Abstract
In Escherichia coli, transcription of the ferric citrate transport genes fecABCDE is controlled by a novel signal transduction mechanism that starts at the cell surface. Binding of ferric citrate to the outer membrane protein FecA initiates a signal that is transmitted by FecR across the cytoplasmic membrane into the cytoplasm where FecI, the sigma factor, is activated. Interaction between the signaling proteins was demonstrated by utilizing two methods. In in vitro binding assays, FecR that was His tagged at the N terminus [(His)10-FecR] and bound to a Ni-nitrilotriacetic acid agarose column was able to retain FecA, and FecR that was His tagged at the C terminus [FecR-(His)6] retained FecI on the column. An N-terminally truncated, induction-negative but transport-active FecA protein did not bind to (His)10-FecR. The in vivo assay involved the determination of the FecA, FecR, and FecI interacting domains with the bacterial two-hybrid Lex-based system. FecA1–79 interacts with FecR101–317 and FecR1–85 interacts with FecI1–173. These data clearly support a model that proposes interaction of the periplasmic N terminus of FecA with the periplasmic C-terminal portion of FecR and interaction of the cytoplasmic N terminus of FecR with FecI, which results in FecI activation.
Escherichia coli takes up ferric citrate through the outer membrane by active transport mediated by the FecA receptor protein and the TonB-ExbB-ExbD energy-transducing device. The TonB-ExbB-ExbD complex (2, 18, 23) transfers the energy required for active transport across the outer membrane (2). Ferric citrate or iron is subsequently released from FecA and binds to FecB in the periplasm. Iron is then transported across the cytoplasmic membrane by an ATP-binding cassette transport system consisting of the FecC, -D, and -E proteins (3, 10, 19, 24, 30). Ferric citrate binding to FecA induces transcription of the fecABCDE transport genes, but does not affect transcription of the fecIR regulatory genes (15, 16). Induction can be uncoupled from transport by point mutations in fecA that lead to constitutive induction of transcription and the inability to transport ferric citrate (9). Furthermore, the deletion of residues 14 to 68 of mature FecA removes the induction function; however, transport activity is fully retained (10). A further vital component for the response to ferric citrate is FecR. FecR is an integral cytoplasmic membrane protein of 317 residues; the N terminus has been localized to the cytoplasm and the C terminus has been localized to the periplasm. A hydrophobic sequence from residues 85 to 100 probably forms the single transmembrane segment (28). The location of FecR in the three subcellular compartments (the periplasm, the cytoplasmic membrane, and the cytoplasm) suggests a structural and functional role in the signaling cascade of the fec system. C- terminally truncated FecR derivatives display a constitutive phenotype and a FecR N-terminal fragment of only 59 amino acids (aa) is able to induce fec transport gene transcription independently of ferric citrate (15). These data led us to propose that the information of ferric citrate binding to FecA is transmitted across the outer membrane by FecA and across the cytoplasmic membrane by FecR. FecR subsequently activates FecI, which binds the RNA polymerase core enzyme and directs it to the fecA promoter to initiate transcription of the fecA, -B, -C, -D, and -E genes. Therefore, signal transduction could involve a series of conformational changes; starting with the binding of ferric citrate to FecA, a signal is then transmitted through the N terminus of FecA to the C terminus of FecR and then across the cytoplasmic membrane to the N terminus of FecR, which interacts with FecI. To support the model of such a signal cascade, we searched for a physical interaction between the proposed Fec signal-transducing proteins. Using N-terminally and C-terminally His-tagged FecR bound to Ni-nitrilotriacetic acid (NTA) agarose columns, we have shown that there is a specific interaction between isolated FecR and isolated FecA and FecI proteins. An alternative approach was undertaken using an in vivo system based on the ability of the Lex repressor to bind to an altered operator placed upstream of lacZ (5). Using this system, we demonstrated heterodimer formation of the N terminus of FecA with the C terminus of FecR and of the N terminus of FecR with FecI. The in vitro and in vivo data demonstrate an interaction of the FecAIR proteins and their subdomains as predicted by the proposed model.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The E. coli strains and plasmids used in this study are listed in Table 1. All strains are derivatives of E. coli K-12, except for the E. coli B derivative BL21(DE3). Cells were grown in tryptone-yeast extract (TY) medium or nutrient broth (NB) medium as previously described (14). Growth on ferric citrate as the sole iron source was tested on Fec agar plates containing NB medium, 1.5% nutrient agar, 0.2 mM 2,2′-dipyridyl, and 1 mM citrate. Antibiotics were used at the following concentrations: ampicillin, 50 μg per ml; tetracycline, 12 μg per ml; and chloramphenicol, 40 μg per ml.
TABLE 1.
E. coli strains and plasmids used in this study
| Strain or plasmid | Genotype | Source or reference |
|---|---|---|
| Strains | ||
| DH5α | Δ(argF lac)U196 endA1 recA1 hsdR17 (rK− mK+) supE44 thi-1 gyrA1 relA1 (F′ φ80 ΔlacZΔM15) | 8 |
| AA93 | Δfec aroB Δ(argF lac)U169 araD139 rspL150 relA1 deoC1 flbB5301 ptsF25 rbsR | 15 |
| BL21(DE3) | F−hsdS galT; phage T7 polymerase under lacUV5 control | 25 |
| SU202 | lexA71::Tn5 sulA211 sulA::lacZ Δ(lacIPOZYA)169 F′ lacIqlacZΔM15::Tn9 | 5 |
| Plasmids | ||
| pT7-7 | Phage T7 promoter; ori (ColE1) Apr | 26 |
| pHSG576 | pSC101 derivative; Cmr | 27 |
| pMLB1034 | ori (ColE1) Apr | 22 |
| pET25b(+) | Phage T7 promoter; C-terminal His-tag; ori (ColE1) Apr | Novagen |
| pET19b | Phage T7 promoter; N-terminal His-tag; ori (ColE1) Apr | Novagen |
| pFecRHis | pET25b(+) fecR | This study |
| pHisFecR | pET19b fecR | This study |
| pIS136 | pHSG576 fecI | 15 |
| pIS135 | pHSG576 fecI fecR | 15 |
| pIRHis | pHSG576 fecI fecRhis | This study |
| pIHisR | pHSG576 fecIhisR | This study |
| pIS1034 | pMLB1034 fecA; fecA-lacZ fusion | 15 |
| pSV66 | pHSG576 fecI fecR fecA | 9 |
| pAA70 | pT7-7 fecR | 29 |
| pAA71 | pT7-7 fecI | 1 |
| pIS711 | pT7-7 fecA | 10 |
| pSP47 | pT7-7 fecAΔ47–101 | 10 |
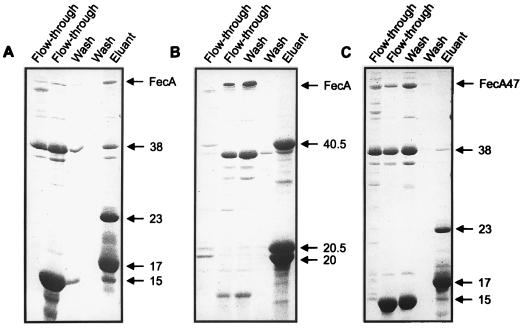
| pDP804 | LexA1–87408-Jun zipper | 5 |
| pMS604 | LexA1–87WT-Fos zipper | 5 |
| pUS10 | LexA1–87WT-FecA1–79a | This study |
| pUS11 | LexA1–87408-FecR101–317a | This study |
| pUS12 | LexA1–87408-FecR151–317a | This study |
| pUS13 | LexA1–87408-FecR101–275a | This study |
| pSM173 | LexA1–87WT-FecI1–173a | This study |
| pSM133 | LexA1–87WT-FecI1–133a | This study |
| pSM4 | LexA1–87WT-FecI82–173a | This study |
| pSM85 | LexA1–87408-FecR1–85a | This study |
| pSM9 | LexA1–87408-FecR9–85a | This study |
| pSM19 | LexA1–87408-FecR19–85a | This study |
| pSM38 | LexA1–87408-FecR1–38a | This study |
| pSM58 | LexA1–87408-FecR1–58a | This study |
Subscripts indicate the amino acid residues contained in the protein deletion derivatives.
Construction of plasmids.
Plasmid pFecRHis was constructed by inserting the fecR gene upstream of the codons encoding six histidine residues of the fusion vector pET25b(+) (Novagen, Schwalbach, Germany) cleaved with NdeI-EcoRI. The fecR gene was amplified by PCR from plasmid pSV66fecIRA′ with primers FecRI and His2701 (Table 2).
TABLE 2.
Deoxyoligonucleotides used in this study
| Deoxyoligonucleotide | Sequence |
|---|---|
| FecRI | 5′-CTGGAGTATGGCATATGAATC-3′ |
| His2701 | 5′-CGGAATTCAGTGGTGAAATGTTTATC-3′ |
| LexFecA1 | 5′-GAACCGGTGACCGGATCTAGAGCACAGGTTAATATCGCA-3′ |
| LexFecA2 | 5′-TTCCCCCTCGAGTCCACTAGTTTCTTTTGGTGCGGGCGC-3′ |
| LexFecR1 | 5′-CGCCTCGAGGGATCTAGATCGGAAACCGGCGAAGGT-3′ |
| LexFecR2 | 5′-GGAAGATCTTCCACTAGTTTACAGTGGTGAAATGTT-3′ |
| LexFecR3 | 5′-CGCCTCGAGGGATGGTACGGTGAAATCGCC-3′ |
| LexFecR7 | 5′-GGAAGATCTTTAAACGGCGGGATCGCAGCG-3′ |
| FecIBstEII | 5′-GATGCAGGTGACCATGTCTGACCGCGCC-3′ |
| FecIPst | 5′-GGTTAACACTGCAGTCATAACCCATACTC-3′ |
| FecRXhoI | 5′-GGAGTACTCGAGATGAATCCTTTGTTAACC-3′ |
| FecRBglII | 5′-CAACAGAATCTTCATTTCATCACACGTGACG-3′ |
| FecI4.2 | 5′-CAGACCATCCTGCAGCGAAAGCAGAAACGC-3′ |
| FecI2 | 5′-CGCGCTGGTGACCGCGTATCTGGAGATGC-3′ |
| FecR58 | 5′-ACCGCCAGATCTGTTGCGCAGGTTTTCAACC-3′ |
| FecR38 | 5′-ATCCTGTTCAGATCTCTGTTGCCAGCGCGC-3′ |
| FecR19 | 5′-GCGTTCAGCTTCCCTCGAGTATGCCGTGCTAAGC-3′ |
| FecR9 | 5′-CCTTTGTTAACCGATTCCCGCCTCGAGGCGCTGCGTTCA-3′ |
Plasmid pHisFecR was obtained as follows. The NdeI-HindIII fragment of fecR from plasmid pAA70 was blunt ended with the Klenow fragment of DNA polymerase I, cloned downstream of the codons encoding 10 histidine residues in the fusion vector pET19b (Novagen), cleaved with NdeI-BamHI, and blunt ended with the Klenow fragment.
The low-copy-number plasmids pIRHis and pIHisR, in which wild-type fecR was replaced by fecRhis or hisfecR, respectively, were created as follows. The fecRhis-containing NdeI-StyI fragment from plasmid pFecRHis was inserted into the NdeI-HindIII-digested and Klenow-treated plasmid pIS135 fecIR, yielding pIRHis. Plasmid pIHisR was obtained by cloning the hisfecR-carrying NdeI-PstI fragment into the NcoI-PstI-cleaved and Klenow-treated plasmid pIS135 fecIR.
The following plasmids were all constructed so as to fuse various regions of FecA, FecR, and FecI in frame with either LexA1–87408 or LexA1–87WT (5). Plasmid pUS10 was constructed by replacing the BstEII-XhoI fragment containing the Fos zipper on pMS604 with the N-terminal region of the mature FecA protein with oligonucleotides LexFecA1 and LexFecA2. Plasmids pUS11 to pUS12 were made by replacing the XhoI-BlgII fragment of pDP804 containing the Jun zipper motif with various parts of the FecR periplasmic domain with oligonucleotides LexFecR1, LexFecR2, LexFecR3, and LexFecR7 (Table 2 and see Fig. 4). Plasmid pSM85 encodes the N-terminal region of FecR representing the region from aa 1 to 85. This region was cloned via XhoI-BglII into pDP804. The plasmids pSM19 and pSM9 represent N-terminal deletions of FecR cloned into pDP804, whereas pSM38 and pSM58 are carboxy-terminal deletions of FecR. Plasmid pSM173 is the entire FecI gene cloned into pMS604 via BstEII-PstI.
FIG. 4.
Diagrammatic representation of the cloning of FecA, FecR, and FecI regions into the plasmids pDP804 and pMS604. Domains of FecA and FecI were fused in frame to LexA1–87WT, by which the Fos zipper domain originally present in pMS604 was deleted. Domains of FecR were all cloned into the XhoI-BglII restriction endonuclease sites present in pDP804. All fecR regions cloned are fused in frame to Lex1–87408, which deleted the Jun zipper motif in pDP804.
The regions used for cloning FecA, FecR, and FecI were PCR-amplified products derived from plasmid pSV66. Oligonucleotide primers and sequences used for the PCR amplification are described in Table 2. All plasmid constructs were confirmed by DNA sequencing.
PCR techniques.
PCR amplification was carried out using Taq polymerase (Qiagen, Hilden, Germany) under standard conditions. DNA was initially denatured by heating to 95°C for 5 min. This was followed by 30 cycles consisting of denaturing at 95°C for 30 sec, annealing at 55°C for 30 sec, and extension at 72°C for 2 min.
Recombinant DNA techniques.
Standard techniques (20) or the protocols of the suppliers were used for the isolation of plasmid DNA, PCR, digestion with restriction endonucleases, ligation, transformation, and agarose gel electrophoresis. DNA was sequenced by the dideoxy chain-termination method (21) using the AutoRead sequencing kit (Pharmacia Biotech, Freiburg, Germany). The reaction products were sequenced on an A.L.F. DNA sequencer (Pharmacia Biotech).
Purification of FecR-(His)6 and (His)10-FecR.
E. coli BL21(DE3) transformed with pFecRHis or pHisFecR was grown at 37°C in TY medium. Transcription of the fusion genes was induced by adding IPTG (isopropyl-β-d-thiogalactopyranoside) (1 mM) when the cultures reached an optical density at 578 nm of 0.5 (26). The culture was incubated for 2 h, and the cells were harvested by centrifugation and suspended in binding buffer (20 mM Tris-HCl, 0.5 M NaCl, 5 mM imidazole [pH 7.9]). Crude cell extracts were obtained by disrupting the cells with a French pressure cell. The inclusion bodies, which contained the overexpressed fusion proteins, were separated by centrifugation (4,000 × g for 20 min) and solubilized by incubating for 1 h at 25°C in binding buffer supplemented with 6 M urea. Undissolved material was removed by centrifugation (30,000 × g for 30 min), and the supernatant fractions were dialyzed against binding buffer to remove the urea. Precipitated material was removed by centrifugation (30,000 × g for 30 min), and the supernatants were applied to Ni-NTA agarose columns previously equilibrated with binding buffer. After two wash steps with 10 bed volumes of binding buffer and 10 bed volumes of wash buffer (20 mM Tris-HCl, 0.5 M NaCl, 60 mM imidazole [pH 7.9]), bound fusion proteins were eluted with 5 bed volumes of elution buffer (20 mM Tris-HCl, 0.5 M NaCl, 1 M imidazole [pH 7.9]). The proteins of each fraction were precipitated with 10% trichloroacetic acid and equal proportions of each fraction were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), with 15% acrylamide gels (11).
Binding assays.
FecI was purified as described previously (1) with the following specifications. E. coli BL21(DE3) transformed with pAA71fecI was grown at 37°C in TY medium to an optical density at 578 nm of 0.5. fecI transcription was induced by adding IPTG to a final concentration of 1 mM, and the culture incubation was continued for 2 h. FecI was solubilized from inclusion bodies by incubating for 1 h at 25°C in binding buffer supplemented with 2 mg of N-dodecyl-N,N-dimethyl-3-ammonio-1-propanesulfonate per ml. Undissolved cell debris was removed by centrifugation (30,000 × g for 30 min), and the supernatant was applied to Ni-NTA agarose columns loaded with FecR-(His)6 or (His)10-FecR. Chromatography was performed as described above.
FecA was overproduced in E. coli BL21(DE3) transformed with pIS711fecA or pSP47fecAΔ47–101 by the addition of IPTG to the cultures (10). The outer membrane fraction was prepared and solubilized by incubating for 1 h at 25°C in binding buffer supplemented with 2 mg of octylglucoside per ml. Undissolved material was removed by centrifugation (30,000 × g for 30 min), and the supernatant was applied to Ni-NTA agarose columns loaded with the FecR fusion proteins. Chromatography was performed as described above.
Immunoblot analysis.
After electrophoresis, the SDS-PAGE gels were electroblotted onto nitrocellulose (Schleicher and Schuell, Dassel, Germany) in transfer buffer (25 mM Tris-HCl, 192 mM glycine, 20% [vol/vol] methanol [pH 8.3]) at 20 V for 12 to 16 h. Nitrocellulose was blocked in wash buffer (20 mM Tris-HCl, 0.5 M NaCl, 0.05% Tween 20 [pH 7.5]) supplemented with 3% bovine serum albumin (BSA). Antisera were incubated in wash buffer supplemented with 1% BSA and 1:5,000-diluted antibodies. Antibody-conjugated alkaline phosphatase with 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium in the detection buffer (100 mM Tris-HCl, 100 mM NaCl, 5 mM MgCl2 [pH 9.5]) was used for identification of the proteins.
Polyclonal antiserum was obtained by immunizing rabbits with the SDS-PAGE-purified 20-kDa fragment of FecR (Eurogentec, Seraing, Belgium).
Determination of β-galactosidase activity.
β-Galactosidase activity was determined according to Miller (14) and Giacomini et al. (7).
For determination of the histidine-tagged FecR in vivo activity, the cells were grown in NB medium supplemented as indicated above. For the LexA-based repression system, the cells were grown in TY medium. Bacterial strains were grown overnight in TY medium supplemented with 1 mM IPTG. The cultures were subcultured (1:50) in TY medium and allowed to grow for an additional 5 h in the presence of 1 mM IPTG.
LexA-based repression assay.
The various constructs of FecA, FecR, and FecI which are fused to the lexA gene were transformed into strain SU202. The lexA gene used for these fusion lacks the domain normally involved in the formation of dimers. The strain SU202 contains the lacZ gene under the control of an altered sulA operator. The altered operator binding site allows only LexA to bind when it has formed heterodimers (5). Differing combinations of plasmids were used to determine which of the Lex-Fec gene products could form heterodimers. The ability to form heterodimers was monitored by measuring the β-galactosidase activity. The amount of β-galactosidase was then determined. The formation of heterodimers and therefore the interaction of the fused protein domains are seen as a reduction of β-galactosidase activity.
RESULTS
Construction and chromatographic characterization of FecR histidine tagged at either the N-terminal or C-terminal end.
If the N-terminal portion of FecA interacts with the C-terminal portion of FecR, and the N-terminal region of FecR contacts FecI, the His tag should thus be located at the noninteracting end of FecR to prevent interference with binding. Therefore, the fecR gene was cloned into the expression vectors pET25b(+) and pET19b to create FecR derivatives containing a C-terminal 6-histidine tag and an N-terminal 10-histidine tag, respectively. FecR-(His)6 contained a linker of 24 amino acids between (His)6 and FecR, and (His)10-FecR contained an 11-amino-acid linker. E. coli BL21(DE3) was transformed with each of the resulting plasmids, pHisFecR and pFecRHis, and the hybrid proteins were overexpressed by the addition of IPTG to the cultures. Both hybrid proteins, like unmodified FecR, accumulated as inclusion bodies. To solubilize the proteins, the isolated inclusion bodies were treated with the binding buffer which was subsequently used for Ni-NTA agarose column chromatography, supplemented with 6 M urea. Removal of urea by dialysis prior to chromatography resulted in a precipitate of presumably incorrectly folded FecR which was removed by centrifugation. Only soluble FecR was used in all further chromatographic analyses.
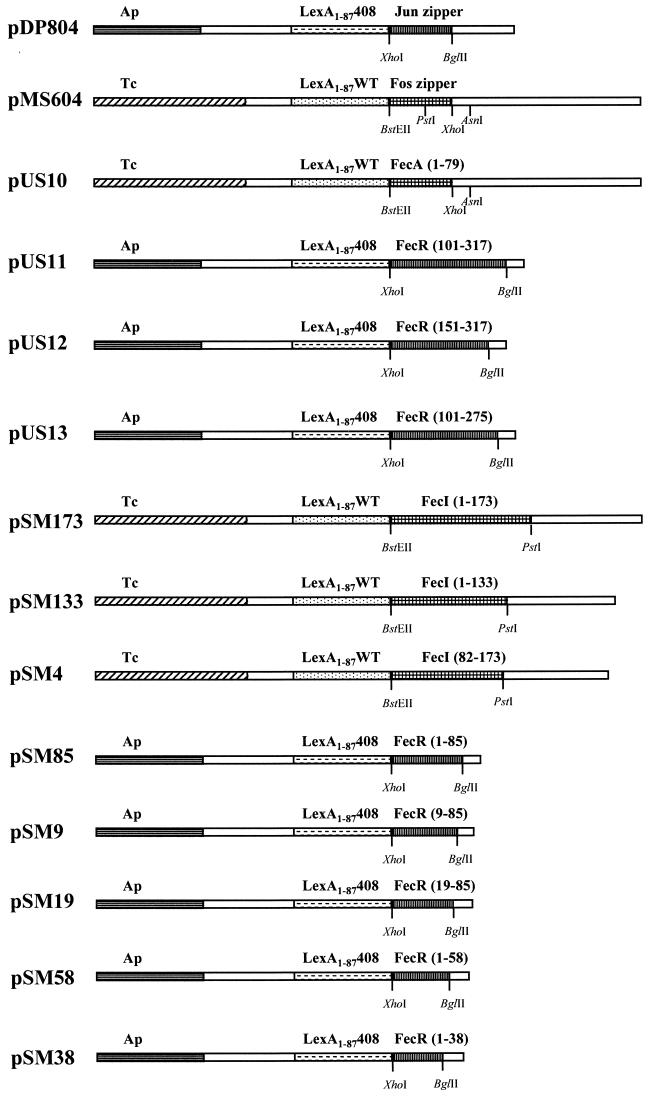
The inclusion bodies contained either FecR-(His)6 or (His)10-FecR and two degradation products, as revealed by SDS-PAGE (data not shown). FecR is proteolytically cleaved after residue 181 into fragments of 20 and 15 kDa (29). Even though full-length FecR can be isolated, it always breaks down into the two proteolytic domains. Figure 1 shows the results of representative chromatographic analyses of the solubilized portions of FecR-(His)6 and (His)10-FecR on a Ni-NTA agarose column. Fractions of each chromatographic step, as indicated in Fig. 1, were analyzed by SDS-PAGE. The uncleaved proteins FecR-(His)6 and (His)10-FecR, containing linkers of 24 and 11 residues, respectively, exhibited the expected molecular masses of 40.5 and 38 kDa. As Fig. 1A shows, the 20.5-kDa fragment resulted from the C-terminal 15-kDa portion of FecR plus the 5.5-kDa histidine tag. In (His)10-FecR, the N-terminal FecR 20-kDa fragment was increased to 23 kDa. The (His)10-FecR contained an additional degradation product of 17 kDa. Immunoblotting with polyclonal FecR antibodies raised to the purified N-terminal 20-kDa FecR degradation product confirmed that all the fragments arose from FecR (Fig. 1C). The immunoblot shows the 40.5-kDa uncleaved FecR-(His)6 and its untagged 20-kDa degradation fragment, as well as the 38-kDa (His)10-FecR and its corresponding degradation product of 23 kDa (Fig. 1C). Western blotting with histidine antibodies revealed essentially the same result as with FecR antibodies, with the exception that the anti-histidine serum could detect the 20.5-kDa FecR-(His)6 fragment instead of the untagged 20-kDa degradation product and the 23-kDa fragment of (His)10-FecR (data not shown). As expected, the 20-kDa fragment of FecR-(His)6 and the 15- and 17-kDa fragments of (His)10-FecR did not contain the His label. The protein band of approximately 38 kDa seen in Fig. 1B (flowthrough) does not represent (His)10-FecR as determined by Western blotting. No histidine-tagged FecR was found in any of the wash steps (Fig. 1). Why the nonhistidine-labeled fragments were retained on the nickel column is unknown, but it is likely that the proteolytic products of FecR stay associated with each other and are thus eluted from the column in conjunction with the histidine-tagged fragment.
FIG. 1.
Affinity chromatography of FecR-(His)6 (A) and (His)10-FecR (B) on a Ni-NTA agarose column. The eluted fractions were subjected to SDS-PAGE (with 15% acrylamide gels) and stained with Coomassie brilliant blue. (C) Western blotting of Ni-NTA agarose column eluant fractions with anti-FecR serum. FecR-(His)6, (His)10-FecR, and their proteolytic cleavage products are indicated by arrows. Numbers indicate molecular masses in kilodaltons.
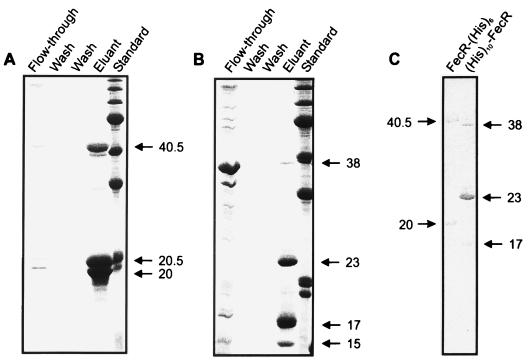
Binding of FecI to FecR-(His)6 fixed to a Ni-NTA agarose column.
Ni-NTA agarose was used as an affinity matrix for FecR-(His)6 and (His)10-FecR to determine the physical interaction of FecR with FecI and FecA. FecI was solubilized from inclusion bodies with binding buffer supplemented with N-dodecyl-N,N-dimethyl-3-ammonio-1-propanesulfonate, which results in active FecI as shown by fecA promoter DNA bandshift caused by FecI in the presence of RNA polymerase (1). This material was then applied to a Ni-NTA agarose column loaded with FecR-(His)6 (Fig. 2A) or (His)10-FecR (Fig. 2B). In this and the following experiments, a surplus of the proteins was used for loading the column and was removed by the two wash steps until no protein was eluted. FecI was retained on the column and was coeluted with FecR-(His)6, which demonstrates binding of FecI to FecR-(His)6. In contrast, FecI was completely eluted in the flowthrough and in the first wash from the column loaded with (His)10-FecR. The (His)10 tag at the N terminus of (His)10-FecR presumably inhibited binding to FecI. This experiment also shows that FecI itself does not bind to the Ni-NTA agarose column.
FIG. 2.
Binding of FecI to FecR-(His)6 (A) and (His)10-FecR (B) on a Ni-NTA agarose column. The eluted fractions were subjected to SDS-PAGE (with 15% acrylamide gels) and stained with Coomassie brilliant blue. FecI, FecR-(His)6, (His)10-FecR, and their proteolytic cleavage products are indicated by arrows. Numbers indicate molecular masses in kilodaltons.
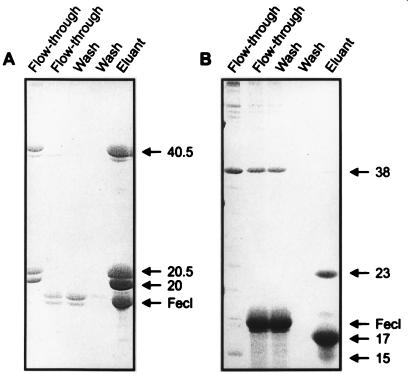
Binding of FecA to (His)10-FecR fixed to a Ni-NTA agarose column.
FecA was solubilized from isolated outer membranes with binding buffer supplemented with octylglucoside and then applied to a Ni-NTA agarose column loaded with (His)10-FecR (Fig. 3A) or FecR-(His)6 (Fig. 3B). The majority of FecA coeluted with (His)10-FecR and only low amounts of FecA were detected in the flowthrough of the (His)10-FecR column and in the first wash. In contrast, FecA was hardly retained on the FecR-(His)6 column. Retention of FecA by (His)10-FecR and failure of retention by FecR-(His)6 support the conclusion that FecA is bound to FecR and that the C-terminal portion of FecR recognizes FecA.
FIG. 3.
Binding of FecA to (His)10-FecR (A) and FecR-(His)6 (B) on a Ni-NTA agarose column and of FecAΔ47 to His10-FecR (C) on a Ni-NTA agarose column. The eluted fractions were subjected to SDS-PAGE (with 15% acrylamide gels) and stained with Coomassie brilliant blue. FecA, FecAΔ47 (designated FecA47), FecR-(His)6, (His)10-FecR, and their proteolytic cleavage products are indicated by arrows. Numbers indicate molecular masses in kilodaltons.
The reliability of FecA binding to (His)10-FecR was tested with FecA47. Previous studies have shown that FecA47, which lacks residues 14 to 68 of mature FecA, displays no induction activity but full transport activity (10). FecA47 was not retained by (His)10-FecR and was eluted in the flowthrough and the first wash (Fig. 3C). The results of this control experiment support a direct and specific role of the periplasmic N terminus of FecA and the C-terminal portion of FecR in FecA-FecR interaction. Control experiments with FecA alone show no binding to the Ni-NTA agarose column (data not shown).
In vivo activity of FecR-(His)6 and (His)10-FecR.
Induction of fec transport gene expression was studied in E. coli AA93 Δfec transformed with plasmid pIS1034, which encodes fecA and a fecA-lacZ promoter fusion. β-Galactosidase activity was determined 2 h after the addition of 1 mM citrate to cells cultivated in NB medium. Plasmids pIRHis and pIHisR were constructed by replacing wild-type fecR by fecRhis or hisfecR on the low-copy-number plasmid pIS135 fecIR. Under these conditions, no inclusion bodies were observed. FecR-(His)6 and (His)10-FecR responded to citrate and displayed approximately 60% wild-type FecR activity (Table 3). No β-galactosidase activity above background level was detected without the addition of citrate; no activity was detected in E. coli AA93 without FecR (pIS136 fecI). These results demonstrate that the addition of histidines to the C terminus or the N terminus had only a minor effect on FecR activity and that the histidine-tagged FecR derivatives were properly inserted into the cytoplasmic membrane.
TABLE 3.
Induction of fecA-lacZ expression in E. coli AA93 Δfec pIS1034 fecA fecA-lacZ synthesizing wild-type FecR, FecR-(His)6, (His)10-FecR, or no FecR
| Plasmid | β-Galactosidase activity (Miller units) in:
|
|
|---|---|---|
| NB medium | NB medium and citrate | |
| pIS136 fecI | 8 | 7 |
| pIS135 fecI fecR | 10 | 446 |
| pIHisR fecI fecRhis | 7 | 296 |
| pIRHis fecIhisR | 8 | 281 |
In vivo interaction of the N terminus of FecA with the C terminus of FecR.
In vivo interaction of the N terminus of FecA with the C terminus of FecR was examined with a bacterial two-hybrid system (5). The two-hybrid system is based on the ability of the LexA protein to form dimers, which then bind to the sulA operator and downregulate transcription. The LexA protein consists of two distinct domains: one domain is required for the dimerization and the other is required for binding to the operator sequence. In this system, the dimerization domain is deleted from LexA and replaced with other domains that are thought to dimerize. The control plasmid pMS604 contains the first 87 aa of LexA-408 and the control plasmid pDP804 contains the first 87 aa of the wild-type LexA (the region involved in operator binding), fused to the Fos and Jun zipper motifs, respectively. In the bacterial strain SU202, an altered sulA hybrid operator containing a site with a wild-type half and a mutated half is placed in front of lacZ, which acts as the reporter gene for LexA binding. This altered operator is only bound if two heterodimeric LexA molecules are formed. The DNA encoding the first 79 aa of the mature FecA protein and aa 101 to 317 of FecR were PCR amplified. These regions were chosen as the most likely interacting domains between FecA and FecR, since the N terminus of FecA reaches into the periplasm as does the C terminus of FecR (10, 28). The FecA region was cloned via the restriction endonuclease sites BstEII-XhoI into pMS604, giving rise to pUS10(FecA1–79), whereas the FecR region was cloned with XhoI-BglII restriction sites into pDP804, giving rise to pUS11(FecR101–317) (Fig. 4). These cloning strategies remove the Fos and Jun zipper motifs present on pMS604 and pDP804. Since the cloned regions were derived from PCR amplification, several independent clones were examined. Plasmids pUS12 and pUS13, also obtained by PCR amplification, represent amino- and carboxy-terminal deletions of FecR. This was done in an attempt to define a minimal region of FecR that interacts with FecA. The plasmid pUS12 encodes aa 151 to 317 of FecR fused to LexA1–87408 and represents the PCR amplification product with oligonucleotides LexFecR2 and LexFecR3, whereas pUS13 encodes aa 101 to 275 of FecR and is derived from oligonucleotides LexFecR1 and LexFecR7 (Fig. 4).
We examined the ability of pUS10(LexA1–87WT-FecA1–79) and pUS11(LexA1–87408-FecR101–317) to interact with the sulA operator and repress the production of β-galactosidase. Initially, we determined if the various proteins alone could repress the lac gene in the absence of an appropriate partner. The plasmids pDP804(LexA1–87408-Jun zipper), pMS604(LexA1–87WT-Fos zipper), pUS10(LexA1–87WT-FecA1–79) and pUS11(LexA1–87408-FecR101–317) alone showed no repression of the lac system. This nonrepressed level was then set as the 100% level. All subsequent β-galactosidase activity is given as a percentage of this level. The combination of the proteins LexA1–87WT-FecA1–79 with LexA1–87408-FecR101–317 gave a residual activity of 9%. β-Galactosidase activity of the control plasmid pMS604(LexA1–87WT-Fos zipper) with pDP804(LexA1–87408-Jun zipper) was on the order of 4 to 7%. The combination of LexA1–87WT-FecA1–79 with LexA1–87408-FecR101–317 showed repression to the same level as the active control. The amino- and carboxy-terminal FecR fragments encoded by pUS12(LexA1–87408-FecR151–317) and pUS13(LexA1–87408-FecR101–275) and combined with pUS10(LexA1–87 WT-FecA1–79) gave β-galactosidase activities on the order of 100% (Table 4). This would indicate that both FecR regions (residues 101 to 150 and 275 to 317) contribute to the interaction with FecA. This does not, however, rule out other sites of interaction with FecA within residues 151 to 274 of FecR. Next, we determined if there is any nonspecific interaction between proteins LexA1–87WT-FecA1–79 and LexA1–87408-Jun zipper and between LexA1–87408-FecR101–317 and LexA1–87 WT-Fos zipper. These protein combinations showed no repression of lacZ (Table 4). It is therefore clear that the repression of lacZ taking place with the protein combinations LexA1–87 WT-FecA1–79 and LexA1–87408-FecR101–317 is dependent on the interaction of the FecA and FecR domains fused to LexA.
TABLE 4.
β-Galactosidase activity by the bacterial two-hybrid Lex-based system in E. coli SU202 sulA::lacZa
| Protein combination | Percentage of β-galactosidase activity ± SD |
|---|---|
| LexA1–87408-Jun zipper | 100 ± 22 |
| LexA1–87WT-Fos zipper | 100 ± 18 |
| LexA1–87408-Jun zipper and LexA1–87WT-Fos zipper | 7 ± 3 |
| LexA1–87WT-FecA1–87 | 100 ± 6 |
| LexA1–87408-FecR101–317 | 100 ± 4 |
| LexA1–87WT-FecA1–79 and LexA1–87408-Jun zipper | 102 ± 6 |
| LexA1–87408-FecR101–317 and LexA1–87WT-Fos zipper | 88 ± 3 |
| LexA1–87WT-FecA1–79 and LexA1–87408-FecR101–317 | 9 ± 2 |
| LexA1–87WT-FecA1–79 and LexA1–87408-FecR151–317 | 124 ± 25 |
| LexA1–87WT-FecA1–79 and LexA1–87408-FecR101–275 | 107 ± 13 |
| LexA1–87WT-FecI1–173 | 85 ± 17 |
| LexA1–87408-FecR1–85 | 96 ± 14 |
| LexA1–87408-Jun zipper and LexA1–87WT-FecI1–173 | 113 ± 19 |
| LexA1–87WT-Fos zipper and LexA1–87408-FecR1–85 | 120 ± 12 |
| LexA1–87WT-FecI1–173 and LexA1–87408-FecR1–85 | 14 ± 4 |
| LexA1–87WT-FecI1–173 and LexA1–87408-FecR9–85 | 12 ± 4 |
| LexA1–87WT-FecI1–173 and LexA1–87408-FecR19–85 | 102 ± 19 |
| LexA1–87WT-FecI1–173 and LexA1–87408-FecR1–58 | 14 ± 5 |
| LexA1–87WT-FecI1–173 and LexA1–87408-FecR1–38 | 107 ± 10 |
| LexA1–87WT-FecI1–133 and LexA1–87408-FecR1–85 | 80 ± 21 |
| LexA1–87WT-FecI82–173 and LexA1–87408-FecR1–85 | 111 ± 23 |
The activity of the various protein combinations is shown as the relative percentage of β-galactosidase activity. An activity of 100% corresponds to approximately 250 Miller units. Percentage indicated is an average over three experiments; the standard deviation is shown.
In vivo interaction of the N terminus of FecR with FecI.
In vivo interaction of the C terminus of FecR with FecI was examined by the same bacterial two-hybrid system used for the FecA-FecR interaction. Prior to cloning the FecI gene fragment into pMS604, the residual Fos zipper was removed via PvuII-AsnI restriction. Since FecI contains a XhoI restriction endonuclease site, it was not possible to clone it in the same manner as FecA, and it was thus cloned via BstEII-PstI into pMS604, giving rise to pSM173. The plasmid pSM173 contains the complete fecI gene of 519 bp (173 aa) (Fig. 4). To define a minimal interacting region on FecI, amino- and carboxy-terminal deletions were constructed. Plasmid pSM133 contains aa 1 to 133 of FecI and was made by PCR amplification with oligonucleotides FecIBstEII and FecI4.2 (Table 2). The N-terminal deletion plasmid designated pSM4 contains aa 82 to 173 of FecI and was constructed by PCR amplification with oligonucleotides FecI2 and FecIPstI. For the construction of LexA1–87408-FecR1–85, the region encoding aa 1 through 85 of FecR was PCR amplified and cloned via XhoI-BglII into pDP804. The resultant plasmid was designated pSM85. This region represents the cytoplasmic domain of FecR, which is most likely where FecR interacts with FecI. In addition, various deletions were constructed in an attempt to define a minimal region of FecR that interacts with FecI. The resultant plasmids, designated pSM9 and pSM19, have the first 9 or 19 aa of FecR deleted, respectively. Plasmids pSM38 and pSM58 encode FecR fragments from aa 1 to 38 and 1 to 58, respectively. All four constructs were cloned into pDP804 via XhoI-BglII (Fig. 4). All FecR constructs are fused in frame with LexA1–87408, whereas the FecI constructs are fused to LexA1–87WT.
For the determination of the interaction between FecR and FecI, the same approach was taken for FecA as for FecR. The combinations of LexA1–87408-FecR1–85(pSM85) and LexA1–87WT-FecI1–173(pSM173) proteins showed clear repression of β-galactosidase activity (residual activity 14%) (Table 4). The deletions of FecR represented by the proteins LexA1–87408-FecR9–85(pSM9) and LexA1–87408-FecR1–58(pSM58) in combination with LexA1–87WT-FecI1–173 also repressed β-galactosidase synthesis to 12 and 14%, respectively (Table 4). The protein LexA1–87408-FecR9–85(pSM9) contains a deletion of 9 aa from the N terminus of FecR, whereas LexA1–87408- FecR1–58(pSM27) is a 27-aa deletion from the periplasmic C terminus. Other FecR deletions represented by LexA1–87408-FecR19–85(pSM19) and LexA1–87408-FecR1–38(pSM38) showed no repression. Furthermore, all deletions affecting FecI represented by LexA1–87WT-FecI1–133(pSM133) and LexA1–87WT-FecI82–173(pSM4) showed no repression. As controls, the proteins LexA1–87WT-FecI1–173(pSM173) and LexA1–87408-FecR1–85(pSM85) alone or in combination with LexA1–87408-Jun zipper(pDP804) and LexA1–87WT-Fos zipper(pMS604), respectively, showed no repression of β-galactosidase activity (Table 4). The data indicate that a region encompassing aa 9 to 58 of FecR is required for the interaction with FecI. Furthermore, it would appear that a large region of FecI is required for the interaction with FecR.
DISCUSSION
The chromosomally encoded FecR regulatory protein is contained in cells in such low amounts that it cannot be detected after radiolabeling and SDS-PAGE or by Western blotting. It has to be overexpressed, and then it precipitates as inclusion bodies (15, 29). Although FecR-(His)6 and (His)10-FecR were more hydrophilic than FecR, they also formed inclusion bodies which were barely contaminated with other proteins, as revealed by SDS-PAGE. The inclusion bodies were not soluble in buffers and buffers supplemented with detergents that usually do not denature proteins, such as octylglucoside, Triton X-100, Tween 20, CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}, and Nonidet P-40; for this reason these detergents are frequently used to solubilize cytoplasmic membrane proteins. Very likely, the small portion of active FecR-(His)6 and (His)10-FecR that was inserted in the cytoplasmic membrane was solubilized, but the amounts were not sufficient for detection by staining after SDS-PAGE and for performing in vitro binding studies. Urea (6 M) had to be used to solubilize the inclusion bodies. This procedure almost certainly denatured FecR-(His)6 and (His)10-FecR, and it is not known to what extent FecR-(His)6 and (His)10-FecR renatured during the dialysis to remove urea. However, retention of FecA and lack of retention of FecAΔ47-101 on the Ni-NTA agarose column and retention of FecI by FecR-(His)6 but not by (His)10-FecR demonstrate binding specificity which suggests correctly folded FecR derivatives are present. In addition, since not only the His-tagged complete proteins and the His-tagged proteolytic fragments but also the untagged fragments were retained by the Ni-NTA column, the untagged fragments were probably bound to the His-tagged fragments. A well-known example of the preservation and restoration of an active protein after the cleavage of a peptide bond is α-complementation of β-galactosidase. FecR is also cleaved by an unknown protease to the same two major products as are the His-tagged FecR derivatives. The requirement for cleavage of FecR for induction of the fec system was studied with a number of FecR mutants (29). Of eight FecR mutants which were inactive or which transcribed fecA-lacZ constitutively, seven were no longer cleaved. This clearly showed that there is no correlation between cleavage and phenotype, and since the mutations were scattered over the entire polypeptide, specific proteolytic processing can be ruled out for FecR activity (29).
The interaction and binding of the FecA N-terminal, but not the C-terminal, region to (His)10-FecR was shown by control experiments which demonstrated that FecA47, lacking residues 14 to 68, did not bind to (His)10-FecR, which clearly shows the specificity for the N terminus of FecA. Furthermore, the specificity of binding to the N terminus of FecR was demonstrated by experiments in which FecA did not bind to FecR-(His)6. The histidine residues at the C terminus of FecR-(His)6 apparently prevented binding of FecA. This lack of binding could be due to steric reasons such that the His tag masks the binding site on FecR or that fixation of FecR-(His)6 on Ni-NTA agarose hinders access to the C-terminal portion of FecR or the partially charged His residues repulse FecA. Since FecR-(His)6 displayed 60% in vivo activity in ferric citrate-dependent regulation of fecA-lacZ transcription, and since this activity should be much lower if binding of FecA to FecR-(His)6 is impaired, it is more likely that fixation of FecR-(His)6 to Ni-NTA agarose is the cause for the lack of FecA–FecR-(His)6 interaction. For the in vivo relevance of the demonstrated interaction between FecA and FecR-(His)6, one has to take into account that the in vitro interactions were not influenced by the addition of ferric citrate (data not shown). This was not unexpected, since transcription initiation in vivo requires not only binding of ferric citrate to FecA but also the electrochemical potential of the cytoplasmic membrane mediated by the Ton system (10). Since the in vitro conditions do not reflect the in vivo situation, these experiments do not reveal whether FecA binds permanently to FecR in vivo. However, the in vitro data clearly demonstrate that the N terminus of FecA and the C terminus of FecR are required for FecA-FecR interaction, which supports the previous in vivo data (10).
Active FecI was solubilized from inclusion bodies with a detergent, as was demonstrated previously (1). Solubilized FecI was shown to direct the RNA polymerase core enzyme to the promoter upstream of the fecA gene, as revealed by bandshift experiments and by in vitro runoff transcription assays (1). Here, FecR-(His)6 but not (His)10-FecR retained FecI on Ni-NTA-agarose; this result supports our previous data, which localized the N terminus of FecR in the cytoplasm and which demonstrated constitutive transcription of the fec transport genes by cytoplasmic N-terminal fragments of FecR (15, 28). The reasons for the failure of (His)10-FecR to bind FecI may be the same as those proposed for the failure of FecA to bind to FecR-(His)6. The relative levels of binding of FecA to (His)10-FecR compared to that seen for FecI binding to FecR-(His)6 may be explained by possible differences in the affinity of FecA compared to FecI for FecR. Alternatively, the binding sites on FecR-(His)6 for FecI are more accessible than those for FecA for binding to (His)10-FecR.
The approximately 60% activity of FecR-(His)6 and (His)10-FecR in ferric citrate-dependent induction of fecA transcription indicates that a fraction of the His-tagged FecR derivatives is properly inserted in the cytoplasmic membrane, receives the signal from FecA, transmits the signal across the cytoplasmic membrane, and activates FecI. Using SDS-PAGE and immunoblotting (see Fig. 1C) of the His-tagged FecR proteins cloned into high-copy-number plasmids and overexpressed, we found no indication that a fraction of the proteins lost the His tags and for this reason were active. The assays carried out to determine if the His-tagged FecR proteins are active were done using low-copy-number plasmids, which should significantly reduce the possibility that there is active FecR without a His tag. We cannot, however, rule out the possibility that very small amounts (below our levels of detection) of the His-tagged FecR proteins have lost their tag. Attempts to maintain FecR in solution by creating a hybrid protein with thioredoxin, which has been shown to work in a number of cases (12), did not prevent the formation of inclusion bodies for FecR (data not shown). The hybrid protein containing thioredoxin fused to the N-terminus of FecR displayed regulatory activities, similar to (His)10-FecR. Upon the addition of ferric citrate to the growth medium, β-galactosidase activity of a fecA-lacZ promoter fusion increased from 9 to 250 U, which amounts to 77% of the β-galactosidase activity obtained by induction with wild-type fecR (data not shown). This high level of activity is not surprising, since we know from our in vivo experiments with the bacterial two-hybrid Lex-based system that the first 9 aa of FecR are dispensable for activity and may serve as a linker between FecR and thioredoxin, thus leaving the cytoplasmic activity domain unaffected to interact with FecI.
The regions of FecA and FecR chosen to examine the interaction with the two-hybrid system were localized to the periplasm according to our previous studies. Moreover, the FecA N-proximal region constitutes a domain whose sole function appears to be important for the induction of fec transport gene transcription but not for transport itself. This has been shown by deletion studies in which residues 14 to 68 of the mature FecA protein were removed; this resulted in an induction-inactive but transport-competent FecA derivative. The structure of the N-terminal region of FecA is quite distinct from those of other TonB-dependent transporters. The TonB box required for transport and induction is located at residues 81 to 84 in FecA, since the FecA N terminus represents an extension when compared to the other TonB-dependent transporters in the outer membrane of E. coli K-12 in which the TonB box is close to the N terminus (10). The extension of FecA is not part of the globular domain that closes the channel of the β barrel in the crystal structure of FhuA (6, 13) and FepA (4), if one assumes an overall structure of FecA that is similar to FhuA and FepA. Rather, the long N terminus of FecA would be contained in the periplasm like the N terminus of FhuA and FepA which are not seen in the crystal structure, probably because they are flexible and assume no fixed structure. The strong allosteric changes in the periplasmically exposed region of the globular domain of FhuA upon ferrichrome binding may occur similarly in FecA upon ferric citrate binding. However, FecA in contrast to FhuA employs the structural transition to initiate a signaling cascade that finally initiates transcription of the fec transport genes. The C-proximal region of FecR and the N-proximal region of FecR used for studying interactions with FecA and FecI also form domains that are separated by the FecR transmembrane region (residues 85 to 100). We therefore employed three domains in the two-hybrid system which display some structural independence but receive signals from the surface-exposed ferric citrate binding site of FecA and transmit signals through the C-proximal region of FecR to the N-terminus of FecR and from there to FecI.
The use of the in vivo LexA-based repression system to look at the interaction of FecA with FecR and FecR with FecI clearly shows that these interactions do take place in vivo and supports the data obtained from the in vitro column binding assays. The LexA system has been successfully used to define very small regions of protein interaction, for example between the Jun and Fos zipper motifs (5). We have been able to show that the first 79 aa of the mature FecA polypeptide interact with the proposed periplasmic domain of FecR, consisting of aa 101 to 317. It has not been possible to reduce this region in FecR and still maintain FecR activity. FecA may interact with FecR over a large region, or FecA may interact with the N and C termini of the 101- to 317-aa region of FecR. It is also feasible that the active conformation of the periplasmic segment of FecR is impaired by the deletions.
Interaction of the cytoplasmic region of FecR with FecI has been reduced to a region between aa 9 and 58. In contrast, it has not been possible to determine the precise region of FecI that interacts with FecR, since both deletion derivatives were unable to form heterodimers. The interaction may take place over large or multiple domains of FecI, or the conformation of FecI may be disturbed by the deletions. We have some initial data based on phage display library biopanning which indicate that the FecR interactions take place over the entire FecI protein. FecI belongs to the family of ECF (extracytoplasmic function) sigma factors, which contain a number of functional domains. The deleted segments represent all of regions 2 and 4.2 of FecI. Initial data show that FecI deletion mutants, which have region 3 removed, are still able to interact with FecR. This would indicate, first, that deletions in FecI do not necessarily lead to instability and, second, that it is likely that there are sites within regions 2 and 4.2 which interact with FecR. The lack of interaction between the various deletion constructs indicates that the interaction of the various components of the Fec system is dependent on secondary structure conservation, and thus many deletions are likely to disrupt the structural framework. Alternatively, the deletions we have constructed in FecR and FecI are unstable, and attempts to show the stability of all of the LexA fusions used were unfortunately unsuccessful. It was not possible to show the presence of these fusion proteins either by Coomassie blue staining in the soluble or insoluble fraction or by immunoblotting with either FecA or FecR antiserum. This would indicate that these proteins are expressed at very low levels or, alternatively, that our antisera do not recognize those regions fused to LexA1–87. This is unlikely in the case of FecR, where large regions of FecR are fused to LexA; however, in the case of FecA, this is a possible explanation since only about 10% of the total length of the protein was fused to LexA.
Both the in vitro and in vivo data show interactions between the N terminus of FecA and the periplasmic domain of FecR and between the cytoplasmic domains of FecR and FecI. However, these interactions occurred in the absence of the ferric citrate inducer. We therefore conclude that the signal transduction from FecA to FecR and then to FecI is not due simply to the interaction itself but possibly to changes in interaction upon binding of ferric citrate to FecA. Alternatively, the Fec proteins may show an interaction pattern similar to that seen in the aspartate receptor in the chemotaxis sensory signaling cascade. In this system, there is a stable complex consisting of the aspartate receptor, CheW, and CheA. The binding of aspartate does not change the association constant of these proteins; in fact, binding causes only a very small change in the aspartate receptor itself. It is now assumed that this change is a piston-like motion of approximately 1 Å of the α-helical transmembrane domains of the receptor. This motion is transmitted from the periplasmic to the cytoplasmic side of the membrane and it is assumed to be perceived by the methylation and phosphorylation enzymes (17). This system exemplifies that protein-protein interactions are able to occur in these systems in the absence of an inducer molecule.
ACKNOWLEDGMENTS
We thank A. Angerer and K. Hantke for helpful discussions and M. Granger-Schnarr for providing the plasmids and strains of the Lex system.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 323, project B1). U.H.S. was the recipient of an Alexander von Humboldt Research Fellowship.
REFERENCES
- 1.Angerer A, Enz S, Ochs M, Braun V. Transcriptional regulation of ferric citrate transport in Escherichia coli K-12. FecI belongs to a new subfamily of ς70-type factors that respond to extracytoplasmic stimuli. Mol Microbiol. 1995;18:163–174. doi: 10.1111/j.1365-2958.1995.mmi_18010163.x. [DOI] [PubMed] [Google Scholar]
- 2.Braun V. Energy-coupled transport and signal transduction through the gram-negative outer membrane via TonB-ExbB-ExbD-dependent receptor proteins. FEMS Microbiol Rev. 1995;16:295–307. doi: 10.1111/j.1574-6976.1995.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 3.Braun V. Surface signaling: novel transcription initiation mechanism starting from the cell surface. Arch Microbiol. 1997;167:325–331. doi: 10.1007/s002030050451. [DOI] [PubMed] [Google Scholar]
- 4.Buchanan S K, Smith B S, Venkatramani L, Xia D, Esser L, Palnitkar M, Chakraborty R, van der Helm D, Deisenhofer J. Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat Struct Biol. 1999;6:56–63. doi: 10.1038/4931. [DOI] [PubMed] [Google Scholar]
- 5.Dmitrova M, Younes-Cauet G, Oertel-Buchheit P, Porte D, Schnarr M, Granger-Schnarr M. A new LexA-based genetic system for monitoring and analyzing protein heterodimerization in Escherichia coli. Mol Gen Genet. 1998;257:205–212. doi: 10.1007/s004380050640. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson A D, Hofmann E, Coulton J W, Diederichs K, Welte W. Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide. Science. 1998;282:2215–2220. doi: 10.1126/science.282.5397.2215. [DOI] [PubMed] [Google Scholar]
- 7.Giacomini A, Corich B, Ollero F J, Squartini A, Nuti M P. Experimental conditions may affect reproducibility of the β-galactosidase assay. FEMS Microbiol Lett. 1992;100:87–90. doi: 10.1111/j.1574-6968.1992.tb14024.x. [DOI] [PubMed] [Google Scholar]
- 8.Hanahan D. Studies in transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 9.Härle C, Kim J, Angerer A, Braun V. Signal transfer through three compartments: transcription initiation of the Escherichia coli ferric citrate transport system from the cell surface. EMBO J. 1995;14:1430–1438. doi: 10.1002/j.1460-2075.1995.tb07129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim I, Stiefel A, Plantör S, Angerer A, Braun V. Transcription induction of the ferric citrate transport genes via the N-terminus of the FecA outer membrane protein, the Ton system and the electrochemical potential of the cytoplasmic membrane. Mol Microbiol. 1997;23:333–344. doi: 10.1046/j.1365-2958.1997.2401593.x. [DOI] [PubMed] [Google Scholar]
- 11.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 12.LaVallie E R, DiBlasio E A, Kovacic S, Grant K L, Schendel P F, McCoy J M. A thioredoxin gene fusion expression system that circumvents inclusion body formation in the Escherichia coli cytoplasm. Bio/Technology. 1992;11:187–193. doi: 10.1038/nbt0293-187. [DOI] [PubMed] [Google Scholar]
- 13.Locher K P, Rees B, Koebnik R, Mitschler A, Moulinier L, Rosenbusch J P, Moras D. Transmembrane signaling across the ligand-gated FhuA receptor: crystal structures of free and ferrichrome-bound states reveal allosteric changes. Cell. 1998;95:771–778. doi: 10.1016/s0092-8674(00)81700-6. [DOI] [PubMed] [Google Scholar]
- 14.Miller J H. Experiments in molecular genetics. Cold Spring HarborNew York, N.Y.: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 15.Ochs M, Veitinger S, Kim I, Welz D, Angerer A, Braun V. Regulation of citrate-dependent iron transport of Escherichia coli: FecR is required for transcription activation by FecI. Mol Microbiol. 1995;15:119–132. doi: 10.1111/j.1365-2958.1995.tb02226.x. [DOI] [PubMed] [Google Scholar]
- 16.Ochs M, Angerer A, Enz S, Braun V. Surface signaling in transcriptional regulation of the ferric citrate transport system of Escherichia coli: mutational analysis of the alternative sigma factor FecI supports its essential role in fec transport gene transcription. Mol Gen Genet. 1996;250:455–465. doi: 10.1007/BF02174034. [DOI] [PubMed] [Google Scholar]
- 17.Ottemann K M, Xiao W, Shin Y-K, Koshland D E., Jr A piston model for transmembrane signaling of the aspartate receptor. Science. 1999;285:1751–1754. doi: 10.1126/science.285.5434.1751. [DOI] [PubMed] [Google Scholar]
- 18.Postle K. TonB protein and energy transduction between membranes. J Bioenerg Biomembr. 1993;25:591–601. doi: 10.1007/BF00770246. [DOI] [PubMed] [Google Scholar]
- 19.Pressler U, Staudenmaier H, Zimmermann L, Braun V. Genetics of the iron dicitrate transport system of Escherichia coli. J Bacteriol. 1988;170:2716–2724. doi: 10.1128/jb.170.6.2716-2724.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 21.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silhavy T S, Bermann M L, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- 23.Skare J T, Ahmer B M M, Seachord C L, Darveau R P, Postle K. Energy transducing between membranes. J Biol Chem. 1993;268:16302–16308. [PubMed] [Google Scholar]
- 24.Staudenmaier H, Van Hove B, Yaraghi Z, Braun V. Nucleotide sequences of the fecBCDE genes and locations of the proteins suggest a periplasmic-binding-protein-dependent transport mechanism for iron(III)dicitrate in Escherichia coli. J Bacteriol. 1989;171:2626–2633. doi: 10.1128/jb.171.5.2626-2633.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Studier F W, Moffat B A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 26.Tabor S, Richardson C C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci USA. 1985;82:1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeshita S, Sato M, Toba M, Masahashi W, Hashimoto-Gotoh T. High-copy-number and low-copy-number plasmid vectors for lacZ α-complementation and chloramphenicol- or kanamycin-resistance selection. Gene. 1987;61:63–74. doi: 10.1016/0378-1119(87)90365-9. [DOI] [PubMed] [Google Scholar]
- 28.Welz D, Braun V. Ferric citrate transport of Escherichia coli: functional regions of the FecR transmembrane regulatory protein. J Bacteriol. 1998;180:2387–2394. doi: 10.1128/jb.180.9.2387-2394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wriedt K, Angerer A, Braun V. Transcriptional regulation from the cell surface: conformational changes in the transmembrane protein FecR lead to altered transcription of the ferric citrate transport genes in Escherichia coli. J Bacteriol. 1995;177:3320–3322. doi: 10.1128/jb.177.11.3320-3322.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zimmermann L, Hantke K, Braun V. Exogenous induction of the iron dicitrate transport system of Escherichia coli. J Bacteriol. 1984;159:271–277. doi: 10.1128/jb.159.1.271-277.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]