Abstract
Purpose
To analyze the success and complication rates and factors associated with technical failure of the ultrasound (US)-guided percutaneous thrombin injection of femoral artery pseudoaneurysms caused by vascular access.
Materials and Methods
Records of 30 patients with post-catheterization femoral artery pseudoaneurysms who had been treated with US-guided percutaneous thrombin injections in the department of radiology between March 2009 and June 2019 were retrospectively analyzed. The lesion was diagnosed based on US or contrast-enhanced CT. The characteristics of the patients and their lesions were analyzed.
Results
The mean patient age was 67.8 years. The mean diameter of the pseudoaneurysmal sac was 20.88 mm (5–40 mm). Twenty patients (66.6%) obtained complete thrombosis after the primary injection, while 10 patients (33.3%) obtained partial thrombosis. The number of patients with a low platelet count (< 130 k/µL) was significantly higher in the partial thrombosis group than in the complete thrombosis group (p = 0.02). No substantial procedure-related complications were found in any patient.
Conclusion
The US-guided percutaneous thrombin injection is considered an initial treatment option for pseudoaneurysms caused by vascular access because of its safety and efficacy.
Keywords: Femoral Artery; Aneurysm, False; Thrombin; Ultrasonography; Interventional Radiology
Abstract
목적
대퇴동맥을 통한 시술 후 발생한 의인성 가성동맥류에 대한 초음파 유도하 경피적 트롬빈 주입 치료의 성공률과 합병증 발생률, 치료의 실패에 관련된 요인 등을 분석해 보고자 한다.
대상과 방법
2009년 3월부터 2019년 6월까지 대퇴동맥에 발생한 의인성 가성동맥류에 대하여 영상의학과에서 초음파 유도하 경피적 트롬빈 주입 치료를 받은 30명의 환자들을 후향적으로 분석하였다. 대퇴동맥의 가성동맥류는 초음파 또는 전산화단층촬영을 사용하여 진단하였으며, 환자와 병변의 특성에 대하여 분석하였다.
결과
환자들의 평균 나이는 67.8세였으며, 가성동맥류의 평균 직경은 20.88 mm (5~40 mm)였다. 첫 치료에서 20명의 환자에서 완전한 혈전형성을 보였으며(66.6%), 10명의 환자에서 부분적 혈전형성을 보였다(33.3%). 혈소판 수가 낮은 환자(< 130 k/µL)에서 부분적 혈전형성의 가능성이 유의하게 높았다. 모든 환자에서 시술 관련 합병증은 보이지 않았다.
결론
대퇴동맥의 의인성 가성동맥류에 대한 초음파 유도하 트롬빈 주입 치료는 안전하고 효과적인 우선적 치료 방법이다.
INTRODUCTION
The frequency of femoral artery pseudoaneurysm caused by vascular access is increasing due to the development and widespread use of minimally invasive percutaneous endovascular procedures. Symptoms include pain, inguinal swelling, skin necrosis, and compression of the surrounding organs (1). If left untreated, the pseudoaneurysm can rupture and cause hemodynamic damage (1,2,3). Treatment options range from simple observation to surgery, ultrasound (US)-guided compression, US-guided thrombin injection, and endovascular management. Surgical repair is invasive and has increased risk of anesthetic complications. Simple or US-guided compression has been shown to be a cost-effective method but time-consuming and painful during procedure (3). US-guided thrombin injection is known to have higher efficacy than US-guided simple compression and has become a standard treatment option for femoral artery pseudoaneurysms (4,5). This study aimed to review the success and complication rates as well as factors associated with technical failure regarding US-guided thrombin injection of femoral artery pseudoaneurysms. Additionally, we briefly introduce the technique of US-guided percutaneous thrombin injection in our institution.
MATERIALS AND METHODS
PATIENTS AND DIAGNOSTIC TOOLS
The medical records and radiologic studies of 30 patients who developed post-catheterization femoral pseudoaneurysm and were treated with US-guided percutaneous thrombin injection in the department of Radiology between March 2009 and June 2019 were retrospectively reviewed. Patients treated with endovascular techniques such as stent-graft placement or balloon-assisted thrombin injection were excluded. Characteristics of the patients and lesions, treatment methods used, and treatment outcomes were analyzed. The diagnosis of femoral pseudoaneurysm was made using either US or contrast-enhanced CT (CECT).
This research was approved by the Institutional Review Board, and informed consent was waived due to the retrospective nature of the study (IRB No. CNUH-2019-300).
US-GUIDED THROMBIN INJECTION
The sonographic diagnosis of pseudoaneurysm was made using gray-scale, color Doppler, or power Doppler sonography. “Yin-yang” and “to-and-fro” are characteristic color and power Doppler signs, which occur as the arterial blood flow enters and exits the pseudoaneurysmal sac (Fig. 1) (3). In CECT, the outpouching sac protruding from the origin artery with contrast filling was demonstrated. If a pseudoaneurysm was found by US or CECT, a detailed evaluation of the origin artery, diameter of the pseudoaneurysmal sac, diameter of the neck, and the presence of multiloculation was evaluated.
Fig. 1. A 58-year-old female with a right common femoral pseudoaneurysm.
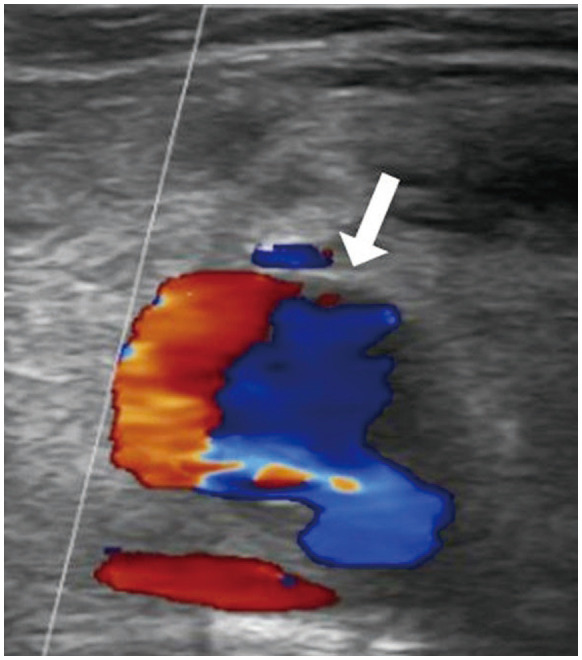
The color Doppler ultrasound image of the femoral pseudoaneurysm shows the “yin-yang” sign (arrow).
Treatment was carried out by four experienced interventional radiologists in our institution. The procedures were performed using US “Affinity 70 Ultrasound machine” (Philips Ultrasound, Bothell, WA, USA) guidance. Convex or linear transducers with 5 MHz or 7.5 MHz were used depending on the patient's condition.
Before starting the procedure, the skin in the inguinal area was sterilized and covered with a sterile drape. The US transducer was also covered with a sterile sleeve. Thrombin lyophilized powder 5000 IU (Reyon Pharmaceutical Company, Seoul, Korea) was mixed with 5 mL of normal saline to make a 1000 IU/mL solution. This solution was then loaded to a 1 mL syringe. The operator sat in a chair next to the bed where the patient was lying and controlled an US transducer with his left hand and a needle with his right hand. Under US guidance, the needle (21 or 22 gauge) was inserted near the neck of the pseudoaneurysmal sac. Once the needle tip was located in the neck of the pseudoaneurysm and blood flowed through the needle, the assistant attached a 1 mL syringe containing thrombin solution to the needle. Then, thrombin solution was slowly injected into the pseudoaneurysmal sac, and extreme care was taken to ensure that the thrombus did not come out of the sac. Thrombin was continuously injected until the pseudoaneurysm became close to being fully thrombosed and the color Doppler flow in the sac disappeared (Fig. 2). When the pseudoaneurysmal sac was multiloculated, thrombin was injected into the proximal locule closest to the origin artery. This is because if the proximal locule was blocked, there would be no blood flow to distal locules, resulting in spontaneous thrombosis of distal locules. When sac obliteration was not complete despite sufficient procedure, the procedure was ended with the expectation of complete thrombosis upon follow-up examination. After the needle was removed from the pseudoaneurysm, US evaluation was performed to confirm that there was no thrombosis around the origin artery (common or superficial femoral artery) and popliteal artery. In addition, the patient was assessed for pain or skin discoloration of the leg. All patients were instructed to remain in supine position for at least 12 hours after the procedure.
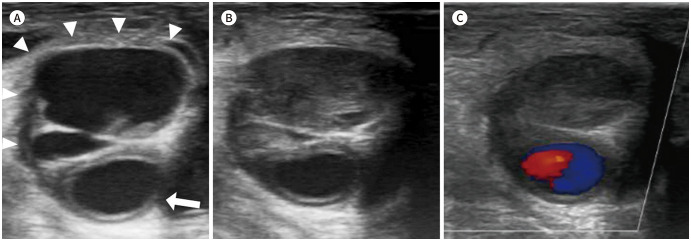
Fig. 2. A 67-year-old male with a right common femoral artery pseudoaneurysm.
A. The gray-scale ultrasound image shows a multiloculated pseudoaneurysm (arrowheads) from the right common femoral artery (arrow).
B, C. Ultrasound images show a pseudoaneurysm after the ultrasound-guided percutaneous thrombin injection. Complete thrombosis of the pseudoaneurysmal sac is achieved (B). There is no internal blood flow on color Doppler ultrasonography (C).
TREATMENT OUTCOME
Imaging follow-up by either US or CECT was performed within 1 month. Complete thrombosis was defined as the complete obliteration of the aneurysm during the injection and follow-up examination. Partial thrombosis was defined as the presence of residual blood flow in the sac during the injection or upon follow-up examination. The primary success was defined as the complete thrombosis of the pseudoaneurysm after initial thrombin injection. Secondary success was defined as the complete thrombosis of the pseudoaneurysm after secondary thrombin injection or spontaneous regression of partial thrombosed pseudoaneurysm without secondary thrombin injection. Technical success was defined as the sum of primary and secondary success.
STATISTICAL ANALYSIS
Statistical analyses were performed using SPSS version 22.0 (IBM Corp., Armonk, NY, USA), and p < 0.05 was considered statistically significant. Categorical variables were compared using Pearson's chi-square test and Fisher's exact test. Continuous variables were compared using Mann-Whitney U test.
RESULTS
The characteristics of the patients and their pseudoaneurysms are shown in Table 1. Arterial closure devices were used in four patients (13.3%). Two patients (6.7%) developed femoral pseudoaneurysms caused by inadvertent puncture of the femoral artery in an attempt to access the femoral vein for an emergency hemodialysis. Five patients had no symptoms, and their pseudoaneurysms were accidentally found by CECT for a separate purpose. Laboratory results obtained closest and prior to the date of thrombin injection were recorded. Five patients had a low platelet count (< 130 K/µL). Seventeen patients (56.7%) were receiving antithrombotic therapy including antiplatelet or anticoagulant drugs at the time of thrombin injection.
Table 1. Patient Demographics and Clinical Characteristics before the Ultrasound-Guided Thrombin Injection.
| Characteristics | Value | |||
|---|---|---|---|---|
| Number of patients | 30 | |||
| Age | 67.8 ± 13.9 (38–88) | |||
| Sex | ||||
| Male | 19 (63.3) | |||
| Female | 11 (36.6) | |||
| Procedure | ||||
| Peripheral angiography and intervention | 15 (50) | |||
| Cardiac catheterization | 8 (26.6) | |||
| CRRT catheterization | 2 (6.7) | |||
| Other transarterial embolization | 5 (16.7) | |||
| BMI (kg/m2) | ||||
| < 25 | 26 (86.7) | |||
| > 25 | 4 (13.3) | |||
| Symptom | ||||
| None | 5 (16.6) | |||
| Inguinal swelling | 21 (70) | |||
| Ecchymosis | 16 (53.3) | |||
| Pain | 7 (23.3) | |||
| Palpable mass | 3 (10) | |||
| Coagulation profile | ||||
| Platelet (K/µL) | 206.38 ± 95.32 (64–439) | |||
| PT (INR) | 1.34 ± 0.37 (0.98–2.51) | |||
| aPTT (second) | 50.82 ± 19.68 (25.7–112.2) | |||
| Antithrombotic medication | ||||
| None | 13 (43.3) | |||
| On medication | 17 (56.7) | |||
| Sac size (mm) | ||||
| Greatest diameter | 20.88 ± 10.02 (5–40) | |||
| Neck width | 2.50 ± 1.24 (1–6) | |||
| Complexity | ||||
| Uniloculated | 14 (46.6) | |||
| Multiloculated | 16 (53.3) | |||
| Site of pseudoaneurysm | ||||
| Common femoral artery | 18 (60) | |||
| Superficial femoral artery | 12 (40) | |||
| Right | 21 (70) | |||
| Left | 9 (30) | |||
| Interval between procedure and treatment (days) | 10.16 ± 9.23 (0–35) | |||
| Thrombin dose (IU) | 437.62 (100–1300) | |||
Data are mean ± standard deviation or n (%) values.
aPTT = activated partial thromboplastin time, BMI = body mass index, CRRT = continuous renal replacement therapy, INR = international normalized ratio, PT = prothrombin time
Procedural outcomes are shown in Table 2. Of the 30 patients, 20 (66.6%) obtained complete thrombosis after primary injection, and 10 (33.3%) obtained partial thrombosis. Of the 10 patients with partial thrombosis, three were treated with secondary thrombin injections. After the secondary US-guided percutaneous thrombin injection, one of the three patients had complete thrombosis. However, two patients showed only partial thrombosis at follow-up US and were not followed up thereafter. Two of the remaining seven patients with partial thrombosis were scheduled for a secondary injection, but spontaneous regression was confirmed at follow-up US. Two patients underwent surgical treatment instead of secondary injection, and the other three patients did not go through follow-up. In brief, the primary success rate was 66.6%, and technical success rate was 76.6% (23 out of 30 patients).
Table 2. Outcomes of Patients Who Were Treated with the Ultrasound-Guided Thrombin Injection.
| Outcome | Overall (n = 30) | ||
|---|---|---|---|
| Complete thrombosis (%) | 20 (66.6) | ||
| Partial thrombosis (%) | 10 (33.3) | ||
| Secondary injection | 3 | ||
| Complete thrombosis | 1 | ||
| Partial thrombosis | 2 | ||
| Spontaneous regression | 2 | ||
| Surgery | 2 | ||
| Follow up loss | 3 | ||
| Complication | 0 | ||
Results of analyses comparing patients with complete and partial thromboses are presented in Table 3. The “low platelet count (< 130 k/µL)” was the only factor associated with partial thrombosis in US-guided percutaneous thrombin injection (p = 0.02). No statistically significant relationship was found between technical success and other parameters mentioned in Table 3.
Table 3. Comparison between Patients Who Underwent Complete Thrombosis and Partial Thrombosis.
| Characteristics | Complete Thrombosis (n = 20) | Partial Thrombosis (n = 10) | p-Value |
|---|---|---|---|
| Age (year) | 69.9 ± 12.7 | 61 ± 15.4 | 0.152 |
| Male | 15 (75) | 7 (70) | 0.548 |
| Platelet < 130 K/µL | 0 (0) | 5 (50) | 0.002 |
| PT (INR) > 1.2 | 7 (35) | 5 (50) | 0.461 |
| aPTT > 33.0 second | 9 (45) | 6 (60) | 0.700 |
| Antithrombotic medication | 14 (70) | 4 (40) | 0.139 |
| Hypertension | 14 (70) | 5 (50) | 0.425 |
| Diabetes | 7 (35) | 2 (20) | 0.675 |
| End-stage renal disease | 3 (15) | 2 (20) | 0.181 |
| BMI (> 25 kg/m2) | 5 (25) | 1 (10) | 0.144 |
| Interval between procedure and treatment (days) | 9.4 ± 7 | 12 ± 12.4 | 0.319 |
| Thrombin dose (IU) | 364.6 ± 317.6 | 564 ± 526.5 | 0.318 |
| Greatest diameter (mm) | 20.3 ± 9.8 | 23.5 ± 10.6 | 0.415 |
| Neck width (mm) | 2.1 ± 1.0 | 3.3 ± 1.2 | 0.144 |
| Multiloculation | 10 (50) | 7 (70) | 0.440 |
| Location (common femoral artery) | 12 (60) | 5 (50) | 0.602 |
| Laocation (right) | 14 (70) | 6 (50) | 0.584 |
aPTT = activated partial thromboplastin time, BMI = body mass index, INR = international normalized ratio, PT = prothrombin time
No substantial procedure-related complications were found in any of the 30 patients. One patient eventually died during hospitalization, but was not associated with pseudoaneurysm treatment. No major complications (i.e. prolonged hospitalization, permanent sequelae, or death) associated with pseudoaneurysm treatment were recorded. Moreover, no arterial ischemic or occlusive events were observed after treatment. One patient showed extension of a small thrombus from the pseudoaneurysm into the underlying superficial femoral artery. This was thought to be due to the relatively large neck diameter of the lesion (5 mm, average 2.57 mm). To prevent distal embolism, aspiration thrombectomy using an 8 Fr guiding catheter was attempted, accessed via the contralateral femoral artery. Subsequent angiography and CECT images showed that the size of the protruding thrombus decreased, but there was still linear density of the thrombus in the superficial femoral artery. As the patient had no related symptom, he was discharged and recommended to visit if any symptoms developed.
DISCUSSION
US-guided percutaneous thrombin injection is now widely used to treat iatrogenic pseudoaneurysms because of its advantages, including high success rates, promptness of the procedure, minimal patient discomfort, and low complication rates (6,7). Usual contraindications include pseudoaneurysms with significant compression of adjacent structures, complicated arteriovenous fistula, associated infection, extremely large defect, or rapidly expanding hematoma (1). There is currently no established guideline for management of iatrogenic femoral pseudoaneurysm, but ongoing anticoagulation reduces the likelihood of spontaneous thrombosis or thrombosis with US-guided compression. Unlike compression repair, anticoagulation does not affect success of thrombin injection. Moreover, compared with US-guided compression, US-guided percutaneous thrombin injection is faster, more effective, and less painful to patients (8,9). Small pseudoaneurysms can be resolved spontaneously or treated with US-guided compression, but for patients with large pseudoaneurysm, taking antithrombotic drugs, or symptomatic, US-guided thrombin injection is more recommended.
The reported success rates of US-guided thrombin injections ranged from 85% to 100% (5,7,9,10,11,12,13,14,15,16). In this study, the primary success rate was 66.6%, which was relatively low compared with those in other studies. However, in some studies, the success rate includes patients who had complete thrombosis not only after the first injection, but also after the second or third injection. For example, Khoury et al. (14) and Paulson et al. (15) reported success rates of 96.1% and 96.4%, respectively, but the success rates after the first injection were 88% and 90%, respectively. Gürel et al. (9) reported an overall success rate of 96.4%, which included secondary injections, supplemental compression, and spontaneous occlusion at follow-up. Only 69% of the patients had complete thrombosis after a single injection, which is similar to the result of our study (66.6%). In the current study, three patients obtained secondary success. One patient obtained complete thrombosis after the secondary injection, and two patients showed spontaneous regression on follow-up US. The technical success rate, which is the sum of the primary and secondary success rates, was 76.6% (23 out of 30 patients). If this study excluded patients who did not go through follow-up, the success rate would be 85% (23 out of 27 patients).
Nevertheless, our success rate is relatively low compared with those in other studies. Thrombin was injected with the needle tip near the neck of the pseudoaneurysm. This location was chosen in an effort to spread the thrombus evenly within the sac, which is formed by contact between the bloodstream coming through the neck and the thrombin. However, in other studies, the needle tip was placed in the center of the pseudoaneurysmal sac (3,9,11,12,16,17) or away from its neck (1,8,15) to avoid distal embolization. Like in other studies, no patient had any limb ischemic event in the current study. The difference in success rates between this study and previous studies can be due to methodological differences. Therefore, further research regarding this is needed.
In the partial thrombosis group, the number of patients with thrombocytopenia (< 130 k/µL) was significantly higher than that in the complete thrombosis group (p = 0.02; odds ratio 2.0, 95% confidence interval 1.07–3.71), suggesting that US-guided percutaneous thrombin injection is less likely to be successful in patients with a low platelet account. Before invasive procedures, platelet concentrates are usually transfused when the platelet count is less than 50 k/µL (18), so the patients with thrombocytopenia in this study were found to have platelet count between 50 and 130 k/µL. Esterson and Pellerito (5) reported that thrombocytopenia was an independent predictor of pseudoaneurysm recurrence, which is similar to our finding.
Many studies insist that antithrombotic therapy does not affect the success rate of thrombin injections (6,12,15,19,20). Sheiman and Mastromatteo (7) reported no significant differences in the maximum dimensions or neck diameters of pseudoaneurysms or between thrombin doses when comparing successful treatments and failures. Gürel et al. (9) reported no statistically significant differences in pseudoaneurysm size or number of locules. Together, results of these studies were consistent with our findings. Specifically, we found no significant associations between partial thrombosis and antithrombotic medication, the greatest diameter or neck width of the pseudoaneurysm, amount of injected thrombin, or multiloculation of the pseudoaneurysm.
However, Vlachou et al. (11) reported that a pseudoaneurysm size less than 2 cm was predictive of the procedural success, and Esterson and Pellerito (5) reported that a pseudoaneurysm size larger than 2 cm was a significant predictor of pseudoaneurysm recurrence. In addition, Chen et al. (1) and Krueger et al. (21) reported greater rates of incomplete thrombosis with multilocular pseudoaneurysms. However, in the current study, no statistical relationship was found between partial thrombosis and pseudoaneurysm size or multiloculation. Madaric et al. (22) found that pseudoaneurysm recurrence diagnosed at 1-month follow-up was associated with obesity [body mass index (BMI) > 30]. No significant relationship was found between obesity (BMI > 25) and partial thrombosis in this study. Because only two patients had BMI > 30, it would be difficult to reach statistical significance.
No complications occurred in all patients. Therefore, this procedure is considered safe. Webber et al. (13) found that the overall complication rate of US-guided thrombin injection was as low as 1.3%. The most serious procedure-related complication is downstream arterial thrombosis resulting in limb ischemia, which did not occur in this study. Similar to the present study, Vlachou et al. (11), Jargiełło et al. (10), and Mishra et al. (23) did not experience any limb-ischemic events during or after pseudoaneurysm management. In patients with previous exposure to bovine thrombin, allergic or anaphylactic reactions have been reported (13). Pope and Johnston (24) recommended that skin prick testing or alternative treatment such as US-guided compression or surgery should be performed in patients with prior exposure to bovine thrombin. In the current study, no patients had previous thrombin exposure, so this was not considered. Other reported complications include venous thrombosis, pulmonary embolism, vasovagal reaction, and rarely skin necrosis (3,13,15), which we also did not experience.
This study has some limitations. First, this study has a small sample size and retrospective design. Second, as the research extended over a decade, many patients were not followed, and some data were unavailable. A large-scale prospective study is needed to prove that the proposed procedure is more effective than conventional methods.
In conclusion, US-guided percutaneous thrombin injection is considered as an initial treatment option for femoral artery pseudoaneurysms caused by vascular access, due to its simplicity and success rate.
Footnotes
- Conceptualization, K.J.K.
- data curation, C.S.Y.
- formal analysis, C.S.Y.
- investigation, C.S.Y.
- methodology, K.H.O.
- project administration, P.C.
- supervision, P.C.
- validation, L.B.C.
- writing—original draft, C.S.Y.
- writing—review & editing, P.C.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Funding: None
References
- 1.Chen DH, Sammel AM, Jain P, Jepson NS. Cardiologist operated ultrasound guided thrombin injection as a safe and efficacious first line treatment for iatrogenic femoral artery pseudoaneurysms. Heart Lung Circ. 2015;24:165–172. doi: 10.1016/j.hlc.2014.07.066. [DOI] [PubMed] [Google Scholar]
- 2.Tisi PV, Callam MJ. Treatment for femoral pseudoaneurysms. Cochrane Database Syst Rev. 2013;11:CD004981. doi: 10.1002/14651858.CD004981.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saad NE, Saad WE, Davies MG, Waldman DL, Fultz PJ, Rubens DJ. Pseudoaneurysms and the role of minimally invasive techniques in their management. Radiographics. 2005;25(Suppl 1):S173–S189. doi: 10.1148/rg.25si055503. [DOI] [PubMed] [Google Scholar]
- 4.Kontopodis N, Tsetis D, Tavlas E, Dedes A, Ioannou CV. Ultrasound guided compression versus ultrasound guided thrombin injection for the treatment of post-catheterization femoral pseudoaneurysms: systematic review and meta-analysis of comparative studies. Eur J Vasc Endovasc Surg. 2016;51:815–823. doi: 10.1016/j.ejvs.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Esterson YB, Pellerito JS. Recurrence of thrombin-injected pseudoaneurysms under ultrasound guidance: a 10-year retrospective analysis. J Ultrasound Med. 2017;36:1617–1624. doi: 10.7863/ultra.16.09063. [DOI] [PubMed] [Google Scholar]
- 6.Shah KJ, Halaharvi DR, Franz RW, Jenkins Ii J. Treatment of iatrogenic pseudoaneurysms using ultrasound-guided thrombin injection over a 5-year period. Int J Angiol. 2011;20:235–242. doi: 10.1055/s-0031-1295521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheiman RG, Mastromatteo M. Iatrogenic femoral pseudoaneurysms that are unresponsive to percutaneous thrombin injection: potential causes. AJR Am J Roentgenol. 2003;181:1301–1304. doi: 10.2214/ajr.181.5.1811301. [DOI] [PubMed] [Google Scholar]
- 8.Stone PA, Campbell JE, AbuRahma AF. Femoral pseudoaneurysms after percutaneous access. J Vasc Surg. 2014;60:1359–1366. doi: 10.1016/j.jvs.2014.07.035. [DOI] [PubMed] [Google Scholar]
- 9.Gürel K, Gür S, Özkan U, Tekbaş G, Önder H, Oğuzkurt L. US-guided percutaneous thrombin injection of postcatheterization pseudoaneurysms. Diagn Interv Radiol. 2012;18:319–325. doi: 10.4261/1305-3825.DIR.4933-11.1. [DOI] [PubMed] [Google Scholar]
- 10.Jargiełło T, Sobstyl J, Światłowski Ł, Kuczyńska M, Kuklik E, Sojka M, et al. Ultrasound-guided thrombin injection in the management of pseudoaneurysm after percutaneous arterial access. J Ultrason. 2018;18:85–89. doi: 10.15557/JoU.2018.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vlachou PA, Karkos CD, Bains S, McCarthy MJ, Fishwick G, Bolia A. Percutaneous ultrasound-guided thrombin injection for the treatment of iatrogenic femoral artery pseudoaneurysms. Eur J Radiol. 2011;77:172–174. doi: 10.1016/j.ejrad.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 12.Schneider C, Malisius R, Küchler R, Lampe F, Krause K, Bahlmann E, et al. A prospective study on ultrasound-guided percutaneous thrombin injection for treatment of iatrogenic post-catheterisation femoral pseudoaneurysms. Int J Cardiol. 2009;131:356–361. doi: 10.1016/j.ijcard.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 13.Webber GW, Jang J, Gustavson S, Olin JW. Contemporary management of postcatheterization pseudoaneurysms. Circulation. 2007;115:2666–2674. doi: 10.1161/CIRCULATIONAHA.106.681973. [DOI] [PubMed] [Google Scholar]
- 14.Khoury M, Rebecca A, Greene K, Rama K, Colaiuta E, Flynn L, et al. Duplex scanning-guided thrombin injection for the treatment of iatrogenic pseudoaneurysms. J Vasc Surg. 2002;35:517–521. doi: 10.1067/mva.2002.120029. [DOI] [PubMed] [Google Scholar]
- 15.Paulson EK, Nelson RC, Mayes CE, Sheafor DH, Sketch MH, Jr, Kliewer MA. Sonographically guided thrombin injection of iatrogenic femoral pseudoaneurysms: further experience of a single institution. AJR Am J Roentgenol. 2001;177:309–316. doi: 10.2214/ajr.177.2.1770309. [DOI] [PubMed] [Google Scholar]
- 16.La Perna L, Olin JW, Goines D, Childs MB, Ouriel K. Ultrasound-guided thrombin injection for the treatment of postcatheterization pseudoaneurysms. Circulation. 2000;102:2391–2395. doi: 10.1161/01.cir.102.19.2391. [DOI] [PubMed] [Google Scholar]
- 17.Lennox AF, Delis KT, Szendro G, Griffin MB, Nicolaides AN, Cheshire NJ. Duplex-guided thrombin injection for iatrogenic femoral artery pseudoaneurysm is effective even in anticoagulated patients. Br J Surg. 2000;87:796–801. doi: 10.1046/j.1365-2168.2000.01436.x. [DOI] [PubMed] [Google Scholar]
- 18.Squires JE. Indications for platelet transfusion in patients with thrombocytopenia. Blood Transfus. 2015;13:221–226. doi: 10.2450/2014.0105-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krüger K, Zähringer M, Söhngen FD, Gossmann A, Schulte O, Feldmann C, et al. Femoral pseudoaneurysms: management with percutaneous thrombin injections--success rates and effects on systemic coagulation. Radiology. 2003;226:452–458. doi: 10.1148/radiol.2262012107. [DOI] [PubMed] [Google Scholar]
- 20.Olsen DM, Rodriguez JA, Vranic M, Ramaiah V, Ravi R, Diethrich EB. A prospective study of ultrasound scanguided thrombin injection of femoral pseudoaneurysm: a trend toward minimal medication. J Vasc Surg. 2002;36:779–782. [PubMed] [Google Scholar]
- 21.Krueger K, Zaehringer M, Strohe D, Stuetzer H, Boecker J, Lackner K. Postcatheterization pseudoaneurysm: results of US-guided percutaneous thrombin injection in 240 patients. Radiology. 2005;236:1104–1110. doi: 10.1148/radiol.2363040736. [DOI] [PubMed] [Google Scholar]
- 22.Madaric J, Mistrik A, Vulev I, Liska B, Vozar M, Lederer P, et al. The recurrence of iatrogenic femoral artery pseudoaneurysm after occlusion by ultrasound guided percutaneous thrombin injection. EuroIntervention. 2009;5:443–447. doi: 10.4244/eijv5i4a70. [DOI] [PubMed] [Google Scholar]
- 23.Mishra A, Rao A, Pimpalwar Y. Ultrasound guided percutaneous injection of thrombin: effective technique for treatment of iatrogenic femoral pseudoaneurysms. J Clin Diagn Res. 2017;11:TC04–TC06. doi: 10.7860/JCDR/2017/25582.9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pope M, Johnston KW. Anaphylaxis after thrombin injection of a femoral pseudoaneurysm: recommendations for prevention. J Vasc Surg. 2000;32:190–191. doi: 10.1067/mva.2000.106498. [DOI] [PubMed] [Google Scholar]