Abstract
Context
Patients with aggrecan (ACAN) deficiency present with dominantly inherited short stature, often with advanced skeletal maturation and premature growth cessation. There is a paucity of information on the effects of growth-promoting interventions.
Objective
The aim of this study was to evaluate the efficacy and safety of recombinant human growth hormone (rhGH) therapy on linear growth in children with ACAN deficiency.
Methods
Open-label, single-arm, prospective study at Cincinnati Children’s Hospital Medical Center. Ten treatment-naïve patients were recruited. Inclusion criteria were a confirmed heterozygous mutation in ACAN, age ≥2 years, prepubertal, bone age (BA) ≥chronological age (CA), and normal insulin-like growth factor I concentration. Treatment was with rhGH (50 µg/kg/day) over 1 year. Main outcomes measured were height velocity (HV) and change in (Δ) height SD score (HtSDS).
Results
Ten patients (6 females) were enrolled with median CA of 5.6 years (range 2.4-9.7). Baseline median HtSDS was –2.5 (range –4.3 to –1.1). Median baseline BA was 6.9 years (range 2.5-10.0), with median BA/CA of 1.2 (range 0.9-1.5). Median pretreatment HV was 5.2 cm/year (range 3.8-7.1), increased to 8.3 cm/year (range 7.3-11.2) after 1 year of therapy (P = .004). Median ΔHtSDS after 1 year was +0.62 (range +0.35 to +1.39) (P = .002). Skeletal maturation did not advance inappropriately (median ΔBA/CA –0.1, P = .09). No adverse events related to rhGH were observed.
Conclusion
Treatment with rhGH improved linear growth in a cohort of patients with short stature due to ACAN deficiency.
Keywords: aggrecan deficiency, short stature, growth hormone
Short stature is a common reason for referral to pediatric endocrinologists. Patients with negative biochemical and hormonal work-up are often diagnosed as having idiopathic short stature (ISS). Recent advances in the genetic evaluation of short stature have led to the identification of several specific genetic etiologies for short stature previously classified under ISS (1-4). These include aggrecan (ACAN) deficiency, an autosomal dominant condition characterized by short stature often accompanied by advanced bone age (BA) and premature epiphyseal fusion (4-7).
ACAN is a proteoglycan, encoded by the ACAN gene, located in the extracellular matrix of articular and growth plate cartilage (8-11). Animal studies revealed that mutations in ACAN result in altered growth plate morphogenesis leading to premature hypertrophic chondrocyte maturation and compromised long bone elongation (9, 11). In addition to short stature, patients with ACAN deficiency often have joint disease, including early-onset osteoarthritis (5), further suggesting dysfunction at both growth plate and articular cartilage.
Although the number of individuals and families diagnosed with ACAN deficiency has increased since the initial description of the disorder, there is a paucity of information on the effects of growth-promoting interventions. Reports thus far have been limited to retrospective reviews (5, 6, 12, 13). In these, patients were treated with different growth hormone (GH) regimens for varying duration, with or without puberty blockade (5, 6, 12, 13), making it difficult to analyze the efficacy of the treatment.
The purpose of this study was to perform a prospective trial of GH therapy in patients with ACAN deficiency to evaluate its short-term efficacy and safety.
Patients and Methods
Participants
Ten prepubertal patients with ACAN deficiency were treated with recombinant human growth hormone (rhGH) for 12 months in an open-label, single-arm, prospective study. The trial was approved by the Institutional Review Board at Cincinnati Children’s Hospital Medical Center (Cincinnati, OH). Written informed consent was obtained from parents of all subjects and assent from subjects of relevant age.
Study Design
All subjects had a documented heterozygous mutation in ACAN explaining their short stature. A mutation was defined as any 1 of the following: heterozygous deletion of the entire gene or of at least 1 complete exon, any truncating mutation (frameshift, nonsense, splice mutations within 2 bases of the exon/intron boundary, and start loss variants), any missense mutation (if predicted to be damaging by both Polyphen2 and SIFT and is de novo or segregates with the short stature phenotype in the family), and in-frame insertions or deletions of more than 1 amino acid and meeting the same criteria as missense mutations. To be included, patients were required to be at least 2 years old, prepubertal on examination (testicular size <4 mL for males, Tanner I breast development for females), have a BA equal to or greater than chronological age (CA) (determined by the Greulich and Pyle method), and an insulin-like growth factor (IGF)-I) concentration within normal range for age and gender. Patients were excluded if they had received previous growth-altering treatment (rhGH, rhIGF-1, gonadotropin-releasing hormone [GnRH] agonist, aromatase inhibitor, oxandrolone, or any investigational pharmacological intervention targeting growth). Further exclusion criteria were history of malignancy, coexisting chronic conditions known to impact growth (eg, cystic fibrosis, diabetes mellitus, inflammatory bowel and celiac disease, asthma requiring significant inhaled steroid use, etc.), malnutrition (body mass index [BMI] <fifth percentile), and fusion of growth plates as determined on an x-ray of the left hand and wrist.
Patients were brought for study visits at baseline, 6 months, and 12 months. Height and weight data were obtained at all visits and plotted on Centers for Disease Control and Prevention growth charts (www.cdc.gov/growthcharts), in addition to physical examination (including musculoskeletal examination). Laboratory evaluation performed at baseline included a complete blood count, renal and hepatic panel, thyroid function studies, IGF-I, and IGF binding protein-3 (IGFBP-3). IGF-I and IGFBP-3 were measured by the IDS-iSYS chemiluminescent immunoassay (Immunodiagnostics System Inc, Gaithersburg, MD). IGF-I standard deviation scores (SDS) were calculated using the formula , with variables provided for age and sex as in Bidlingmaier et al. (14). Similarly, for IGFBP-3 a modified LMS method (skewness [L], mean [M], and coefficient of variation [S]) was used to calculate SDS (15). After normal screening test results were confirmed, all patients were initiated on rhGH at a dose of 50 µg/kg/day subcutaneously, similar to dosing utilized in other primary skeletal disorders (16). The rhGH was provided by Novo Nordisk, Inc. (Norditropin® and Nordiflex®). IGF-I and IGFBP-3 were monitored while on rhGH (at 3 months, 6 months, and 12 months) and adherence with rhGH administration was monitored via compliance log reviews and tracking of GH pen usage. All patients were to continue on initial rhGH dosing per protocol, unless interval IGF-I concentrations were greater than 2.5 SDS above the mean, at which point the rhGH dose was reduced by 10% to 20%. Furthermore, treatment was to be discontinued if serious adverse drug reactions were reported or if withdrawal was desired by participants. Check-in phone calls occurred 1 week after rhGH initiation and at 3 months and 9 months for adherence review (in addition to adherence logs to be maintained by participants for study team review), interim adverse event inquiry, and discussion of any recommended changes in rhGH dosing. BA x-rays and dual x-ray absorptiometry (Hologic Inc., Bedford, MA) of the lumbar spine and total body were obtained at baseline and 12-month visits. BA studies were read by a single investigator (P.B.). An accompanying detailed phenotyping protocol included magnetic resonance imaging of the knees for patients over the age of 6 years who did not require sedation, as well as x-rays of the knees. Results of this separate phenotyping protocol are reported in another manuscript (Alexandrou E, Dauber A, Tyzinsky L, et al. Clinical phenotype and musculoskeletal characteristics of patients with aggrecan deficiency. Am J Med Genet, under review).
Outcomes
The primary outcome measured was change in (Δ) height standard deviation score (HtSDS) from baseline to 12 months, with as secondary outcomes ΔHV (height velocity) and ΔBA compared with CA. Due to the sample size it was not possible to determine if the outcome variables, which are continuous variables, deviated from the assumption of normality, thus we are reporting both mean with associated SD and median with associated interquartile range (25th and 75th percentiles), and range (maximum and minimum). We are also reporting results from both paired T tests, plus a nonparametric equivalent signed rank test. In order to be conservative we are using results from the signed rank test with a statistically significant difference indicated by P ≤ .05. In addition, the frequency of adverse events was ascertained. All statistical analysis was carried out using SAS®, version 9.4 (SAS Institute, Cary, NC).
Results
Ten patients with a confirmed heterozygous mutation in ACAN and otherwise normal screening laboratory studies were recruited with a median CA of 5.6 years (range 2.4-9.7 years) (Table 1). Baseline median HtSDS was –2.52, ranging from –4.3 to –1.1. Median weight SDS at baseline was –0.67 (range –3.1 to +1.5) and BMI percentile was 86.24 (range 36.2-99.8).
Table 1.
Baseline characteristics for 10 study patients ordered according to chronological age, with mean and median values
| Patient/gender | cDNA | Protein | CA (y) | BA (y) | BA/CA | HV (cm/y) | HtSDS | WtSDS | BMI percentile |
|---|---|---|---|---|---|---|---|---|---|
| P1/F | c0.1172delG | p.Gly391Valfs*7 | 2.4 | 2.5 | 1.0 | 4.0 | –2.55 | –1.11 | 88.71 |
| P2/M | c0.280_336del57 | p.Val94_Ile112del | 3.4 | 5.0 | 1.5 | 4.8 | –2.20 | 0.28 | 99.30 |
| P3/M | c0.2023C>T | p.Arg675* | 3.6 | 5.0 | 1.4 | 5.2 | –2.49 | –0.24 | 98.14 |
| P4/M | c0.280_336del57 | p.Val94_Ile112del | 5.0 | 6.0 | 1.2 | N/A | –1.12 | 1.47 | 99.78 |
| P5/F | c0.7202G>A | p.Trp2401* | 5.2 | 6.8 | 1.3 | 7.1 | –1.07 | –0.42 | 72.47 |
| P6/M | c0.609G>T | p.Trp203Cys | 6.0 | 7.0 | 1.2 | 4.4 | –4.27 | –3.09 | 79.09 |
| P7/F | c0.7202G>A | p.Trp2401* | 6.9 | 7.8 | 1.1 | 6.4 | –2.26 | –0.64 | 81.15 |
| P8/F | c0.492C>A | p.Tyr164* | 7.4 | 9.4 | 1.3 | 5.4 | –3.11 | –0.70 | 88.81 |
| P9/F | c0.1051 + 1G>A | p.Val255_Glu352del | 8.1 | 10.0 | 1.2 | 5.8 | –2.99 | –0.83 | 83.77 |
| P10/F | c0.2023C>T | p.Arg675* | 9.7 | 8.8 | 0.9 | 3.8 | –3.48 | –2.53 | 36.17 |
| Mean (SD) | — | — | 5.8 (2.3) | 6.8 (2.3) | 1.2 (0.2) | 5.2 (1.10) | –2.55 (0.99) | –0.78 (1.3) | 82.74 (18.75) |
| Median(IQR) (range) | — | — | 5.6(3.6, 7.4)(2.4, 9.7) | 6.9(5.0, 8.8)(2.5, 10.0) | 1.2(1.1, 1.3)(0.9, 1.5) | 5.2(4.4, 5.8)(3.8, 7.1) | –2.52(–3.1, –2.2)(–4.3, –1.1) | –0.67(–1.1, –0.2) (–3.1, 1.5) | 86.24(79.6, 98.1)(36.2, 99.8) |
Abbreviations: F, female; M, male; CA, chronological age; BA, bone age; HV, height velocity; HtSDS, height standard deviation score; WtSDS, weight standard deviation score; N/A, not available; IQR, interquartile range (25th, 75th percentiles).
Median baseline BA was 6.9 years (range 2.5-10.0 years), with median BA/CA of 1.2 (range 0.9-1.5). One individual was included in the study with a BA that was approximately 9-12 months below the CA, but with a confirmed mutation in the ACAN gene (Table 1).
Response to rhGH Therapy
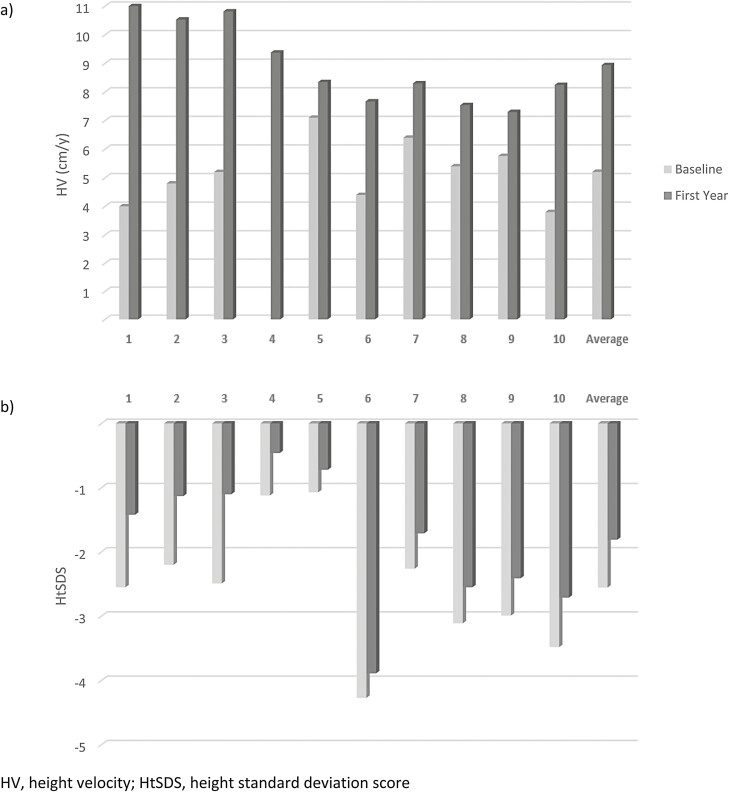
Median pretreatment HV was 5.2 cm/year (range 3.8-7.1 cm/year). This increased to 8.3 cm/year (range 7.3-11.2 cm/year) during the first year of treatment, which was statistically significant (P = .004). HV and HtSDS at baseline and through 1 year of rhGH therapy for each study patient are shown in Fig. 1. As demonstrated in Table 2, the median ΔHtSDS after 1 year was +0.62 (range +0.35 to +1.39) (P = .002). There was a heterogeneous degree of disproportionality in this cohort based on sitting height/height measurements (SH/Ht), but on the whole no consistent disproportionality in comparison to age and gender reference intervals (17), and no significant ΔSH/Ht from baseline of 0.57 to 0.55 after 1 year(P = .16). Weight and BMI data are also described in Table 2.
Figure 1.
(A) Pretreatment height velocity (gray bars) and height velocity (HV) through 1 year of treatment (black bars) with recombinant human growth hormone (rhGH) for 10 study subjects. Median HV increased from 5.2 cm/year (range 3.8-7.1 cm/year) to 8.3 cm/year (range 7.3-11.2 cm/year) after 1 year (P = .004). (B) Height standard deviation score (HtSDS) in 10 subjects, before (gray bars) and after (black bars) 1 year of treatment with rhGH. Median ΔHtSDS after 1 year was +0.62 (range +0.35 to +1.39), P = .002.
Table 2.
Bone age advancement, as well as change in height velocity, height, segmental ratio, weight, BMI, and bone density through 1 year on rhGH
| ΔHeight velocity (cm/y) | ΔHtSDS | ΔBone age/chronological agea | ΔSH/Hta | ΔWtSDS | ΔBMI percentile | ΔLS BMD HAZ | ΔTBLH BMD HAZ | |
|---|---|---|---|---|---|---|---|---|
| P1 | 7.2 | +1.13 | 0.0 (1.0, 1.0) | 0.00 (0.58, 0.58) | +0.97 | +0.78 | N/A | N/A |
| P2 | 5.7 | +1.07 | –0.1 (1.5, 1.4) | +0.08 (0.56, 0.65) | –0.37 | –13.69 | N/A | N/A |
| P3 | 5.6 | +1.39 | –0.2 (1.4, 1.2) | –0.06 (0.62, 0.56) | +0.58 | –3.39 | N/A | N/A |
| P4 | N/A | +0.66 | +0.1 (1.2, 1.3) | –0.09 (0.57, 0.48) | +0.69 | –0.07 | –0.4 | +0.4 |
| P5 | 1.2 | +0.35 | –0.1 (1.3, 1.2) | 0.00 (0.52, 0.52) | +0.55 | +7.61 | +0.3 | –0.1 |
| P6 | 3.3 | +0.38 | –0.1 (1.2, 1.1) | –0.01 (0.57, 0.56) | +0.55 | –5.05 | +0.1 | 0.0 |
| P7 | 1.9 | +0.55 | 0.0 (1.1, 1.1) | –0.02 (0.55, 0.53) | +0.53 | +2.08 | –0.2 | +0.7 |
| P8 | 2.1 | +0.56 | –0.2 (1.3, 1.1) | +0.01 (0.55, 0.56) | +0.31 | –1.83 | +0.8 | 0.0 |
| P9 | 1.5 | +0.58 | 0.0 (0.9, 0.9) | –0.03 (0.54, 0.51) | –0.26 | –19.08 | +1.0 | –0.7 |
| P10 | 4.4 | +0.77 | –0.1 (1.2, 1.1) | –0.02 (0.57, 0.55) | +0.68 | +10.27 | 0.0 | –0.4 |
| Mean (SD)P valueb | 3.7 (2.2)P = .001 | +0.74 (0.34)P < .001 | –0.07 (0.09)P = .11 | –0.01 (0.05)P = .33 | +0.42 (0.42)P = .01 | –2.24 (8.88) P = .45 | +0.21 (0.50)P = .30 | –0.02 (0.45)P = .92 |
| Median(IQR)(range)P valuec | 3.3(1.9, 5.6)(1.2, 7.2)P = .004 | +0.62(0.55, 1.07)(0.35, 1.39)P = .002 | –0.10(–0.10, 0.00)(–0.20, 0.10)P = .09 | –0.02(–0.03, 0.00)(–0.09, 0.08)P = .16 | +0.55(0.31, 0.68)(–0.37, 0.97)P = .01 | –0.95(–5.05, 2.08)(–19.08, 10.27)P = .56 | +0.05(–0.19, 0.75)(–0.40, 0.97)P = .38 | –0.02(–0.40, 0.35)(–0.65, 0.71)P = .84 |
Abbreviations: HtSDS, height standard deviation score; SH, sitting height; WtSDS, weight standard deviation score; BMI, body mass index; LS, lumbar spine; BMD HAZ, bone mineral density height-adjusted Z-score; TBLH, total body less head; N/A, not available; IQR, interquartile range (25th, 75th percentiles).
a Δ in ratio (baseline ratio, ratio at end of 1 year).
b Paired t-test.
c Signed rank test.
Skeletal maturation did not markedly change, as shown in Table 2. Median ΔBA/CA was –0.10, without statistically significant change (P = .09). Only a single subject had an increase in BA of more than 1 year during the 12-month course of the study. This occurred in patient 4 in the context of excessive weight gain in an already obese individual (BMI was greater than the 99th percentile).
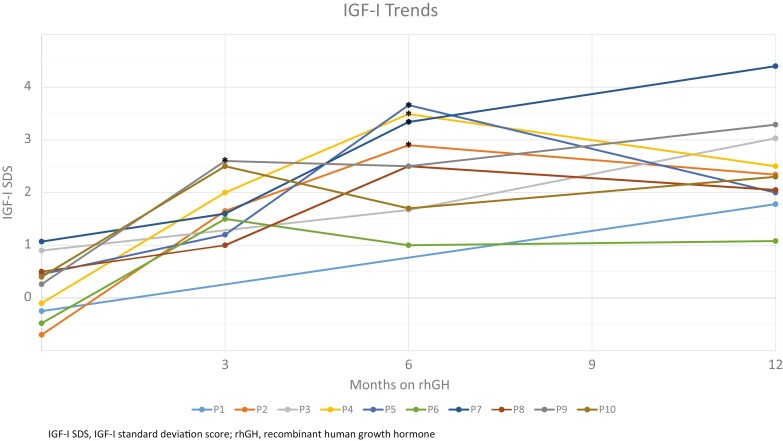
Although all patients were started on an rhGH dose of 50 µg/kg/day subcutaneously, after 1 year of treatment the mean dose was 41 µg/kg/day. Five subjects underwent a protocol-driven dose reduction based on elevated IGF-I concentrations. The median IGF-I SDS at baseline was +0.3 (range –0.7 to +1.1). At 1 year the median IGF-I SDS had increased to +2.3 (range +1.1 to +4.4), P = .002 (Table 1 (18)). The median IGFBP-3 SDS was –0.1 (range –1.4 to +1.0) at baseline and increased to +1.2 (range 0 to +2.2) at 1 year, P = .004 (Table 2 (19)). IGF-I value trend only is presented in Fig. 2.
Figure 2.
Insulin-like growth factor (IGF)-I values for 10 study patients, with protocol-driven recombinant human growth hormone (rhGH) dose reductions (*) at 6 months for patients 2, 4, 5, and 7, as well as dose reduction at 3 months for patient 9. Patients 6 and 10 had weight adjustment of their rhGH at 6 months. HV, height velocity; HtSDS, height standard deviation score.
Joint Findings and Bone Mineralization
At baseline, 3 patients reported intermittent joint discomfort (hip pain in patient 7, knee discomfort/pain in patient 1, stiffness and painful knees in patient 9) or demonstrated weakness (hip weakness in patient 8) on examination. With rhGH therapy there was no report of worsening, other than occasional new reports of knee and hip discomfort in patient 8, and occasional ankle discomfort in patient 7, further with reassuring examinations. Detailed musculoskeletal imaging, bone density, and examination findings are reported elsewhere (Alexandrou E, Dauber A, Tyzinsky L, et al. Clinical phenotype and musculoskeletal characteristics of patients with aggrecan deficiency. Am J Med Genet, under review). Seven patients had bone densitometry by dual x-ray absorptiometry completed at baseline and after 1 year of rhGH treatment. All patients demonstrated normal bone mineral density at baseline. Change in bone mineral density after 1 year of treatment with rhGH is shown in Table 2 with a median change of +0.05 SDS for lumbar spine (P = .38) and –0.02 SDS (P = .84) for total body less head at 1 year.
Adverse Events
No adverse events related to the use of rhGH were reported in any study patient. This includes no concerns for or reports of increased intracranial pressure, slipped capital femoral epiphysis, scoliosis, or appreciable clinical worsening of joint problems.
Discussion
As the evaluation for short stature has evolved over the years to identify new genetic etiologies for patients previously classified as having ISS, it becomes ever more important to recognize the potential benefit of different growth promoting interventions in an attempt to have a more individualized treatment approach for these patients. Responses to existing growth interventions may differ dramatically when parsing out specific subsets of genetic short stature. This study provides novel insights into the utility of a well-known growth promoting agent (rhGH) in a specific short stature population with ACAN deficiency.
Treatment of this ACAN-deficient cohort with rhGH was well tolerated and had beneficial impact on HtSDS after 1 year of therapy. While our cohort number is relatively small, there appeared to be a larger impact on HV in younger patients and in those with more severe short stature at baseline, both of which could be expected. It was reassuring that the BA advancement, often observed in ACAN-deficient patients, did not worsen while on rhGH therapy. This is similar to what has been observed in ISS patients treated with rhGH (20). In 1 patient only, in the context of excessive weight gain, there was accelerated skeletal maturation compared with the remainder of the cohort.
Improvement in linear growth during the first year appeared greater when compared with limited retrospective data from previous reports, in which the HV likely was impacted by the different rhGH doses used (5, 13). Retrospective data from Gkourogianni et al. included patients receiving 1 of 3 different rhGH doses, with a subset also receiving a GnRH agonist. In that study the mean ΔHtSDS was +0.4 after 1 year compared with +0.74 in our study (5). Three patients in a small case series reported by Xu et al. each received a different dose of rhGH, with 2 subjects also treated with a GnRH analog. The ΔHtSDS in this group during the first year ranged only from 0.23 to 0.33 (13). It should also be noted that in both these reports there appeared to be a further decreased benefit after the first year of rhGH therapy, despite the normal onset of puberty in some patients and the use of GnRH agonist therapy to slow skeletal maturation (5, 6, 12, 13). In addition, van der Steen et al. reported 4 patients born small for gestational age with ACAN mutations and treated with rhGH (dose range 1-2 mg/m2/day and duration ranging from approximately 3.5 to 9 years), plus pubertal blockade. Two of the 4 reached adult height without showing significant ΔHtSDS (–0.2 and +0.1). However, they were taller than their affected parents (HtSDS +0.8 and +1.2 compared with the parents after approximately 9 and 6 years of rhGH treatment respectively) (6). In some cases, GnRH agonist therapy was observed to slow BA advancement, but with associated lack of continued improved linear growth, perhaps due to blunting of the pubertal growth spurt (6). It will be interesting to see if patients allowed to progress through puberty normally will have a strong pubertal growth spurt and experience a lasting positive impact on adult height outcome.
Studies in patients with ISS have shown treatment with rhGH has similarly resulted in a more robust response in the first year of treatment, followed by more modest growth in the ensuing years (21, 22). Nevertheless, initiation of catch-up growth early in the treatment course using appropriate rhGH dosing was seen as important for having greater benefit on adult height outcomes in the ISS population (22). Long-term follow-up in the ACAN-deficient patients will help determine whether consistent rhGH dosing leads to sustained catch-up growth or at least a normal HV for age after a period of initial catch-up growth. It should be noted that an increase in HV was observed on a lower than intended rhGH dosing. The ability to achieve good catch-up growth and continued normal growth afterwards on lower doses of GH will be desirable in ACAN-deficient patients, given their underlying propensity to present with BA advancement. Nevertheless, there likely are several factors impacting response to consistent rhGH dosing, perhaps some unrecognized. A few patients in our study showed a more modest response to GH therapy, similar to 2 patients reported by Lin et al. who were treated with the same rhGH dose (48 µg/kg/day): ΔHtSDS +0.23 and +0.48 after 1 year (23).
From a phenotype perspective, this cohort demonstrated relatively proportionate short stature with only subtle clinical indication of disproportionality in certain patients, consistent with previous descriptions of proportionate short stature in ACAN children (5, 23) compared with mildly disproportionate ACAN adults (5). Joint findings, either subjective or objective, were not consistently present within the cohort, but still notable for osteochondritis dissecans in some subjects, and relatively early onset for joint complaints in a subset of this young cohort. Given our experience, it would be difficult at this stage to conclude anything on the potential impact of rhGH on joint complaints.
There certainly exists a phenotypic spectrum for individuals with ACAN deficiency, including the severity of short stature related to the degree of growth plate involvement. This may also have an impact on the treatment response with rhGH. Nevertheless, with 1 year of rhGH treatment there was a clinically significant increase in HV and improvement in HtSDS despite less than projected rhGH dosing used. As additional longitudinal data are needed to assess the long-term efficacy of rhGH in ACAN-deficient patients and the ultimate impact on adult height outcome, the cohort described here was enrolled in an extension trial.
Acknowledgments
Statistical support was graciously provided by Jane Khoury, PhD.
Glossary
Abbreviations
- Δ
change in
- ACAN
aggrecan
- BA
bone age
- BMI
body mass index
- CA
chronological age
- GnRH
gonadotropin-releasing hormone
- HtSDS
height SD score
- HV
height velocity
- IGF
insulin-like growth factor
- IGFBP-3
IGF binding protein-3
- ISS
idiopathic short stature
- rhGH
recombinant human growth hormone
- SH
sitting height
Contributor Information
Gajanthan Muthuvel, Division of Endocrinology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH 45229, USA.
Andrew Dauber, Division of Endocrinology, Children’s National Hospital, Washington, DC 20010, USA; Department of Pediatrics, George Washington University School of Medicine and Health Sciences, Washington, DC 20037, USA.
Eirene Alexandrou, Division of Endocrinology, The University of Iowa Stead Family Children’s Hospital, Iowa City, IA 52242, USA; Department of Pediatrics, University of Iowa, Iowa City, IA 52242, USA.
Leah Tyzinski, Division of Endocrinology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH 45229, USA.
Melissa Andrew, Division of Endocrinology, Children’s National Hospital, Washington, DC 20010, USA.
Vivian Hwa, Division of Endocrinology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH 45229, USA; Department of Pediatrics, University of Cincinnati, Cincinnati, OH 45229, USA.
Philippe Backeljauw, Division of Endocrinology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH 45229, USA; Department of Pediatrics, University of Cincinnati, Cincinnati, OH 45229, USA.
Financial Support
This study was generously supported by a grant from Novo Nordisk Inc (ISS 001152).
Disclosures
This study was conducted under IND #135870 (FDA). A.D. received consulting fees from Novo Nordisk, Ascendis, and BioMarin, as well as research support from Novo Nordisk and BioMarin. P.B. is currently a consultant for, or has received honoraria from, Novo Nordisk, Novartis/Sandoz, Ascendis, BioMarin, EndoPharmaceuticals, Jupiter Bioventures, and Ipsen, and receives research support from Novo Nordisk, Novartis, and Ipsen. The study sponsor, Novo Nordisk, played no role in the design, conduct, data analysis, or manuscript preparation.
Clinical Trial Information
This study is registered on ClinicalTrials.gov (NCT03288103) (registered September 19, 2017).
Data Availability
Data sets generated and/or analyzed during this study are available from the authors upon reasonable request.
References
- 1. Andrade AC, Jee YH, Nilsson O. New genetic diagnoses of short stature provide insights into local regulation of childhood growth. Horm Res Paediatr. 2017;88(1):22-37. [DOI] [PubMed] [Google Scholar]
- 2. Dauber A, Rosenfeld RG, Hirschhorn JN. Genetic evaluation of short stature. J Clin Endocrinol Metab. 2014;( 9):3080-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dauber A, Stoler J, Hechter E, Safer J, Hirschhorn JN. Whole exome sequencing reveals a novel mutation in CUL7 in a patient with an undiagnosed growth disorder. J Pediatr. 2013;162(1): 202-4.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nilsson O, Guo MH, Dunbar N, et al. Short stature, accelerated bone maturation, and early growth cessation due to heterozygous aggrecan mutations. J Clin Endocrinol Metab. 2014;( 8):E1510-E1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gkourogianni A, Andrew M, Tyzinski L, et al. Clinical characterization of patients with autosomal dominant short stature due to aggrecan mutations. J Clin Endocrinol Metab. 2017;102(2):460-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van der Steen, M, Pfundt R, et al. ACAN gene mutations in short children born SGA and response to growth hormone treatment. J Clin Endocrinol Metab. 2017;102(5):1458-1467. [DOI] [PubMed] [Google Scholar]
- 7. Quintos JB, Guo MH, Dauber A. Idiopathic short stature due to novel heterozygous mutation of the aggrecan gene. J Pediatr Endocrinol Metab. 2015;28(7-8):927-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doege KJ, Sasaki M, Kimura T, Yamada Y. Complete coding sequence and deduced primary structure of the human cartilage large aggregating proteoglycan, aggrecan. Human-specific repeats, and additional alternatively spliced forms. J Biol Chem. 1991;266(2):894-902. [PubMed] [Google Scholar]
- 9. Domowicz MS, Cortes M, Henry JG, Schwartz NB. Aggrecan modulation of growth plate morphogenesis. Dev Biol. 2009;329(2):242-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kiani C, Chen L, Wu YJ, Yee AJ, Yang BB. Structure and function of aggrecan. Cell Res. 2002;( 1):19-32. [DOI] [PubMed] [Google Scholar]
- 11. Watanabe H, Yamada Y. Chondrodysplasia of gene knockout mice for aggrecan and link protein. Glycoconj J. 2002;19(4-5):269-73. [DOI] [PubMed] [Google Scholar]
- 12. Liang H, Miao H, Pan H, et al. Growth-promoting therapies may be useful in short stature patients with nonspecific skeletal abnormalities caused by ACAN heterozygous mutations: six Chinese cases and literature review. Endocr Pract. 2020;( 11):1255-1268. [DOI] [PubMed] [Google Scholar]
- 13. Xu D, Sun C, Zhou Z, et al. Novel aggrecan variant, p. Gln2364Pro, causes severe familial nonsyndromic adult short stature and poor growth hormone response in Chinese children. BMC Med Genet. 2018;19(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bidlingmaier M, Friedrich N, Emeny RT, et al. Reference intervals for insulin-like growth factor-1 (IGF-I) from birth to senescence: results from a multicenter study using a new automated chemiluminescence IGF-I immunoassay conforming to recent international recommendations. J Clin Endocrinol Metab. 2014;( 5):1712-1721. [DOI] [PubMed] [Google Scholar]
- 15. Friedrich N, Wolthers OD, Arafat AM, et al. Age- and sex-specific reference intervals across life span for insulin-like growth factor binding protein 3 (IGFBP-3) and the IGF-I to IGFBP-3 ratio measured by new automated chemiluminescence assays. J Clin Endocrinol Metab. 2014;( 5):1675-1686. [DOI] [PubMed] [Google Scholar]
- 16. Blum WF, Crowe BJ, Quigley CA, et al. Growth hormone is effective in treatment of short stature associated with short stature homeobox-containing gene deficiency: Two-year results of a randomized, controlled, multicenter trial. J Clin Endocrinol Metab. 2007;( 1):219-228. [DOI] [PubMed] [Google Scholar]
- 17. Hawkes CP, Mostoufi-Moab S, McCormack SE, Grimberg A, Zemel BS. Sitting height to standing height ratio reference charts for children in the United States. J Pediatr. 2020;226:221-227.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Muthuvel GD, Andrew A, Eirene T, et al. Treatment of short stature in aggrecan deficient patients with recombinant human growth hormone: one-year response. Supplementary Table 1. figshare. Deposited November 15, 2021; Doi: 10.6084/m9.figshare.17013782.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Muthuvel GD, Andrew A, Eirene T, et al. Treatment of short stature in aggrecan deficient patients with recombinant human growth hormone: one-year response. Supplementary table 2. figshare. 2021; Doi: 10.6084/m9.figshare.17014652.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Crowe BJ, Rekers-Mombarg LT, Robling K, Wolka AM, Cutler GB Jr. Effect of growth hormone dose on bone maturation and puberty in children with idiopathic short stature. J Clin Endocrinol Metab. 2006;( 1):169-175. [DOI] [PubMed] [Google Scholar]
- 21. Leschek EW, Rose SR, Yanovski JA, et al. Effect of growth hormone treatment on adult height in peripubertal children with idiopathic short stature: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2004;( 7):3140-3148. [DOI] [PubMed] [Google Scholar]
- 22. Wit JM, Rekers-Mombarg LT. Final height gain by GH therapy in children with idiopathic short stature is dose dependent. J Clin Endocrinol Metab. 2002; 87( 2): 604-611. [DOI] [PubMed] [Google Scholar]
- 23. Lin L, Li M, Luo J, et al. A high proportion of novel ACAN mutations and their prevalence in a large cohort of Chinese short stature children. J Clin Endocrinol Metab. 2021;106(7): e2711-e2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sets generated and/or analyzed during this study are available from the authors upon reasonable request.