Abstract
Study Objective
The aim of this study was to assess sleep behavior, sleep problems and mental health in childhood as possible candidate precursors for the development of delayed sleep phase (DSP) during adolescence.
Methods
A longitudinal cohort study of 2200 children at age 7–9 (T1), 11–13 (T2), and 16–19 (T3) years. DSP was assessed at T3, and mental health problems by the Strength and Difficulties Questionnaire and time in bed and sleep problems at T1 and T2. Logistic regression analyses were used to examine associations between sleep and mental health at T1 and T2, and subsequent DSP at T3. Estimated marginal means were computed to compare mental health at T1 and T2 in adolescents with and without DSP.
Results
Sleeping less than 9 hours per night at age 11–13 was significantly associated with DSP at 16–19 years (adjusted odds ratio = 3.37). Sleep problems at 11–13 years of age were more frequent among those who developed DSP compared to children who did not develop DSP (20% vs. 12%) but the results did not remain significant when controlling for early mental health problems. Sleep problems and mental health at 7–9 years of age was not related to later DSP. In the crude analyses, all Strengths and Difficulties Questionnaire (SDQ) subscales at 11–13 years was significantly associated with later DSP, but in the fully adjusted analysis, only the SDQ total score and hyperactivity subscale remained statistically significant.
Conclusion
Children with DSP in adolescence possess identifiable risk indicators in childhood.
Keywords: DSP, SDQ mental health, longitudinal, sleep
Statement of Significance
Delayed sleep phase (DSP) is associated with health and daily-life functioning in adolescence, but there is a sparsity of longitudinal studies investigating the childhood precursors of DSP in adolescence. This study extends on previous studies by examining mental health and sleep at two time points in school-age children to assess precursors of DSP. While mental health and sleep problems at 7–9 years of age were not significant predictors of later DSP, mental health problems (as indicated by the Strengths and Difficulties Questionnaire [SDQ] total score) and sleep problems at 11–13 years of age were both significant predictors of later DSP. More longitudinal studies are needed to replicate the findings in different samples.
Introduction
A delay in sleep phase usually occurs during puberty [1, 2]. For some adolescents, the circadian delay will be quite pronounced, difficult to reverse, and have negative functional impacts and thus fulfill the diagnostic criteria for Delayed Sleep-Wake Phase Disorder (DSWPD) [3]. DSWPD is a clinical sleep disorder, whereas, delayed sleep phase (DSP) comprises a delay in sleep phase without meeting diagnostic criteria. Still, the functional impact of DSP is often significant for the individual, families and the society with reduced academic performance [4] and school absenteeism as consequences [5–7]. The etiology of DSP is not fully understood, but is most likely a combination of biological, behavioral, psychological, and social factors [6, 8]. Adolescents with DSP have a higher rate of mental health problems than their peers [9, 10] in terms of depressive symptoms, anxiety symptoms [1, 4, 9] as well as co-occurring ADHD-symptoms [9, 11]. Furthermore, a meta-analysis showed that late bedtime, which can be considered as a hallmark of DSP was associated with negative family environment, video gaming, phone, internet and computer use, as well as evening light exposure [12].
The vast majority of previous studies on factors associated with DSP are based on cross-sectional designs. This precludes conclusions about the temporal association between mental health and DSP. Accordingly, mental health problems may both be a cause and a consequence of DSP. Consistently, a Japanese study including 12- to 18-year-old adolescents, with five yearly assessment points, found a reciprocal association between bedtime and mental health [13]. This may also hold true for the association between DSP and mental health. Indeed, in a recent review of the etiology of DSP, prepubescent longitudinal studies were called for to in order to investigate whether those who develop DSP in adolescence are characterized by specific risk indicators in childhood [14], which could be targeted through preventive efforts.
Based on the above considerations, the aim of the present study was to explore sleep problems, time in bed (TIB), and mental health in childhood among adolescents who have DSP in adolescence in comparison to peers without DSP.
Materials and Methods
Participants
Data stems from the first, second and fourth wave of the Bergen Child Study (BCS), carried out in the autumn of 2002, spring of 2006 and winter/spring 2012, respectively. The BCS represents a longitudinal total population study of children in all public and private schools in the city of Bergen, Norway. The last wave of the BCS is also called the youth@hordaland-survey, and included all adolescents in Hordaland County, in which the municipality of Bergen is the largest metropolitan area. The protocol and the population of the BCS have previously been described in detail elsewhere [15]. In short, in the first wave, the target population consisted of 9430 primary school children aged 7 to 9 years, for whom 7007 (74.3%) parents provided informed consent to participate. The second wave was performed 4 years later, and 5683 children, at that time aged 11 to 13 years, participated. The last wave was conducted 6 years later, in which 10254 of the 19439 invited adolescents participated (participation rate of 53%). The longitudinal sample in the current study with complete data on all three waves included a total of 2200 individuals of the original study population, making the participation rate 31.4% of the 7007 children in the first wave, and 38.7% of the children who completed the second wave. For ease of reading, the three waves used in the current study will be labeled T1, T2, and T3, respectively.
Adolescents who completed all three assessments (responders) were more likely to be female, have parents with higher education, and come from a family financially better off than individuals who did not complete all three assessments (nonresponders) (all ps < .001;). There were, however, no significant differences on any of the sleep measures between those who only participated at T3 and the longitudinal sample [16].
Procedure
The questionnaire at T1 was completed by the parents, while the two last waves were completed by the children/adolescents themselves. The data collection at T1 and T2 were paper-based, while T3 used an Internet-based questionnaire. At T3 the adolescents were informed about the study through their official school e-mail, and one school hour (approximately 45 minutes during regular school hours) was allocated for them to complete the electronic questionnaire. A teacher was present to organize the data collection and to ensure confidentiality.
Measures
DSP-T3
The following questions were used to assess DSP: “At what time do you usually go to bed,” “How much time does it take before you fall asleep (hours and minutes),” “When do you usually rise in the morning,” “How many nights per week do you have difficulties falling asleep (0–7),” “How many nights per week do you have problems with nightly awakenings (0–7),” “How often do you oversleep (‘never’, ‘seldom’, ‘sometimes’, ‘mostly’, ‘always’).” The participants provided sleep data separately for weekdays and weekends. No information regarding the time-frame of these symptoms were collected. To establish a proxy for assessing DSP (as close as possible given the available sleep items) in line with the International Classification of Sleep Disorders-3 [3], we employed the following criteria, as specified in Johnson et al. [17] published in Pediatrics (1) minimum 1-hour shift in sleep-onset AND wake times from the weekdays to the weekend, (2) complaint of frequent (≥3 days per week) difficulty falling asleep, (3) report of little or no (≤1 day per week) difficulty maintaining sleep, and (4) frequent difficulty awakening (oversleeping “sometimes” or more often). This operationalization has been used in recent publications from the youth@hordaland-survey [5, 9, 18].
Sleep behavior (T2 and T3)
At T2, the parents reported TIB, operationalized as the difference between the usual bedtime and rise time. No data was available on sleep onset latency and wake after sleep onset for purposes of the present study, TIB was analyzed categorically using the following categories (in hours:minutes): <9:00, 9:00–9:59, 10:00–10:30, and >10:30. Nine hours in bed was chosen as the cutoff for short sleep duration, as the National Sleep Foundation recommends 9–11 hours for school-aged children from 6 to 13 years, and 8–10 hours of sleep per night for teenagers [19].
Difficulties with initiating and/or maintaining sleep—T1–T3
Sleep problems at all three time points were assessed by parent reports with one question encompassing difficulties initiating and/or maintaining sleep, rated on a three-point Likert scale (“not agree”, “partly agree”, and “agree”). To avoid empty/small data cells, which may arise especially in longitudinal samples, dichotomous variables were used such that responding either “agree” or “partly agree” was defined as constituting a sleep problem. This operationalization has previously been applied in the BCS [20]. No data on the severity or duration of difficulties initiating or maintaining sleep (DIMS) were collected.
Mental health problems (T1–T3)
The Strengths and Difficulties Questionnaire (SDQ) is a brief mental health screening questionnaire for children between 4 and 16 years [21]. The SDQ has good psychometric properties [22] and is comprised of five subscales; Emotional Problems, Hyperactivity-inattention, Peer Problems and Prosocial Competence. The first four subscales constitute a composite problem score. Parent report is used in the present study.
Other variables
Gender and date of birth were identified through personal identity number in the Norwegian National Register. Exact age was estimated by calculating the interval of time between date of birth and date of participation. Socioeconomic status (SES) was assessed both by parental education and perceived family financial circumstances. Maternal and paternal education were reported separately with three response options; “primary school,” “secondary school,” and “college or university.” Perceived family financial circumstances (i.e. how well off the adolescent perceived their family to be) was assessed by asking the adolescents how their financial circumstances were compared to most others. Response alternatives were (1) “better financial circumstances,” (2) “approximately like most others,” and (3) “poorer financial circumstances. We have previously shown that ratings of family financial circumstance on this question correlate significantly (r = 0.586, p < .001) with data on taxable monetary income of their parents, which were available for a subsample of 642 participants [23].
Statistics
All analyses were performed using the SPSS statistical software package, version 24 (SPSS Inc., Chicago, IL). Logistic regression analyses were used to examine associations between the sleep and mental health variables at waves 1 and 2, and subsequent DSP at wave 3. Estimated marginal means (EMM) were computed to compare SDQ scores at wave 1 and 2 in adolescents with and without DSP. The analyses were done separately for each of the mental health subscales. Both crude/unadjusted and adjusted analyses were conducted. Adjustment variables included age, gender, socioeconomic status, and previous sleep and mental health problems (depending on the outcome). For the EMM analysis, we controlled for multiple comparisons using the standard false discovery rate (FDR) method with a false-positive rate of 5% (q = 0.05, as outlined by Benjamini and Hochberg, [24]). To assess the clinical significance of mental health problems, Fisher’s Exact test was used to examine if the proportion of high scorers on the SDQ (above 90th percentile on the SDQ total score) at wave 2 differed in adolescents with and without DSP. Missing data were handled using listwise deletion.
Ethics
Each of the study waves were approved by the Regional Committee for Medical and Health Research Ethics in Western Norway (2015/ 800). For the first two waves, written informed consent was obtained from all parents whose child was included in the present study. For T3, the adolescents’ parents were informed about the study, while the adolescents themselves consented to participate as Norwegian regulations state that individuals aged 16 years and older are required to provide consent themselves. No payment was given for participation.
Results
Sample characteristics
The longitudinal sample included in the present study comprised 2200 individuals, of which 57% were girls. The educational level of the parents was high compared with the national average [25]. Specifically, 61% and 57% of the mothers and fathers, respectively, had an educational level beyond high school. The prevalence of DSP at T3 was 3.9%, and there were no significant gender differences.
History of sleep problems and TIB in adolescents with or without DSP
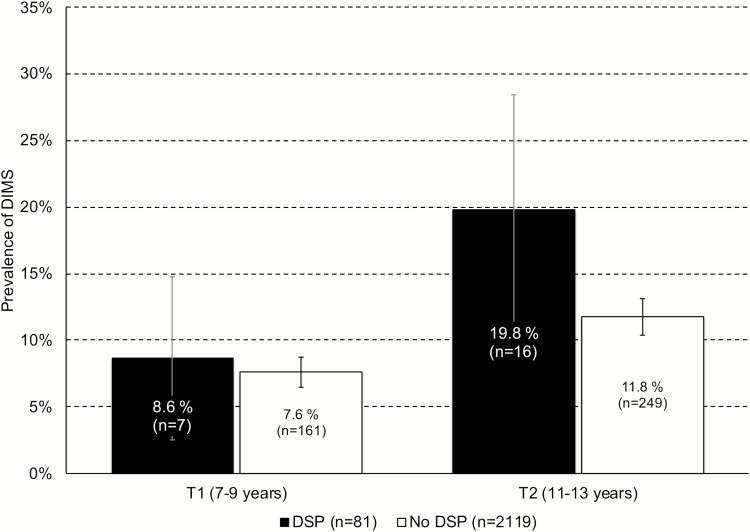
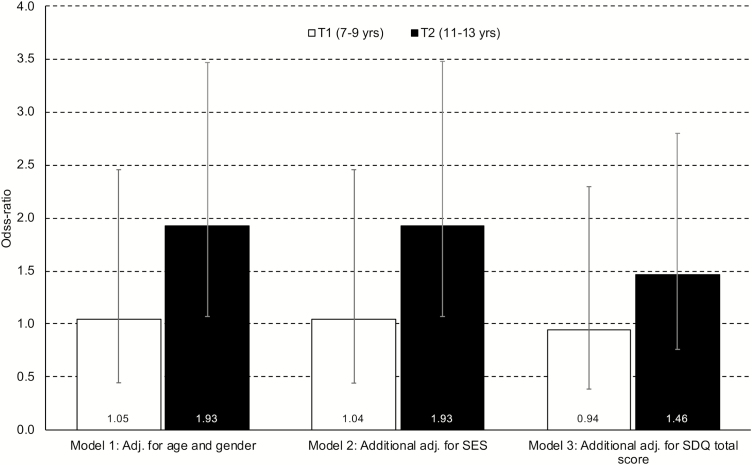
Figure 1 depicts the rate of DIMS at 7–9 and 11–13 years of age in adolescents with and without DSP at age 16–19. Children developing DSP during adolescence had a higher rate of sleep problems at 11–13 years of age compared to children not developing DSP (19.8% vs. 11.8%; p < .030), but not at 7–9 (8.6% and 7.6%, respectively; p < .728). As displayed in Figure 2, adjusting for age and gender only, the odds for DSP in adolescence was significantly higher for children with than without sleep problems at 11–13 (odds ratio [OR] = 1.93 [95% confidence interval [CI]: 1.07–3.48]). While further adjustment for SES did not attenuate this association, additional adjustment for early mental health problems reduced the association to a nonsignificant level (OR = 1.46; 95% CI: 0.76–2.80).
Figure 1.
History of DIMS at T1 (7–9 years) and T2 (11–13 years) in adolescents with and without delayed sleep phase at T3 (16–19 years). Error bars represent 95% confidence intervals.
Figure 2.
DIMS at T1 (7–9 years) and T2 (11–13 years) as risk factor for DSP at T3 (16–19 years). Bars represent odds-ratios and error bars represent 95% confidence intervals.
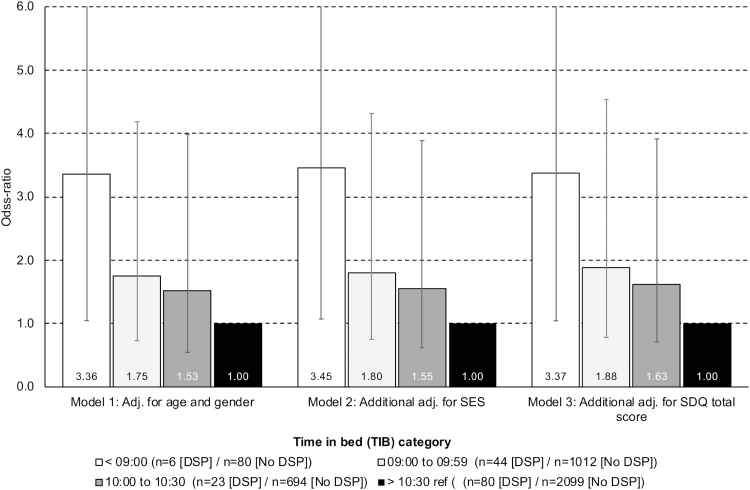
Children with short TIB (less than 9:00) at age 11–13 had significantly higher odds of later DSP (OR = 3.36; 95% CI: 1.05–10.75) compared to those sleeping more than 10:30. Additional adjustment for both SES and early mental health problems did not reduce the magnitude of this association, which remained significant in the fully adjusted analysis (OR = 3.37; 95% CI: 1.04–10.91). The other TIB categories (9:00–9:59 and 10:00–10:30) were not significantly associated with later DSP (see Figure 3 for details).
Figure 3.
Time in bed at T2 (11–13 years) as risk factor for DSP at T3 (16–19 years). Bars represent odds ratios and error bars represent 95% confidence intervals.
Mental health problems in childhood and the association with later DSP
Table 1 details the association between mental health problems at 7–9 and at 11–13 years and later DSP. While no differences in mental health problems between those with and without adolescent DSP were observed at 7–9 years of age, at age 11–13 all subscales of the SDQ were significantly higher (while prosocial subscale was significantly lower, indicating lower prosocial competence) among those with adolescent DSP. When adjusting for SES, the SDQ total score, and emotional and hyperactivity-inattention subscales were still significant, but after further adjustment of co-occurring sleep problems in childhood, only the SDQ total score and hyperactivity-inattention subscale remained significantly higher in the DSP group.
Table 1.
Emotional and behavioral problems (SDQ) at age 7–9 (T1) and 11–13 (T2) in adolescents with and without DSP at age 16–19 (T3)
| Model 1: Adjusted for age and gender | Model 2: Model 1 + adjustment for SES | Model 3: Model 2 + adjustment for DIMS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No DSP | DSP | No DSP | DSP | No DSP | DSP | ||||
| EMM (SE) | EMM (SE) | P-valuea | EMM (SE) | EMM (SE) | P-value | EMM (SE) | EMM (SE) | P-value | |
| T1: 7–9 years | |||||||||
| SDQ Total score | 4.86 (0.09) | 5.14 (0.46) | .579 | 4.69 (0.10) | 5.06 (0.48) | .519 | 4.69 (0.10) | 5.11 (0.46) | .474 |
| SDQ Emotional problems | 1.13 (0.03) | 1.35 (0.18) | .343 | 1.08 (0.04) | 1.29 (0.18) | .380 | 1.08 (0.04) | 1.31 (0.17) | .343 |
| SDQ Conduct problems | 0.77 (0.03) | 0.85 (0.13) | .564 | 0.73 (0.03) | 0.90 (0.13) | .343 | 0.73 (0.03) | 0.91 (0.13) | .343 |
| SDQ Hyperactivity | 2.27 (0.04) | 2.41 (0.21) | .579 | 2.20 (0.05) | 2.31 (0.22) | .630 | 2.20 (0.05) | 2.33 (0.22) | .595 |
| SDQ Peer problems | 0.70 (0.03) | 0.53 (0.14) | .343 | 0.69 (0.03) | 0.56 (0.15) | .503 | 0.69 (0.03) | 0.57 (0.15) | .519 |
| SDQ Prosocial | 8.60 (0.03) | 8.43 (0.16) | .409 | 8.60 (0.04) | 8.43 (0.17) | .433 | 8.60 (0.04) | 8.43 (0.17) | .429 |
| T2: 11–13 years | |||||||||
| SDQ Total score | 5.52 (0.10) | 7.90 (0.50) | <.001 | 5.27 (0.11) | 7.10 (0.52) | .001 | 5.28 (0.11) | 6.91 (0.50) | .006 |
| SDQ Emotional problems | 1.47 (0.04) | 2.07 (0.19) | .006 | 1.38 (0.04) | 1.86 (0.20) | .049 | 1.38 (0.04) | 1.79 (0.19) | .094 |
| SDQ Conduct problems | 0.89 (0.02) | 1.21 (0.13) | .049 | 0.86 (0.03) | 1.10 (0.13) | .176 | 0.86 (0.03) | 1.07 (0.13) | .252 |
| SDQ Hyperactivity | 2.22 (0.04) | 3.25 (0.22) | <.001 | 2.14 (0.05) | 2.90 (0.23) | .001 | 2.15 (0.05) | 2.84 (0.23) | .015 |
| SDQ Peer problems | 0.95 (0.03) | 1.37 (0.15) | .032 | 0.89 (0.03) | 1.24 (0.16) | .094 | 0.89 (0.03) | 1.21 (0.16) | .122 |
| SDQ Prosocial | 8.57 (0.03) | 8.17 (0.16) | .049 | 8.57 (0.04) | 8.22 (0.18) | .122 | 8.57 (0.04) | 8.24 (0.18) | .146 |
SE = standard error.
a P-values are corrected for multiple comparisons, based on FDR, as outlined by Benjamini and Hochberg (1995).
In order to examine the clinical significance of these findings, we compared the rate of high SDQ-scorers (above the 90th percentile on the SDQ total score) in adolescent with and without DSP. These findings showed that significantly more adolescents had an SDQ score in the clinical range compared to adolescents in the no-DSP (20% vs. 7.1%, respectively, p < .001).
Discussion
In this longitudinal study, children who presented with DSP in adolescence had more sleep problems and mental health problems than their peers at 11–13 years of age, but not at 7–9 years of age. In the crude analyses, all SDQ subscales at 11–13 years were significantly associated with later DSP, but in the fully adjusted analysis, only the SDQ total score and hyperactivity-inattention subscale remained statistically significant.
Sleep problems and short TIB were more frequent among children who later developed DSP relative to their peers at 11 and 13 years. This supports the notion of relative stability of sleep problems in general [16] and for sleep timing [26]. The general pattern suggested that there was no association between adolescence DSP and mental health and sleep in prepubescent children (7–9). However, when the children were in early adolescence (11–13) a significant association between the total SDQ scores and DSP emerged. At this age, a significant association between being in the clinical range of mental health problems and DSP was also found. The lack of an association with sleep problems at 7–9 may be due to a number of factors, which the present study cannot answer, either due to lower stability over time, but also qualitatively different processes taking place in late than in middle childhood, of which puberty would be one candidate [2].
The longitudinal association between some of the mental health problems at 11–13 and DSP is in accordance with findings from cross-sectional studies on adolescents in which both depressive and ADHD symptoms co-occur with DSP [9, 10]. The associations reported in the present study were independent of sleep problems in childhood for the total problems score and the hyperactivity-inattention subscale (but not for the other SDQ subscales). The mean SDQ total score for the two groups was both in the normal range based on British norms. However, when comparing the rate of high-scorers on the SDQ total score, the rate was significantly higher in the DSP group, suggesting that they also had more clinically significant mental health problems.
The indication that mental health problems, as indicated by the SDQ total score, were an independent risk factor of later DSP, suggest that also children without manifest sleep problems may be at risk of later DSP, and that preventive efforts should focus on a broad range of variables and include children with other risk indicators than sleep problems. This may also support a model in which psychological factors play a role in the development of the DSP [27]. The reciprocal association between sleep in general and mental health, support the notion that mental health may be an independent predictor as well as a consequence of sleep problems [13]. However, in terms of the specific SDQ subscales, only hyperactivity-inattention problems remained a significant predictor after adjusting for early sleep problems and multiple comparisons, and the results should thus be interpreted with caution.
Although stability of the sleep problem was evident, it is important to note that sleep problems increased from early to late adolescence, and while the risk of developing DSP was higher among those with early sleep problems, many of the adolescents with DSP did not have sleep problems at an earlier age. Sleep problems were no longer significant predictors of later DSP after adjusting for early mental health problems, emphasizing the importance of the interplay between mental health and sleep.
Taking a prevention perspective, it seems that 11–13 years of age and early puberty, may be an important intervention time to prevent the development of DSP. Both mental health problems and sleep problems are possible targets for such interventions. As we did not have sufficient data points, we were precluded from assessing the interaction between these problems at a closer level. Future studies should thus address this issue further.
There are several limitations of the present study that should be highlighted. Firstly, we did not measure DSP during the first two assessment points. Thus, while we accounted for the presence of sleep problems and TIB, we did not have a proxy for DSP at the T1 and T2. Secondly, our definition of DSP is based on questionnaire-based self-report, and consequently lacks clinical evaluation and measurement by actigraphy or sleep diary. Thus, we had no information regarding the desired bedtime or adolescents’ inability to fall asleep at the desired time which, according to the ICSD-3, is required in order to meet the criteria for a clinical diagnosis of DSWPD [3]. Thirdly, we chose to the TIB variables categorically as they represent clinically more meaningful entities, but this approach may have reduced the statistical power of the analysis.
Fourth, DIMS was assessed by parent-report using only a joint variable, precluding separate analysis of each construct separately. In addition, no measures of the severity and duration of the sleep problems were included. Although DIMS constitute the core nocturnal symptoms of insomnia disorder according to the DSM-5, the lack of a validated and psychometric sound measure of sleep problems limits the generalizability of the findings. A more extensive assessment battery of sleep problems would have been preferable. A final and important limitation is the small sample size and the attrition rate, which could impact the generalizability of the results. Some of the relevant groups had very small numbers, which is likely to have reduced the possibility to detect significant differences. The subscale scores may be especially vulnerable for lack of detection of significant differences given the small sample sizes and correction for multiple comparisons, while the composite score might be more robust.
A strength of the present study is the longitudinal design that allowed us to identify the temporal association between study variables. The inclusion of multiple informants, and the assessment of both mental health and sleep patterns as predictors are also noteworthy strengths of the present study. While the longitudinal sample is smaller than cross-sectional samples in this study, a previous study still found no differences in rate of sleep problems among those who completed all three waves compared to those who did not [16]. As school start times in Norway have been stable for years, it is not conceivable that this has acted as a confounder regarding the results.
Funding
Funding was provided by The Norwegian Directorate of Health Institution.
Conflict of interest statement. None declared.
Acknowledgments
We want to thank the Bergen Child Study group and most importantly we are grateful to all participants that made this study possible.
Contributor Information
Mari Hysing, The Regional Centre for Child and Youth Mental Health and Child Welfare, Uni Research Health, Bergen, Norway; Department of Psychosocial Science, University of Bergen, Bergen, Norway.
Allison G Harvey, Department of Psychology, University of California, Berkeley, CA.
Kjell Morten Stormark, The Regional Centre for Child and Youth Mental Health and Child Welfare, Uni Research Health, Bergen, Norway.
Ståle Pallesen, Department of Psychosocial Science, University of Bergen, Bergen, Norway; Norwegian Competence Center for Sleep Disorders, Haukeland University Hospital, Bergen, Norway.
Børge Sivertsen, Department of Health Promotion, Norwegian Institute of Public Health, Bergen, Norway; Department of Research & Innovation, Helse Fonna HF, Haugesund, Norway; Department of Mental Health, Norwegian University of Science and Technology, Trondheim, Norway.
References
- 1. Crowley SJ, et al. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007;8(6):602–612. [DOI] [PubMed] [Google Scholar]
- 2. Carskadon MA, et al. Association between puberty and delayed phase preference. Sleep. 1993;16(3):258–262. [DOI] [PubMed] [Google Scholar]
- 3. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darian, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 4. Saxvig IW, et al. Prevalence and correlates of delayed sleep phase in high school students. Sleep Med. 2012;13(2):193–199. [DOI] [PubMed] [Google Scholar]
- 5. Sivertsen B, et al. Delayed sleep phase syndrome in adolescents: prevalence and correlates in a large population based study. BMC Public Health. 2013;13:1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lovato N, et al. Delayed sleep phase disorder in an Australian school-based sample of adolescents. J Clin Sleep Med. 2013;9(9):939–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Danielsson K, et al. Delayed sleep phase disorder in a Swedish cohort of adolescents and young adults: Prevalence and associated factors. Chronobiol Int. 2016;33(10):1331–1339. [DOI] [PubMed] [Google Scholar]
- 8. Murray JM, et al. Prevalence of circadian misalignment and its association with depressive symptoms in delayed sleep phase disorder. Sleep. 2017;40(1). [DOI] [PubMed] [Google Scholar]
- 9. Sivertsen B, et al. Mental health problems in adolescents with delayed sleep phase: results from a large population-based study in Norway. J Sleep Res. 2015;24(1):11–18. [DOI] [PubMed] [Google Scholar]
- 10. Glozier N, et al. Delayed sleep onset in depressed young people. BMC Psychiatry. 2014;14:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bijlenga D, et al. Associations between sleep characteristics, seasonal depressive symptoms, lifestyle, and ADHD symptoms in adults. J Atten Disord. 2013;17(3):261–275. [DOI] [PubMed] [Google Scholar]
- 12. Bartel KA, et al. Protective and risk factors for adolescent sleep: a meta-analytic review. Sleep Med Rev. 2015;21:72–85. [DOI] [PubMed] [Google Scholar]
- 13. Tochigi M, et al. Annual longitudinal survey at up to five time points reveals reciprocal effects of bedtime delay and depression/anxiety in adolescents. Sleep Med. 2016;17:81–86. [DOI] [PubMed] [Google Scholar]
- 14. Micic G, et al. The etiology of delayed sleep phase disorder. Sleep Med Rev. 2016;27:29–38. [DOI] [PubMed] [Google Scholar]
- 15. Heiervang E, et al. Psychiatric disorders in Norwegian 8- to 10-year-olds: an epidemiological survey of prevalence, risk factors, and service use. J Am Acad Child Adolesc Psychiatry. 2007;46(4):438–447. [DOI] [PubMed] [Google Scholar]
- 16. Sivertsen B, et al. Trajectories of sleep problems from childhood to adolescence: a population-based longitudinal study from Norway. J Sleep Res. 2017;26(1):55–63. [DOI] [PubMed] [Google Scholar]
- 17. Johnson EO, et al. Epidemiology of DSM-IV insomnia in adolescence: lifetime prevalence, chronicity, and an emergent gender difference. Pediatrics. 2006;117(2):e247–e256. [DOI] [PubMed] [Google Scholar]
- 18. Sivertsen B, et al. Academic performance in adolescents with delayed sleep phase. Sleep Med. 2015;16(9):1084–1090. [DOI] [PubMed] [Google Scholar]
- 19. Hirshkowitz M, et al. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health. 2015;1(4):233–243. [DOI] [PubMed] [Google Scholar]
- 20. Sivertsen B, et al. Chronicity of sleep problems in children with chronic illness: a longitudinal population-based study. Child Adolesc Psychiatry Ment Health. 2009;3(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goodman R, et al. Using the Strengths and Difficulties Questionnaire (SDQ) to screen for child psychiatric disorders in a community sample. Br J Psychiatry. 2000; 177:534–539. [DOI] [PubMed] [Google Scholar]
- 22. Stone LL, et al. Psychometric properties of the parent and teacher versions of the strengths and difficulties questionnaire for 4- to 12-year-olds: a review. Clin Child Fam Psychol Rev. 2010;13(3):254–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bøe T, et al. Socioeconomic status and child mental health: the role of parental emotional well-being and parenting practices. J Abnorm Child Psychol. 2014;42(5):705–715. [DOI] [PubMed] [Google Scholar]
- 24. Benjamini Y, et al. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. Series B (Methodological). 1995;289–300. [Google Scholar]
- 25.Statistics Norway. Population’s level of education, 1 October 2013. 2014. https://www.ssb.no/en/utdanning/statistikker/utniv/aar/2014-06-19. Accessed April 21, 2016. [Google Scholar]
- 26. Kuula L, et al. Development of late circadian preference: sleep timing from childhood to late adolescence. J Pediatr. 2018;194:182–189.e1. [DOI] [PubMed] [Google Scholar]
- 27. Richardson CE, et al. Are cognitive “insomnia” processes involved in the development and maintenance of delayed sleep wake phase disorder?Sleep Med Rev. 2016;26:1–8. [DOI] [PubMed] [Google Scholar]