Letter
Prostate-specific membrane antigen (PSMA)-based radionuclide therapy has been shown to be an efficient and well-tolerated option in patients with metastatic castration resistant prostate cancer (mCRPC). Initial results of an ongoing prospective study to assess the intra-arterial (IA) administration of lutetium-177 (177Lu-)-labeled PSMA compared to conventional intravenous (IV) administration in patients with mCRPC is presented. IA administration is promising to increase delivery efficacy and safe. Following approval by the institutional review board, four patients were treated with 177Lu-PSMA (median age, 62.5 years (range, 53–72); median PSA, 89.75 (range, 11.74–173)). Each patient received their treatment dose in two visits (Fig 1). The first half of the dose (3700 MBq) was administered using the routine IV administration route. The second half of the dose was administered a week later selectively from bilateral internal iliac arteries under fluoroscopic guidance (Allura Xper FD20/10 Philips Medical Systems, the Netherlands). Pelvic angiography from the distal aorta was performed using a 4-F pigtail catheter (TEMPO AQUA®, Cordis, Miami, FL). Each internal iliac artery was selectively catheterized using a 4-F cobra catheter (TEMPO AQUA®, Cordis, Miami, FL). With the tip of the Cobra catheter positioned proximal to the anterior branches of the internal iliac artery, the dose was split and each half of the 177Lu-PSMA was infused in the left and right internal iliac arteries successively. SPECT-CT imaging (Siemens Medical Solutions, Erlangen, Germany) of the prostate and metastatic lesion sites following IV and IA administrations, was performed to calculate the absorbed dose. Regions of interest (ROIs) were drawn over the composite image of all axial SPECT slices with increased tracer uptake, with anatomic correlation using low-dose CT.
Figure 1.
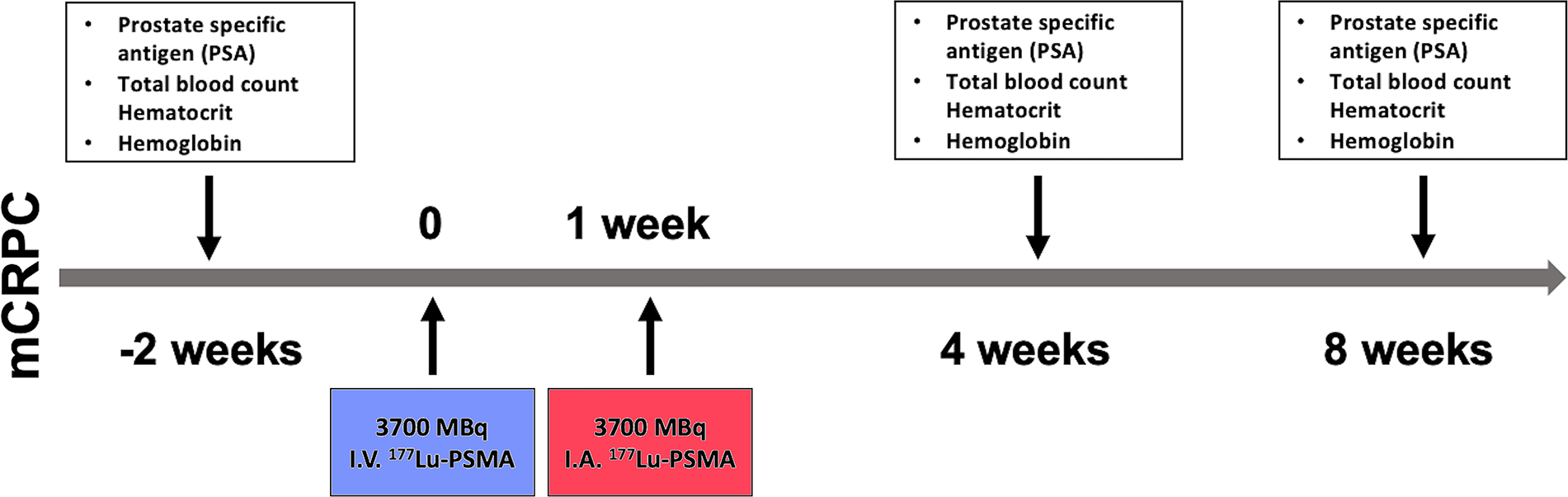
Study design and timeline of Lu-177 PSMA therapy.
Absorbed doses in all metastatic lesions, kidneys, liver, bone marrow (BM), prostate, and whole body (WB) were determined for each patient using the OLINDA/EXM 1.0 software (Vanderbilt University, Nashville, TN) according to the Medical Internal Radiation Dose schema. Mean absorbed dose values of the dominant lesion (i.e., the metastatic lesion with the highest radioactivity count), all lesions and organs were compared between administration routes. Additionally, the absorbed dose value of the dominant lesion and the mean absorbed dose value across all lesions were used to calculate and compare all lesions-to-liver, all lesions-to-BM, and all lesions-to-WB ratios. All comparisons were made using Student’s t-test using Excel.
Absorbed doses following IA administration compared with IV administration are shown in Fig 2. Although dominant lesions had a higher mean absorbed dose with IA versus IV administration (2.23 vs 1.67 MGy/MBq), the difference was not significant (P = 0.10). By contrast, the mean total absorbed dose of all lesions was significantly higher with IA versus IV administration (1.67 vs 1.26 MGy/MBq; P = 0.02). The prostate gland had a lower mean absorbed dose with IA versus IV administration (0.27 vs 0.36 MGy/MBq) but this difference was not significant (P = 0.44). By contrast, the liver mean absorbed dose was significantly lower with IA versus IV administration (0.11 vs 0.13; P = 0.04). Although not statistically significant (0.05 vs 0.07; P = 0.18), patients received lower total body radiation with IA versus IV administration. When lesion-to-liver, lesion-to-BM, lesion-to-prostate, and lesion-to-WB ratios were compared between administration routes, the differences between IA and IV administration were significant (P = 0.039 when only the dominant lesions were used to calculate ratios; P = 0.01 when all lesions were used to calculate ratios).
Figure 2.
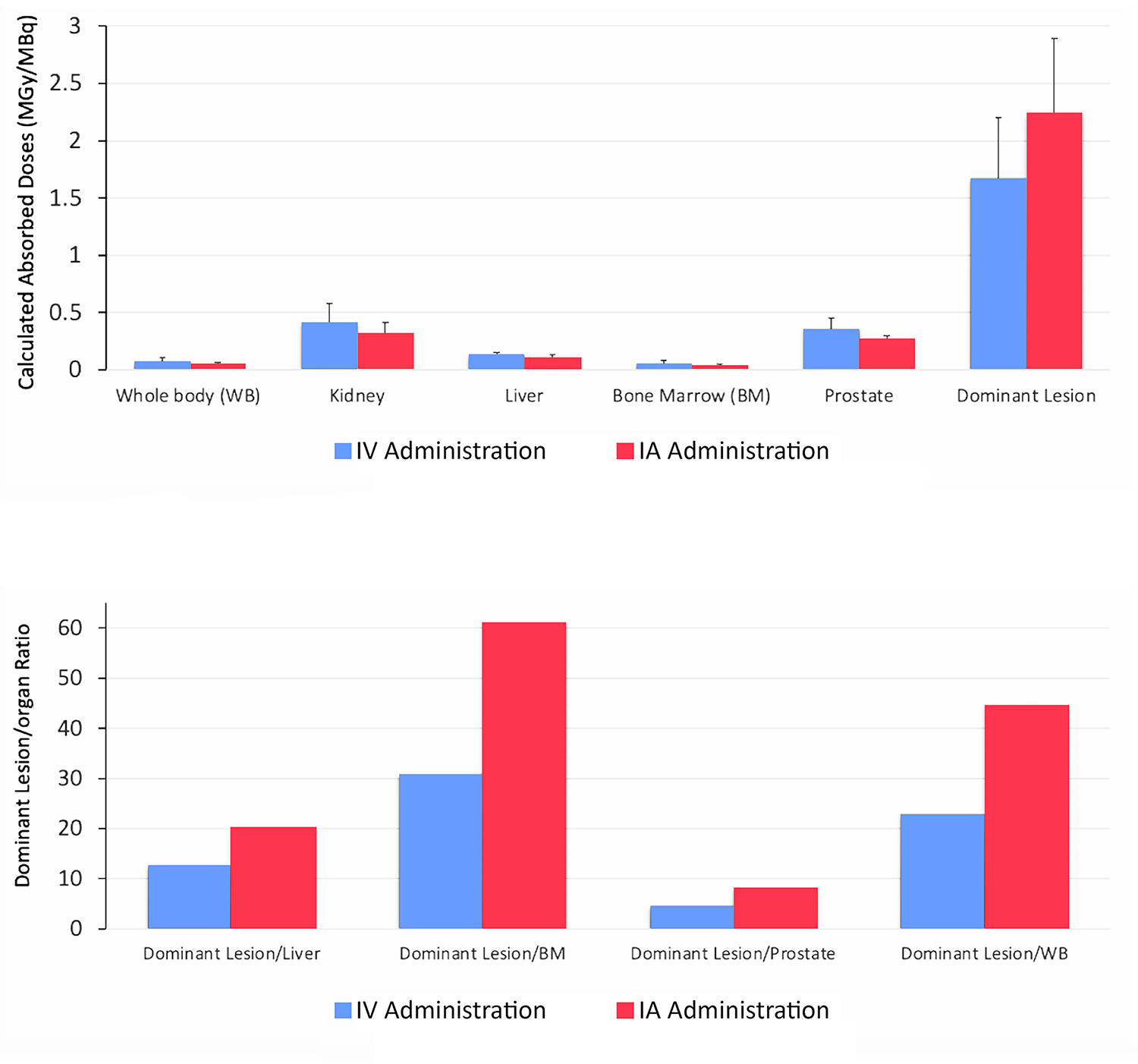
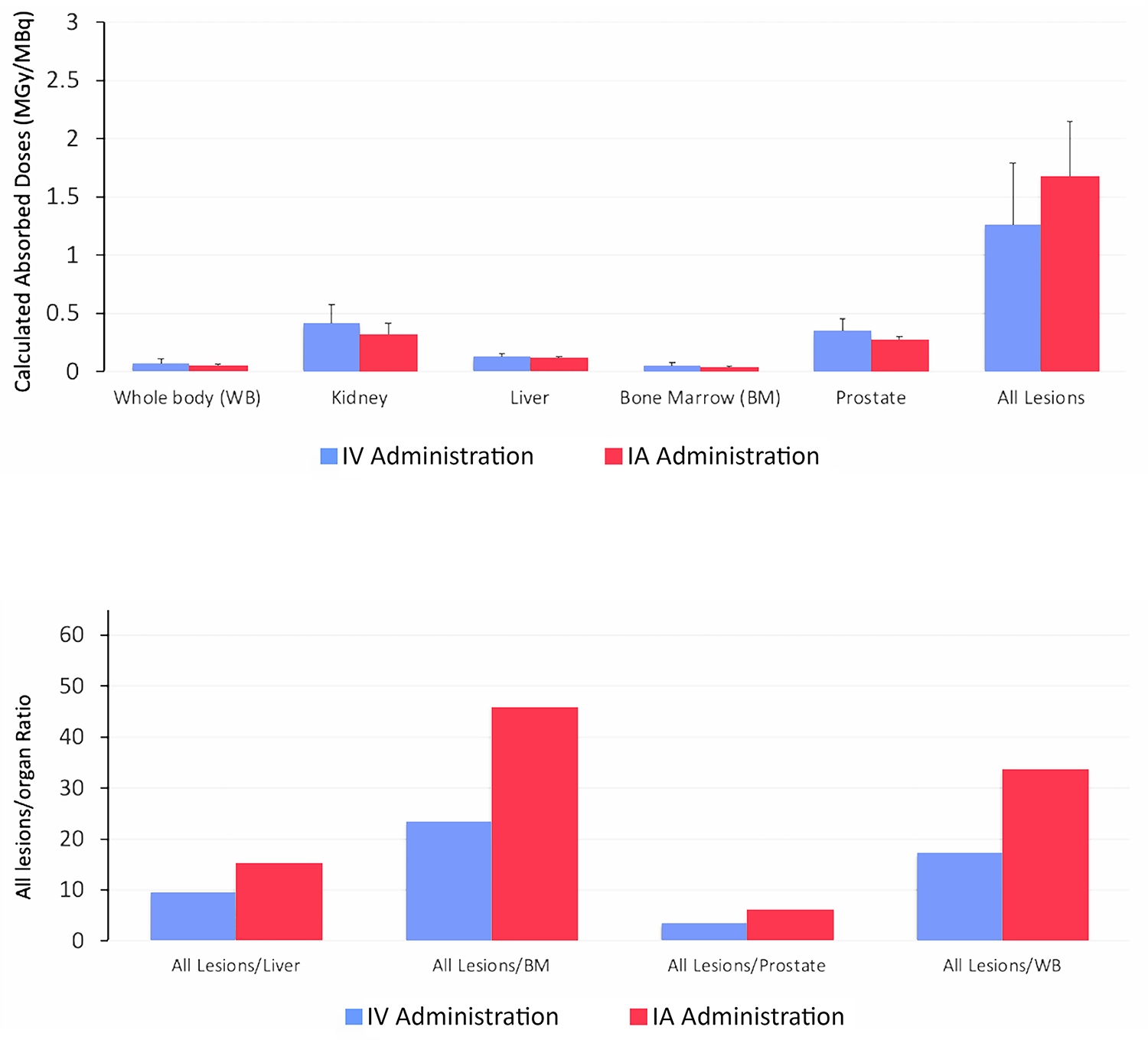
(a) Comparison of intravenous (IV) and intra-arterial (IA) applications by calculated absorbed doses (MGy/MBq) in the dominant lesion with respect to calculated absorbed doses in the the liver, bone marrow (BM), prostate, and whole body (WB). (b). Comparison of intravenous (IV) and intra-arterial (IA) applications by calculated absorbed doses (MGy/MBq) of all lesions with respect to calculated absored doses in the liver, bone marrow (BM), prostate and whole body (WB). Each bar in calculated absorbed doses (MGy/MBq) represents mean ± SEM
There were no unexpected side effects during the early post-therapy period for IA administration in all patients; after a one-night stay, patients were discharged. Several grade 1 adverse effects (xerostomia, nausea, and fatigue) occurred in the first 8–16 weeks of follow-up.
The goal of IA is to increase the local (and subsequently intra-tumoral) concentration of chemotherapeutic agents, decrease systemic adverse effects, and generate greater tumor response.1 In one study, Ga-68 DOTATOC positron emission tomography/computed tomography (PET/CT) was performed after both IV and IA administration; the standardized uptake values of DOTATOC in neuroendocrine tumors were approximately 3.75 times higher after IA administration.2 The LUTIA (Lutetium Intra-Arterial) study, an ongoing study in patients with neuroendocrine tumors, aims to increase the tumor-absorbed dose in liver metastases with IA versus IV administration of 177Lu-Dotate.3
There are limitations in this study. First, there could have been possible saturation of PSMA receptors because the two treatments were performed one week apart. Second, it was difficult to draw ROIs on the prostate due to the low spatial resolution of SPECT/CT images. This, along with high activity crossover from the urinary bladder, could have led to the result that the absorbed dose to the prostate was similar between both administrations. Despite the finding, this study suggests that IA administration could have a lower radiation burden on the liver and more favorable therapeutic effects in distant metastatic lesions. Lastly, it is a small sample size. The study will continue with a larger number of patients, with endpoints such as progression-free survival and overall survival.
Acknowledgments
O.A. was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Funding sources:
Omer Aras was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. The sponsor had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Conflicts of interest: None
Whether the material was presented at an SIR Annual Scientific Meeting or any other conference or posted as a preprint: The material was posted as a preprint in Research Square with the link: https://www.researchsquare.com/article/rs-173258/v1
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.von Scheel J, Golde G. Pharmacokinetics of intra-arterial tumour therapy. An experimental study. Arch Otorhinolaryngol. 1984 [DOI] [PubMed] [Google Scholar]
- 2.Kratochwil C, Giesel FL, López-Benítez R, Schimpfky N, Kunze K, Eisenhut M, et al. Intraindividual comparison of selective arterial versus venous 68Ga-DOTATOC PET/CT in patients with gastroenteropancreatic neuroendocrine tumors. Clin Cancer Res. 2010 [DOI] [PubMed] [Google Scholar]
- 3.Ebbers SC, Braat A, Moelker A, Stokkel MPM, Lam M, Barentsz MW. Intra-arterial versus standard intravenous administration of lutetium-177-DOTA-octreotate in patients with NET liver metastases: study protocol for a multicenter, randomized controlled trial (LUTIA trial). Trials. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]


