil density, in one or more tissue samples.5, 8, 9
Other causes of esophageal eosinophilia, especially gastroesophageal reflux disease (GERD) should be ruled out, as well as infections, connective tissue diseases, Crohn’s disease, and drug hypersensitivity, through a detailed clinical history and physical examination, and through additional specific tests, according to the clinical suspicion.5, 8, 9
The symptoms of EoE and GERD are similar, especially in infants and preschoolers, which makes differential diagnosis between the two entities challenging. The indication for esophageal pH-metry, initially recommended to differentiate the two diseases, no longer plays a valid role, because both conditions can occur in the same patient, and it has also been demonstrated that patients with EoE may have increased acid exposure.8, 9
The absence of histologic response to treatment with proton pump inhibitors (PPIs) for the diagnosis of EoE initially had the intention of ruling out GERD as the cause of esophageal eosinophilia. However, the correlation between eosinophilic infiltration of the esophagus with EoE and GERD was shown to be much more complex. Therefore, a third diagnostic category named PPI-responsive eosinophilic esophagitis (PPIREoE) was created, referring to the subgroup of patients with typical symptoms of EoE, but without GERD and with clinical and histological improvement after treatment with PPIs.10, 11 Additionally, EoE and GERD can coexist in the same patient.
Until recently, it was unclear whether PPIREoE was a subtype of GERD, an EoE subtype, or an independent condition, but it has been shown that at least 50% of patients with suspected EoE may respond to treatment with PPIs.12
The term “PPI-responsive eosinophilic esophagitis” was questioned, because it is based on the response to treatment with a single medication; moreover, patients with eosinophilia in the esophagus, whether or not responsive to PPI, have very similar clinical, endoscopic, and histologic feautures, reinforcing the concept that patients responsive to PPIs constitute a subtype of EoE.8, 9, 13 Therefore, in this study, the group of patients responding to PPI treatment are referred to as patients with PPI-responsive eosinophilic esophagitis (PPIREoE).
The aim of the present study was to compare the endoscopic and histologic characteristics of patients with PPI-non-responsive eosinophilic esophagitis (PPINREoE) with those of patients with PPI-responsive eosinophilic esophagitis (PPIREoE).
Methods
An observational and longitudinal study was performed using the database of the Center forPediatric Gastroenterology and Gastrointestinal Endoscopy of Hospital Pequeno Príncipe -Curitiba, Brazil, where approximately 2,000 procedures are carried out per year. This database contains the summarized clinical, endoscopic, and histopathological information of all patients submitted to endoscopic procedures at the institution.
The authors included patients with symptoms of esophageal dysfunction submitted to upper gastrointestinal endoscopy (UGE) with esophageal biopsies from January 2008 to August 2017 who had eosinophil counts ≥15 per high-power field (×400), without evidence of other possible causes of eosinophilia. Other causes of esophageal eosinophilia were excluded when, during the interview before endoscopy, there was history, symptoms, and/or endoscopic and histologic features of conditions that could lead to esophageal eosinophilia (e.g., history of caustic ingestion, infectious diseases, celiac disease, inflammatory bowel disease, use of medications such as carbamazepine, azathioprine, etc.).
The reports and images of endoscopies of 316 patients were evaluated. Of this group, 85 patients were excluded, of whom 69 who were not submitted to the second endoscopy within the specified period; 5 because they were treated with PPIs before the initial examination and 11 for having received other treatment modalities after the first endoscopy (10 patients on topical corticosteroids and 1 patient receiving dietary treatment). Therefore, 231 patients were included in this study.
Endoscopic examinations were performed, photographed, and reported by four pediatric endoscopists and stored in an electronic image bank. Two fragments from the mid/proximal esophagus and 2 fragments from the distal esophagus were collected for histologic analysis.
A single experienced pathologist examined the biopsy specimens of mid- and distal esophagus, two fragments from each area, and described histologic findings and number of eosinophils/HPF in all patients included in the study.
Patients were treated with a PPI (omeprazole, pantoprazole, or esomeprazole) at a dose of 2 mg/kg/day divided into two doses (12/12 h) for a period of 8 weeks. They were asked about the correct use of the medication before second endoscopy was performed. Patients who showed endoscopic (absence of abnormalities) and histologic (decrease in the number of eosinophils to <15 eos/HPF) responses were considered to be responsive to PPIs.
Data collection (demographics, endoscopic and histologic findings) was started as soon as the presence of ≥15 eos/HPF was identified in any fragment of the esophageal biopsy. After 8 weeks of treatment with PPI, endoscopic and histologica findings were reassessed.
For this study, the endoscopic images were reviewed by two endoscopists, and the findings (edema, rings, whitish exudate, vertical lines or furrows, strictures, and mucosal fragility) were described. These characteristics were described according to the Endoscopic Reference Score for EoE (EREFS), published in 2013, which allows the generation of a numerical score.7 The score (grading) was not used in this study because it comprised an analysis of photographs that allowed the identification of the endoscopic features, but not the precise grading of all the findings.
The histologic findings were classified into three groups (<15 eos/HPF, 15–34 eos/HPF, and ≥35 eos/HPF) in both the mid- and distal esophagus.
The information obtained from the database was entered in a Microsoft Excel® spreadsheet (Microsoft Corporation, Washington, United States) and imported into the IBM SPSS Statistics v.20.0 computer program (IBM Corp., New York, United States) for processing.
Patient age at the diagnosis was described by median, minimum, and maximum values. Categorical variables were described as frequencies and percentages. For the analysis of the association between endoscopic and histologic findings with response to treatment, logistic regression models were adjusted (univariate analysis). Data were analyzed using Student’s t-test, Fisher’s exact test, or the Chi-squared test and the Wald test. The estimated association measure was the odds ratio (OR) with 95% confidence intervals. The normality condition of the quantitative variables was assessed by the Kolmogorov-Smirnov test. Values of p < 0.05 indicated statistical significance.
The study protocol was approved by the Research Ethics Committee of Hospital Pequeno Príncipe, Curitiba-PR (CAAE: 25560113.4.0000.0097, protocol number 094829/2013 on12/17/2013).
Results
Data were collected from 231 patients. Of these, 167 (72.3%) patients were males and 64 (27.7%) were females. The median age of the patients at diagnosis was 6.1 years (9 months–17.6 years). Of the 231 patients, 167 (72.3%) were classified as PPINREoE and 64 (27.7%) were classified as PPIREoE.
The median age at diagnosis of patients with PPINREoE was 6.0 years (9 months–17.3 years)and for patients with PPIREoE, it was 6.2 years (9 months–17.6 years). There was no significant difference between the groups (p = 0.545). In the group of patients with PPINREoE, 123 (74%) were males and 44 (26%) were females, and in the group of patients with PPIREoE, 44 (69%) were males and 20 (31%) were females (p = 0.512).
Of the 231 patients, 34 (14.7%) did not present endoscopic abnormalities, with only histological changes being observed. Of these, 15 (23.4%) patients were from the PPIREoE group and 19 (11.4%) were from the PPINREoE group (p = 0.036).
The comparison of the endoscopic findings between the PPINREoE and PPIREoE groups showed that edema (77.3% vs. 62.5%, respectively, p = 0.031) and vertical lines (69.5% vs. 51.6%, respectively, p = 0.014) were more prevalent in patients with PPINREoE.
Other endoscopic findings showed a similar frequency between the PPINREoE and PPIREoE groups, including the presence of rings (3.6% vs. 1.6% respectively, p = 0.677); whitish exudate (41.3% vs. 29.7%, respectively, p = 0.134); mucosal fragility (3.0% vs. 3.1%, respectively, p = 1) and stricture (2.4% vs. 3.1%, p = 0.670).
The comparison between the histologic findings showed that 25.1% of patients in the PPINREoE group had ≥35 eos/HPF in the mid-esophagus versus 12.5% in the PPIREoE group (p = 0.001). Endoscopic and histologicfindings, prior to the start of PPI treatment, are shown in Table 1, divided into the two groups (PPIREoE and PPINREoE).
Table 1.
Endoscopic and histological findings in patients with PPINREoE (n = 167) and with PPIREoEat the first endoscopy (n = 64).
| Characteristics | Classification | PPINREoE | PPIREoE | p | ||
|---|---|---|---|---|---|---|
| Normal | 19 | 22.4% | 15 | 23.4% | 0.036 | |
| Edema | 129 | 77.3% | 40 | 62.5% | 0.031 | |
| Vertical lines | 116 | 69.5% | 33 | 51.6% | 0.014 | |
| Whitish exudate | <10% | 48 | 28.7% | 16 | 25.0% | |
| >10% | 21 | 12.6% | 3 | 4.7% | 0.134 | |
| Rings | 6 | 3.6% | 1 | 1.6% | 0.677 | |
| Mucosal fragility | 5 | 3.0% | 2 | 3.1% | 1 | |
| Stricture | 4 | 2.4% | 2 | 3.1% | 0.670 | |
| Eos/HPF | <15 | 77 | 46.1% | 47 | 73.4% | |
| Mid-esophagus | 15–34 | 48 | 28.7% | 9 | 14.1% | |
| ≥35 | 42 | 25.1% | 8 | 12.5% | 0.001 | |
| Eos/HPF | <15 | 10 | 6.0% | 4 | 6.3% | |
| Distal esophagus | 15–34 | 90 | 53.9% | 44 | 68.8% | |
| ≥35 | 67 | 40.1% | 16 | 25.0% | 0.095 | |
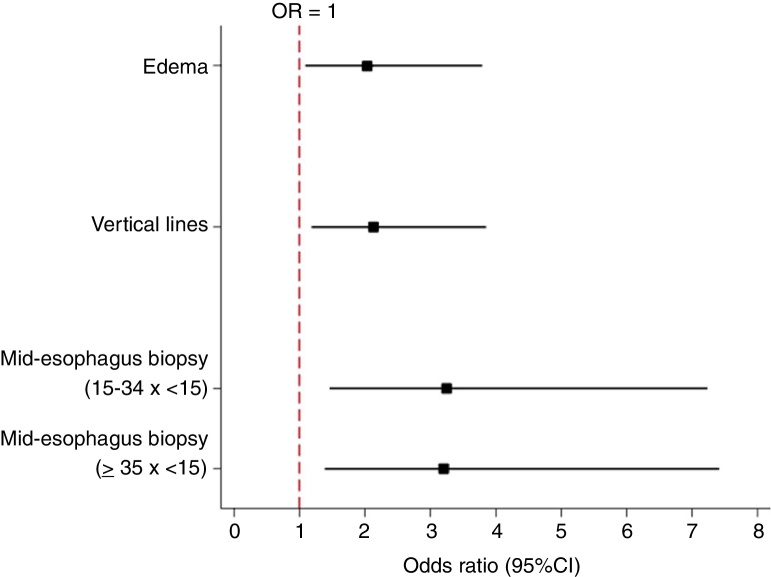
The presence of edema at the initial UGE was a factor significantly associated with histologic non-response to PPI treatment (p = 0.025). Of the patients that had edema, 23.7% showed a response, and of the patients who did not have edema, 38.7% showed a response. Therefore, the absence of edema at the endoscopy was associated with a higher probability of responding to PPI treatment (p = 0.025, OR: 2.04, 95% CI: 1.09–3.79) (Fig. 1).
Figure 1.
Odds ratios and 95% confidence intervals for variables that had statistical significance in the univariate analysis (non-responsive to PPI).
The presence of VL in the initial UGE was a factor associated with non-response to PPI treatment (p = 0.014). Of the patients who had VL, 22.1% showed a response, and of the patients who did not have VL, 37.8% showed a response. Therefore, not having VL was associated with a higher probability of responding to PPI treatment. (p = 0.014, OR: 2.14, 95% CI: 1.18–3.86; Fig. 1).
Patients who had an eosinophil count <15 eos/HPF in the mid-esophagus at the first UGE were more likely to respond to PPI treatment compared to patients with 15–34 eos/HPF (p = 0.004, OR: 3.26, 95% CI: 1.46–7.24) and patients with ≥ 35 eos/HPF (p = 0.006, OR: 3.20, 95% CI: 1.39–7.41; Fig. 1).
The eosinophil counts in the distal esophagus at the first UGE did not show a difference in the probability of responding to PPI treatment between the group with <15 eos/HPF and the group with 15–34 eos/HPF (p = 0.746, OR: 0.82; 95% CI: 0.24–2.76), nor the group with ≥35 eos/HPF (p = 0.430; OR: 1.67; 95% CI: 0.47–6.03).
Discussion
The present study aimed to compare the endoscopic and histologic findings of patients with PPINREoE and PPIREoE. The majority (72.3%) of the assessed patients had a diagnosis of PPINREoE.
It was observed that the age at diagnosis was similar between the groups, and the median age at diagnosis was between 6 and 7 years.
A multicenter American study involving 705 patients with EoE showed that the median age at diagnosis was 8 years.14
There was a higher prevalence of PPINREoE and PPIREoE in male patients, as previously demonstrated by other authors.14, 15
Endoscopic characteristics may appear isolated or in association. In the present study, 85% of the patients had at least one endoscopic abnormality, with a high frequency of edema (73%) and vertical lines (65%) in the study population.
A systematic review and meta-analysis of 100 clinical studies involving adult and pediatric patients described 41% prevalence of edema, 27% prevalence of whitish exudate, 48% prevalence of vertical lines, and 44% prevalence of concentric rings in EoE patients.15 The authors demonstrated that at least one endoscopic abnormality was observed in 93% of the population with EoE. However, substantial heterogeneity was identified between the studies. The exams were normal in 7% of patients with EoE when only prospective studies were analyzed, but this percentage was 17% when considering all analyzed studies (p < 0.05).15 It was also demonstrated that there was a difference in the prevalence of findings according to age. Rings and strictures were more prevalent in adults (57% and 25%, respectively) than in children (11% and 8%, respectively). On the other hand, whitish exudate and edema were more prevalent in children (36% and 58%, respectively) than in adults (19% and 18%).15
These findings are in line with those observed in the present study, which evaluated pediatric patients. It can be speculated that due to the shorter disease duration, the characteristics that suggest the development of fibrosis (rings and strictures) had not yet occurred in most patients.
Among the endoscopic abnormalities, the presence of edema and vertical lines was more frequent in those patients in the PPINREoE group. These findings coincide with those reported in an American study in adults that showed that patients with PPINREoE (n = 41) had a higher prevalence of vertical lines (92% vs. 58%, p = 0.008) and edema (17% vs. 0% %, p = 0.04) when compared to patients with PPIREoE (n = 24).16
Considering the histologicfindings, it was observed that in comparison to patients with PPIREoE, patients with PPINREoE had a higher eosinophil count in the mid- and distal esophagus. A study of 103 adult patients showed that PPI- responsive and non-responsive patients had a similar eosinophil count in the proximal (39 eos/HPF vs. 38 eos/HPF, p = 0.919) and distal (50 vs. 43 eos/HPF, p = 0.285) esophagus.17
However, other authors have identified a difference in eosinophil counts between the groups when evaluating patients in the pediatric age group. Gutiérrez-Junquera et al. showed that children who did not respond to PPI had a higher eosinophil count compared to patients who responded to PPI (74.8 ± 36.2 vs. 46.3 ± 30.7).12 Dranove et al. reported that 50% of children who had eosinophil counts between 15 and 20 eos/HPF responded to PPI treatment, when compared to 29% of those with>20 eos/HPF.18 Therefore, as demonstrated in the present study, children with higher eosinophil counts in the esophagus tend not to respond to PPI treatment.
The limitations of this study include its sample size and the evaluation of only endoscopic and histologic characteristics, since the endoscopy database includes patients followed at other services with summarized clinical information and, therefore, with insufficient data to detail their clinical aspects.
In conclusion, this study demonstrated some endoscopic and histologic differences (edema/vertical lines and eosinophil counts in the mid-esophagus) between the groups, but it cannot confirm that these characteristics are sufficient to differentiate patients with PPINREoE from patients with PPIREoE, since both groups had other similar endoscopic and histologic features. This was a study carried out in pediatric patients in Brazil that disclosed findings similar to those observed in other countries, which may contribute to the management of the disease in Brazil.
The diagnostic criteria for EoE are constantly evolving. PPIs should be considered the first step in the treatment of patients with esophageal dysfunction and eosinophilic infiltration in the esophagus.19 These drugs have been shown to induce and maintain remission in EoE, with a low adverse effect profile and were recently included in the therapeutic arsenal for the disease.9, 12, 19
The safety and effectiveness of the several therapeutic modalities should be continuously discussed, as well as the cost-benefit association. The identification of patients responsive to PPI may increase the use of these drugs, with lower-cost and better adherence totreatment.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
The authors would like to thank the medical student Luiza Feltran Vieira for her collaboration with data collection and tabulation, and professor Márcia Olandoski for her contributions in the development of the statistical analysis.
Footnotes
Please cite this article as: Vieira GG, Ribeiro LB, Truppel SK, Rosário Filho NA, Vieira MC. Endoscopic and histological characteristics in patients with eosinophilic esophagitis responsive and non-responsive to proton pump inhibitors. J Pediatr (Rio J). 2020;96:638–43.
Study conducted at Hospital Pequeno Príncipe, Curitiba, Brazil; Pontifícia Universidade Católica do Paraná, Curitiba, Brazil; Universidade Federal do Paraná, Curitiba, Brazil.
References
- 1.Liacouras C.A., Spergel J.M., Ruchelli E., Verma R., Mascarenhas M., Semeao E., et al. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol. 2005;3:1198–1206. doi: 10.1016/s1542-3565(05)00885-2. [DOI] [PubMed] [Google Scholar]
- 2.Blanchard C., Wang N., Rothenberg M.E. Eosinophilic esophagitis: pathogenesis, genetics, and therapy. J Allergy Clin Immunol. 2006;118:1054–1059. doi: 10.1016/j.jaci.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 3.Furuta G.T., Liacouras C.A., Collins M.H., Gupta S.K., Justinich C., Putnam P.E., et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342–1363. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Liacouras C.A. Clinical presentation and treatment of pediatric patients with eosinophilic esophagitis. Gastroenterol Hepatol (N Y) 2011;7:264–267. [PMC free article] [PubMed] [Google Scholar]
- 5.Ferreira C.T., Vieira M.C., Furuta G.T., Barros F.C., Chehade M. Eosinophilic esophagitis — where are we today? J Pediatr (Rio J) 2019;95:275–281. doi: 10.1016/j.jped.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigues M., D’Amico M.F., Patiño F.R., Barbieri D., Damião A.O., Sipahi A.M. Clinical manifestations, treatment, and outcomes of children and adolescents with eosinophilic esophagitis. J Pediatr (Rio J) 2013;89:197–203. doi: 10.1016/j.jped.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Hirano I., Moy N., Heckman M.G., Thomas C.S., Gonsalves N., Achem S.R. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2013;62:489–495. doi: 10.1136/gutjnl-2011-301817. [DOI] [PubMed] [Google Scholar]
- 8.Lucendo A.J., Molina-Infante J., Arias A., von Arnim U., Bredenoord A.J., Bussmann C., et al. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendation for diagnosis and management in children and adults. United Eur Gastroenterol J. 2017;5:335–358. doi: 10.1177/2050640616689525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dellon E.S., Liacouras C.A., Molina-Infante J.A., Furuta G.T., Spergel J.M., Zevit N., et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: proceedings of the AGREE Conference. Gastroenterology. 2018;155:1022–1033. doi: 10.1053/j.gastro.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molina-Infante J., Van Rhijn B.D. Interactions between gastro-oesophageal reflux disease and eosinophilic oesophagitis. Best Pract Res Clin Gastroenterol. 2015;29:749–758. doi: 10.1016/j.bpg.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Molina-Infante J., Bredenoord A.J., Cheng E., Dellon E.S., Furuta G.T., Gupta S.K., et al. Proton pump inhibitor-responsive oesophageal eosinophilia: an entity challenging current diagnostic criteria for eosinophilic oesophagitis. Gut. 2016;65:524–531. doi: 10.1136/gutjnl-2015-310991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutierrez-Junquera C., Fernandez-Fernandez S., Cilleruelo M.L., Rayo A., Echeverria L., Quevedo S., et al. High prevalence of response to proton-pump inhibitor treatment in children with esophageal eosinophilia. J Pediatr Gastroenterol Nutr. 2016;62:704–710. doi: 10.1097/MPG.0000000000001019. [DOI] [PubMed] [Google Scholar]
- 13.Molina-Infante J., Gonzalez-Cordero P.L., Lucendo A.J. Proton pump inhibitor-responsive esophageal eosinophilia: still a valid diagnosis? Curr Opin Gastroenterolol. 2017;33:285–292. doi: 10.1097/MOG.0000000000000371. [DOI] [PubMed] [Google Scholar]
- 14.Chehade M., Jones S.M., Pesek R.D., Burks A.W., Vickery B.P., Wood R.A., et al. Phenotypic characterization of eosinophilic esophagitis in a large multicenter patient population from the Consortium for Food Allergy Research. J Allergy Clin Immunol Pract. 2018;6:1534–1544. doi: 10.1016/j.jaip.2018.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim H.P., Vance R.B., Shaheen N.J., Dellon E.S. The prevalence and diagnostic utility of endoscopic features of eosinophilic esophagitis: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:988–996. doi: 10.1016/j.cgh.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dellon E.S., Speck O., Woodward K., Gebhart J.H., Madanick R.D., Levinson S., et al. Clinical and endoscopic characteristics do not reliably differentiate PPI-responsive esophageal eosinophilia and eosinophilic esophagitis in patients undergoing upper endoscopy: a prospective cohort study. Am J Gastroenterol. 2013;108:1854–1860. doi: 10.1038/ajg.2013.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moawad F.J., Schoepfer A.M., Safroneeva E., Ally M.R., Chen Y.J., Maydonovitch C.L., et al. Eosinophilic oesophagitis and proton pump inhibitor-responsive oesophageal eosinophilia have similar clinical, endoscopic and histological findings. Aliment Pharmacol Ther. 2014;39:603–608. doi: 10.1111/apt.12636. [DOI] [PubMed] [Google Scholar]
- 18.Dranove J.E., Horn D.S., Davis M.A., Kernek K.M., Gupta S.K. Predictors of response to proton pump inhibitor therapy among children with significant esophageal eosinophilia. J Pediatr. 2009;154:96–100. doi: 10.1016/j.jpeds.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 19.Spergel J.M., Dellon E.S., Liacouras C.A., Hirano I., Molina-Infante J., Bredenoord A.J., et al. Summary of the updated international consensus diagnostic criteria for eosinophilic esophagitis — AGREE conference. Ann Allergy Asthma Immunol. 2018;121:218–224. doi: 10.1016/j.anai.2018.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]