Abstract
Genetic factors are involved in the development, progression, and severity of NAFLD. Polymorphisms in genes regulating liver functions may increase liver susceptibility to NAFLD. Therefore, we conducted this literature study to present recent findings on NAFLD-associated polymorphisms from published articles in PubMed from 2016 to 2021. From 69 selected research articles, 20 genes and 34 SNPs were reported to be associated with NAFLD. These mutated genes affect NAFLD by promoting liver steatosis (PNPLA3, MBOAT7, TM2SF6, PTPRD, FNDC5, IL-1B, PPARGC1A, UCP2, TCF7L2, SAMM50, IL-6, AGTR1, and NNMT), inflammation (PNPLA3, TNF-α, AGTR1, IL-17A, IL-1B, PTPRD, and GATAD2A), and fibrosis (IL-1B, PNPLA3, MBOAT7, TCF7L2, GATAD2A, IL-6, NNMT, UCP, AGTR1, and TM2SF6). The identification of these genetic factors helps to better understand the pathogenesis pathways of NAFLD
Key Words: Fibrosis, Inflammation, Non-alcoholic fatty liver disease, Polymorphism
INTRODUCTION
Non-alcoholic fatty liver disease is a term commonly used to cover an array of clinical manifestations in the liver that are not induced by secondary causes such as alcohol or drug consumption and defined genetic disorders. These manifestations involve steatosis, inflammation, and fibrosis, which can lead to cirrhosis and even hepatocellular carcinoma[1-3]. Histologically, NAFLD is classified into NAFL and NASH. In NAFL, steatosis is seen in more than 5% of the parenchyma, while in NASH, necroinflammatory is present alongside steatosis. Obesity and insulin resistance drive the accumulation of TGs and FFAs in the liver, contributing to the growing epidemic of NAFLD[2]. The average global prevalence rate of NAFLD is 25.24%, with the highest rates reported in the Middle East and South American countries reaching up to 30%. In Asia, the incidence of NAFLD is 50.9 cases per 1,000 person-years. The global prevalence of NAFLD has increased from 15% in 2005 to 25% in 2010, and it keeps increasing steadily[4]. More noticeable growth in NAFLD prevalence has been observed in Asia and Pacific countries, which might be correlated with the increasing rate of obesity, type 2 diabetes, and metabolic syndromes in this region[4,5].
It has been established that genetic factors, along with environmental factors, are involved in the development, progression, and severity of NAFLD[6]. Certain genetic variants confer susceptibility to NAFLD. Several SNPs have been reported to be associated with specific phenotypes of NAFLD. Identifying the genetic factors in NAFLD will help to better understand the pathogenesis pathways of the disease. It also serves as a potential solution for future NAFLD genetic screening, the development of new genetic-based treatments, as well as the development of genetically modified animal models to facilitate studies in the field[6].
Similar reviews have previously been conducted on NAFLD-associated polymorphisms. Duvnjak et al.[7] reviewed the genetic polymorphisms in NAFLD published between 2002 and 2009 and discussed their involvement in NAFLD development and progression. Severson et al.[6] also reported the genetic factors affecting NAFLD from studies between 2012 and 2016, emphasizing certain genes and polymorphisms. A more recent article from Trépo and Valenti[8] has reviewed several selected gene polymorphisms and their implications for NAFLD pathobiology, drug discovery, and risk prediction. In this narrative review, we aimed to present recent findings on NAFLD-associated polymorphisms from published articles in PubMed from 2016 to 2021 and focused on discussing their roles in three main NAFLD spectrums: steatosis, inflammation, and fibrosis.
MATERIALS AND METHODS
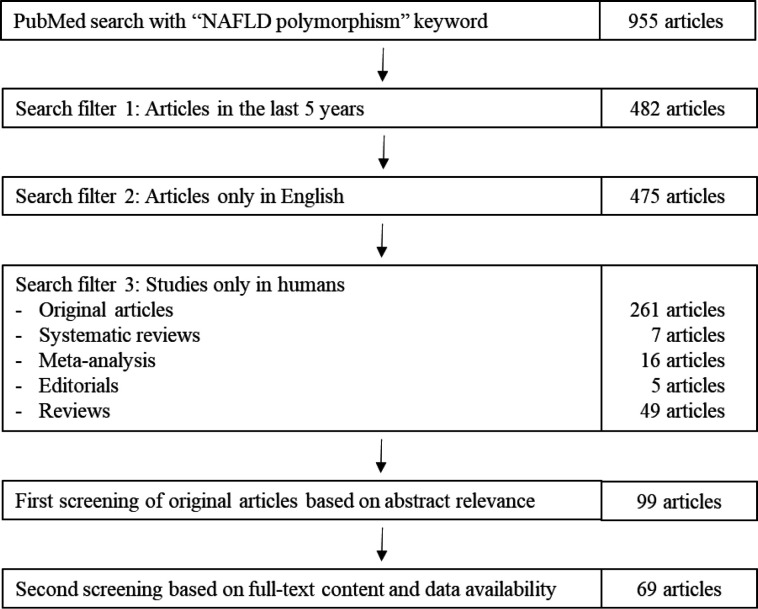
We conducted a search in PubMed to identify the relevant articles. The detailed selection process is shown in Figure 1. The search term used was “NAFLD polymorphism” with the following search filters: published in the last five years (2016-2021), only in humans, and only articles in English. The search yielded 338 published references, which were then sorted by authors for relevance. Review articles and editorials were excluded from this study. Relevant research articles without complete data were also excluded. In the end, 69 published references were selected for this study, and the summarized data are presented in Table 1.
Fig. 1.
Article selection process.
Table 1.
NAFLD-associated SNPs published between 2016 and 2021
| Genes | SNP ID | Risk allele | Associations with NAFLD | |
|---|---|---|---|---|
| PNPLA3 | ||||
| rs738409 | G | Aggravate hepatosteatosis[9-17] | ||
| Development of NAFLD[14,18-34] | ||||
| Elevated alanine aminotransferase levels[10,17,35-38] | ||||
| Associated with NASH[22,39-43] | ||||
| Associated with hepatic fat fractions[44] | ||||
| Associated with hepatocyte ballooning[41] | ||||
| Lobular and portal inflammation[41] | ||||
| Increased liver graft fat content[45] | ||||
| Elevated level of TGs[21,37] | ||||
| Increased liver fibrosis[13,14,17,22,34,36,42,46-50] | ||||
| Associated with cirrhosis[22] | ||||
| Increased AST levels[13,34,37,38] | ||||
| Higher body mass index[37] | ||||
| Higher serum level of γ-glutamyltransferase, ALP, total cholesterol, LDL, and uric acid [37] | ||||
| Higher serum level of CK18-M30[14] | ||||
| Increased severity of liver histology[33,49] | ||||
| Increased steatohepatitis, low level of high-density lipoprotein, and higher insulin resistance[17] | ||||
| rs4823173 | A | Associated with increased AST levels[51] | ||
| rs2896019 | G | Associated with increased AST levels[51,52] | ||
| Associated with NAFLD[52,53] | ||||
| Associated with increased ALT levels and decreased serum TGs and higher levels of LDL[52] | ||||
| rs2281135 | A | Associated with AST levels[51] | ||
| Associated with hepatocyte ballooning and NASH[41] | ||||
| Lobular and portal inflammation[41] | ||||
| Associated with NAFLD[27,54] | ||||
| Associated with advanced fibrosis[50] | ||||
| rs3810622 | T | Associated with NAFLD, increased ALT levels, and higher level of blood glucose[52] | ||
| Elevated ALT levels[35] | ||||
| rs12483959 | A | Associated with NAFLD[27] | ||
| rs143392071 | G | Increased NAFLD risk[55] | ||
| rs2143571 | A | Associated with advanced fibrosis[50] | ||
| MBOAT7 | ||||
| rs626283 | C | Associated with NAFLD and may affect glucose metabolism by modulating intrahepatic fat content[56] | ||
| rs641738 | T | Contributes to hepatic inflammation[57] | ||
| Increased fibrosis[13,57,58] | ||||
| Higher ALT levels[58,59] | ||||
| Associated with increased liver injury[13] | ||||
| Associated with NAFLD risk[14,24] | ||||
| Associated with severe hepatic steatosis[14,58] | ||||
| TM6SF2 | ||||
| rs58542926 | T | Associated with or independent risk factors of hepatic steatosis[13,60,61] | ||
| Elevated ALT levels[13,61] | ||||
| Independent predictors of NASH[60] | ||||
| Increased levels of aminotransferases[36] | ||||
| Associated with advanced fibrosis[32] | ||||
| Associated with the risk of NAFLD[23,24,37,61,62] | ||||
| Associated with liver injury, deleterious effects on liver health, modulate hepatic fat accumulation, and Increased serum AST[13] | ||||
| IL-17A | rs2275913 | A | Development of NAFLD in obese patients[63] | |
| COL13A1 | rs1227756 | A | Higher risk of elevated ALT levels[35] | |
| SAMM50 | rs3761472 | G | Associated with hepatocyte ballooning, lobular and portal inflammation, and NASH[41] | |
| Significant associations with NAFLD[27] | ||||
| rs2143571 | A | Significant associations with NAFLD[27] | ||
| rs2073080 | T | Significant associations with NAFLD[27] | ||
| IL-6 | rs1800795 | C | Associated with the development of NASH[64] | |
| Higher risk of steatosis with less parenchymal damage[65] | ||||
| Increased risk of NAFLD, higher BMI, fat mass, % body fat, waist circumference, serum TGs, total cholesterol, ALP, AST, and fasting insulin levels[66] | ||||
| rs10499563 | C | Associated with the presence of definitive NASH, increased ballooning, and Mallory bodies[65] | ||
| IL-1B | rs1143634 | T | Associated with advanced fibrosis and increased Mallory bodies[65] | |
| FNDC5 | rs3480 | G | More severe steatosis[67] | |
| AGTR1 | rs5186 | C | Predictor of NAFLD incidence and severity[68] | |
| PPARGC1A | rs8192678 | A | Risk factor for the development of NAFLD[69] | |
| CD82 | rs2303861 | G | Involved in the development and progression of NAFLD[70] | |
| UCP2 | rs659366 | A | Higher risk of NAFLD[71] | |
| T | Determinant of fibrosis severity[15] | |||
| TNF-α | rs1800629 | A | Higher risk of NASH development[72] | |
| rs1799964 | C | Independent risk factors contributing to histological progression of NASH[73] | ||
| NNMT | rs694539 | A | Risk factor for developing NAFLD and NASH, correlated with the steatosis degree[74] | |
| HSD11B1 |
rs2235543
rs12565406 rs4844880 |
C G T |
Increased risk of NAFLD development and higher liver fat content[75] | |
| PTPRD | rs35929428 | A | Associated with the development of NAFLD, play a role in hepatic lipid accumulation and fibrosis[76] | |
| GATAD2A | rs4808199 | A | Associated with NAFLD[53] | |
| TCF7L-2 | rs7903146 | T | Independently associated with NAFLD[77] | |
| TLL1 | rs17047200 | T | Higher risk of advanced fibrosis[46] | |
BMI, body mass index; ALP, alkaline phosphatase; LDL, low-density lipoprotein
RESULTS AND DISCUSSION
GWAS has contributed to the identification of potential SNPs in NAFLD. These studies provided insights into the pathogenesis and the long-term prognosis of NAFLD[78]. There were 20 genes and 34 SNPs reported to be associated with NAFLD in studies published in the last five years, which matched our search parameters as presented in Table 1. The majority of the literature we used in this review has investigated the association of NAFLD with three arguably major genetic factors of NAFLD: PNPLA3 rs738409, TM6SF2 rs58542926, and MBOAT7 rs641738. Each SNP has its roles in the development and progression of NAFLD, with the most reported association including the independent risk of NAFLD, aggravated steatosis, increased liver fibrosis, as well as elevated ALT and AST levels. Even though the association of the SNPs and NAFLD has been established in those genetic studies presented in Table 1, the involvement of each polymorphism in NAFLD is often unclear. In this review, we discuss the possible involvement of the genes and/or the variants in three NAFLD spectrums (steatosis, inflammation, and fibrosis) based on the published studies. We also drafted the possible relationships of the discussed genes in those NAFLD spectrums as shown in Figures 2 and 3.
PNPLA3
The Human PNPLA3 gene is located on chromosome 22, encoding a protein called adiponutrin. The gene acts as a lipid droplet regulator in hepatocytes, HSCs, and adipocytes. Since 1998, the rs738409 C>G variant has been identified to be associated with NAFLD[79,80]. The variant was reported to be involved in hepatic steatosis, inflammation, and fibrosis. It is unclear how the variant affects liver TG content, but it has been demonstrated that the variant is associated with the loss of TG hydrolase activities, eventually increasing intrahepatic TG accumulation[81]. Accordingly, the variant was linked to higher levels of circulating TGs, corroborating the impaired TG hydrolysis by lipoprotein lipase[21]. The hepatic fat content in individuals carrying the variant has also shown an increase in n-6 polyunsaturated fatty acids, indicating a pro-inflammatory condition that promotes de novo lipogenesis in the liver[81]. rs738409 was the only variant of PNPLA3 associated with hepatic steatosis in this review. The rs738409 SNP, as well as rs2281135 and rs2143571, are also involved in hepatic fibrosis. PNPLA3 has been reported to activate HSCs and promote migration, proliferation, and the pro-fibrogenic activities of HSCs[82]. Patients with NAFLD carrying the G allele of the rs738409 variant have displayed elevated serum ferritin levels, as well. Iron can cause oxidative stress by interacting with oxygen radicals. Oxidative stress is implicated in mediating the progression of fibrosis. Iron can also induce fibrosis by activating Kupffer cells to release pro-fibrogenic mediators[83]. Chatterjee et al.[41] have reported the association of PNPLA3 variants, rs738409 and rs2281135, with portal and lobular inflammation. The variants are correlated with the release of pro-inflammatory and pro-fibrogenic cytokines such as chemokine ligand 5, monocyte chemoattractant protein-1, IL-8, granulocyte-macrophage colony-stimulating factor, and TNF-α[81]. Individuals harbouring the rs738409 variant had greater inflammatory infiltration than individuals with wild- type genotypes[84]. Accordingly, the culture medium of cells expressing the genetic variants was also shown to recruit more immune cells than the wild-type carriers[81]. To summarize, the polymorphisms in PNPLA3 gene affect NAFLD development and progression by promoting steatosis (rs738409), inflammation (rs738409 and rs2281135), and fibrosis (rs738409, rs2281135, and rs2143571). However, there was limited information on the involvement of the other PNPLA3 variants (rs4823173, rs2896019, rs3810622, rs12483959, and rs143392071) reported in this review on those three NAFLD spectrums.
Fig. 2.
NAFLD-associated SNPs involved in liver steatosis. Polymorphisms in NAFLD-related genes cause TG accumulation in the liver through impaired TG hydrolase activities, increased lipogenesis, increased TG synthesis, reduced secretion of TG-rich VLDL, increased lipid droplet formation, STAT3 inactivation, increased liver supply of FFAs, decreased fatty acid oxidation, and decreased irisin secretion. Each gene and its polymorphisms have specific pathways in causing TG accumulation. For instance, polymorphisms in the PNPLA3 gene can impair the TG hydrolase activities, as well as cause an increase in n-6 PUFA level, which stimulate lipogenesis in the liver, resulting in steatosis. Mutations in TCF7L2, SAMM50, IL-6, and AGTR1 promote lipolysis in adipose tissue and skeletal muscle, leading to increased supply of FFAs to the liver, increased de novo lipogenesis, and eventually increased TG accumulation. Changes in MBOAT7 and IL-1B genes cause increased TG synthesis and lipid droplet formation. Meanwhile, the UCP2 gene seems to possess protective effects against steatosis by inducing fatty acid oxidation, lowering the supply of FFAs to the liver. (→: promote; ─|: inhibit; : mutated genes; ↓: decreased; ↑: increased)
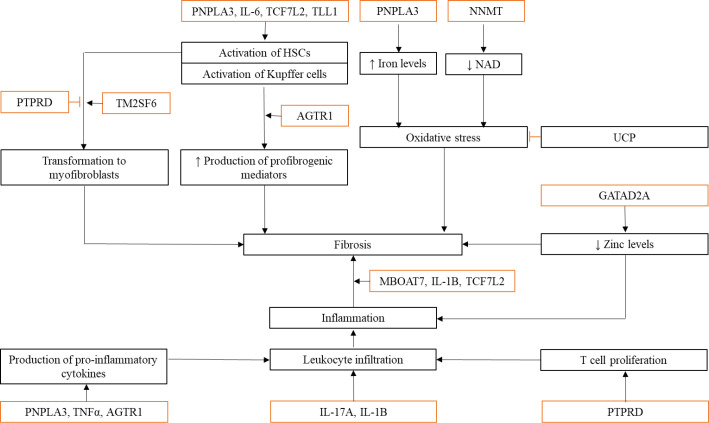
Fig. 3.
NAFLD-associated SNPs involved in liver inflammation and fibrosis. Inflammation is a contributing factor in fibrogenesis. Changes in genes involved in both processes can affect the development and progression of NAFLD. Mutations in PTPRD, PNPLA3, TNF-α, AGTR1, IL-17A, IL-1B, and GATAD2A indirectly cause fibrosis by inducing inflammatory responses through increased production of pro-inflammatory cytokines, increased immune cell proliferation, and leukocyte recruitment. Other polymorphisms are involved in fibrogenesis by either activating HSCs and Kupffer cells or inducing oxidative stress in liver tissue. Activated HSCs can transform into myofibroblasts that will then produce excess collagen, resulting in tissue scarring. (→: promote; ─|: inhibit; : mutated genes; ↓: decreased; ↑: increased)
MBOAT7
MBOAT7 protein, so-called lysophosphatidylinositol acyltransferase 1, is involved in acyl remodelling of phosphatidylinositols in the Lands cycle[58]. The carriers of rs641738 T allele have indicated lower hepatic MBOAT7 mRNA and protein expression[57]. Lower MBOAT7 expression is correlated with severe hepatic inflammation, advanced fibrosis, and higher ALT levels[57-59]. However, MBOAT7 involvement in hepatic inflammation is still unclear. It has previously demonstrated that the strong expression of MBOAT7 is found in immune cell subsets such as neutrophils, peripheral blood mononuclear cells, lymphocytes (B and T), monocytes, macrophages, natural killer cells, and dendritic cells[85]. The protein is also involved in eicosanoid production by neutrophils and myeloid cells, as well as the stimulation of T lymphocyte proliferation[57]. These findings suggest that MBOAT7 plays a role in inflammatory activities. Also, MBOAT7-mediated inflammation is thought to be associated with the progression to fibrosis, possibly independent of lipid accumulation and insulin resistance as the rs641738 variant was not associated with steatosis in chronic hepatitis B and C patients, as well as in obese Taiwanese children[10,57,85]. However, other studies have reported that the variant is also associated with steatosis[14,58]. In cultured human hepatocytes, reduced MBOAT7 expression caused by the rs641738 variant resulted in higher phosphatidylinositols turnover. This condition leads to the constant production of diacylglycerol, resulting in increased synthesis of hepatocyte TG[86]. TGs are known to be the main form of lipid stored in hepatic steatosis. In diet-induced steatotic mice, inhibition of TG synthesis through diacylglycerol acyltransferase 2-knockout could lower hepatic TGs by ~70%, with no significant changes in liver inflammation, fibrosis, and insulin-glucose metabolism[87]. This finding supports the hypothesis that inflammation and fibrosis caused by the rs641738 variant are independent of lipid accumulation. Another variant of the MBOAT7 gene, rs626283, has been exhibited to be related to liver fat content and impaired insulin sensitivity in obese Caucasian youth but not in African American and Hispanic populations[56]. Buch et al.[88] have denoted that rs626283 had the strongest association with severe liver damage at the MBOAT7 locus in European descent individuals with alcohol-related cirrhosis. The variant was also in high linkage disequilibrium with the rs641738 variant, the functional variant affecting MBOAT7 expression[88]. However, the involvement of the rs626283 variant in NAFLD remains unclear. Taken together, both MBOAT7 variants contribute to NAFLD through inflammation-mediated fibrosis and steatosis.
TM6SF2
The TM6SF2 rs58542926 SNP was identified to be associated with NAFLD, hepatic steatosis, elevated ALT and AST levels, and advanced fibrosis. A meta-analysis by Liu et al.[89] has also pointed out that the rs58542926 variant is associated with fibrosis and steatosis in individuals with chronic hepatitis C. Interestingly, the variant was not linked to inflammation. rs58542926 is known to decrease TM6SF2 expression. The variant causes reduced secretion of TG-rich VLDL, leading to lower serum TG levels and increased intrahepatic TG accumulation[6,89]. It is unclear whether the fibrosis is driven by lipid accumulation or not. An in vitro study has demonstrated that the rs58542926 SNP might increase the sensitivity of HSC activation. In the liver, HSCs are activated by TGF-β1, which is secreted by HSCs or Kupffer cells. TGF-β1 stimulates HSC transformation into myofibroblasts. TM6SF2-knockdown LX2 cells have shown increased mRNA expression of αsmooth muscle actin following TGF-β1 treatment, indicating that the variant promotes fibrosis[90]. The role of TM6SF2 in fibrosis still requires further investigations. Altogether, TM6SF2 rs58542926 SNP is involved in NAFLD pathogenesis by promoting steatosis and fibrosis.
PPARGC1A
The A allele of rs8192678 SNP in PPARGC1A gene is a risk factor for NAFLD development in adult Iranian and Chinese Han populations[69,91]. However, the SNP was not associated with the biochemical and physiological parameters investigated in the study, including body mass index, fasting blood sugar, creatine, TGs, plasma lipid levels, HbA1c, and microalbumin levels. PPARGC1A is a transcriptional factor involved in lipid and energy metabolism[69]. The gene encodes peroxisome proliferator-activated receptor PGC-1α, which is highly expressed in the liver. PGC-1α promotes fatty acid oxidation in fasting condition[91]. Liang and Ward[92] have reported that the downregulation of this gene increased lipogenesis and steatosis in the liver. Accordingly, the rs8192678 A allele was found to significantly lower the expression of PPARGC1A, resulting in reduced PGC-1α activities and altered PGC-1α interactions in regulating oxidative stress and lipid metabolism which will eventually lead to NAFLD development[69,91]. Overall, the rs8192678 A allele contributes to NAFLD development through steatosis induction.
IL-17A
IL-17, especially IL-17A, is involved in NAFLD pathogenesis[93]. IL-17 induces the production of IL-6, which is important for Th17 cell differentiation. The rs2275913 (A) allele polymorphism is associated with elevated IL-17A levels[94]. Overexpression of IL-17A resulted in NAFLD progression and worsened liver injury in obese mice[92]. The IL-17A/IL-17RA axis is important in the progression of NAFL to NASH in high fat and methionine choline-deficient diets. Massive infiltration of IL-17+ cells was also found in NASH liver[95]. In conclusion, IL-17A SNP contributes to NAFLD development through its role in inflammation.
IL-6
Upregulation of serum and hepatic IL-6 was observed in patients with NAFLD and animal models. In the liver, IL-6 is produced by hepatocytes and Kupffer cells, and its expression in hepatocytes is correlated with the disease severity. IL-6 has protective roles in the liver due to its antiapoptotic action and its involvement in improving hepatic regeneration and repair. However, prolonged overexpression of IL-6 might increase liver susceptibility to injury and apoptosis. IL-6 is recently known to be a mediator of fibrogenesis in HSCs. IL-6 also promotes the release of FFAs from the adipose tissue, increasing the supply of FFAs to the liver[96]. Both rs1800795 (C) and rs10499563 (C) alleles are polymorphisms in the promoter region of the IL-6 gene. The former polymorphism is frequently associated with lower IL-6 expression even though there were reports of its association with higher serum IL-6 levels[66,97,98]. Further studies are required to confirm the effects of these polymorphisms on IL-6 levels. Mutations in the IL-6 gene weaken its hepatoprotective effect, making the liver more susceptible to NAFLD through inflammation, steatosis, and fibrosis.
IL-1B
IL-1B is involved in NAFLD development through the IL-1 receptor signaling pathway. The rs1143634 polymorphism in IL-1B gene is suggested to be associated with higher IL-1B expression. The presence of IL-1B induces lipid droplet formation in hepatocytes. IL-1B also promotes the recruitment of neutrophils in the liver by upregulating the expression of intercellular adhesion molecule 1 in endothelial cells. IL-1B, IL-6, and TNF-α cause chronic inflammation in the liver by activating local immune cells and attracting other immune cells to the liver. IL-1B also contributes to the progression from liver inflammation to liver fibrosis[99]. IL-1B involvement in steatosis, inflammation, and fibrosis contributes to NAFLD development and progression.
TNF-α
TNF-α is involved in the development and progression of NAFLD by inducing the production of lipid metabolism enzymes, proinflammatory cytokines, and fibrosis-associated proteins[100]. It activates proinflammatory pathways such as c-Jun N-terminal kinase and nuclear factor-κB and indirectly blocks the anti-inflammatory effect of insulin by contributing to the development of insulin resistance[101,102]. Studies have reported the overexpression of circulating TNF-α among patients with NAFLD. Both rs1800629 A allele and rs1799964 C allele are associated with higher TNF-α expression. The increased circulating TNF-α is correlated with NAFLD severity[103]. As a result, the SNPs in the TNF-α gene facilitate the progression to NASH through its role in inflammation, steatosis, and fibrosis.
FNDC5
Metwally et al.[67] have reported an association between the FNDC5 rs3480 variant and advanced steatosis. The variant affects hepatic FNDC5 expression and provides a binding site for miR-135a-5P that regulates several pathways involved in liver disease. FNDC5 is known to secrete irisin, which can ameliorate steatosis. A study by Canivet et al.[104] have shown that FNDC5 could prevent fat accumulation in hepatocytes in vitro. The genetic variant was found to downregulate FNDC5 expression[67]. Therefore, the lower expression of FNDC5 due to the polymorphism can lead to more severe steatosis. This observation suggests that without the polymorphism, liver tissue would express higher FNDC5 for its protective properties[104]. In summary, the FNDC5 variant is involved in NAFLD by causing advanced steatosis.
COL13A1
Larrieta-Carrasco et al.[35] have reported that the carriers of rs1227756 variant in COL13A1 gene expressed elevated ALT and AST levels, even though only the elevated AST level was significantly associated with rs1227756. However, the mechanism underlying the condition is still unclear. The variant was also reported to be associated with lobular inflammation in patients with NAFLD and T2DM[105]. Increased aminotransferase levels often indicate the presence of inflammation[106]. It is possible that changes in COL13A1 gene may influence the levels of liver enzymes through inflammatory response and/or T2DM-related pathways. Further studies are required to elucidate the involvement of COL13A1 in elevating the transaminase levels. To summarize, COL13A1 polymorphism may contribute to NAFLD through inflammation.
CD82
A variant of CD82 was found to be associated with the development and progression of NAFLD. The mechanism by which the rs2303861 polymorphism influences NAFLD pathophysiology is still unclear due to the limited availability of studies on the topic. It is theorized that the polymorphism in CD82 gene promotes hepatic steatosis based on the evidence that CD82-knockout mice exhibit increased adipogenic potential. The rs2303861 SNP is also in linkage disequilibrium with rs7942159 of the PNPLA2 gene, which is involved in fat mobilization in adipose tissue[70]. Further studies are needed to investigate the effects of CD82 on NAFLD. However, it is thought that the CD82 variant plays a role in the development and progression of NAFLD through steatosis.
AGTR1
The AGTR1 rs5186 C allele can predict the risk and severity of NAFLD in Caucasian and Iranian populations[68,107]. The polymorphism promotes fat-induced proinflammatory response and enhances NF-κB activation in mononuclear cells. Activated NF-κB induces the release of pro-inflammatory and pro-fibrogenic adipokines and chemokines, resulting in inflammation, adipose tissue dysfunction, and hepatic injury in NASH. The C allele of the polymorphism causes insulin resistance in skeletal muscle and adipose tissue, increasing the supply of FFAs to the liver and the release of mainly pro-inflammatory adipokines and chemokines[68,108]. The C allele is also responsible for VLDL accumulation, which is rich in TGs and cholesterol[68]. Collectively, the AGTR1 rs5186 C allele can be a predictor of NAFLD incidence and severity due to its involvement in inflammation, steatosis, and fibrosis in NAFLD.
UCP2
The rs659366 G>A and C>T of the UCP2 gene are correlated with NAFLD susceptibility and fibrosis severity, respectively[15,71]. The carriers of rs659366 A allele are at higher risk of developing NAFLD in Iranian population with NAFLD[71]. The AA genotype shows the high expression of UCP2 and oxidative stress markers, as well as reduced insulin production[109]. However, the involvement of UCP2 in the development and progression of NAFLD is still unclear. Theoretically, UCP2 may have protective activities against NAFLD. High plasma fatty acid supply in the liver induces higher expression of UCP2. UCP2 will then promote fatty acid oxidation through several mechanisms: (1) increasing beta-oxidation of fatty acid in the mitochondria, (2) translocating non-esterified fatty acids to prevent accumulation in the mitochondrial matrix, (3) releasing FFAs from the mitochondrial matrix and allowing re-entry as acyl-CoA required for beta-oxidation, and (4) activating AMP-activated protein kinase, promoting the use of fatty acids in energy metabolism. Nevertheless, it has not been proven that UCP2 can prevent steatosis. Controversy also arises over the involvement of UCP2 in oxidative stress. UCP2 is thought to be able to prevent ROS formation, but there is not enough evidence to support this claim. Increased UCP2 expression is still unable to reduce oxidative stress and ROS formation in NAFLD animal models[110]. Ultimately, polymorphisms in the UCP2 gene may disrupt its protective roles in the liver and contribute to NAFLD development through steatosis and fibrosis.
TCF7L-2
The rs7903146 T allele in the TCF7L-2 gene was found to be strongly associated with NAFLD in Asian Indian population[77]. The T allele of this polymorphism is correlated with the increased expression of TCF7L-2[111]. TCF7L-2 modulates the activation of HSCs and fibrogenesis in the liver through β-catenin/TCF pathway. TCF7L-2 is also expressed in adipose tissue. TCF7L-2 activation in adipose tissue leads to inflammation, lipolysis, and lower adiponectin production[112]. Increased lipolysis following TCF7L-2 activation results in higher serum FFAs. Reduced adiponectin production will disrupt glucose and fatty acid metabolism. In line with these findings, the rs7903146 polymorphism was reported to be associated with a high level of serum FFAs[113]. TCF7L-2 also regulates glucose homeostasis, the rs7903146 variant impaired insulin secretion, making the carriers of the polymorphism at risk of developing T2DM[77]. This condition causes insulin resistance that contributes to the development of NAFLD by increasing de novo lipogenesis in the liver and promoting lipolysis in other tissues, leading to a higher FFA supply to the liver[114]. In short, the rs7903146 variant is involved in the development and progression of NAFLD through steatosis, inflammation, and fibrosis.
SAMM50
The rs738491 T allele, rs2143571 A allele, and rs3761472 G allele of SAMM50 gene are associated with NAFLD in Korean population[27]. The association of SAMM50 polymorphisms and NAFLD has also been reported in Japanese, Asian Indian (only rs3761472 SNP), and Chinese Han populations[41,115,116]. The rs3761472 variant was reported to be associated with hepatocyte ballooning, lobular and portal inflammation, and NASH[41]. The gene itself, SAMM50, plays a role in the progression of NAFL to NASH. SAMM50 encodes Sam50, which is important in maintaining the structure of mitochondrial cristae and the assembly of mitochondrial respiratory chain complexes[116]. Downregulation of Sam50 in the liver can cause mitochondrial dysfunction, which is known to contribute to the development of insulin resistance and hepatic steatosis in obese rat model[117]. Liver biopsy from patients with NASH has also shown mitochondrial abnormalities[118]. The three SAMM50 variants may cause the lower production of Sam50, leading to mitochondrial dysfunction-mediated steatosis. To sum up, those variants may be responsible for NAFLD development and progression through inflammation (rs3761472 only), steatosis, and insulin resistance.
TLL1
The rs17047200 T allele is associated with a higher risk of developing advanced fibrosis in Japanese patients with NAFLD[46]. The SNP leads to the elevated expression of TLL1, which has been found to activate HSCs in animal models and humans, indicating its involvement in fibrogenesis[119]. Activated HSCs have a myofibroblast-like phenotype, contributing to fibrogenesis through cell proliferation and upregulation of matrix production[120]. However, a contradicting study by Bayoumi et al.[121] has reported that rs17047200 is not associated with fibrosis in Caucasian patients with biopsy-proven metabolic-associated fatty liver disease. That study has also demonstrated that the overexpression of TLL1 in HSCs is detected in patients with metabolic steatohepatitis, in a murine fibrosis model fed with methionine choline-deficient diet and in an in vitro human fibrosis model. Further studies are needed to elucidate the roles of TLL1 in both steatohepatitis and fibrosis, as well as to confirm the effects of rs17047200 SNP on NAFLD. However, it is theorized that the SNP affects NAFLD through its involvement in steatosis and fibrosis.
NNMT
The AA genotype of NNMT rs694539 variant is related to the increased risk of NASH in obese Egyptians. The SNP is associated with steatosis but it is not considered a fibrosis marker[74]. Komatsu et al.[122] have reported that the overexpression of NNMT depleted nicotinamide adenine dinucleotide (NAD) and S-adenosylmethionine, inducing the genes involved in steatosis and fibrosis in the liver of transgenic mice overexpressing NNMT. NAD has protective effects on ROS and also facilitates hydrogen transfer in reductive or oxidative metabolic reactions. NAD depletion reduces fatty acid oxidation, leading to the accumulation of TGs in hepatocytes. Therefore, inhibiting NNMT activities may prevent progression to NASH. Briefly, changes in the NNMT gene may contribute to NAFLD through steatosis and ROS-mediated fibrosis.
HSD11B1
The rs2235543, rs12565406, and rs4844880 polymorphisms in HSD11B1 gene are associated with the liver fat content. Accordingly, the HSD11B1 mRNA expression positively correlates with the liver fat content, suggesting the involvement of 11β-HSD1 in hepatic fat accumulation. The homozygous major allele carriers of the three SNPs also have shown elevated expression of HSD11B1 gene, and they are also twice at risk of developing NAFLD[75]. Overexpression of 11β-HSD1 in a high-fat diet leads to steatosis, while its deficiency is protective against steatosis. Lower expression of 11β-HSD1 is observed in the early stages of NAFLD, but increased 11β-HSD1 levels are required for the progression to NASH. Inhibition of 11β-HSD1 caused reduced lipid content, making it a potential therapeutic target for steatosis[123]. To summarize, the HSD11B1 variants contribute to NAFLD through steatosis.
PTPRD
Polymorphism in PTPRD gene may be related to hepatic lipid accumulation and fibrosis progression in Japanese patients with NAFLD. More advanced steatosis and fibrosis have been observed in the GA genotype of the rs35929428 variant. PTPRD mainly dephosphorylates STAT3. Based on this evidence, Nakajima et al.[76] observed an association between rs35929428 SNP and STAT3 dephosphorylation and found that the SNP enhanced STAT3 dephosphorylation and strongly suppressed its phosphorylation in hepatocytes. However, dephosphorylation is known to negatively regulate STAT3 activation[124]. STAT3 inactivation leads to TG accumulation and worsens steatosis and hepatocellular damage. STAT3 inactivation also inhibits fibroblast-to-myofibroblast transition in cultured fibroblasts, preventing the development of fibrosis[125], which is in contrast to the results of Nakajima et al.’s[76] study. The rs35929428 polymorphism may exacerbate fibrosis through other signalling pathways. In conclusion, the PTPRD variant has a role in the development and progression of NAFLD through steatosis and fibrosis.
GATAD2A
GATAD2A has been reported to be associated with the increased risk of NAFLD in Japanese patients with NAFLD. However, the function of this gene in NAFLD development is still vague[53,126]. GATAD2A gene is located at 19p12, along with TM6SF2 and NCAN, which are known to be associated with NAFLD[126]. GATAD2A enables zinc ion binding. In NASH, the serum zinc level is lower than in normal condition[53]. Zinc deficiency is a common pathogenesis pathway of NAFLD. Low zinc level correlates with more severe fibrosis and lobular nflammation[126]. The rs4808199 polymorphism might cause the overexpression of GATAD2A, resulting in higher zinc ion binding and lower zinc serum level. Taken together, the variant might be involved in NAFLD pathogenesis through zinc-related inflammation and fibrosis.
Conflicting findings on NAFLD-associated polymorphisms and study limitations
While the presented NAFLD-associated SNPs harbor potential benefits as therapeutic targets, conflicting results arise from several studies. For instance, no association was observed between NAFLD and the rs58542926 variant of TM6SF2 gene in Brazilian patients with NAFLD[127]. MBOAT7 rs641738 variant also did not show any correlation with steatosis in chronic hepatitis B and C patients, as well as in obese Taiwanese children[10,57,85]. These results might be due to the limitations of GWAS. In conventional GWAS, the association is only significant when it reaches the p < 5 × 10-8 threshold. Owing to this high level of significance, the association might be undetected in studies with small sample sizes. The use of larger sample size is preferable even though it is not always possible to assemble a large sample size. Besides, GWAS cannot identify the causal variants and genes[128]. Therefore, further investigation of the SNPs and genes of interest in vitro and in vivo is important to fully understand their involvement in the development and progression of the disease.
NAFLD is a complex disease. Many factors are involved in its development and progression. This review only presented the association of single genetic variants with NAFLD. NAFLD is known to be multigenic, involving the synergistic and antagonistic actions of several genes, along with environmental factors. Providing the information on combined NAFLD-associated SNPs would be a point of interest for future studies. We also could only infer the possible involvement of the SNPs and genes with their associated features in NAFLD from published literature, further studies are required to investigate the nature of their association.
CONCLUSION
Genetic factors are involved in the development and progression of NAFLD. Identifying genetic factors in NAFLD help to better understand the pathogenesis pathways of the disease. The SNPs presented in this review affect NAFLD through their involvement in three NAFLD spectrums (steatosis, fibrosis, and inflammation). Mutations in PNPLA3, MBOAT7, TM2SF6, PTPRD, FNDC5, IL-1B, PPARGC1A, UCP2, and NNMT directly induce steatosis in the liver, while polymorphisms in TCF7L2, SAMM50, IL-6, and AGTR1 genes indirectly promote liver steatosis by increasing lipolysis in adipose tissue and skeletal muscle, resulting in a higher supply of FFAs to the liver. SNPs in PNPLA3, TNF-α, AGTR1, IL-17A, IL-1B, PTPRD, and GATAD2A cause liver inflammation. Inflammation, along with mutations in IL-1B, PNPLA3, MBOAT7, TCF7L2, GATAD2A, IL-6, NNMT, UCP, AGTR1, and TM2SF6 also contributes to liver fibrosis. On the contrary, polymorphism in the PTPRD gene can inhibit fibrosis by preventing the transformation of HSCs to myofibroblasts. Even though these NAFLD-associated SNPs show potential benefits as therapeutic targets, conflicting findings from similar studies arise due to the limitations of GWAS. Therefore, further investigation of those genes and SNPs in vitro and in vivo is important to fully understand their involvement in the development and progression of NAFLD.
DECLARATIONS
Acknowledgments
The authors would like to thank the Ministry of Research and Technology, the Republic of Indonesia for the financial support by providing the Penelitian Dasar Unggulan Perguruan Tinggi (PDUPT) grant (reference number: 1674/UN1/DITLIT/DIT-LIT/PT/2021 and 1642/UN1/DITLIT/DIT-LIT/PT.01.03/2022).
Ethical statement
Not applicable.
Data availability
All data presented in the manuscript can be accessed on PubMed.
Author contributions
FDA, NR, and WW contributed equally to the study conception, design, material preparation, data collection, data analysis, manuscript drafting, and manuscript writing. All authors have read and approved the final manuscript.
Conflict of interest
None declared.
Funding/support
This study has been supported by Penelitian Dasar Unggulan Perguruan Tinggi (PDUPT) grant (reference number: 1674/UN1/DITLIT/DIT-LIT/PT/2021 and 1642/UN1/DITLIT/DIT-LIT/PT.01.03/2022) from the Ministry of Research and Technology, Republic of Indonesia.
References
- 1.Kudaravalli P, John S. Nonalcoholic fatty liver. In: Editorial Board., editor. StatPearls. Florida: StatPearls Publishing; 2021. [PubMed] [Google Scholar]
- 2.Benedict M, Zhang X. Non-alcoholic fatty liver disease: An expanded review. World journal of hepatology. 2017;9(16):715–732. doi: 10.4254/wjh.v9.i16.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomic D, Kemp WW, Roberts SK. Nonalcoholic fatty liver disease: current concepts, epidemiology and management strategies. European journal of gastroenterology and hepatology. 2018;30(10):1103–1115. doi: 10.1097/MEG.0000000000001235. [DOI] [PubMed] [Google Scholar]
- 4.Mitra S, De A, Chowdhury A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Translational gastroenterology and hepatology. 2020;5:16. doi: 10.21037/tgh.2019.09.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashtari S, Pourhoseingholi MA, Zali MR. Non-alcohol fatty liver disease in Asia: Prevention and planning. World journal of hepatology. 2015;7(13):1788–1796. doi: 10.4254/wjh.v7.i13.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Severson TJ, Besur S, Bonkovsky HL. Genetic factors that affect nonalcoholic fatty liver disease: A systematic clinical review. World journal of gastroenterology. 2016;22(29):6742–6756. doi: 10.3748/wjg.v22.i29.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duvnjak M, Barsić N, Tomasić V, Lerotić I. Genetic polymorphisms in non-alcoholic fatty liver disease: clues to pathogenesis and disease progression. World journal of gastroenterology. 2009;15(48):6023–6027. doi: 10.3748/wjg.15.6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trépo E, Valenti L. Update on NAFLD genetics: From new variants to the clinic. Journal of hepatology. 2020;72(6):1196–1209. doi: 10.1016/j.jhep.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Enomoto H, Aizawa N, Hasegawa K, Ikeda N, Sakai Y, Yoh K, Takata R, Yuri Y, Kishino K, Shimono Y, Ishii N, Takashima T, Nishimura T, Nishikawa H, Iwata Y, Iijima H, Nishiguchi SH. Possible Relevance of PNPLA3 and TLL1 Gene Polymorphisms to the Efficacy of PEG-IFN Therapy for HBV-Infected Patients. International journal of molecular sciences. 2020;21(9):3089. doi: 10.3390/ijms21093089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin YC, Chang PF, Chang MH, Ni YH. Genetic determinants of hepatic steatosis and serum cytokeratin-18 fragment levels in Taiwanese children. Liver international. 2018;38(7):1300–1307. doi: 10.1111/liv.13689. [DOI] [PubMed] [Google Scholar]
- 11.Xia MF, Lin HD, Chen LY, Wu L, Ma H, Li Q, Aleteng Q, Hu Y, He WY, Gao J, Bian H, Li XY, Gao X. The PNPLA3 rs738409 C>G variant interacts with changes in body weight over time to aggravate liver steatosis, but reduces the risk of incident type 2 diabetes. Diabetologia. 2019;62(4):644–654. doi: 10.1007/s00125-018-4805-x. [DOI] [PubMed] [Google Scholar]
- 12.Lang S, Martin A, Zhang X, Farowski F, Wisplinghoff H, Vehreschild MJGT, Krawczyk M, Nowag A, Kretzschmar A, Scholz C, Kasper P, Roderburg Ch, Mohr R, Lammert F, Tacke F, Schnabl B, Goeser T, Steffen H-M, Demir M. Combined analysis of gut microbiota, diet and PNPLA3 polymorphism in biopsy-proven non-alcoholic fatty liver disease. Liver international. 2021;41(7):1576–1591. doi: 10.1111/liv.14899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krawczyk M, Rau M, Schattenberg JM, Bantel H, Pathil A, Demir M, Kluwe J, Boettler T, Lammert F, Geier A, NAFLD Clinical Study Group. Combined effects of the PNPLA3 rs738409, TM6SF2 rs58542926, and MBOAT7 rs641738 variants on NAFLD severity: a multicenter biopsy-based study. Journal of lipid research. 2017;58(1):247–255. doi: 10.1194/jlr.P067454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krawczyk M, Bantel H, Rau M, Schattenberg JM, Grünhage F, Pathil A, Demir M, Kluwe J, Boettler T, Weber SN, Geier A, Lammert F, NAFLD CSG. Could inherited predisposition drive non-obese fatty liver disease? Results from German tertiary referral centers. Journal of human genetics. 2018;63(5):621–626. doi: 10.1038/s10038-018-0420-4. [DOI] [PubMed] [Google Scholar]
- 15.Hudert CA, Selinski S, Rudolph B, Bläker H, Loddenkemper CH, Thielhorn R, Berndt N, Golka K, Cadenas C, Reinders J, Henning S, Bufler P, Jansen PLM, Holzhütter HG, Meierhofer D, Hengstler JG, Wiegand S. Genetic determinants of steatosis and fibrosis progression in paediatric non-alcoholic fatty liver disease. Liver international. 2019;39(3):540–556. doi: 10.1111/liv.14006. [DOI] [PubMed] [Google Scholar]
- 16.Valentini D, Mosca A, Di Camillo C, Crudele A, Sartorelli MR, Scoppola V, Tarani L, Villani A, Raponi M, Novelli A, Alisi A. PNPLA3 gene polymorphism is associated with liver steatosis in children with Down syndrome. Nutrition, metabolism, and cardiovascular diseases. 2020;30(9):1564–1572. doi: 10.1016/j.numecd.2020.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Martínez LA, Larrieta E, Kershenobich D, Torre A. The Expression of PNPLA3 Polymorphism could be the Key for Severe Liver Disease in NAFLD in Hispanic Population. Annals of hepatology. 2017;16(6):909–915. doi: 10.5604/01.3001.0010.5282. [DOI] [PubMed] [Google Scholar]
- 18.Akkiz H, Taskin E, Karaogullarindan U, Delik A, Kuran S, Kutlu O. The influence of RS738409 I148M polymorphism of patatin-like phospholipase domain containing 3 gene on the susceptibility of non-alcoholic fatty liver disease. Medicine. 2021;100(19):e25893. doi: 10.1097/MD.0000000000025893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosca A, De Cosmi V, Parazzini F, Raponi M, Alisi A, Agostoni C, Nobili V. The role of genetic predisposition, programing during fetal life, family conditions, and post-natal diet in the development of pediatric fatty liver disease. The Journal of pediatrics. 2019;211:72–77. doi: 10.1016/j.jpeds.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 20.Akkiz H, Taskin E, Karaogullarindan U, Delik A, Kuran S, Kutlu O. The influence of RS738409 I148M polymorphism of patatin-like phospholipase domain containing 3 gene on the susceptibility of non-alcoholic fatty liver disease. Medicine. 2021;100(19):e25893. doi: 10.1097/MD.0000000000025893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narayanasamy K, Karthick R, Panneerselvam P, Mohan N, Ramachandran A, Prakash R, Rajaram M. Association of metabolic syndrome and patatin-like phospholipase 3-rs738409 gene variant in non-alcoholic fatty liver disease among a Chennai-based south Indian population. The journal of gene medicine. 2020;22(4):e3160. doi: 10.1002/jgm.3160. [DOI] [PubMed] [Google Scholar]
- 22.Alam S, Islam MS, Islam S, Mustafa G, Saleh AA, Ahmad N. Association of single nucleotide polymorphism at PNPLA3 with fatty liver, steatohepatitis, and cirrhosis of liver. Indian journal of gastroenterology. 2017;36(5):366–372. doi: 10.1007/s12664-017-0784-y. [DOI] [PubMed] [Google Scholar]
- 23.Zusi C, Mantovani A, Olivieri F, Morandi A, Corradi M, Giudice EMD, Dauriz M, Valenti L, Byrne CD, Targher G, Maffeis C. Contribution of a genetic risk score to clinical prediction of hepatic steatosis in obese children and adolescents. Digestive and liver disease. 2019;51(11):1586–1592. doi: 10.1016/j.dld.2019.05.029. [DOI] [PubMed] [Google Scholar]
- 24.Di Costanzo A, Belardinilli F, Bailetti D, Sponziello M, D'Erasmo L, Polimeni L, Baratta F, Pastori D, Ceci F, Montali A, Girelli G, Masi BD, Angeloni A, Giannini G, Ben MD, Angelico F, Arca M. Evaluation of polygenic determinants of non-alcoholic fatty liver disease (NAFLD) by a candidate genes resequencing strategy. Scientific reports. 2018;8(1):3702. doi: 10.1038/s41598-018-21939-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalafati IP, Dimitriou M, Borsa D, Vlachogiannakos J, Revenas K, Kokkinos A, Ladas S D, Dedoussis G V. Fish intake interacts with TM6SF2 gene variant to affect NAFLD risk: results of a case-control study. European journal of nutrition. 2019;58(4):1463–1473. doi: 10.1007/s00394-018-1675-4. [DOI] [PubMed] [Google Scholar]
- 26.Jain V, Kumar A, Ahmad N, Jana M, Kalaivani M, Kumar B, Shastri Sh, Jain O, Kabra M. Genetic polymorphisms associated with obesity and non-alcoholic fatty liver disease in Asian Indian adolescents. Journal of pediatric endocrinology and metabolism. 2019;32(7):749–758. doi: 10.1515/jpem-2018-0543. [DOI] [PubMed] [Google Scholar]
- 27.Chung GE, Lee Y, Yim JY, Choe EK, Kwak MS, Yang JI, Park B, Lee JE, Kim JA, Kim JS. Genetic polymorphisms of PNPLA3 and SAMM50 are associated with Nonalcoholic fatty liver disease in a Korean population. Gut and liver. 2018;12(3):316–323. doi: 10.5009/gnl17306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niriella MA, Pathmeswaran A, De Silva ST, Kasturiratna A, Perera R, Subasinghe CE, Kodisinghe K, Piyaratna CH, Rishikesawan V, Dassanayaka AS, Silva APD, Wickramasinghe R, Takeuchi F, Kato N, Silva HJ. Incidence and risk factors for non-alcoholic fatty liver disease: A 7-year follow-up study among urban, adult Sri Lankans. Liver international. 2017;37(11):1715–1722. doi: 10.1111/liv.13478. [DOI] [PubMed] [Google Scholar]
- 29.Oniki K, Watanabe T, Kudo M, Izuka T, Ono T, Matsuda K, Sakamoto Y, Nagaoka K, Imafuku T, Ishima Y, Watanabe H, Maruyama T, Otake K, Ogata Y, Saruwatari J. Modeling of the weight status and risk of non-alcoholic fatty liver disease in elderly individuals: the potential impact of the disulfide bond-forming oxidoreductase A-like protein (DsbA-L) polymorphism on the weight status. CPT pharmacometrics and systems pharmacology. 2018;7(6):384–393. doi: 10.1002/psp4.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chinchilla-López P, Ramírez-Pérez O, Cruz-Ramón V, Canizales-Quinteros S, Domínguez-López A, Ponciano-Rodríguez G, Sánchez-Muñoz F, Méndez-Sánchez N. More evidence for the genetic susceptibility of mexican population to non-alcoholic fatty liver disease through PNPLA3. Annals of hepatology. 2018;17(2):250–255. doi: 10.5604/01.3001.0010.8644. [DOI] [PubMed] [Google Scholar]
- 31.Wang S, Song J, Shang X, Chawla N, Yang Y, Meng X, Wang H, Ma J. Physical activity and sedentary behavior can modulate the effect of the PNPLA3 variant on childhood NAFLD: a case-control study in a Chinese population. BMC medical genetics. 2016;17(1):90. doi: 10.1186/s12881-016-0352-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petta S, Di Marco V, Pipitone RM, Grimaudo S, Buscemi C, Craxì A, Buscemi S. Prevalence and severity of nonalcoholic fatty liver disease by transient elastography: Genetic and metabolic risk factors in a general population. Liver international. 2018;38(11):2060–2068. doi: 10.1111/liv.13743. [DOI] [PubMed] [Google Scholar]
- 33.Uygun A, Ozturk K, Demirci H, Oztuna A, Eren F, Kozan S, Yilmaz Y, Kurt O, Turker T, Vatansever S, Alper E, Unsal B. The association of nonalcoholic fatty liver disease with genetic polymorphisms: a multicenter study. European journal of gastroenterology and hepatology. 2017;29(4):441–447. doi: 10.1097/MEG.0000000000000813. [DOI] [PubMed] [Google Scholar]
- 34.Mazo DF, Malta FM, Stefano JT, Salles APM, Gomes-Gouvea MS, Nastri ACS, Almeida JR, Pinho JRR, Carrilho FJ, Oliveira CP. Validation of PNPLA3 polymorphisms as risk factor for NAFLD and liver fibrosis in an admixed population. Annals of hepatology. 2019;18(3):466–471. doi: 10.1016/j.aohep.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Larrieta-Carrasco E, Flores YN, Macías-Kauffer LR, Ramírez-Palacios P, Quiterio M, Ramírez-Salazar EG, León-Mimila P, Rivera-Paredez B, Cabrera-Álvarez G, Canizales-Quinteros S, Zhang Z-F, López-Pérez TV, Salmerón J, Velázquez-Cruz R. Genetic variants in COL13A1, ADIPOQ and SAMM50, in addition to the PNPLA3 gene, confer susceptibility to elevated transaminase levels in an admixed Mexican population. Experimental and molecular pathology. 2018;104(1):50–58. doi: 10.1016/j.yexmp.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kallwitz ER, Tayo BO, Kuniholm MH, Cai J, Daviglus M, Cooper RS, Cotler S. American ancestry is a risk factor for suspected nonalcoholic fatty liver disease in hispanic/latino adults. Clinical gastroenterology and hepatology. 2019;17(11):2301–2309. doi: 10.1016/j.cgh.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Chen LZ, Ding HY, Liu SS, Jiang XJ, Xin YN, Xuan SY. Combining I148M and E167K variants to improve risk prediction for nonalcoholic fatty liver disease in Qingdao Han population, China. Lipids in health and disease. 2019;18(1):45. doi: 10.1186/s12944-019-0992-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Unalp-Arida A, Ruhl CE. Patatin-like phospholipase domain-containing protein 3 I148M and liver fat and fibrosis scores predict liver disease mortality in the U. S. population. Hepatology. 2020;71(3):820–834. doi: 10.1002/hep.31032. [DOI] [PubMed] [Google Scholar]
- 39.Mosca A, Fintini D, Scorletti E, Cappa M, Paone L, Zicari A-M, Nobili V, Byrne CD. Relationship between non-alcoholic steatohepatitis, PNPLA3 I148M genotype and bone mineral density in adolescents. Liver international. 2018;38(12):2301–2308. doi: 10.1111/liv.13955. [DOI] [PubMed] [Google Scholar]
- 40.Fracanzani AL, Petta S, Lombardi R, Pisano J, Russello M, Consonni D, Marco VD, Cammà C, Mensi L, Dongiovanni P, Valenti L, Craxì A, Fargion S. Liver and cardiovascular damage in patients with lean nonalcoholic fatty liver disease, and association With visceral obesity. Clinical gastroenterology and hepatology. 2017;15(10):1604–1611. doi: 10.1016/j.cgh.2017.04.045. [DOI] [PubMed] [Google Scholar]
- 41.Chatterjee A, Basu A, Das K, Chowdhury A, Basu P. Exome-wide scan identifies significant association of rs4788084 in IL27 promoter with increase in hepatic fat content among Indians. Gene. 2021;775:145431. doi: 10.1016/j.gene.2021.145431. [DOI] [PubMed] [Google Scholar]
- 42.Mehta R, Jeiran K, Koenig AB, Otgonsuren M, Goodman Z. The role of mitochondrial genomics in patients with non-alcoholic steatohepatitis (NASH) BMC medical genetics. 2016;17(1):63. doi: 10.1186/s12881-016-0324-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nobili V, Mantovani A, Cianfarani S, Alisi A, Mosca A, Sartorelli MR, Maffeis C, Loomba R, Byrne CD, Targher G. Prevalence of prediabetes and diabetes in children and adolescents with biopsy-proven non-alcoholic fatty liver disease. Journal of hepatology. 2019;71(4):802–810. doi: 10.1016/j.jhep.2019.06.023. [DOI] [PubMed] [Google Scholar]
- 44.Stanislawski MA, Shaw J, Litkowski E, Lange EM, Perng W, Dabelea D, Lange LA. Genetic risk for hepatic fat among an ethnically diverse cohort of youth: the exploring perinatal outcomes among children study. The Journal of paediatrics. 2020;220:146–153. doi: 10.1016/j.jpeds.2020.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trunečka P, Míková I, Dlouhá D, Hubáček JA, Honsová E, Kolesár L, Lánská V, Fraňková S, Šperl J, Jirsa M, Poledne R. Donor PNPLA3 rs738409 genotype is a risk factor for graft steatosis A post-transplant biopsy-based study. Digestive and liver disease. 2018;50(5):490–495. doi: 10.1016/j.dld.2017.12.030. [DOI] [PubMed] [Google Scholar]
- 46.Seko Y, Yamaguchi K, Mizuno N, Okuda K, Takemura M, Taketani H, Hara T, Umemura A, Nishikawa T, Moriguchi M, Yasui K, Kamaguchi M, Nishioji K, Mochizuki N, Kobayashi M, Mori K, Tanaka S, Matsuura K, Tanaka Y, Itoh Y. Combination of PNPLA3 and TLL1 polymorphism can predict advanced fibrosis in Japanese patients with nonalcoholic fatty liver disease. Journal of gastroenterology. 2018;53(3):438–448. doi: 10.1007/s00535-017-1372-8. [DOI] [PubMed] [Google Scholar]
- 47.Bruschi FV, Tardelli M, Herac M, Claudel T, Trauner M. Metabolic regulation of hepatic PNPLA3 expression and severity of liver fibrosis in patients with NASH. Liver international. 2020;40(5):1098–1110. doi: 10.1111/liv.14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Machado CM, Leite NC, França PH, Cardoso CR, Salles GF, Villela-Nogueira CA. PNPLA3 gene polymorphism in Brazilian patients with type 2 diabetes: A prognostic marker beyond liver disease? Nutrition, metabolism, and cardiovascular diseases. 2019;29(9):965–971. doi: 10.1016/j.numecd.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Liu W, Anstee QM, Wang X, Gawrieh S, Gamazon E R, Athinarayanan S, Liu Y-L, Darlay R, Cordell HJ, Daly AK, Day CP, Chalasani N. Transcriptional regulation of PNPLA3 and its impact on susceptibility to nonalcoholic fatty liver Disease (NAFLD) in humans. Aging. 2016;9(1):26–40. doi: 10.18632/aging.101067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Serper M, Vujkovic M, Kaplan DE, Carr RM, Lee KM, Shao Q, Miller DR, Reaven PD, Phillips LS, O'Donnell CJ, Meigs JB, Wilson PWF, Vickers-Smith R, Kranzler HR, Justice AC, Gaziano JM, Muralidhar S, Pyarajan S, DuVall SL, Assimes TL, Lee JS, Tsao PS, Rader DJ, Damrauer SM, Lynch JM, Saleheen D, Voight BF, Chang K-M, VA Million Veteran Program. Validating a non-invasive, ALT-based non-alcoholic fatty liver phenotype in the million veteran program. PLoS One. 2020;15(8):e0237430. doi: 10.1371/journal.pone.0237430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Young KA, Palmer ND, Fingerlin TE, Langefeld CD, Norris JM, Wang N, Xiang AH, Guo X, Williams A H, Chen Y-D, Taylor KD, Rotter JI, Raffel LJ, Goodarzi MO, Watanabe RM, Wagenknecht LE. Genome-wide association study identifies loci for liver enzyme concentrations in Mexican Americans: the GUARDIAN Consortium. Obesity (silver spring) 2019;27(8):1331–1337. doi: 10.1002/oby.22527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song G, Xiao C, Wang K, Wang Y, Chen J, Yu Y, Wang Z, Deng G, Sun X, Zhong L, Zhou C, Qi X, Wang S, Peng Z, Wang X. Association of patatin-like phospholipase domain-containing protein 3 gene polymorphisms with susceptibility of nonalcoholic fatty liver disease in a Han Chinese population. Medicine (baltimore) 2016;95(33) doi: 10.1097/MD.0000000000004569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawaguchi T, Shima T, Mizuno M, Mitsumoto Y, Umemura A, Kanbara Y, Tanaka S, Sumida Y, Yasui K, Takahashi M, Matsuo K, Itoh Y, Tokushige K, Hashimoto E, Kiyosawa K, Kawaguchi M, Itoh H, Uto H, Komorizono Y, Shirabe K, Takami S, Takamura T, Kawanaka M, Yamada R, Matsuda F, Okanoue T. Risk estimation model for nonalcoholic fatty liver disease in the Japanese using multiple genetic markers. PLoS One. 2018;13(1) doi: 10.1371/journal.pone.0185490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.James G, Reisberg S, Lepik K, Galwey N, Avillach P, Kolberg L, Mägi R, Esko T, Alexander M, Waterworth D, Loomis AK, Vilo J. An exploratory phenome wide association study linking asthma and liver disease genetic variants to electronic health records from the Estonian Biobank. PLoS One. 2019;14(4):e0215026. doi: 10.1371/journal.pone.0215026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boonvisut S, Yoshida K, Nakayama K, Watanabe K, Miyashita H, Iwamoto S. Identification of deleterious rare variants in MTTP, PNPLA3, and TM6SF2 in Japanese males and association studies with NAFLD. Lipids in health and disease. 2017;16(1):183. doi: 10.1186/s12944-017-0570-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Umano GR, Caprio S, Di Sessa A, Chalasani N, Dykas DJ, Pierpont B, Bale AE, Santoro N. The rs626283 variant in the MBOAT7 gene is associated with insulin resistance and fatty liver in Caucasian obese youth. The American journal of gastroenterology. 2018;113(3):376–383. doi: 10.1038/ajg.2018.1. [DOI] [PubMed] [Google Scholar]
- 57.Thabet K, Chan HLY, Petta S, Mangia A, Berg T, Boonstra A, Brouwer WP, Lorena Abate M, Wong V WS, Nazmy M, Fischer J, Liddle C, George J, Eslam M. The membrane-bound O-acyltransferase domain-containing 7 variant rs641738 increases inflammation and fibrosis in chronic hepatitis B. Hepatology. 2017;65(6):1840–1850. doi: 10.1002/hep.29064. [DOI] [PubMed] [Google Scholar]
- 58.Di Sessa A, Umano GR, Cirillo G, Prete AD, Iacomino R, Marzuillo P, Giudice EMD. The membrane-bound O-acyltransferase7 rs641738 variant in pediatric nonalcoholic fatty liver disease. Journal of pediatric gastroenterology and nutrition. 2018;67(1):69–74. doi: 10.1097/MPG.0000000000001979. [DOI] [PubMed] [Google Scholar]
- 59.Viitasalo A, Eloranta AM, Atalay M, Romeo S, Pihlajamäki J, Lakka TA. Association of MBOAT7 gene variant with plasma ALT levels in children: the PANIC study. Pediatric research. 2016;80(5):651–655. doi: 10.1038/pr.2016.139. [DOI] [PubMed] [Google Scholar]
- 60.Raimondo G, Saitta C, Lombardo D, Giraudi PJ, Rosso N, Ieni A, Lazzara S, Palmisano S, Bonazza D, Alibrandi A, Navarra G, Tiribelli C, Pollicino T. Occult hepatitis B virus infection predicts non-alcoholic steatohepatitis in severely obese individuals from Italy. Liver international. 2020;40(7):1601–1609. doi: 10.1111/liv.14473. [DOI] [PubMed] [Google Scholar]
- 61.Xu M, Li Y, Zhang S, Wang X, Shen J, Zhang S. Interaction of TM6SF2 E167K and PNPLA3 I148M variants in NAFLD in northeast China. Annals of hepatology. 2019;18(3):456–460. doi: 10.1016/j.aohep.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 62.Li Y, Liu S, Gao Y, Ma H, Zhan S, Yang Y, Xin Y, Xuan S. Affiliations expand Association of TM6SF2 rs58542926 gene polymorphism with the risk of non-alcoholic fatty liver disease and colorectal adenoma in Chinese Han population. BMC biochemistry. 2019;20(1):3. doi: 10.1186/s12858-019-0106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Akbulut UE, Emeksiz HC, Citli S, Cebi AH, Korkmaz HAA, Baki G. IL-17A, MCP-1, CCR-2, and ABCA1 polymorphisms in children with non-alcoholic fatty liver disease. Jornal de pediatria. 2019;95(3):350–357. doi: 10.1016/j.jped.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 64.Kurbatova IV, Topchieva LV, Dudanova OP. Caspase 3, 6, 8, and 9 gene expression in peripheral blood leukocytes and plasma concentrations of IL-6 and TNF-α in carriers of different polymorphic marker -174G>C genotypes of IL-6 gene associated with the risk of nonalcoholic steatohepatitis. Bulletin of experimental biology and medicine. 2017;162(3):370–374. doi: 10.1007/s10517-017-3618-0. [DOI] [PubMed] [Google Scholar]
- 65.Nelson JE, Handa P, Aouizerat B, Wilson L, Vemulakonda LA, Yeh MM, Kowdley KV, NASH Clinical Research Network. Increased parenchymal damage and steatohepatitis in Caucasian non-alcoholic fatty liver disease patients with common IL1B and IL6 polymorphisms. Alimentary pharmacology and therapeutics. 2016;44(11-12):1253–1264. doi: 10.1111/apt.13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bhatt SP, Guleria R, Vikram NK, Vivekanandhan S, Singh Y, Gupta AK. Association of inflammatory genes in obstructive sleep apnea and non alcoholic fatty liver disease in Asian Indians residing in North India. PLoS One. 2018;13(7):e0199599. doi: 10.1371/journal.pone.0199599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Metwally M, Bayoumi A, Romero-Gomez M, Thabet K, John M, Adams LA, Huo X, Aller R, García-Monzón C, Arias-Loste MT, Bugianesi E, Miele L, Gallego-Durán R, Fischer J, Berg T, Liddle C, Qiao L, George J, Eslam M. A polymorphism in the Irisin-encoding gene (FNDC5) associates with hepatic steatosis by differential miRNA binding to the 3'UTR. Journal of hepatology. 2019;70(3):494–500. doi: 10.1016/j.jhep.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 68.Musso G, Saba F, Cassader M, Paschetta E, Michieli FD, Pinach S, Framarin L, Berrutti M, Leone N, Parente R, Ayoubi Khajekini MT, Zarovska A, Gambino R. Angiotensin II Type 1 Receptor rs5186 Gene Variant Predicts Incident NAFLD and Associated Hypertension: Role of Dietary Fat-Induced Pro-Inflammatory Cell Activation. The American journal of gastroenterology. 2019;114(4):607–619. doi: 10.14309/ajg.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 69.Saremi L, Lotfıpanah S, Mohammadi M, Hosseinzadeh H, Hosseini-Khah Z, Johari B, Saltanatpour Z. Association between PPARGC1A single nucleotide polymorphisms and increased risk of nonalcoholic fatty liver disease among Iranian patients with type 2 diabetes mellitus. Turkish journal of medical sciences. 2019;49(4):1089–1094. doi: 10.3906/sag-1808-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Habibzadeh P, Honarvar B, Silawi M, Bahramjahan Sh, Kazemi A, Faghihi MA, Lankarani K. Association between rs2303861 polymorphism in CD82 gene and non-alcoholic fatty liver disease: a preliminary case-control study. Croatian medical journal. 2019;60(4):361–368. doi: 10.3325/cmj.2019.60.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rezapour S, Khosroshahi SA, Farajnia H, Mohseni F, Khoshbaten M, Farajnia S. Association of 45-bp ins/del polymorphism of uncoupling protein 2 (UCP2) and susceptibility to nonalcoholic fatty liver and type 2 diabetes mellitus in North-west of Iran. BMC research notes. 2021;14(1):169. doi: 10.1186/s13104-021-05586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kurbatova IV, Topchieva LV, Dudanova OP. Gene TNF Polymorphism -308G>A (rs1800629) and Its Relationship with the Efficiency of Ursodeoxycholic Acid Therapy in Patients with Nonalcoholic Stetohepatitis. Bulletin of experimental biology and medicine. 2017;164(2):181–185. doi: 10.1007/s10517-017-3953-1. [DOI] [PubMed] [Google Scholar]
- 73.Daijo K, Nakahara T, Inagaki Y, Nanba M, Nishida Y, Uchikawa S, Kodama K, Oya K, Morio K, Fujino H, Ono A, Murakami E, Yamauchi M, Kawaoka T, Miki D, Tsuge M, Hiramatsu A, Hayes CN, Imamura M, Aikata H, Ochi H, Chayama K. Risk factors for histological progression of non-alcoholic steatohepatitis analyzed from repeated biopsy cases. Journal of gastroenterology and hepatology. 2020;35(8):1412–1419. doi: 10.1111/jgh.14968. [DOI] [PubMed] [Google Scholar]
- 74.Hasan EM, Abd Al Aziz RA, Sabry D, Darweesh SK, Badary HA, Elsharkawy A, Abouelkhair MM, Yosry A. Genetic Variants in nicotinamide-N-methyltransferase (NNMT) gene are related to the stage of non-alcoholic fatty liver disease diagnosed by controlled attenuation parameter (CAP)-fibroscan. Journal of gastrointestinal and liver diseases. 2018;27(3):265–272. doi: 10.15403/jgld.2014.1121.273.wsh. [DOI] [PubMed] [Google Scholar]
- 75.Lutz SZ, Peter A, Machicao F, Lamprinou A, Machann J, Schick F, Königsrainer I, Königsrainer A, Fritsche A, Staiger H, Häring H-U, Stefan N, Kantartzis K. Genetic Variation in the 11β-hydroxysteroid-dehydrogenase 1 Gene Determines NAFLD and Visceral Obesity. The Journal of clinical endocrinology and metabolism. 2016;101(12):4743–4751. doi: 10.1210/jc.2016-2498. [DOI] [PubMed] [Google Scholar]
- 76.Nakajima S, Tanaka H, Sawada K, Hayashi H, Hasebe T, Abe M, Hasebe C, Fujiya M, Okumura T. Polymorphism of receptor-type tyrosine-protein phosphatase delta gene in the development of non-alcoholic fatty liver disease. Journal of gastroenterology and hepatology. 2018;33(1):283–290. doi: 10.1111/jgh.13820. [DOI] [PubMed] [Google Scholar]
- 77.Bhatt SP, Misra A, Pandey RM. rs7903146 (C/T) polymorphism of Transcription factor 7 like 2 (TCF7L-2) gene is independently associated with non-alcoholic fatty liver disease in Asian Indians. Diabetes and metabolic syndrome. 2020;14(3):175–180. doi: 10.1016/j.dsx.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 78.Anstee QM, Darlay R, Cockell S, Meroni M, Govaere O, Tiniakos D D Burt A, Bedossa P, Palmer J, LinLiu Y P, Aithal G, Allison M, Yki-Järvinen H, Vacca M, Dufour J-F, Invernizzi P, Prati D. Genome-wide association study of non-alcoholic fatty liver and steatohepatitis in a histologically characterised cohort. Journal of hepatol. 2020;73(3):505–515. doi: 10.1016/j.jhep.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 79.Yuan L, Terrrault NA. PNPLA3 and nonalcoholic fatty liver disease: towards personalized medicine for fatty liver. Hepatobiliary surgery and nutrition. 2020;9(3):353–356. doi: 10.21037/hbsn.2019.10.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu R, Tao A, Zhang S, Deng Y, Chen G. Association between patatin-like phospholipase domain containing 3 gene (PNPLA3) polymorphisms and nonalcoholic fatty liver disease: a HuGE review and meta-analysis. Scientific reports. 5:9284. doi: 10.1038/srep09284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bruschi FV, Tardelli M, Claudel T, Trauner M. PNPLA3 expression and its impact on the liver: current perspectives. Hepatic medicine. 2017;9:55–66. doi: 10.2147/HMER.S125718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pingitore P, Romeo S. The role of PNPLA3 in health and disease. Biochimica et biophysica acta. Molecular and cell biology of lipids. 2019;1864(6):900–906. doi: 10.1016/j.bbalip.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 83.Hotta K, Yoneda M, Hyogo H, Ochi H, Mizusawa S, Ueno T, Chayama K, Nakajima A, Nakao K, Sekine A. Association of the rs738409 polymorphism in PNPLA3 with liver damage and the development of nonalcoholic fatty liver disease. BMC medical genetics. 2010;11:172. doi: 10.1186/1471-2350-11-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Salari N, Darvishi N, Mansouri K, Ghasemi H, Hosseinian-Far M, Darvishi F, Mohammadi M. Association between PNPLA3 rs738409 polymorphism and nonalcoholic fatty liver disease: a systematic review and meta-analysis. BMC endocrine disorders. 2021;21(1):125. doi: 10.1186/s12902-021-00789-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thabet K, Asimakopoulos A, Shojaei M, Romero-Gomez M, Mangia A, Irving W, Berg T, Dore GJ, Gronbæk H, Sheridan D, Abate ML, Bugianesi E, Weltman M, Mollison L, Cheng W, Riordan S, Fischer J, Spengler U, Nattermann J, Wahid A, Rojas A, White R, Douglas MW, McLeod D, Powell E, Liddle C, Poorten DV, George G, Eslam M. MBOAT7 rs641738 increases risk of liver inflammation and transition to fibrosis in chronic hepatitis C. Nature communications. 2016;7:12757. doi: 10.1038/ncomms12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tanaka Y, Shimanaka Y, Caddeo A, Kubo T, Mao Y, Kubota T, Kubota N, Yamauchi T, Margherita Mancina R, Baselli G, Luukkonen P, Pihlajamäki J, Yki-Järvinen H, Valenti L, Arai H, Romeo S, Kono N. LPIAT1/MBOAT7 depletion increases triglyceride synthesis fueled by high phosphatidylinositol turnover. Gut. 2021;70(1):180–193. doi: 10.1136/gutjnl-2020-320646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gluchowski NL, Gabriel KR, Chitraju C, Bronson RT, Mejhert N, Boland S, Wang K, Weng Lai Z, Farese Jr RV, Walther TC. Hepatocyte Deletion of Triglyceride-Synthesis Enzyme Acyl CoA: Diacylglycerol Acyltransferase 2 Reduces Steatosis Without Increasing Inflammation or Fibrosis in Mice. Hepatology. 2019;70(6):1972–1985. doi: 10.1002/hep.30765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Buch S, Stickel F, Trépo E, Way M, Herrmann A, Dieter Nischalke H, Brosch M, Rosendahl J, Berg T, Ridinger M, Rietschel M, McQuillin A, Frank J, Kiefer F, Schreiber S, Lieb W, Soyka M, Semmo N, Aigner E, Datz C, Schmelz R, Brückner S, Zeissig S, Stephan A-M, Wodarz N, Devière J, Clumeck N, Sarrazin C, Lammert F, Gustot T, Deltenre P, Völzke H, Lerch MM, Mayerle J, Eyer F, Schafmayer C, Cichon S, M Nöthen M, Nothnagel M, Ellinghaus D, Huse K, Franke A, Zopf S, Hellerbrand C, Moreno C, Franchimont D, Morgan MY, Hampe J. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nature genetics. 2015;47(12):1443–1448. doi: 10.1038/ng.3417. [DOI] [PubMed] [Google Scholar]
- 89.Liu Z, Que S, Zhou L, Zheng S, Romeo S, Mardinoglu A, Valenti L. The effect of the TM6SF2 E167K variant on liver steatosis and fibrosis in patients with chronic hepatitis C: a meta-analysis. Scientific reports. 2017;7(1):9273. doi: 10.1038/s41598-017-09548-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu S, Murakami E, Nakahara T, Ohya K, Teraoka Y, Makokha GN, Uchida T, Morio K, Fujino H, Ono A, Yamauchi M, Kawaoka T, Miki D, Tsuge M, Hiramatsu A, Abe-Chayama H, Hayes NC, Imamura M, Aikata H, Chayama K. In vitro analysis of hepatic stellate cell activation influenced by transmembrane 6 superfamily 2 polymorphism. Molecular medicine reports. 2021;23(1):16. doi: 10.3892/mmr.2020.11654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang RN, Shen F, Pan Q, Cao HX, Chen GY, Fan JG. PPARGC1A rs8192678 G>A polymorphism affects the severity of hepatic histological features and nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease. World journal of gastroenterology. 2021;27(25):3863–3876. doi: 10.3748/wjg.v27.i25.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liang H, Ward WF. PGC-1alpha: a key regulator of energy metabolism. Advances in physiology education. 2006;30(4):145–151. doi: 10.1152/advan.00052.2006. [DOI] [PubMed] [Google Scholar]
- 93.Giles DA, Moreno-Fernandez ME, Divanovic S. IL-17 Axis Driven Inflammation in Non-Alcoholic Fatty Liver Disease Progression. Current drug targets. 2015;16(12):1315–1323. doi: 10.2174/1389450116666150531153627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lang X, Liu W, Hou Y, Zhao W, Yang X, Chen L, Yan Q, Cheng W. IL-17A polymorphism (rs2275913) and levels are associated with preeclampsia pathogenesis in Chinese patients. BMC medical genomics. 2021;14(1):5. doi: 10.1186/s12920-020-00840-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tang Y, Bian Z, Zhao L, Liu Y, Liang S, Wang Q, Han X, Peng Y, Chen X, Shen L, Qiu D, Li Z, Ma X. Interleukin-17 exacerbates hepatic steatosis and inflammation in non-alcoholic fatty liver disease. Clinical and experimental immunology. 2011;166(2):281–290. doi: 10.1111/j.1365-2249.2011.04471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. The American journal of gastroenterology. 2008;103(6):1372–1379. doi: 10.1111/j.1572-0241.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- 97.Fang M, Huang Y, Zhang Y, Ning Z, Zhu L, Li X. Interleukin-6 -572C/G polymorphism is associated with serum interleukin-6 levels and risk of idiopathic pulmonary arterial hypertension. Journal of the American society of hypertension. 2017;11(3):171–177. doi: 10.1016/j.jash.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 98.Bittar MN, Carey JA, Barnard J, Fildes JE, Pravica V, Yonan N, Hutchinson IV. Interleukin 6 G-174C polymorphism influences outcome following coronary revascularization surgery. The heart surgery forum. 2005;8(3):E140–E145. doi: 10.1532/HSF98.20041120. [DOI] [PubMed] [Google Scholar]
- 99.Mirea AM, Tack CJ, Chavakis T, Joosten LAB, Toonen EJM. IL-1 family cytokine pathways underlying NAFLD: towards new treatment strategies. Trends in molecular medicine. 2018;24(5):458–471. doi: 10.1016/j.molmed.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kakino S, Ohki T, Nakayama H, Yuan X, Otabe S, Hashinaga T, Wada N, Kurita Y, Tanaka K, Hara K, Soejima E, Tajiri Y, Yamada K. Pivotal role of TNF-α in the development and progression of nonalcoholic fatty liver disease in a murine model. Hormone and metabolic research. 2018;50(1):80–87. doi: 10.1055/s-0043-118666. [DOI] [PubMed] [Google Scholar]
- 101.Seo YY, Cho YK, Bae JC, Hae Seo M, Park SE, Rhee EJ, Park CY, Oh KW, Park SW, Lee WY. Tumor necrosis factor-α as a predictor for the development of nonalcoholic fatty liver disease: A 4-year follow-up study. Endocrinology and metabolism (Seoul, Korea) 2013;28(1):41–45. doi: 10.3803/EnM.2013.28.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cheng Y, An B, Jiang M, Xin Y, Xuan S. Association of tumor necrosis factor-alpha polymorphisms and risk of coronary artery disease in patients with non-alcoholic fatty liver disease. Hepatitis monthly. 2015;15(3):e26818. doi: 10.5812/hepatmon.26818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Potoupni V, Georgiadou M, Chatzigriva E, Polychronidou G, Markou E, Zapantis Gakis C, Filimidou I, Karagianni M, Anastasilakis D, Evripidou K, Ftergioti A, Togkaridou M, Tsaftaridis N, Apostolopoulos A, Polyzos SA. Circulating tumor necrosis factor-α levels in non-alcoholic fatty liver disease: A systematic review and a meta-analysis. Journal of gastroenterology and hepatology. 2021;10:15631. doi: 10.1111/jgh.15631. [DOI] [PubMed] [Google Scholar]
- 104.Canivet CM, Bonnafous S, Rousseau D, Leclere PS, Lacas-Gervais S, Patouraux S, Sans A, Luci C, Bailly-Maitre B, Iannelli A, Tran A, Anty R, Gual P. Hepatic FNDC5 is a potential local protective factor against non-alcoholic fatty liver. Biochimica et biophysica acta. Molecular basis of disease. 2020;1866(5):165705. doi: 10.1016/j.bbadis.2020.165705. [DOI] [PubMed] [Google Scholar]
- 105.Karimov DD, Erdman VV, Nasibullin TR, Tuktarova IA, Somova RSH, Timasheva YR, Mustafine OE. Alu insertion-deletion polymorphism of COL13A1 and LAMA2 genes: The analysis of association with longevity. Genetika. 2016;52(10):1185–1193. [PubMed] [Google Scholar]
- 106.Sjogren MH. Transaminase levels and vigorous exercise. Gastroenterology and hepatology. 2007;3(12):913–914. [PMC free article] [PubMed] [Google Scholar]
- 107.Eshraghian A, Iravani S, Azimzadeh P. The association between angiotensin II type 1 receptor gene A1166C polymorphism and non-alcoholic fatty liver disease and its severity. Middle East journal of digestive diseases. 2018;10(2):96–104. doi: 10.15171/mejdd.2018.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.de Gracia Hahn D, Duret A, Mann JP. An AGTR1 variant worsens nonalcoholic fatty liver disease and the metabolic syndrome. The American journal of gastroenterology. 2019;114(4):556–559. doi: 10.14309/ajg.0000000000000193. [DOI] [PubMed] [Google Scholar]
- 109.Hou G, Jin Y, Liu M, Wang C, Song G. UCP2-866G/A polymorphism is associated with prediabetes and type 2 diabetes. Archives of medical research. 2020;51(6):556–563. doi: 10.1016/j.arcmed.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 110.Cortez-Pinto H, Machado MV. Uncoupling proteins and non-alcoholic fatty liver disease. Journal of hepatology. 2009;50(5):857–860. doi: 10.1016/j.jhep.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 111.Lyssenko V, Lupi R, Marchetti P, Guerra SD, Orho-Melander M, Almgren P, Sjögren M, Ling C, Eriksson KF, Lethagen A-L, Mancarella R, Berglund G, Tuomi T, Nilsson P, Prato SD, Groop L. Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. The Journal of clinical investigation. 2007;117(8):2155–2163. doi: 10.1172/JCI30706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Musso G, Gambino R, Pacini G, Pagano G, Durazzo M, Cassader M. Transcription factor 7-like 2 polymorphism modulates glucose and lipid homeostasis, adipokine profile, and hepatocyte apoptosis in NASH. Hepatology. 2009;49(2):426–435. doi: 10.1002/hep.22659. [DOI] [PubMed] [Google Scholar]
- 113.Kaminska D, Kuulasmaa T, Venesmaa S, Käkelä P, Vaittinen M, Pulkkinen L, Pääkkönen M, Gylling H, Laakso M, Pihlajamäki J. Adipose tissue TCF7L2 splicing is regulated by weight loss and associates with glucose and fatty acid metabolism. Diabetes. 2012;61(11):2807–2813. doi: 10.2337/db12-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Smith GI, Shankaran M, Yoshino M, Schweitzer GG, Chondronikola M, Beals JW, Okunade AL, Patterson BW, Nyangau E, Field T, Sirlin CB, Talukdar S, Hellerstein MK, Klein S. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. The Journal of clinical investigation. 2020;130(3):1453–1460. doi: 10.1172/JCI134165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kitamoto T, Kitamoto A, Yoneda M, Hyogo H, Ochi H, Nakamura T, Teranishi H, Mizusawa S, Ueno T, Chayama K, Nakajima A, Nakao K, Sekine A, Hotta K. Genome-wide scan revealed that polymorphisms in the PNPLA3, SAMM50, and PARVB genes are associated with development and progression of nonalcoholic fatty liver disease in Japan. Human genetics. 2013;132(7):783–792. doi: 10.1007/s00439-013-1294-3. [DOI] [PubMed] [Google Scholar]
- 116.Chen L, Lin Z, Jiang M, Lu L, Zhang H, Xin Y, Jiang X, Xuan S. Genetic variants in the SAMM50 gene create susceptibility to nonalcoholic fatty liver disease in a Chinese Han population. Hepatitis monthly. 2015;15(10):e31076. doi: 10.5812/hepatmon.31076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rector RS, Thyfault JP, Uptergrove GM, Morris EM, Naples SP, Borengasser SJ, Mikus CR, Laye MJ, Laughlin MH, Booth FW, Ibdah JA. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. Journal of hepatology. 2010;52(5):727–736. doi: 10.1016/j.jhep.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, ContosMJ , Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120(5):1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 119.Matsuura K, Sawai H, Ikeo K, Ogawa S, Iio E, Isogawa M, Shimada N, Komori A, Toyoda H, Kumada T. Genome-wide association study identifies TLL1 variant associated with development of hepatocellular carcinoma after eradication of hepatitis C virus infection. Gastroenterology. 2017;152(6):1383–1394. doi: 10.1053/j.gastro.2017.01.041. [DOI] [PubMed] [Google Scholar]
- 120.Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best practice and research. Clinical gastroenterology. 2011;25(2):195–206. doi: 10.1016/j.bpg.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bayoumi A, Jalil I, Metwally M, Adams LA, Aller R, García-Monzón C, Arias-Loste MT, Miele L, Petta S, Craxì A, Gallego-Durán R. Genetic variation in the TLL1 gene is not associated with fibrosis in patients with metabolic associated fatty liver disease. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0243590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Komatsu M, Kanda T, Urai H, Kurokochi A, Kitahama R, Shigaki S, Ono T, Yukioka H, Hasegawa K, Tokuyama H, Kawabe H, Wakino S, Itoh H. NNMT activation can contribute to the development of fatty liver disease by modulating the NAD+ metabolism. Scientific reports. 2018;8(1):8637. doi: 10.1038/s41598-018-26882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zou X, Ramachandran P, Kendall TJ, Pellicoro A, Dora E, Aucott RL, Manwani K, Yung Man T, Chapman KE. 11Beta-hydroxysteroid dehydrogenase-1 deficiency or inhibition enhances hepatic myofibroblast activation in murine liver fibrosis. Hepatology. 2018;67(6):2167–2181. doi: 10.1002/hep.29734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim M, Morales LD, Jang IS, Cho YY, Kim DJ. Protein tyrosine phosphatases as potential regulators of STAT3 signaling. International journal of molecular sciences. 2018;19(9):2708. doi: 10.3390/ijms19092708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chakraborty D, Šumová B, Mallano T, Chen C-W, Distler A, Bergmann C, Ludolph I, Horch RE, Gelse K, Ramming A, Distler O, Schett G, Šenolt L, Distler JHW. Activation of STAT3 integrates common profibrotic pathways to promote fibroblast activation and tissue fibrosis. Nature communications. 2017;8(1):1130. doi: 10.1038/s41467-017-01236-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim MC, Lee JI, Kim JH, Kim HJ, Cho YK, Jeon WK, Kim BI, Sohn W. Serum zinc level and hepatic fibrosis in patients with nonalcoholic fatty liver disease. PLoS One. 2020;15(10):e0240195. doi: 10.1371/journal.pone.0240195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lisboa QC, Nardelli MJ, Pereira PA, Miranda DM, s Ribeiro SN, Neves Costa RS, Azevedo Versiani C, Teixeira Vidigal PV. PNPLA3 and TM6SF2 polymorphisms in Brazilian patients with nonalcoholic fatty liver disease. World journal of hepatology. 2020;12(10):792–806. doi: 10.4254/wjh.v12.i10.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tam V, Patel N, Turcotte M, Bossé Y, Paré G, Meyre D. Benefits and limitations of genome-wide association studies. Nature reviews. Genetics. 2019;20(8):467–484. doi: 10.1038/s41576-019-0127-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data presented in the manuscript can be accessed on PubMed.