Abstract
Objectives
To describe the ontogeny of the immune system and the adaptive mechanisms of the immune system in the neonatal period, with an emphasis on transplacental antibody transport and breastfeeding.
Source of data
Non-systematic literature review in the PubMed database.
Summary of the findings
The last two decades have witnessed a great advance in the knowledge of the immune system since conception. Several investigation tools have provided insight on phenomena that were previously inadequately understood. Still expanding, the functional and molecular investigation of various aspects of the immune system will make it possible to understand how intra-uterus maternal-fetal exchanges, the maternal microbiota interacting with the fetus and newborn, and the acquisition of immunological competence occur in healthy and disease scenarios.
Conclusions
In-depth knowledge of the development of the immune system and of the adaptive mechanisms that allow a safer transition to the extrauterine environment are fundamental components of optimizing maternal and young infant vaccination, as well as the strategies associated with full postnatal development, and the early diagnosis and treatment of innate errors of immunity.
Keywords: Immune system, Immunocompetence, Host-pathogen interactions, Vaccination, Breastfeeding, Maternal-fetal relations
Immune system ontogeny
The immune system is constantly developing from conception onwards, including in the neonatal period and in the first years of life. This is an ongoing process in which both accelerated and delayed development can harm the individual1.
The fetus and newborn face a complex set of immunological demands, including protection against infection and prevention of inflammatory and harmful immune responses that can lead to preterm birth, as well as balancing the transition from a protected intrauterine environment to a world rich in foreign antigens2.
Until recently, it was thought that the intrauterine environment was completely sterile. Recent studies have contested this concept, as they have described a very small microbial biomass in the placental tissue, umbilical cord blood, and meconium. In turn, methodological challenges, contradictory results, and the current immunological knowledge still cast doubt on the interpretation of these findings3.
However, it is after birth that the immune system needs to act more intensely, as it is exposed to different microorganisms1. The limited immune memory and the developing immune system increases the newborn's vulnerability to infectious agents4. In this sense, innate immunity plays a major role in the first years of life, as the adaptive response is still maturing and is only completed after the first decade.
Knowledge of the various stages of the immune system’s ontogeny is essential both for understanding the increased risk of infections and their complications in the pediatric age group and for suspecting defects in immunological competence.
Embryogenesis
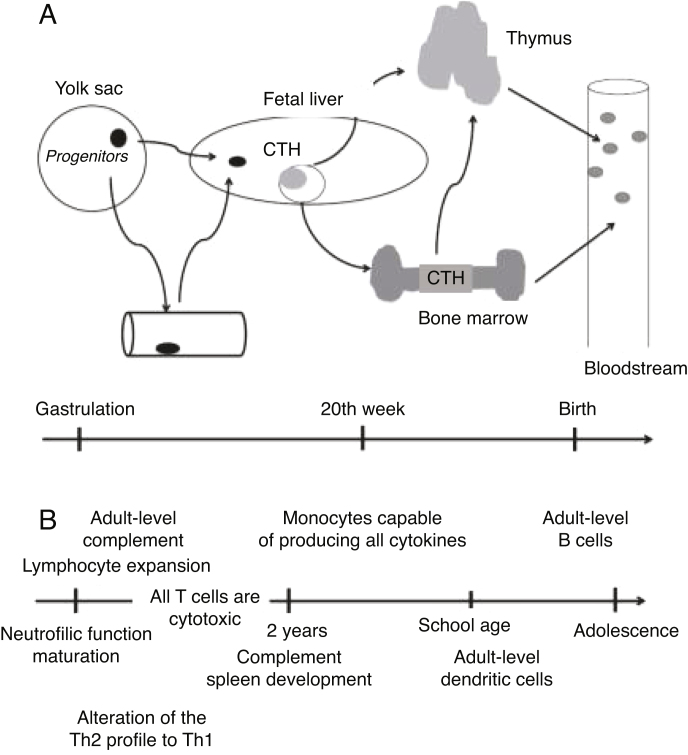
During embryonic and fetal development, there is a continuous modulation of lymphoid tissue. The hematopoietic system, along with the vascular and cardiac systems, is one of the first to appear during embryogenesis. The first blood cells, derived from cells of the mesoderm, are located in the yolk sac in the embryonic phase. These primitive cells migrate to the anterior region of the primitive line in the embryo, forming erythroid progenitors. These early progenitors are also believed to be the source of granulocytes, macrophages, and megakaryocytes1.
Then, the fetal liver becomes responsible for hematopoiesis, which later occurs in the bone marrow. After seven weeks of gestation, T-cell progenitor cells that express CD34 receptors migrate to the thymus, where they differentiate and mature into T cells with the αβ receptor (TCR)5.
Smaller portions of T-cell progenitors in the fetal liver have γδ TCRs from the sixth to the eight week of pregnancy, and do not migrate to the thymus for maturation. Studies with umbilical cord blood demonstrated that multipotent lymphoid progenitors differentiate to become B cells4. Figure 1 schematically presents the immune system’s ontogeny.
Figure 1.
Immune system ontogeny. Adapted from Ygberg and Nilsson1.
Maturation and differentiation of fetal B cells involves the gradual activation of transcription factors and V(D)J recombination to the origin of IgD and IgM molecules on the surface of the B cell2.
Immune cells seed other lymphoid or peripheral organs – including lymph nodes, skin, intestines, kidneys and lungs– and adapt to the environment of each organ. Several types of immune cells develop and mature at different gestational stages, which is necessary to establish tolerance and functional response based on developmental needs. This prepares the developing embryo and fetus for exposure to the antigen during pregnancy and after birth5.
Epigenetics
Exposure to allergens and pathogens causes a change in the intrauterine environment, with an impact on both immunity at birth and immune maturation during the early life of children4. Maternal nutritional imbalance, whether deficient or excessive, can also have a considerable effect on neonatal immunity and immune maturation early in life. Nutritional stress in mothers induces high stimulation of the hypothalamic-pituitary-adrenal axis, which results in a reduction in the weight of the fetal thymus, which in turn leads to apoptosis of thymocytes and immature B and Tcells. Disorders in the neonatal immune system development caused by maternal nutritional imbalance can result in susceptibility to infections at birth and/or late risk of immune-mediated or inflammatory diseases4, 6.
Innate immunity
The innate immune system consists of granulocytes (mainly neutrophils), antigen presenting cells, natural killer cells (NK), and γδ T-cells. These cells are immediately available to act efficiently on a wide range of pathogens. Given the limited exposure to antigens in the intrauterine environment and the immature neonatal adaptive immune response, newborns rely heavily on their innate immune response for protection against infection4.
Neutrophils are the main component of the innate immune system and are responsible for the destruction of pathogens during infection. Most human blood cells are neutrophils (70%–75%). However, neonatal neutrophils have both quantitative and qualitative defects. At birth, the number of neutrophils ranges from 1.5 to 28 × 109 cells/L of blood, compared to 4.4 × 109/L in adults2, 4.
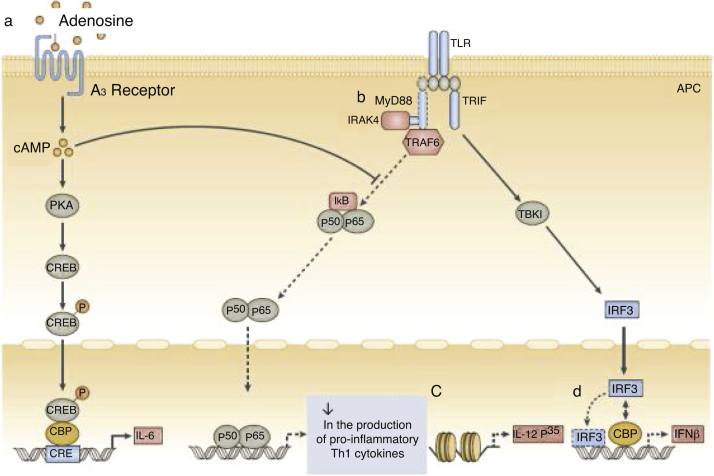
In addition to quantitative deficiencies, neonatal neutrophils express TLR4 at lower levels than adult neutrophils; the expression of TLR2 by neutrophils in neonates is similar to that of adult cells. Signaling via the MyD88 pathways is deficient in neonates after stimulation of both TLR2 and TLR4. This decreased response is attributed to high levels of adenosine in neonatal blood, which increases levels of cyclic AMP (cAMP), leading to inhibition of TLR-stimulated TNF-α secretion (Fig. 2).
Figure 2.
Function of neonatal human monocytes and antigen-presenting cells at different stages of intracellular signaling pathways.
a, High concentrations of adenosine in neonatal blood plasma act through adenosine A3 receptors in neonatal mononuclear cells to induce high intracellular concentrations of cyclic AMP (cAMP). Dependent and independent protein kinase A (PKA) pathways can inhibit the tumor necrosis factor mediated by the Toll-like receptor 2 (TLR2), which preserves the production of interleukin-6 (IL-6). b, Neonatal monocytes have decreased expression of MyD88, a key adapter molecule for TLR-mediated signaling. c, Failure in the nucleosome remodeling of the Il12p35 gene promoter contributes to the decrease in TLR-mediated IL-12 p35 production by neonatal dendritic cells (DCs), an example of distinct regulation of neonatal cytokine production at the chromatin level. d, The lipopolysaccharide (LPS)-induced association of interferon (IFN) with regulatory factor 3 (IRF3) with cAMP-responsive element-binding protein (CREB) - binding protein (CBP) and DNA IRF3 binding are reduced in human neonates, resulting in impaired IFN receptor expression. Adapted from Levy6.
As soon as a neutrophil detects a pathogen, it adheres to the vascular endothelium and migrates, following a chemotactic gradient towards the infection site to phagocytize and destroy the pathogen. These events are followed by neutrophil apoptosis, in order to avoid excessive inflammation.
Neonatal neutrophils express low levels of L-selectin on the cell surface and Mac-1 (CD11b/CD18), which causes a 50% reduction in the transmigration of these cells to infection sites2, 4. This impaired chemotactic response is due to the reduced intracellular calcium influx and altered actin polymerization, limiting the ability of neutrophils to deform and penetrate the vascular endothelial lining. Furthermore, neonatal neutrophils do not adequately produce neutrophil networks (neutrophil extracellular traps [NETs]), which are important in the destruction of extracellular bacteria. In addition, the NADPH oxidase system and the ability to generate hydroxyl radicals also do not work perfectly on neonatal granulocytes.
All these peculiarities of neutrophils early in life make newborns especially susceptible to sepsis2, 4. During the first years of life, these cells undergo a maturation process that is essential for the acquisition of immunological competence in the first years of life6.
The components of the complement system are initially expressed in the fetus during pregnancy and increase, reaching adult levels throughout the first 12–18 months of life. The C proteins found in the fetus under physiological conditions play a critical role in the ability to neutralize antibodies and protect the fetus from the maternal immune system. Newborns express C3 and C4 fractions and total hemolytic complement (CH50). A deficiency of these factors increases the susceptibility to pre- or perinatal infections4.
NK cells play an important role in the resolution of severe acute respiratory viral infections caused by influenza or respiratory syncytial virus. NK cell counts are higher in newborns than in adults, with increased expression of the inhibitory CD94/NKG2A receptor. However, in general, neonatal NK cells have a reduced functional capacity when compared with adult NK cells4.
Γδ Tcells are one of the first to respond to infections by Mycobacterium tuberculosis and Listeria monocytogenes. They release large amounts of IFN-γ and exhibit a cytotoxic function. They are found in the thymus and cord blood2, 4. In general, neonatal γδ Tcells have a low capacity for proliferation and production of cytokines when stimulated, and produce lower proportions of perforin and granzyme B4.
Adaptive immunity
There are two distinct subsets of Tcells that express α/β and γ/δ T-cell receptors (TCRs). Cells that express γ/δ TCRs in the fetal liver do not migrate to the thymus for maturation, but play an important role in the protection against microbial infections at an early stage of development. A/β T-cells migrate to the thymus for maturation, resulting in TCR + thymocytes of the CD4 + T or CD8 + T lineage, which is associated with later antigen recognition and T-cell activation4, 6.
In humans, neonatal CD4 + T cord blood cells proliferate in response to IL-7 in the absence of TCR stimulation. Experimental studies of neonatal CD4 + T cells demonstrate polarization to T-helper 2 (Th2) responses (IL-4, IL-5, IL-10) with decreased production of Th1 cytokines (IFN-γ, IL-2, and TNF-α). In turn, Th17 cells play an important role in the development of immunity to bacterial and fungal infections at the level of mucosa and skin. Experiments using umbilical cord blood cells have shown that neonates have a very low frequency or complete absence of Th17 cells2, 4.
Th1, Th2, and Th17 cells play an important role in the development of immunity to intracellular pathogens and extracellular parasites, while regulatory T cells (Treg) are essential for immune tolerance and play a crucial role in limiting excessive immune responses exerted by cells Th1, Th2, and Th172, 4.
Neonatal B cells show no evidence of antigenic exposure and have only a partially developed surface immunoglobulin (Ig) repertoire. The deficiencies observed in the production of neonatal antibodies may be due to several intrinsic characteristics, such as B cell immaturity, poor B cell repertoire, or reduced B cell receptor (BCR) signaling strength2, 4.
In summary, generally, naïve B and T lymphocytes are programmed differently in neonates when compared with their counterparts found in adults. Newborns present an increased susceptibility to infections due to the immaturity of their lymphocytes, including a low number of effector memory T cells, reduced Th1 cytokine secretion, and reduced B cell receptor signaling strength. For a few months after conception, the infant is under the influence of maternal IgG, which decreases over the first few months. After the age of 2 years, the adaptive response begins to manifest, becoming fully functional after the first decade of life.
Adaptive immune system mechanisms in the neonatal period
Several aspects of the newborn's immune system characterize it as that of a developing being. Although the neonatal innate immune response is less efficient than that of the adult, when compared with the neonatal adaptive response, it appears to be more "complete" at birth.
Several examples of this still developing stage of the adaptive immune response can be described: the finding of circulating CD4 + CD8 + T cells 7, the preponderance of the naive phenotype (CD45RA + CCR7+)8, and the reduced humoral immune response to infection or vaccination9.
In this sense, it is important to assess the expressive increase in circulating polymorphonuclear cells immediately after birth10. Maternal antibody-mediated transplacental immunity11, 12, and the immunity conferred by breast milk and, especially, by colostrum, with transfer of immunoglobulins and cells13.
These would be the three adaptive mechanisms that, during a transitional period – which ranges from two to three days, in the case of polymorphonuclear elevations to many months, in the case of breast milk – would facilitate the survival of the newborn and young infant in the extrauterine environment. Perhaps it is not without reason that two of these three factors, colostrum/breast milk and antibody-mediated transplacental immunity, are mainly made up of elements of the maternal adaptive immune system, precisely the least mature at birth.
While little has been studied regarding the importance of the serum polymorphonuclear peak, much more is already known about the role played by the two other factors: colostrum/breast milk and antibody-mediated transplacental immunity.
Antibody-mediated transplacental immunity
The first evidence regarding the transmission of immunoglobulins from the mother to the fetus came with Brambell et al.14, when studying rabbits. Those authors noted that the fluid that filled the yolk sac of the young rabbit embryo was similar in its protein content to that of plasma. Among the proteins in this fluid were immunoglobulins of maternal origin, which passed through the trophoblast and the endoderm of the embryonic yolk sac, suggesting that immunity was transmitted from the mother to the fetus through the yolk sac, instead of the placenta. The rate of transmission did not vary with the antigenic specificity of the antibody, but there was a clear selection in relation to different serum protein fractions: the gamma globulin fraction was transmitted more efficiently than albumin15.
In 1958, Brambell et al.16 formulated a hypothesis for the immunoglobulin transmission mechanism, which was later refined17.
According to Brambell's hypothesis17, receptors on the surface of the microvilli would be invaginated during the phagocytosis process, covering the internal wall of the phagosomes. Part of the protein would bind to these receptors, being subsequently released from the cells in an intact form into the intercellular space; the part of the protein in the phagosomes that did not bind to the receptors would be degraded by lysosomal enzymes, not reaching the circulation of the fetus/newborn.
Thus, the amount of gamma globulin that could be transmitted in its intact form to circulation would be limited by the population of available receptors. The rate of transmission would depend on the rates of binding and release of the protein by the receptors. The selective character of the transmission would depend on one or both rates, and could vary with different types of gamma globulin.
From maternal circulation to fetal blood, the IgG molecule must cross at least two cell barriers: the syncytiotrophoblast and the fetal capillary endothelium. It is known that parts of the transported IgG, especially alloantibodies directed against antigens expressed in the placenta, do not reach the fetal circulation. The antigen-antibody complexes formed in the villi stroma would be eliminated by phagocytosis by fetal macrophages, known as Hofbauer cells. The receptor responsible for the transport of IgG through the syncytiotrophoblast is FcRn18. All IgG subclasses cross the placenta, but it is known that IgG1 and IgG3 pass more efficiently, followed by IgG4 and, lastly, IgG2. Although there is evidence of transport of IgG through the placenta in early stages of pregnancy, it is known that it occurs efficiently in the third trimester of pregnancy19.
Some factors are known to be associated with a reduction in transplacental antibody transport. Among them, the following are noteworthy: maternal concentrations of total IgG, especially above 15 g/L20, 21, 22; prematurity11, low birth weight11, placental malaria infection22, 23, and maternal HIV infection21, 22, 24.
After birth, maternal antibodies decline. Studies vary with regard to the half-life of total serum IgG, but on average it ranges from 24 to 30 days25.
Around the fourth month of life, total IgG concentrations begin to rise: this is the moment when the child's IgG production exceeds the maternal IgG consumption. However, the child's IgG concentrations will not reach the adult's concentrations until around 8 years of age.
The knowledge of passive immunization acquired through the transplacental route and the catabolism of maternal IgG in the first months of the child's life are essential to narrow the window of susceptibility to specific pathogens at this stage of life, both for children without comorbidities and for those whose mothers have conditions that predispose to reduced transplacental transport or those who present an impaired immune response in the first months of life9.
Breastfeeding
Breast milk is an unparalleled food, with variable composition and adjusted to the infant's needs; its use is associated with adequate growth and development, and the promotion of child health in the short and long term. The current breastfeeding recommendation by the World Health Organization and adopted in Brazil is exclusive breastfeeding up to 6 months of life and breastfeeding combined with other foods until the age of 2 years or more26.
Breastfeeding provides infants with protection against respiratory and gastrointestinal infections and is associated with reduced risk for inflammatory diseases such as asthma, atopy, obesity, and inflammatory bowel disease13.
The intrauterine and lactation periods are fundamental to modulate the interaction and lead to the maturation of the newborn's immune system27. In the intrauterine period, this interaction occurs through the transfer of maternal antibodies and through the amniotic fluid that comes into contact with the skin and gastrointestinal tract of the fetus28.
Especially after birth, the child comes into contact with new and diverse physical and chemical agents, as well as microbiological agents, which will interact with their immune system. Vaginal delivery, skin-to-skin contact, and breastfeeding in the first hour of life should always be encouraged. For the newborn, this practice is associated with reduced risk of early sepsis, establishment of a protective microbiota, and greater frequency and duration of breastfeeding29.
Breastfed infants show a 47% reduction in the risk of death from infectious diseases, 63% in the risk of death from acute diarrheal disease, and 57% in the risk of death from hospitalizations due to respiratory diseases13.
There are several components present in breast milk that influence the development of the immune system. New substances and interactions are still being discovered. The following are noteworthy: immunoglobulins, human milk oligosaccharides (HMO), lactose, lactalbumin, long-chain polyunsaturated fatty acids (LC-PUFAS), vitamins (A, E, C), interleukins, lactoferrin, lysozyme, lactoperoxidase, TNF-β, food antigens from the maternal diet, soluble CD14 receptors, TLR2, tumor necrosis factor (TNF-α), defense cells (macrophages, neutrophils), and probiotics27.
Although all classes of immunoglobulins can be found in breast milk, IgA is considered the most important. Secretory IgA (IgAs) is produced and transported to human milk by the mammary gland from the proteolytic cleavage of serum IgA30. IgAs correspond to 80%–90% of the total immunoglobulins in breast milk; only 10% is absorbed in the intestine and transferred to the child's bloodstream; its action occurs, predominantly, in the mucosa of the gastrointestinal tract31.
This protective action of breast milk IgA in the gastrointestinal tract occurs through inhibition of pathogen binding in the intestinal mucosa, neutralization of toxins, and stimulation of passive immunity. It is known that IgAs in breast milk is one of the main factors that protects the infant against enteric infections caused by rotavirus, E. coli, poliovirus, and retrovirus. Pregnant women vaccinated for meningococcus, influenza, and pneumococcus have higher concentrations of specific IgAs for these microorganisms in breast milk and reduced risk of developing diseases in infants32.
There are no studies to date that prove the viability and infection capacity of SARS-CoV-2 from the breast milk of infected women33; the transmission of this new coronavirus through breast milk is unknown. However, some authors have already demonstrated the presence of anti-SARS-CoV-2 IgA and neutralizing antibodies in human milk, which suggests that breastfeeding may protect the infant from COVID-19 infection34, 35. In view of this, the recommendation of the World Health Organization and the Brazilian Ministry of Health is that breastfeeding be encouraged and maintained in women with COVID-19, taking into account the maternal clinical condition and adopting precautions regarding contact (hand sanitization and use of a face mask)36, 37.
Colostrum is the first milk that the nursing mother offers to the newborn. It is produced in the first days of life and in small quantities; it contains a high concentration of immunoglobulins, especially IgAs, trophic factors for the gastrointestinal tract such as TGF-β, in addition to proteins. This differentiated composition is important for the continuity of the transfer of passive maternal immunity to the newborn. Over the days, the composition of the milk changes, becoming mature milk, which includes changes in the factors that contribute to mucosal immunity. These are modifications that vary from mother to mother, being influenced by health, microbiota, and maternal diet, as well as by genetic and environmental factors38.
It is known that allergic diseases have increased worldwide39, probably due to changes in the population's lifestyle, especially in relation to nutritional aspects. Breastfeeding is one of the factors that is associated with protection against food allergies40. The breastfed infant ingests small amounts of various food antigens from the maternal diet, which are presented to their gastrointestinal tract in conjunction with protective factors (IgAs, TGF-β, oligosaccharides) from breast milk, which tend to promote a tolerogenic response rather than an allergy3.
In an interesting clinical trial conducted by a Finnish group, breastfeeding mothers who had been on a milk and dairy-restricted diet during the first three months of breastfeeding had lower concentrations of IgAs specific for casein and beta-lactoglobulin in breast milk and IgG4 specific for these proteins in blood. Their newborns presented a higher incidence of allergy to cow's milk when compared with a group of nursing mothers who ingested milk and dairy products without any restriction during pregnancy. The authors concluded that maternal restriction of diet during a critical phase for the development of tolerance in the child can influence the development of their children's immune system, increasing the risk of allergy to cow's milk41. These results corroborate the current Brazilian and international recommendations that contraindicate a restricted diet for foods considered more allergenic (fish, milk, eggs, nuts) during pregnancy and lactation, and recommend that the diet of the pregnant woman and the nursing mother be varied and based on healthy, fresh, and minimally processed foods39, 42.
The microbiota is a component of breast milk that impacts the immune system. During the end of pregnancy and the immediate postnatal period, the translocation of microorganisms from the oral cavity and gastrointestinal tract increases significantly27. Breast milk contains hundreds of different bacterial species – around 1000 colony-forming units per mL. It is estimated that the breastfed infant ingests approximately 800,000 live bacteria a day43. An important feature, which differs from the administration of probiotics orally in food or medicine – usually a single or a few strains with a high amount – is that breast milk consumes small amounts of a wide variety of strains; the bacterial profile is quite variable but very similar to that of the maternal intestinal microbiota. In general, the species most commonly found in breast milk are Streptococcus and Staphylococcus, Bifidobacterium, Lactobacillus, Propionibacterium, Enterococcus, and members of the Enterobacteriaceae family44.
The microbiota of breast milk acts on the infant's immune system both by the presence of viable microorganisms and by the metabolites generated by them that act locally and at a distance. Among them, short chain fatty acids, vitamins K and B complex, and retinoid compounds stand out. In addition, it is known that the production of antibodies (IgAs, IgM, and IgG) is also influenced by the maternal microbiota27.
Human milk oligosaccharides (HMO) are complex carbohydrates synthesized by the breast with prebiotic function, i.e., favoring the proliferation and the establishment of beneficial bacteria in the gastrointestinal tract of breastfed infants. Their composition varies according to maternal blood groups. Due to their complexity and variability, it is not possible to synthesize HMO with a structure similar to those present in breast milk45.
From a clinical standpoint, breast milk prevents necrotizing enterocolitis (NEC) in preterm newborns46. It is known that NEC is a multifactorial disease linked to prematurity, inflammation, hypoxia, and bacterial translocation. The effect of raw milk from the child’s own mother is superior to that of pasteurized human milk, considering that the pasteurization process mainly modifies the human milk microbiota. Nonetheless, both raw and pasteurized milk reduce the risk of NEC. In turn, the use of any infant formula and human milk additives with heterologous protein increases the risk of NEC47. Recent work also suggests that the judicious use of probiotics in medicated form in preterm newborns who do not receive breast milk and are hospitalized in neonatal units with a high rate of NEC can reduce the incidence, severity, and mortality of this condition, demonstrating the influence of the intestinal microbiota on protection and its action on the immune system of the gastrointestinal tract48.
Extracellular vesicles (EVs) or exosomes, recently described as components of breast milk, also influence the integrity of the immune system of young infants. EVs are secreted by the mammary gland, as fat envelopes that carry various components involved in cell signaling, such as messenger RNA, micro-RNAs, cytosolic, and membrane proteins. The exosomes of breast milk have several biological functions related to cell maintenance, DNA synthesis, glucose metabolism, and immunological steps49. One of these micro-RNAs found in breast milk is miR-148ª, which acts on the methylation of DNA methyltransferase 1 (DNMT1), modifying the expression of the forkhead box P3 (FOXP3) gene; among several functions, this gene influences tolerance and allergy to food and environmental antigens50.
All the knowledge acquired about the composition of breast milk and its interaction with the child's immune system shows how complex and diverse the contribution of this food is in the development and promotion of the child’s health in the short and long term.
Final considerations
The in-depth knowledge of the development of the immune system and the adaptive mechanisms that allow a safer transition to the extrauterine environment are fundamental elements for the optimization of maternal and young infant vaccination, as well as the strategies associated with full postnatal development and early diagnosis and treatment of innate errors of immunity. They will also be the basis for revolutionizing stem cell transplantation and tissue engineering for immunotherapy and regenerative medicine in the near future5.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Ygberg S., Nilsson A. The developing immune system - from foetus to toddler. Acta Paediatr. 2012;101:120–127. doi: 10.1111/j.1651-2227.2011.02494.x. [DOI] [PubMed] [Google Scholar]
- 2.Rechavi E., Lev A., Lee Y.N., Simon A.J., Yinon Y., Lipitz S., et al. Timely and spatially regulated maturation of B and T cell repertoire during human fetal development. Sci Transl Med. 2015;7 doi: 10.1126/scitranslmed.aaa0072. 276ra25. [DOI] [PubMed] [Google Scholar]
- 3.Ganal-Vonarburg S.C., Hornef M.W., Macpherson A.J. Microbial-host molecular exchange and its functional consequences in early mammalian life. Science. 2020;368:604–607. doi: 10.1126/science.aba0478. [DOI] [PubMed] [Google Scholar]
- 4.Basha S., Surendran N., Pichichero M. Immune responses in neonates. Expert Rev Clin Immunol. 2014;10:1171–1184. doi: 10.1586/1744666X.2014.942288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park J.E., Jardine L., Gottgens B., Teichmann S.A., Haniffa M. Prenatal development of human immunity. Science. 2020;368:600–603. doi: 10.1126/science.aaz9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 7.Griffiths-Chu S., Patterson J.A., Berger C.L., Edelson R.L., Chu A.C. Characterization of immature T cell subpopulations in neonatal blood. Blood. 1984;64:296–300. [PubMed] [Google Scholar]
- 8.Moraes-Pinto M.I., Ono E., Santos-Valente E.C., Almeida L.C., Andrade P.R., Dinelli M.I., et al. Lymphocyte subsets in human immunodeficiency virus-unexposed Brazilian individuals from birth to adulthood. Mem Inst Oswaldo Cruz. 2014;109:989–998. doi: 10.1590/0074-0276140182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kollmann T.R., Marchant A., Way S.S. Vaccination strategies to enhance immunity in neonates. Science. 2020;368:612–615. doi: 10.1126/science.aaz9447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manroe B.L., Weinberg A.G., Rosenfeld C.R., Browne R. The neonatal blood count in health and disease. I. Reference values for neutrophilic cells. J Pediatr. 1979;95:89–98. doi: 10.1016/s0022-3476(79)80096-7. [DOI] [PubMed] [Google Scholar]
- 11.de Moraes-Pinto I., Hart C.A. Transplacental antibody transfer and neonatal immunity. Br J Hosp Med. 1997;58:317–319. [PubMed] [Google Scholar]
- 12.Palmeira P., Quinello C., Silveira-Lessa A.L., Zago C.A., Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012;2012 doi: 10.1155/2012/985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Victora C.G., Bahl R., Barros A.J., França G.V., Horton S., Krasevec J., et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475–490. doi: 10.1016/S0140-6736(15)01024-7. [DOI] [PubMed] [Google Scholar]
- 14.Brambell F.W., Hemmings W.A. The passage into the embryonic yolk-sac cavity of maternal plasma proteins in rabbits. J Physiol. 1949;108:177–185. doi: 10.1113/jphysiol.1949.sp004321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hemmings W.A., Brambell F.W. Protein transfer across the foetal membranes. Br Med Bull. 1961;17:96–101. doi: 10.1093/oxfordjournals.bmb.a069903. [DOI] [PubMed] [Google Scholar]
- 16.Brambell F.W., Halliday R., Morris I.G. Interference by human and bovine serum and serum protein fractions with the absorption of antibodies by suckling rats and mice. Proc R Soc Lond B Biol Sci. 1958;149:1–11. doi: 10.1098/rspb.1958.0046. [DOI] [PubMed] [Google Scholar]
- 17.Brambell F.W. The transmission of immunity from mother to young and the catabolism of immunoglobulins. Lancet. 1966;2:1087–1093. doi: 10.1016/s0140-6736(66)92190-8. [DOI] [PubMed] [Google Scholar]
- 18.Pyzik M., Rath T., Lencer W.I., Baker K., Blumberg R.S. FcRn: The Architect Behind the Immune and Nonimmune Functions of IgG and Albumin. J Immunol. 2015;194:4595–4603. doi: 10.4049/jimmunol.1403014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kollmann T.R., Marchant A., Way S.S. Vaccination strategies to enhance immunity in neonates. Science. 2020;368:612–615. doi: 10.1126/science.aaz9447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michaux J.L., Heremans J.F., Hitzig W.H. Immunoglobulin levels in cord-blood serum of negroes and Caucasians. TropGeogr Med. 1966;18:10–14. [PubMed] [Google Scholar]
- 21.de Moraes-Pinto M.I., Almeida A.C., Kenj G., Filgueiras T.E., Tobias W., Santos A.M., et al. Placental transfer and maternally acquired neonatal IgG immunity in human immunodeficiency virus infection. J Infect Dis. 1996;173:1077–1084. doi: 10.1093/infdis/173.5.1077. [DOI] [PubMed] [Google Scholar]
- 22.de Moraes-Pinto M.I., Verhoeff F., Chimsuku L., Milligan P.J., Wesumperuma L., Broadhead R.L., et al. Placental antibody transfer: influence of maternal HIV infection and placental malaria. Arch Dis Child Fetal Neonatal Ed. 1998;79:F202–5. doi: 10.1136/fn.79.3.f202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brair M.E., Brabin B.J., Milligan P., Maxwell S., Hart C.A. Reduced transfer of tetanus antibodies with placental malaria. Lancet. 1994;343:208–209. doi: 10.1016/s0140-6736(94)90991-1. [DOI] [PubMed] [Google Scholar]
- 24.de Moraes-Pinto M.I., Farhat C.K., Carbonare S.B., Curti S.P., Otsubo M.E., Lazarotti D.S., et al. Maternally acquired immunity in newborns from women infected by the human immunodeficiency virus. Acta Paediatr. 1993;82:1034–1038. doi: 10.1111/j.1651-2227.1993.tb12805.x. [DOI] [PubMed] [Google Scholar]
- 25.Brambell F.W. In: The transmission of passive immunity from mother to young. Brambell F.W., editor. Amsterdam, North-Holland; 1970. Transmission of immunity in man and in the monkey; pp. 234–276. [Google Scholar]
- 26.Brasil . Ministério da Saúde; Brasília: 2019. Ministério da Saúde. Secretaria de Atenção Primária à Saúde. Departamento de Promoção à Saúde. Guia Alimentar para crianças brasileiras menores de 2 anos; p. 265. p.II. [Google Scholar]
- 27.Le Doare K., Holder B., Bassett A., Pannaraj P.S. Mother’s Milk: A Purposeful Contribution to the Development of the Infant Microbiota and Immunity. Front Immunol. 2018;9:361. doi: 10.3389/fimmu.2018.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kollmann T.R., Kampmann B., Mazmanian S.K., Marchant A., Levy O. Protecting the Newborn and Young Infant from Infectious Diseases: Lessons from Immune Ontogeny. Immunity. 2017;46:350–363. doi: 10.1016/j.immuni.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Abdulghani N., Edvardsson K., Amir L.H. Worldwide prevalence of mother-infant skin-to-skin contact after vaginal birth: A systematic review. PLoS One. 2018;13 doi: 10.1371/journal.pone.0205696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brandtzaeg P. The mucosal immune system and its integration with the mammary glands. J Pediatr. 2010;156:S8–15. doi: 10.1016/j.jpeds.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 31.Brandtzaeg P. Mechanisms of gastrointestinal reactions to food. Environ ToxicolPharmacol. 1997;4:9–24. doi: 10.1016/s1382-6689(97)10036-9. [DOI] [PubMed] [Google Scholar]
- 32.Schlaudecker E.P., Steinhoff M.C., Omer S.B., McNeal M.M., Roy E., Arifeen S.E., et al. IgA and neutralizing antibodies to influenza a virus in human milk: a randomized trial of antenatal influenza immunization. PLoS One. 2013;8 doi: 10.1371/journal.pone.0070867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lackey K.A., Pace R.M., Williams J.E., Bode L., Donovan S.M., Järvinen K.M., et al. SARS-CoV-2 and human milk: What is the evidence? Matern Child Nutr. 2020;16 doi: 10.1111/mcn.13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pace R.M., Williams J.E., Järvinen K.M., Belfort M.B., Pace C.D., Lackey K.A., et al. COVID-19 and human milk: SARS-CoV-2, antibodies, and neutralizing capacity. medRxiv [Preprint] 2020 Sep 18:2020.09.16.20196071. doi: 10.1101/2020.09.16.20196071. PMID: 32995804; PMCID: PMC7523143. [Google Scholar]
- 35.Lebrão C.W., Cruz M.N., Silva M.H., Dutra L.V., Cristiani C., Affonso Fonseca F.L., Suano-Souza F.I. Early Identification of IgA Anti-SARSCoV-2 in Milk of Mother With COVID-19 Infection. J Hum Lact. 2020 doi: 10.1177/0890334420960433. 890334420960433. doi: 10.1177/0890334420960433. Epub ahead of print. PMID: 32985922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization (WHO) Clinical Management of COVID-19. Interim Guidance. 2020. https://www.who.int/publications/i/item/clinical-management-of-covid-19 27 May [cited 2020 October 21st]. Available from:
- 37.Brasil . 2020. Ministério da Saúde. Secretaria de Atenção Primária à Saúde. Departamento de Ações Programáticas Estratégicas. Coordenação-Geral de Ciclos da Vida. Coordenação de Saúde das Mulheres. Nota Técnica nº 9/2020-COSMU/CGCIVI/DAPES/SAPS/MS – Recomendações para o Trabalho de Parto, Parto e Puerpério durante a pandemia da COVID-19. Abr. [Google Scholar]
- 38.Macpherson A.J., de Agüero M.G., Ganal-Vonarburg S.C. How nutrition and the maternal microbiota shape the neonatal immune system. Nat Rev Immunol. 2017;17:508–517. doi: 10.1038/nri.2017.58. [DOI] [PubMed] [Google Scholar]
- 39.Greer F.R., Sicherer S.H., Burks A.W., Committee on Nutrition, Section on Allergy and Immunology The Effects of Early Nutritional Interventions on the Development of Atopic Disease in Infants and Children: The Role of Maternal Dietary Restriction, Breastfeeding, Hydrolyzed Formulas, and Timing of Introduction of Allergenic Complementary Foods. Pediatrics. 2019;143 doi: 10.1542/peds.2019-0281. e20190281. [DOI] [PubMed] [Google Scholar]
- 40.Baker M.G., Nowak-Wegrzyn A. Food allergy prevention: current evidence. CurrOpin Clin NutrMetab Care. 2020;23:196–202. doi: 10.1097/MCO.0000000000000651. [DOI] [PubMed] [Google Scholar]
- 41.Järvinen K.M., Westfall J.E., Seppo M.S., James A.K., Tsuang A.J., Feustel P.J., et al. Role of maternal elimination diets and human milk IgA in the development of cow’s milk allergy in the infants. Clin Exp Allergy. 2014;44:69–78. doi: 10.1111/cea.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brasil . 2 ed. Ministério da Saúde; Brasília: 2014. Ministério da Saúde. Secretaria de Atenção Primária à Saúde. Departamento de Promoção à Saúde. Guia Alimentar para população brasileira / Ministério da Saúde, Secretaria de Atenção Básica; p. 156. p.: iI. [Google Scholar]
- 43.Cabrera-Rubio R., Collado M.C., Laitinen K., Salminen S., Isolauri E., Mira A. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am J Clin Nutr. 2012;96:544–551. doi: 10.3945/ajcn.112.037382. [DOI] [PubMed] [Google Scholar]
- 44.Khodayar-Pardo P., Mira-Pascual L., Collado M.C., Martínez-Costa C. Impact of lactation stage, gestational age and mode of delivery on breast milk microbiota. J Perinatol. 2014;34:599–605. doi: 10.1038/jp.2014.47. [DOI] [PubMed] [Google Scholar]
- 45.Bode L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology. 2012;22:1147–1162. doi: 10.1093/glycob/cws074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee J.K., Hern Tan L.T., Ramadas A., Ab Mutalib N.S., Lee L.H. Exploring the Role of Gut Bacteria in Health and Disease in Preterm Neonates. Int J Environ Res Public Health. 2020;17:E6963. doi: 10.3390/ijerph17196963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quigley M., Embleton N.D., McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2018;6 doi: 10.1002/14651858.CD002971.pub4. CD002971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shelby R.D., Raab R., Besner G.E., McElroy S.J. Hope on the horizon: promising novel therapies for necrotizing enterocolitis. Pediatr Res. 2020;88:30–34. doi: 10.1038/s41390-020-1077-1. [DOI] [PubMed] [Google Scholar]
- 49.Karlsson O., Rodosthenous R.S., Jara C., Brennan K.J., Wright R.O., Baccarelli A.A., et al. Detection of long non-coding RNAs in human breastmilk extracellular vesicles: Implications for early child development. Epigenetics. 2016;11:721–729. doi: 10.1080/15592294.2016.1216285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Melnik B.C., Schmitz G. MicroRNAs: Milk’s epigenetic regulators. Best Pract Res Clin Endocrinol Metab. 2017;31:427–442. doi: 10.1016/j.beem.2017.10.003. [DOI] [PubMed] [Google Scholar]