Abstract
In recent years, the century-old Mycobacterium bovis Bacillus Calmette-Guerin (BCG) vaccine against tuberculosis (TB) has been re-evaluated for its capacity to stem the global tide of TB. There is increasing evidence that the efficacy of BCG can be improved by modified administration methods and schedules. Here, we first discuss recent approaches of vaccine administration, revaccination, or boosting that have been used to try to improve the efficacy of BCG against TB. We then dive deeper into studies investigating the immune correlates of protection, describe studies that have investigated BCG-specific T cell responses, and the influence of environmental exposures. These studies all highlight that there is still a lot to learn about the immune response induced by BCG, both in terms of phenotype and specificity, which has been surprisingly understudied. We argue that several critical gaps in knowledge exist and must be addressed by future research to rationally improve the efficacy of BCG, including comprehensive, proteome-wide understanding of the epitopes derived from BCG recognized by BCG vaccinated individuals, the phenotype of responding antigen-specific T cells, and how previous exposure to environmental mycobacteria affect these parameters and thus influence vaccine efficacy. The development of modern techniques allows us to answer some of these questions to better understand how BCG works both in terms of protection against TB and the immune response that it triggers.
Keywords: Vaccine, Tuberculosis, Immunology
Graphical Abstract
Background
Tuberculosis (TB), caused by infection with Mycobacterium tuberculosis (Mtb), is the leading cause of death from one infectious agent. Mtb’s formidable contribution to worldwide morbidity and mortality since its discovery in 1882 by Robert Koch, coupled with the modern emergence of drug-resistant strains, underscore the urgent need for improved vaccination strategies [1]. Approximately 40 years after the discovery of Mtb, in 1921, the Bacillus Calmette-Guerin (BCG) vaccine, discovered by Drs. Calmette and Guerin, was first used. After a century of use, BCG remains the only approved vaccine against TB today. Having been given to over 4 billion people, it is the most widely used vaccine in the world. However, despite BCG’s widespread use, TB is still the deadliest infectious disease.
To reach the goal of reducing TB deaths by 95% between 2015 and 2035, the End TB strategy relies on three pillars: integrated patient-centered TB care and prevention (including vaccines and improved diagnostics), bold policies and supportive systems, and intensified research and innovation. Importantly, to reach the goals of the End TB Strategy, a new vaccine or improved vaccination strategies against TB is required, preferably one that targets adolescents and adults who represent the vast majority of new cases [2, 3] and are responsible for spreading Mtb infection.
At the time when the BCG vaccine was developed there was little, if any, understanding of the mechanisms of immune protection. Still, several gaps in knowledge must be filled to understand how it works and what limits its efficacy [4]. BCG offers substantial protection against disseminated TB in young children, but its efficacy is very variable in both children and adults with pulmonary TB (the transmittable form of the disease) [5–7]. Though the reason for variable efficacy in adolescents and adults is unclear, some protection against Mtb may be already afforded by exposure to environmental mycobacteria which BCG fails to improve upon [8]. In fact, higher efficacy of BCG is associated with low exposure to environmental mycobacteria or absence of previous Mtb infection [8].
The variable efficacy associated with BCG vaccination has prompted researchers to study the mechanisms by which the vaccine works, to develop research strategies aimed at improving efficacy, and to determine whether it can be used for other applications. In this review, we discuss the efficacy of BCG, strategies for improvement using different routes of administration, revaccination, or boosting, and BCG-specific immune responses. We also highlight some of the current knowledge gaps and offer suggestions for future research directions to understand the mechanism of BCG immunogenicity, specific immune responses triggered and the influence of mycobacterial exposure.
Efficacy
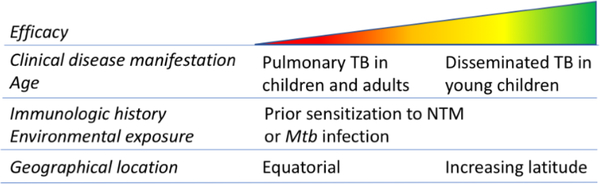
There is a 60–80% decrease in the incidence of active TB through the administration of the BCG vaccine to 100 million newborn children annually. However, in countries with low incidence of Mtb infection, BCG vaccination is not recommended due to its variable efficacy in preventing pulmonary TB in adults. Multiple studies have tried to pinpoint the underlying cause of this variability, which is likely a result of multiple factors such as geographical location, exposure to environmental mycobacteria, age at vaccination, HLA allele variation, genotypic differences in BCG and/or the infecting mycobacteria, and perhaps even UV exposure, levels of vitamin D, helminth infections, or effects of poor nutrition (Figure 1).
Figure 1. Factors affecting BCG efficacy.
At its early stages, BCG, which is a live attenuated vaccine, was derived by serial passage of the bovine TB bacillus, Mycobacterium bovis, and distributed around the world. Continued passaging led to the accumulation of genetic mutations and thus sub-strains with both genetic and phenotypic differences [9, 10]. 129 open reading frames (ORFs) were deleted as a result of the attenuation of BCG [11], divided into 16 genomic regions of difference (designated RD1-RD16). There is not complete deletion of all of these regions in every BCG sub-strain. The lack of the RD1 region, which contains ESAT-6 and CFP-10, in BCG was taken advantage of for the development of the Interferon Gamma Release Assay (IGRA) tests used for diagnosis of Mtb infection. The efficacy of BCG in humans is not significantly affected by the sub-strain used [8]. However, variations in efficacy have been observed in animal models, which has been reviewed previously [10].
Unlinke BCG sub-strains, several other factors are known to influence BCG efficacy including the geographical location, with better protection at increasing latitudes, and previous exposure to mycobacteria [12, 13]. Analyses have also found higher efficacy in geographical locations with colder climate compared to warmer [8]. Thus, unfortunately, in areas with higher incidences of TB where BCG is most needed, it is the least effective. In addition, warmer, regions also have the highest HIV infection rates, which is a risk factor for TB and a contraindication for BCG vaccination.
Increased exposure to environmental mycobacteria (non-tuberculous mycobacteria; NTM) appears to reduce reactivity to BCG [6, 8]. Studies suggest that the species, not the overall burden, of NTM varies geographically, i.e. different NTM species prefer different climates and environments [14]. The impact of prior sensitization by NTM is difficult to discern due to the lack of specific reagents able to distinguish between Mtb and NTM [14]. Some studies have tried to use tuberculin skin testing with NTM preparations to detect prior sensitization by NTM [15]. However, due to the lack of NTM-specific reagents it is not possible to measure sensitization to all environmental mycobacteria [8]. Additionally, detection of prior NTM sensitization does not provide indication on how it affects BCG efficacy. There are two hypotheses on the effect of NTM on BCG vaccine efficacy: masking and blocking [16–18]. The masking hypothesis postulates that prior exposure to NTM generates a background immunity that can mask any added benefit of subsequent BCG vaccination, i.e. NTM induce protection that BCG cannot improve upon [17]. Alternatively, the blocking hypothesis states that pre-existing immune responses to antigens that are shared between NTM and Mtb result in the rapid clearance of BCG and block antigen availability to trigger a sufficient immune response [18, 19]. Thus, the presence of pre-existing immune responses to NTM prevent the establishment of BCG vaccine-induced protective responses.
The length of time an individual is protected after being vaccinated with BCG is also highly variable depending on the study, with reports ranging between 10–60 years [12, 13]. Some studies have shown waning of protection after 10–15 years, which corresponds to the age in early adulthood at which the rate of TB increases [20]. In contrast, other studies have identified long-lived protection. These studies tend to be based in locations in the Northern hemisphere: 15 years in the UK [21], 30–40 years in Norway [22], and as long as 50–60 years in Alaska [23].
Administration
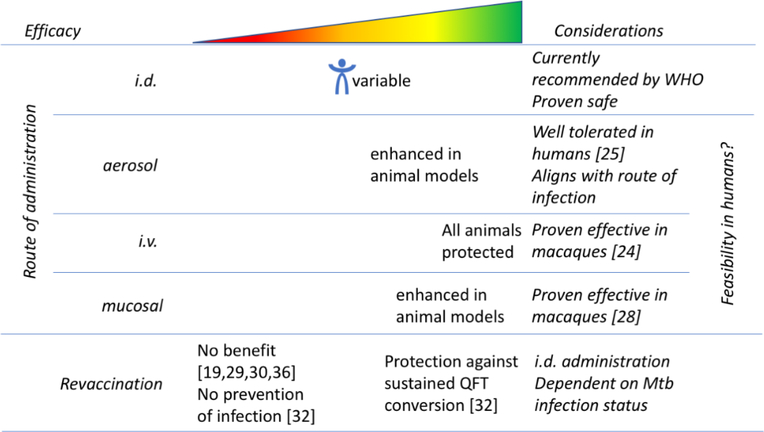
The route of BCG administration currently recommended by the WHO is intradermal injection. However, several studies have tested modified administration methods to try to improve the efficacy of BCG (Figure 2). Most recently, in a study by Darrah et al., the efficacy of BCG administered to rhesus macaques via various routes and doses: intravenous (i.v.), low- or high-dose intradermal (i.d.), aerosol, or combined aerosol with low-dose i.d. was compared. BCG administered by i.v. injection led to unprecedented protection from Mtb infection, with 9 out of 10 animals showing no signs of disease and 6 out of 10 showing no signs of infection [24]. A higher magnitude of BCG-specific CD4 and CD8 T cell responses in blood, spleen, bronchoalveolar lavage (BAL), and lung lymph nodes were detected following i.v. immunization in comparison to i.d. or aerosol immunization. Mechanisms by which i.v. BCG mediates protection include rapid elimination of Mtb by a high levels of T cells located in the lung, control mediated by antibodies, and epigenetically modified macrophages with enhanced capacity to protect against Mtb infection.
Figure 2. Route and frequency of administration to improve BCG efficacy.
Another route that has been considered for improving the efficacy of BCG is aerosol delivery. Infection with Mtb is established primarily in the lung through inhalation of aerosolized droplets containing the Mtb bacteria. Consequently, a TB vaccine that facilitates recruitment of cells to the lung may offer a physiological and immunological advantage. Aerosol delivery is of particular interest due to the potential of using a route of vaccination which matches the route of infection for Mtb. In addition to aligning with the primary route of Mtb infection, a functional aerosol vaccine would not require needles and syringes.
Aerosol vaccination has been studied for a long time. In 1968, a study involving aerosol nebulization of BCG in guinea pigs, school children, and medical students [25] was found to be well-tolerated and feasible. Other studies on aerosol delivery to mice, guinea pigs, and rhesus macaques have also shown enhanced protection against Mtb infection [26, 27]. However, as described previously, aerosol delivery was not as effective as i.v. administration [24]. White et al. investigated the mechanisms behind the protection mediated by BCG delivered via aerosol [26]. They identified an induction of Th1 and Th17 cytokine responses, as well as CD4 T cell responses in BAL fluid cells producing distinct cytokines. Additionally, they found a significant increase in frequencies of peripheral central memory T cells and in PPD-specific IFNγ-producing cells 10–13 weeks following aerosol vaccination [26].
In addition to intravenous and aerosol delivery, routes like mucosal administration of BCG have also been shown to provide high-level protection against Mtb challenges in non-human primates (NHP) [28]. Additional studies are still required to determine whether a route of administration other than intradermal of BCG is safe, practical, and effective in humans. There also needs to be specific efforts to determine the practicality and feasibility of these alternate administration routes for use in humans; for example since both aerosol and i.v. administration may be challenging in infants.
BCG revaccination strategies
Another interesting strategy to improve the BCG efficacy is revaccination. Variable benefits and inconsistent levels of protection against TB disease with BCG revaccination in adolescents and other age groups have been observed in many countries with diverse TB incidence [19, 29, 30]. While some studies saw promising results [31, 32], others showed that there was no additional protection provided after revaccination with BCG and it may result in a higher number of adverse effects [29, 30]. These studies differ in geographical location and likely in exposure to environmental mycobacteria as well as HLA allele frequencies, all of which may influence vaccine efficacy.
In a study by Bekker et al., BCG was administered intradermally to healthy Quantiferon-TB (QFT) negative adolescents with previous BCG vaccination, which elicited a robust, polyfunctional BCG-specific CD4+ T cell response [31]. This was also shown in a recent BCG revaccination study by Nemes et al. in which QFT- adolescents with previous neonatal BCG vaccination were studied [32]. BCG revaccination had 45.4% efficacy against sustained QFT conversion, indicating that revaccination reduced sustained Mtb infection in Cape Town, South Africa, a high TB transmission setting [32]. Thus, BCG did not prevent Mtb infection as measured by initial QFT conversion. This study also observed efficacy for protection against conversion at an IFNγ level of more than 4.0 IU per milliliter, a threshold that is associated with an increased risk of TB disease in infants and adults [32]. The efficacy against sustained QFT conversion highlights the importance of considering the differences between a vaccine that prevents TB disease and one that prevents Mtb infection. Clinical trials and vaccination strategies that are currently considered include: prevention of infection (pre-exposure vaccination), prevention of disease in individuals with latent TB (LTBI), and prevention of recurrence (in regions where TB is endemic, recurrence of infection is common in patients who were previously cured by canonical drug treatments) [33].
A study by Rakshit et al. aimed to explore how BCG revaccination impacts immunity against TB by studying healthy adolescents, both IGRA+ and IGRA-, who were BCG vaccinated at birth [34]. Similar to the other studies, they found that BCG revaccination increases Mtb-specific CD4 and CD8 T cell responses in whole blood above responses detected prior to vaccination [34]. Revaccination not only boosted adaptive immune responses, but also induced NK, γδ T, and NKT cells expressing IFNγ, in comparison to age and health-matched controls that were not revaccinated [34]. These results are congruent with a study by Suliman et al. in which they showed a transient boost of BCG-specific cytokine-expressing Th1 CD4, CD8, and γδ T cells, as well as durable boosts of CD3+CD56+ NKT-like and NK cell responses upon BCG revaccination [35]. These levels remained elevated up to one year after revaccination.
These promising results from preliminary studies of the immune effects of BCG revaccination provide hope for a relatively simple strategy that could help reduce the global TB burden. However, two other previous randomized trials showed no benefit of BCG revaccination [19, 30, 36]. The recent studies in South Africa suggest that it is important to enroll participants on the basis of Mtb infection status and measure new Mtb infection during follow-up when studying BCG revaccination because an effective vaccine may target individuals at different stages of TB from infection to active disease [32].
Improved BCG strains or booster vaccines
Much effort has been spent on reengineering BCG to improve its immunogenicity and on producing live attenuated vaccine strains of Mtb that can serve in place of BCG. While some TB vaccine candidates are developed to replace BCG, others aim to be used as a boosting vaccine in conjunction or soon after regular BCG vaccination.
Booster vaccines aim to enhance the immunity generated by BCG and generate primary immunity to antigens absent in BCG, thereby increasing the protective efficacy. This strategy avoids withholding the BCG vaccine and thus allows the non-specific beneficial health effects mediated by BCG to persist. Several of the existing candidates have been reviewed in detail elsewhere [37].
Unfortunately, not all candidates for boosting BCG have been successful. One such example is the candidate MVA85A, which was developed as a heterologous boost for BCG [38, 39]. It expresses Ag85A (Rv3804c), an antigen conserved across mycobacterial species, including BCG [40]. Pre-existing anti-mycobacterial immune responses induced by either BCG vaccination or NTM were boosted by MVA85A [39]. Importantly, the phase 2b infant efficacy trial of MVA85A in a high-burden setting illustrated the achievability of a large efficacy trial of a new TB vaccine for the first time in over 50 years [41]. The trial has and will continue to serve as a crucial template for protocol development and design of future vaccine trials and strategies. Unfortunately, MVA85A vaccination did not reduce the occurrence of Mtb infection in infants. One hypothesis to explain this lack of efficacy is that due to the broad conservation of Ag85A in mycobacterial species, it is not possible to improve the immunity already induced by exposure to NTM or BCG by boosting with this antigen.
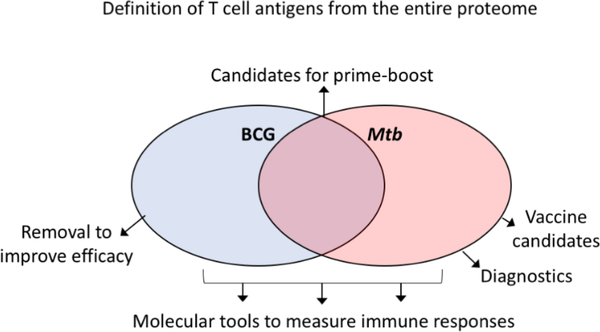
The identification of T cell reactive antigens following BCG vaccination can help identify new strategies for boosting, especially when compared to antigens recognized during different stages of Mtb infection (Figure 3). Boosting immune responses against antigens expressed during the active phase of Mtb infection might translate into reduced incidence or reactivation of infection. Conversely, boosting responses against antigens exclusively recognized in the latent phase might be of limited value towards the prevention of infection or reactivation. Moreover, identification of dominant antigens induced by BCG vaccination, that are not recognized in Mtb infection, could potentially lead to an improved BCG vaccine; removal of BCG-specific antigens from a prospective vaccine may be able to induce a more potent cross-reactive response. Conversely, antigens dominantly recognized following BCG vaccination, as well as in TB might be ideal candidates for BCG prime/antigen-boosting regimens. Despite the fact that BCG prime/antigen-boosting regimens are at the forefront of vaccine development, there has been no evaluation on what antigens are dominantly recognized in BCG vaccinated individuals.
Figure 3. Identification of antigen-specificity can inform vaccination strategies.
Proteome-wide definition of T cell antigens and epitopes following BCG vaccination (blue) and Mtb infection (red). If antigens dominantly recognized following BCG vaccination only are removed vaccine efficacy may be improved. Antigens that are recognized following BCG vaccination and in Mtb infection are candidates for prime-boost regimens. Antigens only recognized in Mtb infection can be used as diagnostics (for example IGRA antigens) or are vaccine candidates to induce immune responses beyond what BCG triggers to improve efficacy. All separate categories can be used as molecular tools to measure immune responses.
Other avenues to improve BCG besides boosting include modification of BCG. Recombinant BCGs come in many different varieties; some express adjuvants or other specific proteins with the goal of providing increased protection through their specific immune effects [37]. There are strains that express Mtb antigens, cytokines, or host resistance factors. Others have insertions of adjuvants derived from bacterial toxins or manipulations of bacterial genes with the goal of enhancing immune responses, antigen presentation, and/or immune activation [37].
One recombinant BCG vaccine that has shown much promise is VPM1002. To improve immunogenicity of VPM1002, the gene encoding urease C was replaced with a gene from Listeria monocytogenes encoding listerolysin [42]. Listerolysin mediates the escape of bacterial products to the cytosol by perturbating the phagosomal membrane. [42]. VPM1002 has shown promising results in preclinical studies and many hope to use it to prevent TB in newborns as well as immunize adults after they have been exposed to Mtb to prevent recurrence of infection [42].
Immune correlates of protection
Our knowledge regarding immune correlates of protection against TB is incomplete. The long-held paradigm for the primary mechanism of action of BCG vaccination based on multiple studies is Th1 cell mediated immunity through the production of IFNγ [43–46]. Therefore, IFNγ produced by CD4 T cells is the gold standard immune marker to determine protective immunity against BCG or other candidate vaccines [4]. In support of the importance of IFNγ in BCG, in vaccinated infants an association was found over the next 3 years of life between IFNγ-producing BCG-specific T cells and the reduced risk of TB disease [47]. However, a single immune marker is unlikely to predict protection provided by BCG vaccination. The measurement of multiple host factors in a systems biology approach will provide a more complete picture, which could lead to identification of better correlates of protection.
In fact, not all studies evaluating mechanisms of immune protection of BCG even show a correlation between IFNγ-production by CD4 T cells and BCG-induced immune protection [48–50]. Attempts to identify immune correlates of protection other than IFNγ has proved difficult and when identified, often are non-specific in nature. Kagina et al. found no differences in polyfunctional cytokine responses between protected and unprotected infants [51]. BCG-specific CD4 T cell responses in the infants peaked at 6–10 weeks following vaccination, and then gradually waned over the next 40 weeks [52]. After the peak, effector phase BCG-specific CD4 T cells expressed markers that suggest the development of long-lived memory characteristics [52].
In addition to cytokines, induction of activation-induced cell surface expression has also been considered as an immune correlate of protection. For example, in BCG vaccinated infants, the presence of activated HLA-DR+ CD4+ T cells was associated with increased risk of TB [47].
Importantly, BCG-specific T cell reactivity can be used as an immune correlate of TB infection and disease, but it does not mediate protection alone [47, 51]. Moreover, efficacy in humans cannot be predicted by the presence and size of BCG scar or delayed-type hypersensitivity [47, 51]. A specific population of immune cells has not yet been shown to mediate the mechanisms of protection. The constant immune re-stimulation mediated by exposure to NTM complicates the identification of mycobacteria-specific immune cells that are present due to a historic BCG vaccination. The different outcomes of BCG vaccination and TB disease susceptibility may be explained by this immunologic cross-reactivity. We have recently reviewed this in the context of NTM [14].
Interestingly, particularly high levels of antigen-specific BAL CD4 T cells of the Th1/Th17 phenotype were observed upon i.v. BCG administration [24]. Our group has also identified cells of a similar phenotype as being increased in humans with LTBI as compared to TB negative controls [53], and has found that the vast majority of Mtb-specific cells are found in this subset [54]. Other NHP studies also showed this cell subset to be associated with protection against Mtb [28, 55]. Thus, antigen-specific Th1/Th17 (Th1* cells) may be a promising candidate for an immune correlate of protection against TB.
BCG-specific T cell responses
The relative benefits of using a live attenuated vaccine like BCG over a non-live vaccine include that it can be administered without the need for an adjuvant, induces immune responses against multiple antigens, stimulates monocytes, which boost innate immunity, and persists longer. Some of the immune responses triggered by BCG have already been discussed above. Studying the cellular response induced by BCG vaccination is a key component of understanding how it mediates protection and what immune responses are triggered. In humans, BCG induces higher CD4+ T cell responses than CD8+ responses [56, 57]. Studies have consistently found a highly variable BCG-specific response, but always with increased levels of BCG-specific cells above those observed pre-vaccination [52, 56]. However, the simple model that an exposure to a single species elicits a species-specific response is not valid in humans due to the complex interplay between the environment and the immune system.
The phenotype and strength of antigen-specific T cell responses elicited by BCG, as well as the breadth of antigens recognized remains to be defined. Previous work from our group has demonstrated that Mtb-specific T cells are primarily from a specific Th subset called Th1* which express CXCR3 and CCR6 [54]. Moreover, T cells elicited by Mtb and NTM cross-reactive epitopes in TB negative (BCG-naïve) individuals were found mainly in the same memory subset [58]. Thus, both Mtb and NTM (and likely BCG) appear to elicit a phenotypically similar T cell response. More work remains necessary to clarify the involvement of different Th subsets in the immune response to BCG.
Significant knowledge gaps surrounding the mechanism of BCG immunogenicity remain. Some of the antigen-specific human T cell responses recognized following BCG vaccination have been investigated previously [51, 59, 60], but the full proteome-wide breadth of epitopes and antigens still remains to be determined. First, as mentioned above, knowledge of antigens that are recognized during Mtb infection and following BCG vaccination could lead to improved vaccination strategies (Figure 3), such as the identification of prime-boosting antigen candidates recognized in both scenarios, or improved efficacy through the removal of antigens that are exclusively recognized following BCG vaccination. Second, knowledge of the antigen-specific T cell phenotypes can indicate the most preferable immune response and suggest modulations that could be made to other vaccine strategies. Finally, BCG-specific antigens and epitopes can be used as molecular tools to monitor immune responses and assess other vaccine candidates and strategies.
Systems immunology approaches have been used to obtain a global picture of the immune responses to vaccination in humans. In the study by Darrah et al. described above, a systems biology approach was used to understand the effects of i.v. BCG vaccination [24]. Additionally, a study by Hoft et al. applied systems immunology to investigate the difference in immune responses between oral and intradermal BCG vaccination [61]. Oral and intradermal BCG induced distinct molecular signatures, suggesting that it may be possible to identify genes that should be differentially targeted by vaccines designed to induce both optimal mucosal and systemic TB immunity. Intradermal BCG induced the strongest Mtb-specific systemic immune responses, while oral vaccination induced the strongest Mtb-specific mucosal immune responses. This suggests that a combination of intradermal and oral vaccination may induce an optimal combination of immune responses relevant for protection against TB. Another study that investigated transcriptional signatures in response to BCG is Cortes et al. who conducted a transcriptomic analysis in mice comparing BCG vaccinated and naïve mice before and after M. bovis challenge [62]. Differentially expressed genes in the lung revealed a specific pulmonary gene expression signature related to connective tissue development and function that predicted vaccine success before M. bovis challenge [62]. The protection following M. bovis infection also correlated with a Th17-related cytokine profile [62]. However, no studies have investigated the effects of BCG in humans using a comprehensive systems biology approach, which would generate an unprecedented global picture of the immune responses triggered by BCG. Similar to what we have previously described for individuals with LTBI as compared to TB-uninfected controls [63, 64], future studies employing transcriptomic strategies can also be used to determine a unique gene signature for BCG vaccination in humans.
As discussed above, microbes in the environment shape the repertoire of Mtb-specific reactivity. Exposure to NTM might provide both occasional boosting to specific antigens and preexisting immunity, which is important to consider when vaccination strategies are developed. This environmental exposure may complicate the evaluation of vaccination strategies, but may also be providing immunity against Mtb.
A large fraction of Mtb-derived peptides are conserved in M. bovis BCG, as the two bacterial strains are closely related and both members of the Mtb complex. However, not all of the Mtb reactivity identified in a given immunized individual is expected to be due to BCG vaccination, as conservation of Mtb peptides also extends more broadly to NTM [58]. We have previously shown that TB negative (BCG naïve) individuals cross-recognize Mtb-derived T cell epitopes and this recognition correlates with sequences conserved in NTM [58]. Even epitopes with little sequence identity may induce cross-reactive T cell responses, making these types of responses difficult to predict [65]. In this respect it is important to consider whether amino acid substitutions are conservative, target anchor residues for MHC binding or influence T cell recognition, which will affect cross-reactivity differently.
With the current tools available to measure Mtb-specific immune responses, we cannot determine which responses are induced by BCG vaccination versus exposure to other mycobacteria. Given the interest in boosting adults with BCG, it is especially important to understand how pre-existing immunity based on mycobacterial exposure will impact the vaccine induced response. Thus, there is a need to study how pre-existing immunity shaped by exposure and infections with environmental mycobacteria impacts the epitope specificity and/or phenotype of T cells following BCG vaccination. Furthermore, given the wide-spread use of BCG, many vaccine candidates, aimed at either replacing or augmenting the efficacy of BCG [4, 66, 67], will be given to recipients who have been BCG immunized at birth. Therefore, understanding the immune response triggered by BCG will provide important information and can help direct design of vaccines and diagnostic tests.
Summary and future research directions
Since the creation of BCG, many important discoveries have been made to improve its efficacy and to understand the adaptive and innate immune responses it triggers. However, important knowledge gaps still remain, such as which parts of the BCG induced immune response correlates with protection against TB, and needs to be boosted. It seems unlikely that a single immune marker can predict protection provided by BCG vaccination. The measurement of multiple host factors in a systems biology/multi-omics approach will provide a more complete picture, which could lead to the identification of novel correlates of protection. An immune signature for BCG will serve as an important comparator to novel vaccination strategies, facilitating their design and evaluation. Additionally, to rationally improve upon BCG, its immune signature must be well characterized.
As mentioned above, there is a limited number of studies aiming at identifying the particular antigens that trigger BCG-specific immune responses. A proteome-wide screen for reactivity would allow for unbiased identification of such antigens. Additionally, as outlined by our group elsewhere, more specific reagents to measure immune responses are urgently needed, not only for BCG, but also NTM-specific ones [14]. Moreover, defining the overlap in antigenic reactivity between cohorts at various Mtb infection disease states or vaccinations remains a high priority in order to develop reagents that can be used to measure disease-state and antigen-specific immune responses, as well as monitor and evaluate different vaccine candidates, or different vaccination strategies.
Understanding why BCG has low efficacy, what the best strategy is for boosting, and what correlates of protection are can all be addressed by additional targeted research into the immune response induced by BCG. Therefore, despite being a century old, we predict many more studies of BCG induced immunity in the years to come.
Acknowledgments
Financial support. This work was supported by the National Institutes of Health grant number 75N93019C00067 to B.P. and C.S.L.A.
Footnotes
Conflict of interest statement
All authors: No reported conflicts of interest.
References
- 1.WHO, Global Tuberculosis Report 2018. 2018. [Google Scholar]
- 2.Schrager LK, et al. , WHO preferred product characteristics for new vaccines against tuberculosis. Lancet Infect Dis, 2018. 18(8): p. 828–829. [DOI] [PubMed] [Google Scholar]
- 3.WHO, The End TB Strategy. 2017. [Google Scholar]
- 4.Abebe F, Is interferon-gamma the right marker for bacille Calmette–Guérin-induced immune protection? The missing link in our understanding of tuberculosis immunology. Clinical & Experimental Immunology, 2012. 169(3): p. 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colditz GA, et al. , The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics, 1995. 96(1 Pt 1): p. 29–35. [PubMed] [Google Scholar]
- 6.Fine PE, Variation in protection by BCG: implications of and for heterologous immunity. Lancet, 1995. 346(8986): p. 1339–45. [DOI] [PubMed] [Google Scholar]
- 7.Trunz BB, Fine P, and Dye C, Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet, 2006. 367(9517): p. 1173–80. [DOI] [PubMed] [Google Scholar]
- 8.Mangtani P, et al. , Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis, 2014. 58(4): p. 470–80. [DOI] [PubMed] [Google Scholar]
- 9.Brosch R, et al. , Genome plasticity of BCG and impact on vaccine efficacy. Proceedings of the National Academy of Sciences, 2007. 104(13): p. 5596–5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ritz N, et al. , Influence of BCG vaccine strain on the immune response and protection against tuberculosis. FEMS microbiology reviews, 2008. 32(5): p. 821–41. [DOI] [PubMed] [Google Scholar]
- 11.Behr M, et al. , Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science, 1999. 284: p. 1520–1523. [DOI] [PubMed] [Google Scholar]
- 12.Dockrell HM and Smith SG, What Have We Learnt about BCG Vaccination in the Last 20 Years? Front Immunol, 2017. 8: p. 1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dye C, Making wider use of the world’s most widely used vaccine: Bacille Calmette-Guerin revaccination reconsidered. J R Soc Interface, 2013. 10(87): p. 20130365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah JA, et al. , Nontuberculous Mycobacteria and Heterologous Immunity to Tuberculosis. J Infect Dis, 2019. 220(7): p. 1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan K, Wang J, and Marras TK, Nontuberculous mycobacterial sensitization in the United States: national trends over three decades. Am J Respir Crit Care Med, 2007. 176(3): p. 306–13. [DOI] [PubMed] [Google Scholar]
- 16.Andersen P and Doherty TM, The success and failure of BCG - implications for a novel tuberculosis vaccine. Nat Rev Microbiol, 2005. 3(8): p. 656–62. [DOI] [PubMed] [Google Scholar]
- 17.Black GF, et al. , BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet, 2002. 359(9315): p. 1393–401. [DOI] [PubMed] [Google Scholar]
- 18.Brandt L, et al. , Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect Immun, 2002. 70(2): p. 672–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barreto ML, et al. , Causes of variation in BCG vaccine efficacy: examining evidence from the BCG REVAC cluster randomized trial to explore the masking and the blocking hypotheses. Vaccine, 2014. 32(30): p. 3759–64. [DOI] [PubMed] [Google Scholar]
- 20.Abubakar I, et al. , Systematic review and meta-analysis of the current evidence on the duration of protection by bacillus Calmette-Guerin vaccination against tuberculosis. Health Technol Assess, 2013. 17(37): p. 1–372, v-vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterne JA, Rodrigues LC, and Guedes IN, Does the efficacy of BCG decline with time since vaccination? Int J Tuberc Lung Dis, 1998. 2(3): p. 200–7. [PubMed] [Google Scholar]
- 22.Nguipdop-Djomo P, et al. , Duration of BCG protection against tuberculosis and change in effectiveness with time since vaccination in Norway: a retrospective population-based cohort study. Lancet Infect Dis, 2016. 16(2): p. 219–26. [DOI] [PubMed] [Google Scholar]
- 23.Aronson NE, et al. , Long-term efficacy of BCG vaccine in American Indians and Alaska Natives: A 60-year follow-up study. JAMA, 2004. 291(17): p. 2086–91. [DOI] [PubMed] [Google Scholar]
- 24.Darrah PA, et al. , Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature, 2020. 577(7788): p. 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manjaly Thomas ZR and McShane H, Aerosol immunisation for TB: matching route of vaccination to route of infection. Trans R Soc Trop Med Hyg, 2015. 109(3): p. 175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White AD, et al. , Evaluation of the Immunogenicity of Mycobacterium bovis BCG Delivered by Aerosol to the Lungs of Macaques. Clin Vaccine Immunol, 2015. 22(9): p. 992–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price DN, et al. , Oral Tolerance to Environmental Mycobacteria Interferes with Intradermal, but Not Pulmonary, Immunization against Tuberculosis. PLoS Pathog, 2016. 12(5): p. e1005614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dijkman K, et al. , Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques. Nat Med, 2019. 25(2): p. 255–262. [DOI] [PubMed] [Google Scholar]
- 29.Dourado I, et al. , Rates of adverse reactions to first and second doses of BCG vaccination: results of a large community trial in Brazilian schoolchildren. Int J Tuberc Lung Dis, 2003. 7(4): p. 399–402. [PubMed] [Google Scholar]
- 30.Rodrigues LC, et al. , Effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: the BCG-REVAC cluster-randomised trial. Lancet, 2005. 366(9493): p. 1290–5. [DOI] [PubMed] [Google Scholar]
- 31.Bekker LG, et al. , A phase 1b randomized study of the safety and immunological responses to vaccination with H4:IC31, H56:IC31, and BCG revaccination in Mycobacterium tuberculosis-uninfected adolescents in Cape Town, South Africa. EClinicalMedicine, 2020. 21: p. 100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nemes E, et al. , Prevention of M. tuberculosis Infection with H4:IC31 Vaccine or BCG Revaccination. N Engl J Med, 2018. 379(2): p. 138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaufmann SHE, Vaccination Against Tuberculosis: Revamping BCG by Molecular Genetics Guided by Immunology. Front Immunol, 2020. 11: p. 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rakshit S, et al. , BCG revaccination boosts adaptive polyfunctional Th1/Th17 and innate effectors in IGRA+ and IGRA- Indian adults. JCI Insight, 2019. 4(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suliman S, et al. , Bacillus Calmette-Guerin (BCG) Revaccination of Adults with Latent Mycobacterium tuberculosis Infection Induces Long-Lived BCG-Reactive NK Cell Responses. J Immunol, 2016. 197(4): p. 1100–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Randomised controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Karonga Prevention Trial Group. Lancet, 1996. 348(9019): p. 17–24. [PubMed] [Google Scholar]
- 37.Nieuwenhuizen NE and Kaufmann SHE, Next-Generation Vaccines Based on Bacille Calmette-Guerin. Front Immunol, 2018. 9: p. 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scriba TJ, et al. , Dose-finding study of the novel tuberculosis vaccine, MVA85A, in healthy BCG-vaccinated infants. The Journal of infectious diseases, 2011. 203(12): p. 1832–43. [DOI] [PubMed] [Google Scholar]
- 39.McShane H, et al. , Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nature medicine, 2004. 10(11): p. 1240–4. [DOI] [PubMed] [Google Scholar]
- 40.D’Souza S, et al. , Mapping of murine Th1 helper T-Cell epitopes of mycolyl transferases Ag85A, Ag85B, and Ag85C from Mycobacterium tuberculosis. Infection and Immunity, 2003. 71(1): p. 483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tameris MD, et al. , Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet, 2013. 381(9871): p. 1021–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nieuwenhuizen NE, et al. , The Recombinant Bacille Calmette-Guerin Vaccine VPM1002: Ready for Clinical Efficacy Testing. Front Immunol, 2017. 8: p. 1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andersen P and Smedegaard B, CD4(+) T-cell subsets that mediate immunological memory to Mycobacterium tuberculosis infection in mice. Infect Immun, 2000. 68(2): p. 621–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hengel RL, et al. , Increasing CD4+ T cells specific for tuberculosis correlate with improved clinical immunity after highly active antiretroviral therapy. AIDS Res Hum Retroviruses, 2002. 18(13): p. 969–75. [DOI] [PubMed] [Google Scholar]
- 45.Lammas DA, Casanova JL, and Kumararatne DS, Clinical consequences of defects in the IL-12-dependent interferon-gamma (IFN-gamma) pathway. Clin Exp Immunol, 2000. 121(3): p. 417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vallinoto AC, et al. , IFNG +874T/A polymorphism and cytokine plasma levels are associated with susceptibility to Mycobacterium tuberculosis infection and clinical manifestation of tuberculosis. Hum Immunol, 2010. 71(7): p. 692–6. [DOI] [PubMed] [Google Scholar]
- 47.Fletcher HA, et al. , T-cell activation is an immune correlate of risk in BCG vaccinated infants. Nat Commun, 2016. 7: p. 11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mittrucker HW, et al. , Poor correlation between BCG vaccination-induced T cell responses and protection against tuberculosis. Proc Natl Acad Sci U S A, 2007. 104(30): p. 12434–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vordermeier HM, et al. , Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect Immun, 2002. 70(6): p. 3026–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sutherland JS, et al. , Pattern and diversity of cytokine production differentiates between Mycobacterium tuberculosis infection and disease. Eur J Immunol, 2009. 39(3): p. 723–729. [DOI] [PubMed] [Google Scholar]
- 51.Kagina BM, et al. , Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette-Guerin vaccination of newborns. Am J Respir Crit Care Med, 2010. 182(8): p. 1073–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soares AP, et al. , Bacillus Calmette-Guerin Vaccination of Human Newborns Induces T Cells with Complex Cytokine and Phenotypic Profiles. J Immunol, 2008. 180(5): p. 3569–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arlehamn CL, et al. , Transcriptional Profile of Tuberculosis Antigen-Specific T Cells Reveals Novel Multifunctional Features. J Immunol, 2014. 193(6): p. 2931–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lindestam Arlehamn CS, et al. , Memory T cells in latent Mycobacterium tuberculosis infection are directed against three antigenic islands and largely contained in a CXCR3+CCR6+ Th1 subset. PLoS Pathog, 2013. 9(1): p. e1003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cadena AM, et al. , Concurrent infection with Mycobacterium tuberculosis confers robust protection against secondary infection in macaques. PLoS Pathog, 2018. 14(10): p. e1007305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodo MJ, et al. , A comparison of antigen-specific T cell responses induced by six novel tuberculosis vaccine candidates. PLoS Pathog, 2019. 15(3): p. e1007643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murray RA, et al. , Bacillus Calmette Guerin vaccination of human newborns induces a specific, functional CD8+ T cell response. J Immunol, 2006. 177(8): p. 5647–51. [DOI] [PubMed] [Google Scholar]
- 58.Lindestam Arlehamn CS, et al. , Immunological consequences of intragenus conservation of Mycobacterium tuberculosis T-cell epitopes. Proc Natl Acad Sci U S A, 2015. 112(2): p. E147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boesen H, et al. , Human T-cell responses to secreted antigen fractions of Mycobacterium tuberculosis. Infect. Immun, 1995. 63(4): p. 1491–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ravn P, et al. , Human T cell responses induced by vaccination with Mycobacterium bovis bacillus Calmette-Guerin. Journal of immunology, 1997. 158(4): p. 1949–55. [PubMed] [Google Scholar]
- 61.Hoft DF, et al. , PO and ID BCG vaccination in humans induce distinct mucosal and systemic immune responses and CD4(+) T cell transcriptomal molecular signatures. Mucosal Immunol, 2018. 11(2): p. 486–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aranday Cortes E, et al. , Mycobacterium bovis-BCG vaccination induces specific pulmonary transcriptome biosignatures in mice. PLoS One, 2010. 5(6): p. e11319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burel JG, et al. , Transcriptomic Analysis of CD4(+) T Cells Reveals Novel Immune Signatures of Latent Tuberculosis. J Immunol, 2018. 200(9): p. 3283–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pomaznoy M, et al. , Quantitative and Qualitative Perturbations of CD8(+) MAITs in Healthy Mycobacterium tuberculosis-Infected Individuals. Immunohorizons, 2020. 4(6): p. 292–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Birnbaum ME, et al. , Deconstructing the peptide-MHC specificity of T cell recognition. Cell, 2014. 157(5): p. 1073–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hanekom WA, The immune response to BCG vaccination of newborns. Annals of the New York Academy of Sciences, 2005. 1062: p. 69–78. [DOI] [PubMed] [Google Scholar]
- 67.Orme IM, The Achilles heel of BCG. Tuberculosis, 2010. 90(6): p. 329–32. [DOI] [PubMed] [Google Scholar]