Abstract
Objective
To develop a nomogram for predicting post-hepatectomy liver failure (PHLF) in patients with resectable hepatocellular carcinoma (HCC) based on portal hypertension, the extent of resection, ALT, total bilirubin, and platelet count.
Methods
Patients with HCC hospitalized from January 2015 to December 2020 were included in a retrospective cohort study. 595 HCC patients were divided into a training cohort (n=416) and a validation cohort (n=179) by random sampling. Univariate and multivariable analyses were performed to identify the independent variables to predict PHLF. The nomogram models for predicting the overall risk of PHLF and the risk of PHLF B+C were constructed based on the independent variables. Comparisons were made by using receiver operating characteristic (ROC) curve, calibration curve, and decision curve analysis (DCA) with traditional models, such as FIB-4 score, APRI score, CP class (Child-Pugh), MELD score (model of end-stage liver disease), and ALBI score (albumin-bilirubin) to analyze the accuracy and superiority of the nomogram.
Results
We discovered that portal hypertension (yes vs no) (OR=1.677,95% CI:1.817–4.083, p=0.002), the extent of liver resection (OR=1.872,95% CI:3.937–47.096, p=0.001), ALT (OR=1.003,95% CI:1.003–1.016, P=0.003), total bilirubin (OR=1.036,95% CI:1.031–1.184, p=0.005), and platelet count (OR= 1.004, 95% CI:0.982–0.998, p=0.020) were independent risk factors for PHLF using multifactorial analysis. The nomogram models were constructed using well-fit calibration curves for each of these five covariates. When compared to the FIB4, ALBI, MELD, and CP score, our nomogram models have a better predictive value for predicting the overall risk of PHLF or the risk of PHLF B+C. The validation cohort’s results were consistent. DCA also confirmed the conclusion.
Conclusion
Our models, in the form of static nomogram or web application, were developed to predict PHLF overall risk and PHLF B+C risk in patients with HCC, with a high prediction sensitivity and specificity performance than other commonly used scoring systems.
Keywords: post-hepatectomy liver failure, hepatocellular carcinoma, nomogram, predictive model
Introduction
Liver cancer is the fifth most frequent malignancy and the second leading cause of cancer death worldwide, with China accounting for 49% of new liver cancer cases in the world each year.1 In China, liver cancer is ranked as the fourth most common malignancy and the third leading cause of cancer-related death. Hepatocellular carcinoma (HCC) accounts for 75–85% of all liver cancers.1–3 Hepatectomy with a negative margin is still the first choice in treating HCC. However, chronic liver disease, especially cirrhosis, often concurs with HCC in about 86% of patients4 and increases the risk of developing post-hepatectomy liver failure (PHLF). The reported incidence of PHLF in the literature is wide ranging, ranging from 1.2–32%, attributed to different etiologies and surgical procedures.5 Various clinical composite scoring systems can help predict PHLF after radical hepatectomy in patients with HCC, but there are some issues. The current study shows that the clinical composite score system can predict PHLF, but does not have high sensitivity and specificity. Thus, establishing and improving a PHLF prediction system remains a hot topic in hepatobiliary surgery.
At present, there are many existing clinical methods for assessing liver function, including the Child-Pugh scoring system, the Albumin-Bilirubin score (ALBI) scoring system, the Model for Prognosis of End-stage Liver Disease (MELD) scoring system,6 Studies have confirmed that intraoperative events also affect the risk of PHLF, and none of the above models includes surgical-related factors, such as the extent of liver resection and intraoperative blood loss and blood transfusion, to predict the probability of postoperative PHLF.7 A scoring system solely designed for liver function assessment (Child-Pugh Score or MELD score) has certain errors and is not conducive to more accurate judgment of prognosis after hepatectomy. Therefore, composite metrics combining multiple indicators have become more popular. Additionally, the nomogram prediction model is an intuitive and convenient tool. Thus, the construction of a nomogram prediction model, which can determine PHLF more intuitively, thereby replacement of traditional scoring systems, will likely facilitate the assessment of liver function and reduce the incidence of PHLF and postoperative mortality after radical hepatectomy.
Patients and Methods
Patients
We retrospectively collected data from 595 patients with HCC undergoing partial hepatectomy from Xingtai People’s Hospital, The Second Affiliated Hospital of Nanjing Medical University, Fifth Medical Center of PLA General Hospital, The First Affiliated Hospital of Dalian Medical University, and Tongji Hospital Affiliated to Huazhong University of Science and Technology. 416 patients were selected as the training cohort through random sampling, and another 179 patients were selected as the validation cohort. The study was approved by the Ethics Committee, in compliance with the Declaration of Helsinki. Written informed consent was obtained from all patients to use their data in this study. The Barcelona Clinical Liver Cancer (BCLC) criteria were used to select HCC patients for hepatectomy.
Inclusion criteria were as follows: 1) histology of hepatocellular carcinoma on post-operative pathology; 2) complete perioperative clinical data; and 3) informed consent from the patient.
The exclusion criteria were as follows: 1) postoperative pathological results of cholangiocarcinoma or other malignant tumors; 2) preoperative locoregional therapy, radiotherapy, other systemic anti-tumor treatment or previous hepatectomy; 3) emergency admission due to hepatocellular carcinoma rupture and bleeding.
Clinicopathologic Variables
We collected demographic data, including age, gender, hypertension, portal hypertension, and type of liver disease. Patients’ radiological data included using contrast-enhanced MRI, contrast-enhanced CT, and ultrasound to determine the number of tumor nodules, tumor size (largest nodule diameter), cirrhosis, and ascites. We also collected the time of surgery and blood loss. Serum α-fetoprotein (AFP), creatinine (Cr), albumin (ALB), total bilirubin (TBIL), direct bilirubin (DBIL), alanine transaminase (ALT), aspartate transaminase (AST), prothrombin time (PT), red blood cells (RBC), platelets (PLT), and liver extent of resection, blood loss, and the transfusion were all examined preoperatively.
Diagnosis and Definitions
We calculated the ALBI score8 using the formula 0.66×lg (TBIL, umol/L)–0.085×(ALB, g/L). MELD score9 was calculated by 11.2×ln (INR) +9.57×ln (Cr, mg/dL)+3.78×ln (TBIL, mg/dL)+6.43. APRI score10 was calculated as [AST level (/ULN)/Platelet counts (109/L)] ×100. Finally, FIB-4 score11 was obtained using the formula AST (U/L) ×age (years)/[platelet count (×109/L) ×alanine aminotransferase ALT (U/L) 1/2]. Three continuous factors (total bilirubin, albumin, and prothrombin time) and two categorical variables (ascites, hepatic encephalopathy) comprise the CP scoring system.12 According to their CP scores, patients were categorized into three grades: grade A (5–6 points), grade B (7–9 points), and grade C (10–15 points). Portal hypertension was detected in patients with esophageal varices visible during endoscopy or those with a low platelet count (100×109/L) associated with splenomegaly (diameter larger than 12 cm on ultrasonography, CT, or MRI images).13 The International Study Group of Liver Surgery defined PHLF as a total serum bilirubin level more than 50 µmol/L and a prothrombin time index of 50% (corresponding to an international normalized ratio (INR) greater than 1.7) on post-operative day 5 or later (ISGLS).5 The extent of hepatic resection was defined by the number of Couinaud liver segments removed: a major hepatectomy was defined as the removal of three or more Couinaud liver segments, whereas a minor resection was defined as the removal of fewer than three segments.14
Statistical Analysis
Our study was conducted using SPSS 26.0 (SPSS Inc, Chicago, IL, USA) and R 4.0.3 (Institute for Statistics and Mathematics). The level test of 0.05 is used for both univariate and multivariable analyses. The mean and standard deviation of continuous variables were calculated and compared using the Mann–Whitney U-test or the Student’s t-test. Retain statistically significant indicators from univariate analysis and incorporate multivariable analysis. Variables associated with PHLF were assessed using logistic regression analysis. We built a nomogram prediction model by generating a consistency index and evaluating the calibration curve. We then used the quantitative nomogram to predict PHLF correctness. Simultaneously, the AUC was calculated using the ROC curves of the nomogram prediction model and other established liver function scoring methods. We compared and contrasted the predictive value of the nomogram model with that of existing scores predicting PHLF. To determine the accuracy of our nomogram, a calibration plot with 1000 bootstrap samples was used. The DCA was used to measure the nomogram’s clinical utility by calculating net benefits at various threshold probabilities. P< 0.05 was considered statistically significant.
Results
Clinicopathological Characteristics
A total of 595 patients meeting the inclusion criteria were enrolled in the study, divided into training and validation cohorts, of whom 500 (84.03%) were male, and 95 (15.97%) were female. The entire cohort’s median age is 54 years. Most patients (87.39%) had hepatitis B virus (HBV), 16 (2.69%) were infected with hepatitis C virus, 59 (9.92%) had other liver diseases. Five hundred and twelve patients had liver cirrhosis, of which Child-Pugh class A 94.34% (483/512) and Child-Pugh class B 5.66% (29/512). Also, 80 patients (30.25%) had portal hypertension. Among all patients, 521 (87.56%) had minor hepatectomy, 399 patients (67.06%) had blood loss less than 400 mL, and 134 (22.52%) patients received a transfusion. AFP was greater than or equal to 400 ng/mL in 138 (23.19%) patients. The overall incidence of PHLF was 24.03% (143/595), of which 16.9% (101/595) for PHLF grade A, 6.2% (37/595) for PHLF grade B, and 0.8% (3/595) for PHLF grade C. The clinicopathologic characteristics of the patients are listed in Table 1.
Table 1.
Characteristics of Patients in Training Cohort and Validation Cohort
| Charcateristics | Total (n=595) | Training (n=416) | Validation (n=179) | P value |
|---|---|---|---|---|
| PHLF | ||||
| No | 452 (75.97) | 320 (76.92) | 132 (73.74) | 0.405 |
| Yes | 143 (24.03) | 96 (23.08) | 47 (26.26) | |
| Age, years | 54 (46–61) | 54 (47–61.2) | 52 (45–60.5) | 0.012 |
| Gender | ||||
| Female | 95 (15.97) | 67 (16.11) | 28 (15.64) | 0.999 |
| Male | 500 (84.03) | 349 (83.89) | 151 (84.36) | |
| Causes of liver disease | ||||
| Hepatitis B virus | 520 (87.39) | 365 (87.74) | 155 (86.59) | 0.902 |
| Hepatitis C virus | 16 (2.69) | 11 (2.64) | 5 (2.79) | |
| Other | 59 (9.92) | 40 (9.62) | 19 (10.61) | |
| Cirrhosis | ||||
| No | 83 (13.95) | 63 (15.14) | 20 (11.17) | 0.245 |
| Yes | 512 (86.05) | 353 (84.86) | 159 (88.83) | |
| Portal hypertension | ||||
| No | 415 (69.75) | 291 (69.95) | 124 (69.27) | 0.923 |
| Yes | 180 (30.25) | 125 (30.05) | 55 (30.73) | |
| Extent of resection | ||||
| Minor | 521 (87.56) | 367 (88.22) | 154 (86.03) | 0.499 |
| Major | 74 (12.44) | 49 (11.78) | 25 (13.97) | |
| Blood loss, mL | ||||
| <400 | 399 (67.06) | 283 (68.03) | 116 (64.8) | 0.448 |
| ≥400 | 196 (32.94) | 133 (31.97) | 63 (35.2) | |
| Transfution | ||||
| No | 461 (77.48) | 325 (78.12) | 136 (75.98) | 0.593 |
| Yes | 134 (22.52) | 91 (21.88) | 43 (24.02) | |
| ALT, U/L | 30 (21–44) | 29 (21–44) | 30.4 (21–43) | 0.015 |
| AST, U/L | 29 (22–43) | 29 (22–43) | 29 (22.5–40.5) | <0.001 |
| Albumin, g/L | 39.9 (37–42.5) | 40 (37–42.8) | 39.4 (36.5–42) | <0.001 |
| Total bilirubin, μmol/L | 13.8 (10.6–18.5) | 13.8 (10.6–18.7) | 14 (11.1–18.4) | <0.001 |
| Creatinine, μmol/L | 75 (64–82) | 74 (64–83) | 75 (63–81.5) | 0.001 |
| PT, s | 12.7 (11.6–13.8) | 12.6 (11.5–13.7) | 13 (11.8–14.1) | 0.003 |
| RBC, 1012/L | 4.5 (4.2–4.9) | 4.5 (4.2–4.9) | 4.5 (4.2–5) | 0.011 |
| Platelet, 109/L | 143 (102–191) | 139.5 (96–184.5) | 150 (112–199.5) | <0.001 |
| AFP, ng/mL | ||||
| <400 | 457 (76.81) | 323 (77.64) | 134 (74.86) | 0.460 |
| ≥400 | 138 (23.19) | 93 (22.36) | 45 (25.14) | |
| Child-Pugh class | ||||
| Class-A | 483/512 (94.34) | 333/353 (94.33) | 150/159 (94.34) | 0.999 |
| Class-B | 29/512 (5.66) | 20/353(5.67) | 9/159 (5.66) | |
| MELD score | 4.6 (2.9–6.6) | 4.6 (2.7–6.7) | 4.6 (3.1–6.2) | 0.002 |
| ALBI score | −2.6 (−2.9 - −2.4) | −2.6 (−2.9 - −2.4) | −2.6 (−2.9 - −2.4) | <0.001 |
| APRI | 0.5 (0.3–0.9) | 0.6 (0.4–1) | 0.5 (0.3–0.9) | <0.001 |
| FIB-4 score | 2.1 (1.4–3.4) | 2.1 (1.4–3.9) | 2 (1.3–3.3) | <0.001 |
NoteS: Categorical variables are expressed as frequency (percentage). Continuous variables are expressed as median (interquartile range).
Abbreviations: PHLF, post-hepatectomy liver failure, ALT, alanine transaminase; AST, aspartate transaminase; PT, prothrombin time; INR, international normalized ratio; RBC, red blood cell; AFP, a-fetoprotein; MELD, Model For End-Stage Liver Disease; ALBI, Albumin-bilirubin; APRI, AST-to-platelet ratio index; FIB-4, Fibrosis 4.
Univariate and Multivariable Analysis of PHLF Factors
Cirrhosis (p=0.049), portal hypertension (p<0.001), extent of resection (p=0.043), ALT (P=0.022), albumin (p<0.001), total bilirubin (p<0.001), PT (p<0.001), RBC (p=0.003), platelet (p<0.001), as well as Child-Pugh score (p<0.001), MELD score (p<0.001), ALBI (p<0.001), APRI (p=0.015), FIB-4 (p=0.001) were identified as possible risk factors for PHLF in the training cohort using univariate analysis (Table 2). Subsequently, a multivariable logistic analysis was performed with all of these potential risk factors. Only portal hypertension (p=0.002), the extent of resection (p=0.001), ALT (p=0.003), total bilirubin (p=0.005), and platelet (p=0.020) were found to be independent risk factors for PHLF (Table 2).
Table 2.
Univariate and Multivariable Analysis of Post-Hepatectomy Liver Failure Factors
| Characteristics | Univariable Logistic Regression | Multvariable Logistic Regression | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| (Intercept) | 6.558(0.021–14.153) | 0.618 | ||
| Age, years | 1.017(0.984–1.052) | 0.320 | ||
| Gender, (Female vs male) | 0.606(0.247–1.643) | 0.294 | ||
| Hypertension, (Yes vs No) | 0.181(0.01–0.901) | 0.099 | ||
| Causes of cirrhosis, (HBV vs HCV vs Others) | 1.6(0.82–2.933) | 0.138 | ||
| Cirrhosis, (Yes vs No) | 7.622(1.556–137.786) | 0.049 | ||
| Portal hypertension, (Yes vs No) | 6.774(3.322–14.172) | <0.001 | 3.344(1.762–6.398) | <0.001 |
| Extent of resection, (Major vs Minor) | 2.243(1.002–4.832) | 0.043 | 3.528(1.607–7.715) | 0.002 |
| Blood loss, (≥400 vs <400 mL) | 1.903(0.954–3.771) | 0.065 | ||
| Transfution, (Yes vs No) | 2.067(0.928–4.423) | 0.066 | ||
| ALT, U/L | 1.006(1.001–1.011) | 0.022 | 1.003(1.002–1.011) | 0.013 |
| AST, U/L | 1.003(0.999–1.007) | 0.195 | ||
| Albumin, g/L | 0.846(0.776–0.917) | <0.001 | ||
| Total bilirubin, μmol/L | 1.123(1.067–1.188) | <0.001 | 1.036(1.031–1.184) | 0.005 |
| Creatinine, μmol/L | 0.982(0.958–1.005) | 0.138 | ||
| PT, s | 2.163(1.582–3.032) | <0.001 | ||
| RBC, 1012/L | 0.387(0.202–0.719) | 0.003 | ||
| Platelet, 109/L | 0.987(0.98–0.993) | <0.001 | 0.994(0.988–0.999) | 0.028 |
| AFP, (≥400 vs <400 ng/mL) | 1.186(0.577–2.372) | 0.634 | ||
| Child-Pugh class, (class-B vs class-A) | 4.133(1.651–10.611) | 0.002 | ||
| MELD score | 1.331(1.162–1.538) | <0.001 | ||
| ALBI score | 11.469(4.505–32.143) | <0.001 | 1.678(0.762–8.022) | 0.156 |
| APRI | 1.32(1.082–1.674) | 0.015 | ||
| FIB-4 score | 1.167(1.074–1.28) | 0.001 | ||
Abbreviations: PHLF, post-hepatectomy liver failure, ALT, alanine transaminase; AST, aspartate transaminase; PT, prothrombin time; INR, international normalized ratio; RBC, red blood cell; AFP, a-fetoprotein; MELD, Model For End-Stage Liver Disease; ALBI, Albumin-bilirubin; APRI, AST-to-platelet ratio index; FIB-4, Fibrosis 4.
Constructing a Nomogram Prediction Model for the Incidence of PHLF
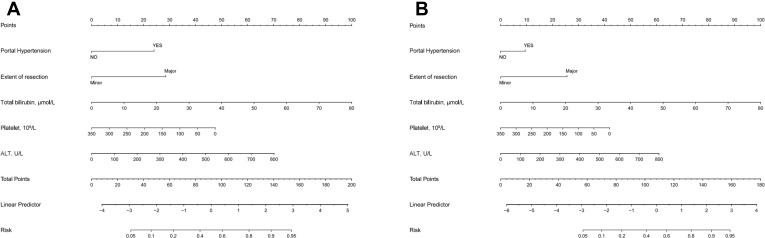
We discovered that portal hypertension, the extent of resection, ALT, total bilirubin, and platelet were all independent risk factors for PHLF using multivariable analysis. The independent factors identified in the preceding multivariable analysis were included in a nomogram prediction model for the occurrence of PHLF utilizing the R software’s rms package. The value is rounded to obtain the total score, which is then subtracted from the chance of developing PHLF, yielding the anticipated probability of occurrence of PHLF (Figure 1A).
Figure 1.
In the training cohort, the nomogram models for predicting the overall risk of post-hepatectomy liver failure (A) and the risk of post-hepatectomy liver failure class B+C (B) was constructed by combining portal hypertension, resection extent, alanine transaminase, total bilirubin, and platelet risk variables.
The Nomogram is Highly Predictive of PHLF in the Training Group
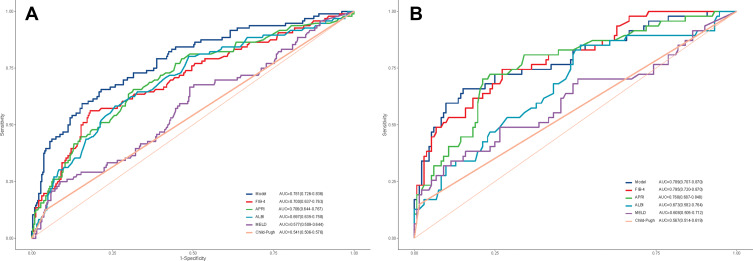
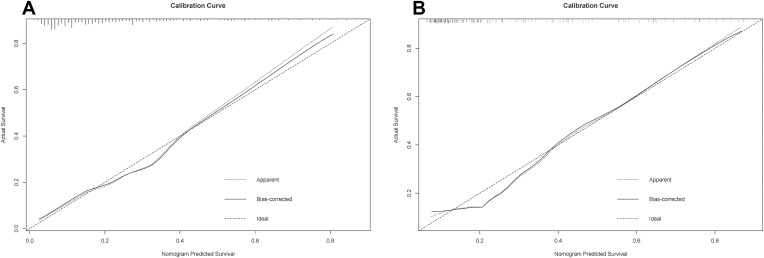
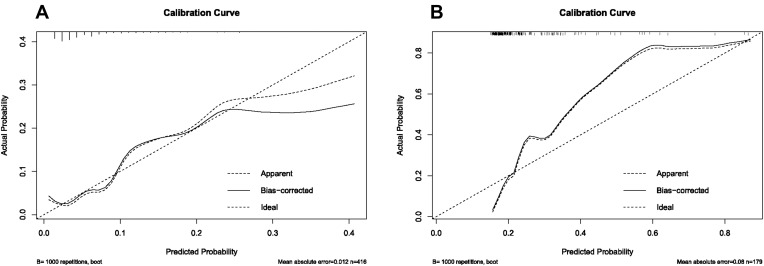
In the training cohort, our nomogram demonstrated a higher predictive accuracy for PHLF compared to other models. Our nomogram’s C-index was 0.857 (95% CI, 0.789–0.925), significantly higher than the FIB-4 score C-index of 0.742 (95% CI, 0.646–0.838), the APRI score C-index of 0.748(95% CI, 0.658–0.838), the ALBI score C-index of 0.751 (95% CI, 0.669–0.832), and the MELD score C-index of 0.681 (95% CI, 0.586–0.776) (Figure 2A). Simultaneously, the calibration curves for PHLF prediction demonstrated a high degree of agreement between the nomogram and observed values. Our nomogram-based prediction model accurately predicts the occurrence of PHLF in the training cohort (Figure 3A).
Figure 2.
By (A) training cohort and (B) validation cohort, we compared the Model’s prediction accuracy for post-hepatectomy liver failure to that of conventional models (FIB-4 score, APRI, Child-Pugh class, MELD score, and ALBI score).
Abbreviations: FIB-4, Fibrosis 4; APRI, AST-to-platelet ratio index; MELD, Model For End-Stage Liver Disease; ALBI, Albumin-bilirubin.
Figure 3.
Calibration curves for the purpose of developing a nomogram for predicting the overall risk of post-hepatectomy liver failure in (A) training cohort and (B) validation cohort. The dashed line depicts the ideal curve that would exist if the expected result and the actual circumstance were identical. If the anticipated occurrence rate of the calibration curve is closer to the dashed line than the measured occurrence rate, the model’s prediction ability is more accurate. The x-axis depicts the expected incidence of post-hepatectomy liver failure for this nomogram, whereas the y-axis depicts the actual incidence of PHLF.
The Nomogram is Highly Predictive of PHLF in the Validation Group
The nomogram also demonstrated superior accuracy for PHLF prediction in the Validation cohort, with a C-index of 0.753 (95% CI, 0.696–0.809), higher than the FIB-4 score C-index of 0.721 (95% CI, 0.663–0.78), the APRI score C-index of 0.713 (95% CI, 0.653–0.772), the ALBI score C-index of 0.661 (95% CI, 0.597–0.725), MELD score C-index of 0.555 (95% CI, 0.487–0.623), CTP score C-index of 0.526 (95% CI, 0.496–0.556) (Figure 2B). Meanwhile, the calibration curves for PHLF prediction demonstrated a high degree of agreement between the nomogram and observed values. It demonstrates that the nomogram-based prediction model accurately predicts the occurrence of PHLF in the training cohort (Figure 3B).
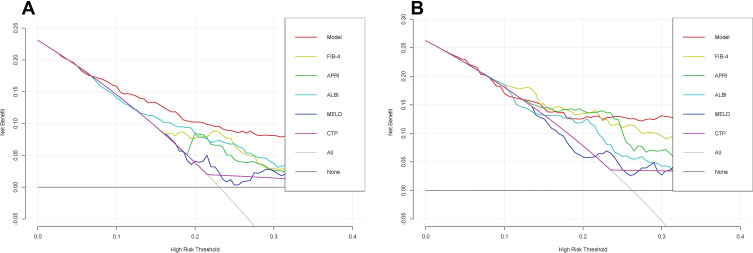
DCA Curve Comparison of Nomogram and Conventional Models for PHLF Prediction Accuracy
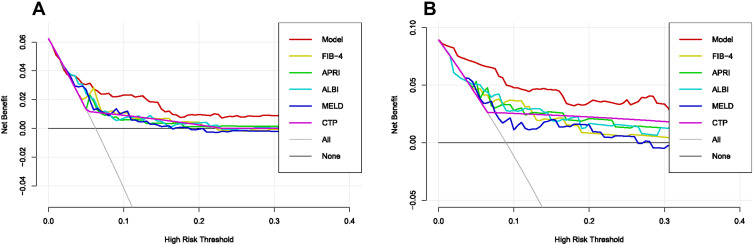
The DCA curves indicated that, for most cohorts, the nomogram of PHLF prediction added more value than the FIB4 score, APRI score, ALBI score, MELD score, or CTP score (Figure 4A). We can draw the same conclusion from the validation cohort. DCA of the validation cohort demonstrated that this nomogram was also more reliable than conventional models (Figure 4B). The above results indicated that our nomogram outperforms existing models.
Figure 4.
In (A) training cohort and (B) validation cohort, decision curve analysis were performed on the nomogram and conventional models for post-hepatectomy liver failure.
Constructing a Nomogram Prediction Model for the Incidence of PHLF B+C
Considering that PHLF B+C group require clinical interventions, rather than PHLF grade A or non-PHLF group, we divided the entire cohort into PHLF B+C and non-PHLF B+C groups. A new nomogram model for predicting PHLF B+C was constructed based on the existing independent risk factors (Figure 1B).
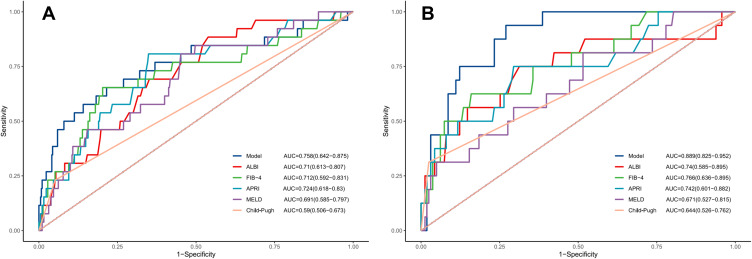
Compared with other noninvasive models in the training and validation cohorts, our nomogram for predicting PHLF B+C also demonstrated superior accuracy for PHLF prediction, with a C-index of 0.758 (95% CI, 0.642–0.875) and 0.889 (95% CI, 0.825–0.952), higher than the FIB-4 score, the APRI score, the ALBI score, the MELD score, and the CTP score (Figure 5). The DCA curves also confirmed that our PHLF B+C prediction model outperformed other common non-invasive models, both in the training cohort and the validation cohort (Figure 6). Calibration curves for PHLC B+C probabilities showed the best agreement between actual observations and nomogram-based model predictions in both training and validation cohorts (Figure 7).
Figure 5.
By (A) training cohort and (B) validation cohort, we compared the Model’s prediction accuracy for post-hepatectomy liver failure B+C grade to that of conventional models (FIB-4 score, APRI, Child-Pugh class, MELD score, and ALBI score).
Abbreviations: FIB-4, Fibrosis 4; APRI, AST-to-platelet ratio index; MELD, Model For End-Stage Liver Disease; ALBI, Albumin-bilirubin.
Figure 6.
In (A) training cohort and (B) validation cohort, decision curve analysis were performed on the nomogram and conventional model for post-hepatectomy liver failure class B+C.
Figure 7.
Calibration curves for the purpose of developing a nomogram for predicting the risk of post-hepatectomy liver failure class B+C in (A) training cohort and (B) validation cohort. The dashed line depicts the ideal curve that would exist if the expected result and the actual circumstance were identical. If the anticipated occurrence rate of the calibration curve is closer to the dashed line than the measured occurrence rate, the model’s prediction ability is more accurate. The x-axis depicts the expected incidence of post-hepatectomy liver failure B+C for this nomogram, whereas the y-axis depicts the actual incidence of PHLF.
Web Calculator Applications
The online calculators based on our nomogram models were developed for clinicians and researchers by simply entering clinical data (portal hypertension, extent of hepatectomy, platelet count, bilirubin level, ALT level) to prediction of PHLF overall risk (https://xingtai.shinyapps.io/DynNomapp_for_PHLF/) and PHLF B+C risk (https://xingtai.shinyapps.io/DynNomapp_for_PHLF_B-C/) (Figures S1 and S2).
Discussion
Among the treatment options for HCC, radical hepatectomy remains the first choice. The safety of hepatectomy has been improving significantly with newer techniques in surgery, the widespread use of innovative surgical instruments, and the progress in critical care medicine. As a result, the perioperative mortality rate after hepatectomy has decreased by about 15%.15 However, hepatocellular carcinoma is commonly associated with other complications, increasing the likelihood of PHLF after surgery,4 which accounts for a prominent cause of death after radical hepatectomy in HCC patients. Post-hepatectomy liver failure is one of the most dreadful complications after hepatectomy in HCC patients. It is crucial to identify HCC patients with PHLF risk proactively. Therefore, establishing a predictive model for PHLF is necessary to improve clinical decision-making. Published research has demonstrated that several clinical composite scoring systems have predictive values for PHLF. However, those clinical scores have limited predictive value and are still evolving.16 Therefore, there are continuing efforts to improve the predictive system for PHLF.
Our study identified portal hypertension, the extent of resection, ALT, total bilirubin, and platelet count as independent risk factors for PHLF in HCC patients by multivariable logistic regression. As shown in previous studies, all these risk factors by themselves were predictive of PHLF. Berzigotti et al17 have demonstrated that portal hypertension increased the risk for clinical decompensation in HCC patients undergoing liver resection. Besides, liver resection in HCC patients with portal hypertension showed significantly increased postoperative complications, including PHLF, resulting in shorter long-term survival than non-portal hypertensive patients.18 Several reports have also demonstrated that patients with a smaller residual liver after major hepatectomy have a greater chance of developing PHLF.19,20 In addition, Heng Zou and his team found that the residual liver was a good predictor of PHLF.21 The same study identified that the kinetics of the transaminases can predict lethal PHLF and that ALT is a factor that indicates an increased risk of death in HBV-related HCC patients who develop PHLF.22 Similarly, total bilirubin and platelet count play an important role in predicting PHLF.23–26 We will integrate these 5 risk factors to verify whether it can better predict PHLF.
Nomogram is a visual and intuitive statistical tool that can be used in clinical practice to provide information to facilitate decision making. It has been shown that the aforementioned existing scoring systems, including Child-Pugh score, MELD score, ALBI score, APRI score, have reasonable predictive values in the prediction of PHLF.6,8,27 However, because many confounding factors contribute to the development of PHLF, and some indicators in the scores mentioned earlier are subjective, the above systems do not predict PHLF accurately. For example, the Child-Pugh score is the most widely used scoring system for assessing liver function, but its value in predicting PHLF is limited.28 According to the Child-Pugh scale, most patients are classified as Grade A, but there may still be significant liver function heterogeneity among Grade A patients. For example, studies have shown that patients with Child-Pugh Grade A can be divided into two groups with significantly different hepatic functions.8 In addition, some of the variables in Child-Pugh classification are correlated with each other. Therefore, the limitations of the Child-Pugh class to assess the preoperative liver reserve function of HCC patients and determine the occurrence of PHLF have been widely acknowledged.
The MELD, commonly used to predict mortality in patients with end-stage liver disease after transjugular intrahepatic portosystemic shunts, incorporates international standardized ratios, creatinine, and serum bilirubin and is now the most widely used metrics for assessing liver function before liver transplantation,29 allowing for a more accurate assessment of liver reserve function and severity. The higher the MELD value, the higher the risk and the lower the survival rate. Chin et al found that the MELD score has a significant predictive value for PHLF.30 A MELD score ≥10 indicates a higher risk of PHLF, but the MELD prediction model does not accurately predict the severity of liver failure after hepatectomy.31 The MELD prediction model is often used to study patients with end-stage cirrhosis rather than patients with HCC who underwent hepatectomy. Therefore, the MELD prediction model is still partially flawed as a judgment of PHLF. Noninvasive liver fibrosis assessment indexes such as aspartic aminotransferase-platelet ratio index (APRI) and liver fibrosis-4 index (FIB-4) can be used to assess liver function in patients with HCC reserve and prognosis in HCC patients.32,33 Some studies suggest that FIB-4 and APRI have a role in predicting the prognosis of hepatectomy in HCC patients.34 Still, the predictive effect of both in liver function after hepatectomy remains yet to be confirmed. Several studies35–37 have recently demonstrated the predictive value of ALBI scores for PHLF. It has been reported38 that an ALBI >-2.6 indicates a higher risk of PHLF. Although the ALBI score only includes two indicators, serum bilirubin and serum albumin, without “ceiling effect”, the ALBI score has not been widely used and accepted in clinical practice at this stage, and further validation and research are needed.
Therefore, we attempted to develop the nomogram models to better predict the occurrence of adequate PHLF and PHLF B+C. Our nomogram models performed well in predicting PHLF or PHLF B+C compared to CP scores, FIB-4, APRI, ALBI scores, and MELD scores. The ROC curves of the nomogram prediction model, Child-Pugh score, ALBI score, MELD score, and APRI score were plotted, and the C-index values were calculated to compare the predictive ability of the line graph prediction model and the existing relevant composite liver function scores for PHLF. The C-index value of this prediction model is the highest in the training and validation groups, which confirms that this nomogram prediction model has the best prediction effect. In terms of validation, the Bootstrap self-sampling method was used to calculate the consistency index, and the results proved that the prediction model has good accuracy. The calibration curve fits well with the ideal curve, indicating that the nomogram prediction model has a good prediction effect on the occurrence of PHLF. Traditionally, nomograms have been evaluated using diagnostic performance metrics for which clinical value cannot be determined. DCA is a widely used tool for assessing the benefits of various patient-preferred diagnostic tests to identify the risk of under- and over-treatment to facilitate decisions about test selection and use. DCA demonstrated that our nomogram models provide more benefits than other models in our study’s training and validation cohorts. Therefore, our nomogram model can be used consistently in clinical practice. More importantly, we provide two easy-to-use free online calculation applications for predicting overall risk (https://xingtai.shinyapps.io/DynNomapp_for_PHLF/) and PHLF B+C risk (https://xingtai.shinyapps.io/DynNomapp_for_PHLF_B-C/). By entering five clinical variables, a patient’s probability of PHLF or PHLF B+C with 95% CI can be quickly obtained.
Although the PHLF nomogram prediction model constructed in this study has been proven to have a good predictive effect, it still has its shortcomings and limitations. (1) This study is a retrospective analysis, and although it includes multicenter subjects, the overall sample size is still small; (2) Some studies have included relevant preoperative and intraoperative indicators such as residual liver volume, imaging histology score, and liver elasticity value to construct the prediction model, and due to the lack of this part of data, this study was not able to comprehensively analyze its correlation with the occurrence of PHLF, and the inclusion of these indicators may improve the ability to predict the occurrence of PHLF; (3) With the development of individualized treatment, the treatment pattern of PHLF may be different so that the prediction model may have bias. With the inclusion of large sample data, more accurate clinical prediction models for PHLF may emerge.
In conclusion, we demonstrated that portal hypertension, extent of resection, ALT, total bilirubin, and platelet counts are the independent risk factors for predicting PHLF overall risk and PHLF B+C risk. We present the static prediction nomograms or online prediction calculation applications by combining the independent risk factors. The nomogram models showed a good predictive performance and would be a convenient tool to facilitate clinical decisions.
Acknowledgments
We wish to particularly acknowledge the patients enrolled in this study for their participation.
Funding Statement
The authors received no financial support for the research, authorship, and/or publication of this article.
Data Sharing Statement
The datasets used in this study are available from the corresponding authors on reasonable requests.
Ethics Statement
Clinical data passed the ethics committees of Xingtai People’s Hospital, Second Affiliated Hospital of Nanjing Medical University, Fifth Medical Center of PLA General Hospital, First Affiliated Hospital of Dalian Medical University and Tongji Hospital Affiliated to Huazhong University of Science and Technology.
Author Contributions
Jitao Wang, Zhanguo Zhang, Dong Shang, Yong Liao and Peng Yu share first authorship. All authors made a significant contribution to the work whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; agreed to submit to the current journal; and agreed to be accountable for all aspects of the work.
Disclosure
The authors disclose no potential conflicts of interest.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 4.Allaire M, Goumard C, Lim C, Le Cleach A, Wagner M, Scatton O. New frontiers in liver resection for hepatocellular carcinoma. JHEP Rep. 2020;2(4):100134. doi: 10.1016/j.jhepr.2020.100134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149(5):713–724. doi: 10.1016/j.surg.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 6.Søreide JA, Deshpande R. Post hepatectomy liver failure (PHLF) - Recent advances in prevention and clinical management. Eur J Surg Oncol. 2021;47(2):216–224. doi: 10.1016/j.ejso.2020.09.001 [DOI] [PubMed] [Google Scholar]
- 7.Kauffmann R, Fong Y. Post-hepatectomy liver failure. Hepatobiliary Surg Nutr. 2014;3(5):238–246. doi: 10.3978/j.issn.2304-3881.2014.09.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-The ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi: 10.1200/JCO.2014.57.9151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman RB Jr. Model for end-stage liver disease (MELD) for liver allocation: a 5-year score card. Hepatology. 2008;47(3):1052–1057. doi: 10.1002/hep.22135 [DOI] [PubMed] [Google Scholar]
- 10.Shiha G, Ibrahim A, Helmy A, et al. Asian-Pacific Association for the Study of the Liver (APASL) consensus guidelines on invasive and non-invasive assessment of hepatic fibrosis: a 2016 update. Hepatol Int. 2017;11(1):1–30. doi: 10.1007/s12072-016-9760-3 [DOI] [PubMed] [Google Scholar]
- 11.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317–1325. doi: 10.1002/hep.21178 [DOI] [PubMed] [Google Scholar]
- 12.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–649. doi: 10.1002/bjs.1800600817 [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Zhai J, Cai X, et al. Severity of portal hypertension and prediction of postoperative liver failure after liver resection in patients with Child-Pugh grade A cirrhosis. Br J Surg. 2012;99(12):1701–1710. doi: 10.1002/bjs.8951 [DOI] [PubMed] [Google Scholar]
- 14.Kang SH, Kim KH, Shin MH, et al. Surgical outcomes following laparoscopic major hepatectomy for various liver diseases. Medicine. 2016;95(43):e5182. doi: 10.1097/MD.0000000000005182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russell MC. Complications following hepatectomy. Surg Oncol Clin N Am. 2015;24(1):73–96. doi: 10.1016/j.soc.2014.09.008 [DOI] [PubMed] [Google Scholar]
- 16.Dasari B, Hodson J, Roberts KJ, et al. Developing and validating a pre-operative risk score to predict post-hepatectomy liver failure. HPB (Oxford). 2019;21(5):539–546. doi: 10.1016/j.hpb.2018.09.011 [DOI] [PubMed] [Google Scholar]
- 17.Berzigotti A, Reig M, Abraldes JG, Bosch J, Bruix J. Portal hypertension and the outcome of surgery for hepatocellular carcinoma in compensated cirrhosis: a systematic review and meta-analysis. Hepatology. 2016;63(1):349. doi: 10.1002/hep.28351 [DOI] [PubMed] [Google Scholar]
- 18.Zheng YW, Wang KP, Zhou JJ, et al. Portal hypertension predicts short-term and long-term outcomes after hepatectomy in hepatocellular carcinoma patients. Scand J Gastroenterol. 2018;53(12):1562–1568. doi: 10.1080/00365521.2018.1538386 [DOI] [PubMed] [Google Scholar]
- 19.Kim HJ, Kim CY, Park EK, et al. Volumetric analysis and indocyanine green retention rate at 15 min as predictors of post-hepatectomy liver failure. HPB (Oxford). 2015;17(2):159–167. doi: 10.1111/hpb.12295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kishi Y, Abdalla EK, Chun YS, et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250(4):540–548. doi: 10.1097/SLA.0b013e3181b674df [DOI] [PubMed] [Google Scholar]
- 21.Safiri S, Ayubi E. Comments on combining albumin-bilirubin score with future liver remnant predicts posthepatectomy liver failure in HBV-associated HCC patients. Liver Int. 2018;38(4):761. doi: 10.1111/liv.13542 [DOI] [PubMed] [Google Scholar]
- 22.Yu LH, Yu WL, Zhao T, Wu MC, Fu XH, Zhang YJ. Post-operative delayed elevation of ALT correlates with early death in patients with HBV-related hepatocellular carcinoma and Post-hepatectomy Liver Failure. HPB (Oxford). 2018;20(4):321–326. doi: 10.1016/j.hpb.2017.10.001 [DOI] [PubMed] [Google Scholar]
- 23.Xu Y, Hu X, Li J, Dong R, Bai X. An improved scoring system based on platelet-albumin-bilirubin in predicting posthepatectomy liver failure outcomes. Dig Dis. 2021;39(3):258–265. doi: 10.1159/000511138 [DOI] [PubMed] [Google Scholar]
- 24.Meyer J, Balaphas A, Combescure C, Morel P, Gonelle-Gispert C, Bühler L. Systematic review and meta-analysis of thrombocytopenia as a predictor of post-hepatectomy liver failure. HPB (Oxford). 2019;21(11):1419–1426. doi: 10.1016/j.hpb.2019.01.016 [DOI] [PubMed] [Google Scholar]
- 25.Mai RY, Ye JZ, Long ZR, et al. Preoperative aspartate aminotransferase-to-platelet-ratio index as a predictor of posthepatectomy liver failure for resectable hepatocellular carcinoma. Cancer Manag Res. 2019;11:1401–1414. doi: 10.2147/CMAR.S186114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mai RY, Wang YY, Bai T, et al. Combination of ALBI and APRI To predict post-hepatectomy liver failure after liver resection for HBV-related HCC patients. Cancer Manag Res. 2019;11:8799–8806. doi: 10.2147/CMAR.S213432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30(1):61–74. doi: 10.1055/s-0030-1247133 [DOI] [PubMed] [Google Scholar]
- 28.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245–1255. doi: 10.1016/S0140-6736(11)61347-0 [DOI] [PubMed] [Google Scholar]
- 29.Zheng Y, Yang H, He L, et al. Reassessment of different criteria for diagnosing post-hepatectomy liver failure: a single-center study of 1683 hepatectomy. Oncotarget. 2017;8(51):89269–89277. doi: 10.18632/oncotarget.19360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chin KM, Allen JC, Teo JY, et al. Predictors of post-hepatectomy liver failure in patients undergoing extensive liver resections for hepatocellular carcinoma. Ann Hepatobiliary Pancreat Surg. 2018;22(3):185–196. doi: 10.14701/ahbps.2018.22.3.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahbari NN, Reissfelder C, Koch M, et al. The predictive value of postoperative clinical risk scores for outcome after hepatic resection: a validation analysis in 807 patients. Ann Surg Oncol. 2011;18(13):3640–3649. doi: 10.1245/s10434-011-1829-6 [DOI] [PubMed] [Google Scholar]
- 32.Dong J, Xu XH, Ke MY, et al. The FIB-4 score predicts postoperative short-term outcomes of hepatocellular carcinoma fulfilling the milan criteria. Eur J Surg Oncol. 2016;42(5):722–727. doi: 10.1016/j.ejso.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 33.Akiyama T, Miyamoto Y, Imai K, et al. Fibrosis-4 index, a noninvasive fibrosis marker, predicts survival outcomes after hepatectomy for colorectal cancer liver metastases. Ann Surg Oncol. 2020;27(9):3534–3541. doi: 10.1245/s10434-020-08828-5 [DOI] [PubMed] [Google Scholar]
- 34.Dong J, Zhang XF, Zhu Y, et al. The value of the combination of fibrosis index based on the four factors and future liver remnant volume ratios as a predictor on posthepatectomy outcomes. J Gastrointest Surg. 2015;19(4):682–691. doi: 10.1007/s11605-014-2727-6 [DOI] [PubMed] [Google Scholar]
- 35.Wang YY, Zhong JH, Su ZY, et al. Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br J Surg. 2016;103(6):725–734. doi: 10.1002/bjs.10095 [DOI] [PubMed] [Google Scholar]
- 36.Fagenson AM, Gleeson EM, Pitt HA, Lau KN. Albumin-bilirubin score vs model for end-stage liver disease in predicting post-hepatectomy outcomes. J Am Coll Surg. 2020;230(4):637–645. doi: 10.1016/j.jamcollsurg.2019.12.007 [DOI] [PubMed] [Google Scholar]
- 37.Andreatos N, Amini N, Gani F, et al. Albumin-bilirubin score: predicting short-term outcomes including bile leak and post-hepatectomy liver failure following hepatic resection. J Gastrointest Surg. 2017;21(2):238–248. doi: 10.1007/s11605-016-3246-4 [DOI] [PubMed] [Google Scholar]
- 38.Shi JY, Sun LY, Quan B, et al. A novel online calculator based on noninvasive markers (ALBI and APRI) for predicting post-hepatectomy liver failure in patients with hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2021;45(4):101534. doi: 10.1016/j.clinre.2020.09.001 [DOI] [PubMed] [Google Scholar]