Abstract
In order to extend the limited knowledge about crenarchaeal DNA polymerases, we cloned a gene encoding a family B DNA polymerase from the hyperthermophilic crenarchaeon Pyrobaculum islandicum. The enzyme shared highest sequence identities with a group of phylogenetically related DNA polymerases, designated B3 DNA polymerases, from members of the kingdom Crenarchaeota, Pyrodictium occultum and Aeropyrum pernix, and several members of the kingdom Euryarchaeota. Six highly conserved regions as well as a DNA-binding motif, indicative of family B DNA polymerases, were identified within the sequence. Furthermore, three highly conserved 3′-5′ exonuclease motifs were also found. The gene was expressed in Escherichia coli, and the DNA polymerase was purified to homogeneity by heat treatment and affinity chromatography. Activity staining after sodium dodecyl sulfate-polyacrylamide gel electrophoresis revealed an active polypeptide of approximately 90 kDa. For the recombinant DNA polymerase from P. islandicum, activated calf thymus DNA was used as a substrate rather than primed single-stranded DNA. The enzyme was strongly inhibited by monovalent cations and N-ethylmaleimide; it is moderately sensitive to aphidicolin and dideoxyribonucleoside triphosphates. The half-life of the enzyme at 100 and 90°C was 35 min and >5 h, respectively. Interestingly, the pH of the assay buffer had a significant influence on the 3′-5′ exonuclease activity of the recombinant enzyme. Under suitable assay conditions for PCR, the enzyme was able to amplify λ DNA fragments of up to 1,500 bp.
While our knowledge about DNA replication in Eucarya and Bacteria is quite advanced (22), only very limited information is available on the replication system in Archaea (14, 24). Deduced from their amino acid sequences, DNA polymerases can be classified into the four families A, B, C, and X (4). The majority of archaeal DNA polymerases described to date, as well as the eucaryotic replicative DNA polymerases α, δ, and ɛ, belongs to the family B (28). Genome sequence analyses have indicated that many euryarchaeal genomes encode a single family B DNA polymerase (5, 16, 21). Additionally, a new heterodimeric DNA polymerase was detected in several members of the kingdom Euryarchaeota, and the biochemical properties indicate that this enzyme could play a role in replication (7, 20, 43). Comparatively little information is available on DNA polymerases from hyperthermophilic members of the kingdom Crenarchaeota. Recent investigations have revealed that Euryarchaeota and Crenarchaeota differ in their DNA replication mechanisms. A sequence homologous to the heterodimeric DNA polymerase found in several Euryarchaeota has so far not been detected in Crenarchaeota. The finding of two family B DNA polymerase genes in Pyrodictium occultum (42) and Aeropyrum pernix (6) and three in Sulfolobus solfataricus (10, 15, 32, 33) reveals that, in contrast to Euryarchaeota, several B-type DNA polymerases are involved in crenarchaeal DNA replication. Phylogenetic analysis has indicated that these multiple crenarchaeal family B DNA polymerases fall into three distinct clusters, designated as the B1, B2, and B3 groups (15). Interestingly, none of the currently described crenarchaeal DNA polymerases has been successfully applied to PCR.
The hyperthermophilic crenarchaeon Pyrobaculum islandicum was isolated in 1987 from an islandic hot spring (19) and was assigned to the order Thermoproteales. Due to the difficulties in cultivating this strictly anaerobic microorganism, P. islandicum has been poorly investigated with respect to physiology, protein chemistry, and molecular biology. Our understanding of the crenarchaeal replication process would be enhanced by a comparative analysis of DNA polymerases from a Thermoproteales species with those from phylogenetically distant Crenarchaeota and Euryarchaeota species.
In this study, we present for the first time detailed data on the cloning and expression of a gene from P. islandicum and the first description of a DNA polymerase within the order Thermoproteales. A sequence comparison of the encoded DNA polymerase to other archaeal proteins indicates that the enzyme belongs to the B3 group of DNA polymerases. Phylogenetic analysis of the sequence contributes to our knowledge on the propagation and evolution of archaeal DNA polymerases. The purified, recombinant enzyme was biochemically characterized, and its application in PCR was successfully demonstrated.
MATERIALS AND METHODS
Strains, enzymes, vectors, and chemicals.
P. islandicum DSM 4184T (19) was obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen, GmbH (DSMZ; Braunschweig, Germany). Escherichia coli BL21 cells were obtained from Novagen. Unless otherwise mentioned, enzymes, chemicals, and kits for manipulations of DNA and for the characterization of the DNA polymerase were obtained from Roche Diagnostics. Kits for purification and gel extraction of DNA were products of Qiagen. A kit for the preparation of plasmid and cosmid DNA was obtained from Bio-Rad. The expression vector pASK-IBA 3, the inductor anhydrotetracycline, and the Streptactin Sepharose columns were purchased from IBA (Göttingen, Germany). N-ethylmaleimide (NEM) was obtained from Serva (Heidelberg, Germany). Other chemicals for the preparation of media and buffers were obtained from Difco, Serva, Sigma, and Merck (Darmstadt, Germany).
Isolation of DNA.
P. islandicum cells were grown anaerobically at 98°C in 1.5 liters of medium 390 as recommended by DSMZ. Cells were harvested, washed with TE buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA), and suspended in 3 ml of 10 mM Tris-HCl (pH 8.0) containing 20% (wt/vol) sucrose. Triton X-100 was added to a final concentration of 0.3% (vol/vol). After incubation at 20°C for 1 h, 3 ml of SET buffer (150 mM NaCl, 20 mM Tris-HCl [pH 8.0], 1 mM EDTA), 0.32 ml of 10% (wt/vol) sodium dodecyl sulfate (SDS), and 0.16 ml of proteinase K (10 mg/ml) were added. The mixture was incubated for 1 h at 37°C. After subsequent phenol-chloroform extraction, DNA was recovered by ethanol precipitation (25).
PCR and preparation of probes.
For the amplification of a P. islandicum DNA polymerase gene fragment, two degenerated primers were designed based on the conserved regions I and II of family B-like DNA polymerases (45). The forward primer was 5′-GTI(CT)TIGATTT(CT)GCI(AT)(GC)I(CT)TITA(CT)CC-3′, and the reverse primer was 5′-GAAIAGIGAGTCIGTATCICCAT-3′, using deoxyinosine, which is complementary to all four nucleotides. Primers were custom synthesized by ARK Scientific, GmbH Biosystems (Darmstadt, Germany). Approximately 1 ng of P. islandicum DNA and 100 pmol of each primer were added to a PCR mixture containing 200 μM deoxyribonucleoside triphosphates and 2 U of Expand High Fidelity enzyme (Roche Diagnostics). After an initial denaturation step at 94°C for 2 min, 30 cycles with a temperature profile of 10 s at 94°C, 30 s at 46°C, and 90 s at 68°C were performed with a DNA thermal cycler (GeneAmp PCR System 2400; Perkin-Elmer). Following the last cycle, the samples were incubated for an additional 7 min at 68°C to ensure completion of the extension step. The PCR product was purified and sequenced.
The same PCR mixture, also containing the template and primers described above and 200 μM (each) dATP, dGTP, and dCTP, but 190 μM dTTP and 10 μM digoxigenin (DIG)-11-dUTP (DIG-labeled dUTP), was used to prepare a nonradioactive probe.
PCRs with P. islandicum DNA polymerase were carried out with 1 U of enzyme in the following buffer: 15 mM Tris-HCl (pH 8.6), 12.5 mM KCl, 2.5 mM (NH4)2SO4, 1.25 mM MgCl2, and 20 μg of bovine serum albumin (BSA) per ml. λ DNA (10 ng) was used as template. The forward primer 5′-GATGAGTTCGTGTCCGTACAACT-3′ was combined with the following reverse primers: 5′-GGTTATCGAAATCAGCCACAGCG-3′, for amplification of 500 bp; 5′-GTTAACTTTGATTCTGGCCTGCG-3′, for amplification of 1,000 bp; and 5′-GTGAGATAAACGGCAACTGCCGG-3′, for amplification of 1,500 bp. PCR cycles were carried out as described before with a temperature profile of 10 s at 94°C, 30 s at 56°C, and 1.5 min at 75°C. Expand High Fidelity enzyme was used as a control under PCR conditions as recommended by the manufacturer.
Genomic Southern blot hybridization.
P. islandicum DNA was digested overnight with several restriction enzymes and resolved on a 0.7% agarose gel. The DNA was transferred to a positively charged nylon membrane. The DIG-labeled probe described before was used to perform hybridizations at 68°C. DNA fragments which hybridized to the probe were immunologically detected according to the manufacturer's instructions (DIG System User's Guide for Filter Hybridization, Roche Diagnostics).
Cosmid cloning.
Following Southern blot hybridization, the restriction enzyme SacI was chosen to perform a preparative digestion of P. islandicum DNA. DNA fragments between the sizes of 6.5 and 7.5 kbp were extracted from an agarose gel and cloned using the Expand Cloning Kit (Roche Diagnostics). The fragments were blunt ended with T4 DNA polymerase and ligated into the cosmid vector, and the constructs were packed into bacteriophages. An E. coli DH5α magnesium culture included in the kit was subsequently infected. Positive clones were selected by the vector-mediated ampicillin resistance. A cosmid clone containing the DNA polymerase gene was identified by colony filter hybridization using the probe described before. Cosmid DNA from a positive clone was prepared and sequenced.
DNA sequencing.
Purified PCR products and cosmid DNA preparations were custom sequenced by SeqLab (Göttingen, Germany). The nucleotide sequences were determined by the dideoxy chain termination method (34).
RNA isolation and determination of transcription start.
In order to examine the transcription start site of the detected DNA polymerase gene, RNA from freshly cultivated P. islandicum cells was prepared, following a protocol published by DiRuggiero and Robb (13). A 5′ rapid amplification of cDNA end (RACE) PCR was performed with the 5′/3′ RACE Kit (Roche Diagnostics). For first-strand cDNA synthesis, cDNA amplification and control PCR reverse primers deduced from the gene sequence were used. The amplified cDNA was gel extracted and directly sequenced.
Alignment of the amino acid sequences.
Except for the DNA polymerase sequence of P. islandicum, all other sequences were retrieved from public databases. To determine sequence identities, we carried out a pairwise alignment using the program SPADE (Search Procedure for All Diagonal Elements; C. Vorgias and K. Paliakasis, personal communication). The program is based on the diagonal plot known as dot blot concept. It was designed to avoid difficulties in alignment of sequences that share short areas of high identities and/or long areas of low or no identities. In contrast to dynamic programming, which returns the best overall alignment for a given scoring scheme, SPADE presents only the parts of sequences which are achieved in a reliable, gap-free alignment.
The software program Clustal W (Higgins, European Molecular Biology Laboratory, Heidelberg, Germany) was used for multiple sequence alignments and the identification of conserved regions within the DNA polymerase.
Expression and purification of P. islandicum DNA polymerase.
The DNA polymerase gene was expressed in E. coli using the vector pASK-IBA 3. The expression cassette of this vector is transcriptionally controlled by the chemically inducible tetracycline promoter (38). For convenient purification of the recombinant protein, the vector C terminally contained an eight-amino-acid coding sequence, called Strep-tag. The Strep-tag allows the purification of the resulting fusion protein by affinity chromatography on Sepharose-coupled streptavidin or Streptactin (37, 44). Two primers, Exforw-BsaI (5′-GCTGATGGTCTCGAATGGAACTAAAAGTTTGGCCTCT-3′) and Exrev-BsaI (5′-GCTGATGGTCTCCGCGCTACTTAGAAAATCAAGAAGCGACCT-3′), were used to amplify the polymerase gene from chromosomal P. islandicum DNA. Both primers were designed with a BsaI restriction site. The start codon, encoding valine, was replaced by the vector-encoded methionine. The stop codon of the polymerase gene was removed. The PCR product was purified by gel extraction, cleaved with BsaI, gel extracted, and ligated into BsaI-restricted pASK-IBA 3 vector. Expression of the polymerase gene was performed in E. coli BL21. Transformed cells were cultivated at 37°C in 1 liter of Luria-Bertani medium containing 100 μg of ampicillin per ml. At an optical density at 600 nm of 0.5, expression was induced by the addition of 100 μl of anhydrotetracycline (2 mg/ml in dimethyl formamide; final concentration, 200 μg/liter). The culture incubation was continued for a further 15 h. After cooling, the cells were harvested by centrifugation at 4°C, washed with buffer W (50 mM Tris-HCl [pH 8.0], 1 mM EDTA plus Complete mini, EDTA-free protease inhibitor cocktail [Roche Diagnostics]), and resuspended in 5 ml of the same buffer. The cells were disrupted by sonication, and after removing the cell debris by centrifugation, the cell-free crude extract was heat treated at 80°C for 20 min and centrifuged again. The supernatant was applied onto a Sepharose-coupled Streptactin column (4 ml), equilibrated with buffer W. After washing the column twice with 20 ml of buffer W, proteins were eluted by the stepwise application of 20 ml of buffer W containing 2.5 mM desthiobiotin (Sigma). Active fractions were pooled and dialyzed against 1,000 volumes of 50 mM Tris-HCl (pH 7.3) and 10% (vol/vol) glycerol.
Polymerase activity assay.
A nonradioactive colorimetric enzyme immunoassay (DNA Polymerase Assay, nonradioactive; Roche Diagnostics) was performed, based on the incorporation of DIG- and biotin-labeled dUTP into the same DNA. The detection and quantification of the synthesized DNA as a parameter for DNA polymerase activity followed a sandwich enzyme-linked immunosorbent assay protocol, as described by the manufacturer. Unless otherwise mentioned, the standard reaction mixture contained, in a 100-μl volume, 50 mM Tris-HCl (pH 7.3), 0.5 mM MgCl2, 60 μg of BSA, 0.36 μM DIG-11-dUTP, 18 nM biotin-11-dUTP, 18 μM (each) dGTP, dCTP, and dATP, 1.8 μM dTTP, 1.2 μg of DNase I-activated calf thymus DNA as a template, and the DNA polymerase sample. The reaction mixture was incubated at 75°C for 1 h. One unit of DNA polymerase was defined as the amount of enzyme required to incorporate 120 fmol of DIG-11-dUTP per 30 min at 75°C. DNA polymerases from Thermus aquaticus (Taq polymerase; Life Technologies) was used for the production of calibration curves, with 50 mM Tris-HCl (pH 7.9), 5 mM MgCl2, and 50 mM KCl in the reaction mixture. In order to determine the thermostability of the DNA polymerase from P. islandicum, preincubations were performed in a reaction mix without BSA, labeled or nonlabeled deoxynucleoside triphosphate (dNTP), and template DNA, followed by the addition of the missing components and standard incubation of 1 h at 75°C. The template specificity was investigated in the standard reaction mixture, using 240 ng of activated calf thymus DNA, the same amount of circular single-stranded M13 DNA (Pharmacia) primed with a 10-fold molar excess of a 45-mer primer (5′-CCGGAACCGCCTCCCTCAGAGCCGCCACCCTCAGAACCGCCACCC-3′), or 200 ng of a poly(A)-oligo(dT)15 template (Roche Diagnostics). For the determination of all biochemical properties of the DNA polymerase, reaction mixtures contained 0.2 U of enzyme.
DNA polymerase activity gel.
The detection of DNA polymerase activities on SDS polyacrylamide gels was performed according to a modified version (27) of the method described by Spanos and Hübscher (39). Taq polymerase was used as a positive control. E. coli Klenow enzyme (Roche Diagnostics) and a sonicated crude extract of E. coli BL21 cells were used as negative controls. Crude extracts of E. coli BL21 and P. islandicum were prepared in 50 mM Tris-HCl (pH 7.3), 0.5 mM MgCl2, and 10% glycerol. Sample concentrations of 0.5 to 1 U of each DNA polymerase were run on SDS–10% polyacrylamide gels. Sample preparation and gel electrophoresis were carried out as described by Laemmli (23). In addition, the resolving gel contained 150 μg of DNAse I-activated calf thymus DNA per ml as template for the DNA polymerases. SDS was removed by immersing the gel four times in 50 mM Tris-HCl (pH 7.3), 3 mM β-mercaptoethanol, 1 mM EDTA, and 5% (vol/vol) glycerol. Renaturation of the proteins was performed at 4°C overnight using 1 mM MgCl2 and 1 μM dATP added to the same buffer. The gel was then preincubated for 30 min at 70°C in a buffer containing 50 mM Tris-HCl (pH 7.3), 3 mM β-mercaptoethanol, 1 mM dithiothreitol, 1 mM MgCl2, and 5% glycerol, transferred into activity buffer containing 50 mM Tris-HCl (pH 7.3), 3 mM β-mercaptoethanol, 1 mM dithiothreitol, 1 mM MgCl2, 5% (vol/vol) glycerol, 5 μM (each) dGTP, dATP, and dCTP, 2.5 μM dTTP, and 1 μM DIG-11-dUTP, and incubated at 70°C for 4 h. DNA was transferred to a nylon membrane, and incorporated DIG-11-dUTP was detected immunologically as previously described.
Assay for exonuclease activity.
For 3′-5′ exonuclease activity on single strands, the degradation of a synthetic primer (22-mer), 5′ labeled with DIG for immunological detection, was analyzed. 3′-5′ exonuclease activity on double strands was measured using the same primer, hybridized to a template (34-mer). (For illustrations of the primer and template sequences, see Fig. 7.) The assay principle was described previously (27). Labeled substrate (0.5 pmol) and purified DNA polymerase (0.1 U) were incubated in 10 μl of different buffers with and without increasing amounts of dNTPs (up to 1 μM) at 70°C for 1 h. To ensure that no contaminating exonuclease activity from E. coli was measured, the assay was further carried out at 37°C. The optimal DNA polymerase buffer containing 50 mM Tris-HCl (pH 7.3) and 0.5 mM MgCl2 was used as well as a buffer containing 50 mM Tris-HCl (pH 8.6) and 0.5 mM MgCl2. The reaction was stopped on ice with formamide buffer, and the samples were electrophoresed on a 12% polyacrylamide sequencing gel containing 8 M urea in Tris-borate-EDTA (TBE) buffer. The degradation or polymerization products were visualized by immunological detection. Pwo polymerase in a buffer containing 10 mM Tris-HCl (pH 8.9), 25 mM KCl, 2 mM MgSO4, and 5 mM (NH4)2SO4 and Taq polymerase in activity buffer (described above) were used as controls.
FIG. 7.
Single- and double-strand-dependent 3′-5′ exonuclease activity of DNA polymerases from P. islandicum (P. isl. pol), T. aquaticus (Taq pol), and P. woesei (Pwo pol). The assay was carried out, as described in Materials and Methods, at 70°C for 1 h with 0.1 U of each polymerase. A DIG-labeled primer as a single-stranded substrate (ss) and a primer-template complex as a double-stranded substrate (ds) were used. Concentrations of dNTP (micromolar) in each reaction are indicated. The reaction products were separated on a 12% polyacrylamide gel containing 8 M urea and visualized by immunological detection. The sizes (nucleotides [nt]) of the nondegraded primer and the reaction products are indicated. Lane P, DIG-labeled primer in buffer without dNTPs; buffer 1, 50 mM Tris-HCl (pH 7.3), 0.5 mM MgCl2; buffer 2, 50 mM Tris-HCl (pH 8.6), 0.5 mM MgCl2. Taq and Pwo polymerases were tested using buffers according to Materials and Methods. Primer and template sequences are illustrated on the right.
Nucleotide sequence accession number.
The sequence of the DNA polymerase from P. islandicum described in this study has been deposited in GenBank under accession number AF195019.
RESULTS AND DISCUSSION
Cloning and nucleotide sequencing of a DNA polymerase gene from P. islandicum.
Based on the most conserved amino acid sequences found in archaeal family B DNA polymerases, two degenerated primers were designed. The PCR was performed with chromosomal DNA from P. islandicum, and a reaction product of 445 bp was obtained. The sequence of the PCR product showed the expected identity to already known archaeal DNA polymerase regions in a BLAST alignment. The PCR product was used as a probe to obtain the whole DNA polymerase gene from P. islandicum. In Southern blot hybridizations, SacI-digested DNA fragments with sizes of 6.5 to 7.5 kbp were chosen to be cloned in a cosmid vector system. In a cosmid clone that was identified by colony filter hybridization, an open reading frame of 2,358 bp encoding a protein of 785 amino acids was detected (Fig. 1). The open reading frame starts with GTG, encoding valine. It has been shown that approximately 25% of archaeal proteins appear to start at GTG codons (11). Schleper et al. (35, 36) described several open reading frames in the crenarchaeon Cenarchaeum symbiosum, including a family B DNA polymerase gene, starting with GTG instead of ATG. In order to determine the transcription start site of the DNA polymerase gene from P. islandicum, a 5′ RACE PCR was performed, and the amplified cDNA was sequenced. The 5′ terminus of the mRNA sequence corresponds to the 5′ guanosine of the GTG start codon. This suggests that the polymerase mRNA is synthesized without a leader, as was already reported for several genes of the halophilic archaeon Halobacterium salinarium (2, 29). As shown in Fig. 1, an archaeal promoter sequence TTTATT (box A), essential for transcription initiation (9, 18), could be identified in the 5′ noncoding region of the DNA polymerase gene. Whereas the typical box A consensus sequence of an archaeal promoter seems to be TTTATA, in some promoters, e.g., from Thermoproteus tenax, Desulfurolobus ambivalens, or Thermoplasma acidophilum, a box A sequence TTTATT has been detected (18). The distance between the TA sequence of box A and the transcription start point of the P. islandicum DNA polymerase gene is 23 nucleotides. A relatively small distance of 23 or less nucleotides between the TA sequence of box A and the transcription start point has also been detected in several other archaeal promoters (18). The putative ribosome-binding site overlapping the GTG start codon was detected (Fig. 1). Datukishvili et al. (10) have also reported on a ribosome-binding site overlapping the ATG start codon of a DNA polymerase gene from Sulfolobus acidocaldarius. Although no common pattern of secondary structure is present among archaeal transcription termination regions, these sites appear to be indicated by a T-rich polypyrimidine sequence (9). As shown in Fig. 1, a putative transcription termination site was identified downstream of the stop codon of the DNA polymerase gene from P. islandicum.
FIG. 1.
Nucleotide sequence of the polymerase gene and deduced amino acid sequence (785 amino acids) of the family B DNA polymerase from P. islandicum. The GTG start codon, encoding valin, is illustrated in bold. The transcription start site, determined by 5′ RACE, is marked with a bullet above it (•). An archaeal consensus box A motif (double underlined) is indicated. The putative ribosome-binding site is underlined and in italic. A T-rich polypyrimidine sequence, indicating a putative transcription termination site, is underlined.
Comparison of the sequence to other known DNA polymerases.
The DNA polymerase sequence was aligned with those of archaeal DNA polymerases available in public databases. The six conserved motifs indicative of family B DNA polymerases (4) were identified in the P. islandicum DNA polymerase sequence, including those residues that are invariant in all DNA polymerases and have been shown to be functionally important (Fig. 2) (8). The three 3′-5′ exonuclease motifs (3) were also localized in the sequence (Fig. 2). The DNA-binding motif Y-G(G/A), which is highly conserved among family B DNA polymerases (4), was also found in the DNA polymerase sequence from P. islandicum. This motif is situated between the Exo III and Pol II regions and is involved in switching the primer terminus between the polymerase and the 3′-5′ exonuclease active sites (30, 31, 40, 41).
FIG. 2.
Amino acid alignment of archaeal family B DNA polymerases. Only selected representatives belonging to the B3 group (15) are shown. A total of 573 residues from the P. islandicum polymerase (785 amino acids) is represented in this alignment. A total of 127 residues at its N terminus and 85 residues at the C terminus have been omitted. Pol I through Pol VI, the conserved regions of family B DNA polymerases (4); Exo I through Exo III, conserved motifs of the 3′-5′ exonuclease domain (3); and the DNA-binding motif Y-G(G/A) (31, 40, 41) are marked. Letters boxed and shaded in gray represent identical positions found in four or more of the six sequences. Gaps within the alignment are indicated by dashes.
In a pairwise alignment, the DNA polymerase sequence from P. islandicum showed highest identities to the following family B DNA polymerases: P. occultum Pol B (accession no. D38574), A. pernix Pol II (accession no. AB017501), Archaeoglobus fulgidus Pol B3 (accession no. AE001070), Pfu polymerase (accession no. D12983), and Thermococcus sp. 9°N-7 DNA polymerase (accession no. U47108) (Table 1). In contrast, the sequence identities of the DNA polymerase from P. islandicum to other crenarchaeal family B DNA polymerases were comparatively lower. This result was in agreement with recent reports concerning the phylogenetic relationships of archaeal DNA polymerases. Edgell et al. (15) found that all known crenarchaeal family B DNA polymerases fall into three distinct groups. One group comprises exclusively crenarchaeal DNA polymerases, which have been designated B1 DNA polymerases. The second group includes one of the three DNA polymerases from S. solfataricus, designated B2 DNA polymerase, and a DNA polymerase from the euryarchaeon A. fulgidus (16). The third group, termed as B3 DNA polymerases, groups together with most euryarchaeal family B DNA polymerases (15). As indicated in Table 1, the DNA polymerase from P. islandicum shows highest identities to archaeal DNA polymerases of the B3 group. Interestingly, the B3 DNA polymerase from S. solfataricus (accession no. Y08257) shows comparatively low levels of identities to the DNA polymerases from P. islandicum and other members of this group. This result is in agreement with the previous reports (15) that the S. solfataricus B3 DNA polymerase, which until now has not been successfully expressed, contains a number of unusual amino acid substitutions in functional important polymerase and exonuclease domains (Fig. 2). To date, only the B3 DNA polymerases from three Crenarchaeota, A. pernix (6), P. occultum (42), and P. islandicum (this work), have been successfully expressed and characterized.
TABLE 1.
Amino acid sequence comparison of family B DNA polymerases
| Sourceb | % Identity of sequence froma: (identical amino acids/common length)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P. islandicum B3 (785 aa) | P. occultum B3 (803 aa) | A. pernix B3 (772 aa) | S. solfataricus B3 (764 aa) | A. fulgidus B3 (781 aa) | P. furiosus B3 (771 aa) | Thermococcus sp. 9°N-7 B3 (775 aa) | P. occultum B1 (914 aa) | A. pernix B1 (923 aa) | S. solfataricus B1 (882 aa) | C. symbiosum B1 (844 aa) | |
| P. islandicum B3 | 100 | ||||||||||
| P. occultum B3 | 53 | 100 | |||||||||
| (416/780) | |||||||||||
| A. pernix B3 | 53 | 59 | 100 | ||||||||
| (391/745) | (452/768) | ||||||||||
| S. solfataricus B3 | 32 | 34 | 35 | 100 | |||||||
| (244/753) | (259/758) | (253/734) | |||||||||
| A. fulgidus B3 | 42 | 45 | 44 | 31 | 100 | ||||||
| (317/758) | (342/759) | (320/728) | (231/738) | ||||||||
| P. furiosus B3 | 37 | 39 | 38 | 29 | 41 | 100 | |||||
| (267/724) | (290/744) | (260/704) | (197/674) | (299/731) | |||||||
| Thermococcus sp. 9°N-7 B3 | 36 | 37 | 39 | 28 | 40 | 80 | 100 | ||||
| (263/725) | (277/744) | (273/704) | (195/688) | (293/730) | (621/774) | ||||||
| P. occultum B1 | 33 | 32 | 35 | 24 | 31 | 30 | 32 | 100 | |||
| (231/707) | (206/642) | (237/677) | (139/580) | (206/672) | (215/722) | (208/645) | |||||
| A. pernix B1 | 31 | 32 | 31 | 23 | 31 | 29 | 29 | 49 | 100 | ||
| (220/731) | (218/679) | (206/669) | (147/629) | (199/651) | (194/681) | (197/675) | (438/889) | ||||
| S. solfataricus B1 | 30 | 31 | 33 | 22 | 30 | 31 | 30 | 52 | 46 | 100 | |
| (204/671) | (215/684) | (207/636) | (153/689) | (210/708) | (210/674) | (216/721) | (439/838) | (400/867) | |||
| C. symbiosum B1 | 27 | 30 | 31 | 22 | 28 | 28 | 28 | 42 | 40 | 38 | 100 |
| (177/647) | (180/609) | (196/637) | (126/567) | (189/671) | (187/661) | (165/595) | (335/802) | (329/825) | (316/829) | ||
| S. solfataricus B2 | 30 | 30 | 27 | 23 | 29 | 26 | 27 | 28 | 27 | 24 | 27 |
| (122/402) | (120/395) | (135/501) | (96/421) | (103/351) | (119/459) | (103/387) | (118/416) | (137/505) | (129/534) | (102/381) | |
Identity values were calculated by the program SPADE (C. Vorgias and K. Paliakasis, personal communication) in pairwise comparisons. The values are indicated as percent identities in common length of both aligned sequences. aa, amino acids.
Nomenclature of the polymerases (B1, B2, and B3) is that of Edgell et al. (15).
Expression and purification of the P. islandicum DNA polymerase.
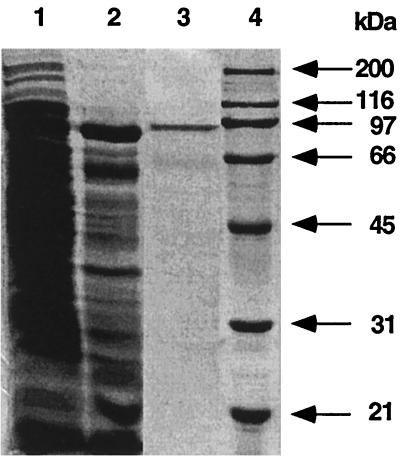
After determining the sequence of the P. islandicum DNA polymerase, the gene was cloned into the vector pASK-IBA 3, and the enzyme was expressed as a fusion protein with a C-terminal Streptactin-binding oligopeptide. The recombinant DNA polymerase was purified to homogeneity by a heat denaturation step (20 min at 80°C) followed by affinity chromatography on Streptactin. Starting from 1 liter of E. coli BL21 culture, 0.4 mg of recombinant DNA polymerase with a specific activity of 1,255 U/mg was purified (Table 2). The purity of the DNA polymerase after each purification step was monitored by SDS-polyacrylamide gel electrophoresis (Fig. 3). The activity gel shows that the purified recombinant protein of approximately 90 kDa in the Streptactin preparation possesses DNA polymerase activity (Fig. 4). In crude extracts of P. islandicum, a DNA polymerase activity band with a molecular mass of approximately 90 kDa could be detected (Fig. 4). No activity could be detected in the E. coli Klenow enzyme and the crude extract of E. coli BL21 cells, indicating that the activity gel specifically showed thermoactive DNA polymerase activities.
TABLE 2.
Purification scheme of the DNA polymerase from P. islandicum
| Purification step | Total protein (mg) | Total activity (U) | Sp act (U/mg) | Purification (fold) | Recovery (%) |
|---|---|---|---|---|---|
| Crude extract | 45.0 | 1,138 | 25.3 | 1 | 100 |
| Heat denaturation | 11.5 | 768 | 66.8 | 2.6 | 68 |
| Streptactin chromatography | 0.4 | 502 | 1,255.0 | 49.6 | 44 |
FIG. 3.
SDS-polyacrylamide gel electrophoresis of enzymatic fractions during purification of the recombinant polymerase. The SDS–10% polyacrylamide gel was stained with Coomassie brilliant blue R-250 after electrophoresis. Lane 1, sonicated crude extract; lane 2, crude extract after heat treatment (20 min at 80°C) and centrifugation; lane 3, protein fraction eluted from the Streptactin column; lane 4, broad-range marker (Bio-Rad).
FIG. 4.
Activity gel analysis of the thermoactive DNA polymerase from P. islandicum. Polymerizing activity of renatured protein after separation on an SDS–10% polyacrylamide gel was detected by in situ incorporation of DIG-labeled dUTP at 70°C, as described in Materials and Methods. Lane 1, Taq polymerase (0.5 U); lane 2, E. coli Klenow enzyme (0.5 U); lane 3, purified P. islandicum DNA polymerase (1 U); lane 4, sonicated crude extract of E. coli BL21 (100 μg); lane 5, sonicated crude extract of P. islandicum (100 μg). Unit definitions for Taq polymerase and Klenow fragment are as given by the suppliers. The molecular mass of Taq polymerase is indicated in kilodaltons.
Biochemical properties.
To determine the biochemical properties of the purified recombinant DNA polymerase, the nonradioactive assay described in Materials and Methods was carried out under different reaction conditions.
In the assay performed at 75°C, the DNA polymerase was most active with activated calf thymus DNA as a substrate. The activity obtained with 240 ng of activated DNA was taken as a standard value of 100%. The enzyme was able to utilize the same amount of primed single-stranded M13 DNA as a template, with a relative activity of 28%. In contrast to Taq polymerase (a family A DNA polymerase), the family B DNA polymerases from P. occultum and Pyrococcus furiosus also prefer activated calf thymus DNA as a template (42). The DNA polymerase from P. islandicum was inactive on the unprimed single-stranded M13 DNA. Almost no activity (relative activity of 1.5%) was detected when poly(A)-oligo(dT)15 was used as a DNA-primed RNA template.
The optimal conditions for the DNA polymerase activity were tested in the presence of activated calf thymus DNA as a template. The DNA polymerase was most active at pH 7.3. At pHs 6.0 and 9.0 the enzyme revealed 10 and 15% of residual polymerase activity, respectively. The optimal temperature for polymerase activity could not be measured because activated DNA was not stable above 80°C. The enzyme required extremely low concentrations of divalent cations for activity; concentrations above 0.5 mM MgCl2 were inhibitory (Fig. 5). No activity was detected in the absence of divalent cations. As illustrated in Fig. 5, monovalent potassium ions as well as ammonium ions strongly inhibited the enzyme. Only 7% of residual activity could be detected in the presence of 100 mM KCl, and 22% could be detected in the presence of 50 mM (NH4)2SO4.
FIG. 5.
Influence of potassium ions (A), ammonium ions (B), and magnesium ions (C) on the activity of the DNA polymerase from P. islandicum. The standard assay as described in the text was carried out with 0.2 U of purified DNA polymerase in the presence of various concentrations of KCl, (NH4)2SO4, and MgCl2.
To determine the thermostability of the DNA polymerase, a preincubation of the enzyme in 50 mM Tris-HCl (pH 7.3) was performed at 90 and 100°C, followed by an incubation at 75°C under standard assay conditions. The half-life of the enzyme was 35 min at 100°C and >5 h at 90°C (Fig. 6).
FIG. 6.
Thermostability of the DNA polymerase from P. islandicum at 90 and 100°C. The purified polymerase (0.2 U) was preincubated without template, dNTPs, and BSA for various time intervals. Afterward, the missing components were added and residual activity was detected in the standard assay at 75°C. Activity values are indicated relative to the activity present without preincubation.
Influence of inhibitors.
The influence of representative inhibitors on the DNA polymerase activity was investigated (Table 3). The sulfhydryl blocking reagent NEM strongly inhibited the DNA polymerase activity. In the presence of 1 mM NEM, 40% residual activity was determined. A total loss of activity was detected after a preincubation with 5 mM NEM. The DNA polymerase from P. islandicum was sensitive to the tetracyclic diterpenoid aphidicolin, which competes with each dNTP for binding to DNA polymerase. This compound inhibits eucaryotic and several archaeal family B DNA polymerases. Similar to the B3 DNA polymerases from A. pernix, P. occultum, and P. furiosus (6, 42), the DNA polymerase from P. islandicum was inhibited by high concentrations of aphidicolin. Fifty-five percent of residual activity could be detected in the presence of 500 μM aphidicolin, and 21% could be detected in the presence of 2,000 μM aphidicolin. Another criterion which distinguishes family B DNA polymerases from other DNA polymerases is the reasonable resistance of the former enzymes to dideoxyribonucleoside triphosphates. A very low sensitivity of the DNA polymerase to ddGTP was detected. No inhibition was found at a ddGTP/dGTP ratio of 2. When the ratio was raised to 10, 80% of residual activity was detected.
TABLE 3.
Influence of inhibitors on the DNA polymerase activity from P. islandicum
| Inhibitor | Concentrationa | DNA polymerase activity (%)b |
|---|---|---|
| NEMc | 0 | 100 |
| 1 | 40 | |
| 2 | 30 | |
| 5 | 0 | |
| Aphidicolin | 0 (0) | 100 |
| 50 (17) | 100 | |
| 200 (68) | 70 | |
| 500 (170) | 55 | |
| 2,000 (680) | 21 | |
| ddGTP | 5 | 95 |
| 10 | 80 | |
| 20 | 53 |
The concentrations of the NEM inhibitor are millimolar, and the concentrations of the aphidicolin inhibitor are micromolar and, in parentheses, micrograms per milliliter. The concentrations of the ddGTP inhibitor are ratios of ddGTP/dGTP.
The influence of different inhibitors was detected at standard assay conditions (pH 7.3; 0.5 mM MgCl2; 0 mM KCl; 75°C) using 0.2 U of purified DNA polymerase.
A preincubation of the enzyme with NEM was performed at 90°C for 20 min.
Exonuclease activity.
Almost all archaeal family B DNA polymerases are known to have associated 3′-5′ exonuclease activity, which is responsible for correction of mismatched dNTPs. Three domains (Exo I, Exo II, and Exo III) have been proposed to be essential for this activity (3). Highly conserved amino acids (12) in all these domains could be identified within the deduced DNA polymerase sequence from P. islandicum (Fig. 2). To investigate if a 3′-5′ exonuclease activity on single- and double-stranded DNA could be detected, we used a 5′-DIG-labeled primer and a primer-template complex. 3′-5′ exonuclease activity was shown by the degradation of the primer after incubation with the DNA polymerase at 70°C for 1 h. As indicated in Fig. 7, the rate of P. islandicum DNA polymerase-associated 3′-5′ exonuclease activity strongly depends on the pH of the reaction buffer. Using the optimal polymerase buffer (pH 7.3), the degradation of the primer by the enzyme from P. islandicum was significantly lower than that with Pwo polymerase, whereas in buffer 2 (pH 8.6) the value for exonuclease activity was comparable to that of Pwo polymerase. Both on single-stranded primer and double-stranded primer-template the DNA polymerase from P. islandicum showed 3′-5′ exonuclease activities in the absence of dNTPs. Even in the presence of 0.01 and 0.1 μM dNTPs a significant degradation of the primer-template substrate at pH 8.6 was detected. In contrast, Taq polymerase did not show any degradation of the primer, neither on single-stranded nor on double-stranded DNA substrate (Fig. 7). As expected for DNA polymerases with 3′-5′ exonuclease activity, the degradation of the primer-template substrate by the DNA polymerases from P. islandicum and Pyrococcus woesei was reduced in the presence of higher amounts (1 μM) of dNTPs. Neither degradation nor elongation of the primer by the DNA polymerase from P. islandicum was detectable when the assay was performed at 37°C (data not shown). This result clearly indicates that the enzyme preparations did not contain a contaminating exonuclease activity from E. coli which could interfere with the test.
PCR performed with P. islandicum DNA polymerase.
Thermostable DNA polymerases are particularly used for in vitro amplification of DNA fragments by PCR and for DNA sequencing (26, 28). Due to the associated 3′-5′ exonuclease activity, archaeal DNA polymerases offer the possibility to amplify DNA fragments with high fidelity (low mutation frequency). To date, all archaeal DNA polymerases used in PCR are derived from Euryarchaeota. So far, no reports are available concerning the effective use of crenarchaeal DNA polymerases in PCR.
To verify the suitability of the recombinant DNA polymerase from P. islandicum in DNA amplification, PCR was performed using 1 U of enzyme and several primer combinations. As shown in Fig. 8, up to 1,500 bp of a λ DNA template could be amplified. This is the first report on a successful application of a crenarchaeal DNA polymerase in PCR.
FIG. 8.
Application of DNA polymerase from P. islandicum in PCR. Reactions were carried out in a buffer containing 15 mM Tris-HCl (pH 8.6), 12.5 mM KCl, 2.5 mM (NH4)2SO4, 1.25 mM MgCl2, and 20 μg of BSA per ml. One unit of DNA polymerase from P. islandicum was used to amplify 500 bp (lane 1), 1,000 bp (lane 2), and 1,500 bp (lane 3) of a λ DNA template. Control PCR was performed with 2.5 U of High Fidelity enzyme (lane 4). Twenty microliters of each PCR product was applied on an 1% agarose gel. The corresponding molecular sizes of the marker (lane 5) are indicated in kilobase pairs.
In vivo function of family B DNA polymerases.
Regardless of the interesting in vitro activity of the B3 DNA polymerase from P. islandicum, studies of the in vivo function of crenarchaeal family B DNA polymerases are still in their infancy. However, the existence of multiple DNA polymerases in Crenarchaeota may indicate that the DNA replication mechanism in these organisms depends, analogous to that of Eucarya, on more than one single DNA polymerase. Recently, we detected a second family B DNA polymerase gene in P. islandicum. The deduced amino acid sequence shows highest identities to B1 DNA polymerases from the Crenarchaeota A. pernix, P. occultum, S. solfataricus, and C. symbiosum (data not shown). In correspondence to the eucaryotic replication mechanism (1), it could be assumed that both family B DNA polymerases, B1 and B3, play a role in the replication fork of P. islandicum. It is likely that additional replication proteins such as single-stranded DNA-binding proteins or processivity factors are involved in archaeal replication. The B1 DNA polymerase from S. solfataricus was found to be activated by two sliding clamp proteins homologous to eucaryotic proliferating cell nuclear antigen, indicating that this polymerase is involved in DNA replication (17). To date, the influence of a DNA polymerase sliding clamp on a crenarchaeal B3 DNA polymerase has not been investigated. In order to identify all the components involved in the DNA replication of Crenarchaeota, it would be of interest to search for replication factors in P. islandicum that are similar to the ones detected in Eucarya and to study the interaction of these proteins and the multiple family B DNA polymerases.
ACKNOWLEDGMENTS
We are grateful to Constantinos E. Vorgias and Konstantinos D. Paliakasis for creating the pairwise alignment. We acknowledge Bruno Frey from Roche Diagnostics and Frank Niehaus for many helpful discussions. We are obliged to Francesca Pisani for her critical reading of the manuscript. We thank Raimond D. Michaelis and Ralf Grote for computational assistance.
Part of this work was supported by the Commission of European Communities (the Biotech Generic project Extremophiles as Cell Factories, contract BIO4CT975058).
Footnotes
Dedicated to Gerhard Gottschalk on his 65th birthday.
REFERENCES
- 1.Bambara R A, Murante R S, Henricksen L A. Enzymes and reactions at the eucaryotic DNA replication fork. J Biol Chem. 1997;272:4647–4650. doi: 10.1074/jbc.272.8.4647. [DOI] [PubMed] [Google Scholar]
- 2.Betlach M, Friedman J, Boyer H, Henricksen L A. Enzymes and reactions at the eucaryotic DNA replication fork. J Biol Chem. 1997;272:7949–7959. doi: 10.1074/jbc.272.8.4647. [DOI] [PubMed] [Google Scholar]
- 3.Blanco L, Bernad A, Blasco M A, Salas M. A general structure for DNA-dependent DNA polymerases. Gene. 1991;100:27–38. doi: 10.1016/0378-1119(91)90346-d. [DOI] [PubMed] [Google Scholar]
- 4.Braithwaite D K, Ito J. Compilation, alignment, and phylogenetic relationships of DNA polymerases. Nucleic Acids Res. 1993;21:787–802. doi: 10.1093/nar/21.4.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bult C J, White O, Olsen G J, Zhou L, Fleischmann R D, Sutton G G, Blake J A, Fitzgerald L M, Clayton R A, Gocayne J D, Kerlavage A R, Dougherty B A, Tomb J-F, Adams M D, Reich C I, Overbeek R, Kirkness E F, Weinstock K G, Merrick J M, Glodek A, Scott J L, Geoghagen N S M, Weidman J F, Fuhrmann J L, Nguyen D, Utterback T R, Kelley J M, Peterson J D, Sadow P W, Hanna M C, Cotton M D, Roberts K M, Hurst M A, Kain B P, Borodovsky M, Klenk H-P, Fraser C M, Smith H O, Woese C R, Venter J C. Complete genome sequence of the methanogenic archaeon Methanococcus jannashii. Science. 1996;273:1058–1073. doi: 10.1126/science.273.5278.1058. [DOI] [PubMed] [Google Scholar]
- 6.Cann I K O, Ishino S, Nomura N, Sako Y, Ishino Y. Two family B DNA polymerases from Aeropyrum pernix, an aerobic hyperthermophilic crenarchaeote. J Bacteriol. 1999;181:5984–5992. doi: 10.1128/jb.181.19.5984-5992.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cann I K O, Komori K, Toh H, Kanai S, Ishino Y. A heterodimeric DNA polymerase: evidence that members of euryarchaeota possess a distinct DNA polymerase. Proc Natl Acad Sci USA. 1998;95:14250–14255. doi: 10.1073/pnas.95.24.14250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Copeland W C, Dong Q, Wang T S-F. Rationale for mutagenesis of DNA polymerase active sites: DNA polymerase α. Methods Enzymol. 1995;262:294–303. doi: 10.1016/0076-6879(95)62025-7. [DOI] [PubMed] [Google Scholar]
- 9.Dalgard J Z, Garret R A. Archaeal hyperthermophilic genes. In: Kates M, Kushner D J, Matheson A T, editors. The biochemistry of archaea (archaebacteria). Amsterdam, The Netherlands: Elsevier Science Publishers; 1993. pp. 535–563. [Google Scholar]
- 10.Datukishvili N, Pokholok D, Lottspeich F, Prangishvili D, Rechinsky V. The DNA polymerase encoding gene from a thermoacidophilic archaeon Sulfolobus acidocaldarius. Gene. 1996;177:271–273. doi: 10.1016/0378-1119(96)00298-3. [DOI] [PubMed] [Google Scholar]
- 11.Dennis P P. Ancient ciphers: translation in archaea. Cell. 1997;89:1007–1010. doi: 10.1016/s0092-8674(00)80288-3. [DOI] [PubMed] [Google Scholar]
- 12.Derbyshire V, Pinsonneault J K, Joyce C M. Structure-function analysis of 3′-5′-exonuclease of DNA polymerase. Methods Enzymol. 1995;262:363–385. doi: 10.1016/0076-6879(95)62030-3. [DOI] [PubMed] [Google Scholar]
- 13.DiRuggiero J, Robb F T. RNA extraction from sulfur-utilizing thermophilic archaea. In: Robb F T, Place A R, editors. Archaea—a laboratory manual. Thermophiles. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1995. pp. 97–99. [Google Scholar]
- 14.Edgell D R, Doolittle W F. Archaea and the origin(s) of DNA replication proteins. Cell. 1997;89:995–998. doi: 10.1016/s0092-8674(00)80285-8. [DOI] [PubMed] [Google Scholar]
- 15.Edgell D R, Klenk H-P, Doolittle W F. Gene duplications in evolution of archaeal family B DNA polymerases. J Bacteriol. 1997;179:2632–2640. doi: 10.1128/jb.179.8.2632-2640.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edgell D R, Malik S-B, Doolittle W F. Evidence of independent gene duplications during the evolution of archaeal and eukaryotic family B DNA polymerases. Mol Biol Evol. 1998;15:1207–1217. doi: 10.1093/oxfordjournals.molbev.a026028. [DOI] [PubMed] [Google Scholar]
- 17.Felice M D, Sensen C W, Charlebois R L, Rossi M, Pisani F M. Two DNA polymerase sliding clamps from the hyperthermophilic archaeon Sulfolobus solfataricus. J Mol Biol. 1999;291:47–57. doi: 10.1006/jmbi.1999.2939. [DOI] [PubMed] [Google Scholar]
- 18.Hain J, Reiter W-D, Hüdepohl U, Zillig W. Elements of an archaeal promoter defined by mutational analysis. Nucleic Acids Res. 1993;20:5423–5428. doi: 10.1093/nar/20.20.5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huber B, Kristjansson J K, Stetter K O. Pyrobaculum gen. nov., a new genus of neutrophilic, rod-shaped archaebacteria from continental solfataras growing optimally at 100°C. Arch Microbiol. 1987;149:95–101. [Google Scholar]
- 20.Ishino Y, Komori K, Cann K O, Koga Y. A novel DNA polymerase family found in Archaea. J Bacteriol. 1998;180:2232–2236. doi: 10.1128/jb.180.8.2232-2236.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawarabayasi Y, Sawada M, Horikawa H, Haikawa H, Hino Y, Yamamoto S, Sekine M, Baba S, Kosugi H, Hosoyama A, Nagai Y, Sakai M, Ogura K, Otsuka R, Nakazawa H, Takamiya M, Ohfuku Y, Funahashi T, Tanaka T, Kudoh Y, Yamazaki J, Kushida N, Oguchi A, Aoki K, Yoshizawa T, Nakamura Y, Robb F T, Horikoshi K, Masuchi Y, Shizuya H, Kikuchi H. Complete sequence and gene organization of the genome of a hyper-thermophilic archaebacterium, Pyrococcus horikoshii OT3. DNA Res. 1998;5:55–76. doi: 10.1093/dnares/5.2.55. [DOI] [PubMed] [Google Scholar]
- 22.Kornberg A, Baker D. DNA replication. 2nd ed. New York, N.Y: Freeman and Company; 1992. [Google Scholar]
- 23.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Leipe D D, Aravind L, Koonin E V. Survey and summary—did DNA replication evolve twice independently? Nucleic Acids Res. 1999;27:3389–3401. doi: 10.1093/nar/27.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- 26.Niehaus F, Bertoldo C, Kähler M, Antranikian G. Extremophiles as a source of novel enzymes for industrial application. Appl Microbiol Biotechnol. 1999;51:711–729. doi: 10.1007/s002530051456. [DOI] [PubMed] [Google Scholar]
- 27.Niehaus F, Frey B, Antranikian G. Cloning and characterisation of a thermostable α-DNA polymerase from the hyperthermophilic archaeon Thermococcus sp. TY. Gene. 1997;204:153–158. doi: 10.1016/s0378-1119(97)00536-2. [DOI] [PubMed] [Google Scholar]
- 28.Perler F B, Kumar S, Kong H. Thermostable DNA polymerases. Adv Protein Chem. 1996;48:377–435. doi: 10.1016/s0065-3233(08)60367-8. [DOI] [PubMed] [Google Scholar]
- 29.Pfeifer F, Griffig J, Oesterhelt D. The fdx gene encoding the [2Fe-2S] ferredoxin of Halobacterium salinarium (H. halobium) Mol Gen Genet. 1993;239:66–71. doi: 10.1007/BF00281602. [DOI] [PubMed] [Google Scholar]
- 30.Pisani F M, Felice M D, Manco G, Rossi M. Domain organization and biochemical features of Sulfolobus solfataricus DNA polymerase. Extremophiles. 1998;2:171–177. doi: 10.1007/s007920050057. [DOI] [PubMed] [Google Scholar]
- 31.Pisani F M, Felice M D, Rossi M. Amino acid residues involved in determining the processivity of the 3′-5′ exonuclease activity in a family B DNA polymerase from the thermoacidophilic archaeon Sulfolobus solfataricus. Biochemistry. 1998;37:15005–15012. doi: 10.1021/bi981127s. [DOI] [PubMed] [Google Scholar]
- 32.Pisani F M, Martino C D, Rossi M. A DNA polymerase from the archaeon Sulfolobus solfataricus shows sequence similarity to family B DNA polymerases. Nucleic Acids Res. 1992;20:2711–2716. doi: 10.1093/nar/20.11.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prangishvili D, Klenk H-P. Nucleotide sequence of the gene for a 74 kDa DNA polymerase from the archaeon Sulfolobus solfataricus. Nucleic Acids Res. 1993;21:2768. doi: 10.1093/nar/21.11.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schleper C, DeLong E F, Preston C M, Feldman R A, Wu K-Y, Swanson R V. Genomic analysis reveals chromosomal variation in natural populations of the uncultured psychrophilic archaeon Cenarchaeum symbiosum. J Bacteriol. 1998;180:5003–5009. doi: 10.1128/jb.180.19.5003-5009.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schleper C, Swanson R V, Mathur E J, DeLong E F. Characterization of a DNA polymerase from the uncultivated psychrophilic archaeon Cenarchaeum symbiosum. J Bacteriol. 1997;179:7803–7811. doi: 10.1128/jb.179.24.7803-7811.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt T G M, Skerra A. One-step affinity purification of bacterially produced proteins by means of the “Strep-tag” and immobilized recombinant core streptavidin. J Chromatogr A. 1994;676:337–345. doi: 10.1016/0021-9673(94)80434-6. [DOI] [PubMed] [Google Scholar]
- 38.Skerra A. Use of the tetracycline promoter for the tightly regulated production of a murine antibody fragment in Escherichia coli. Gene. 1994;151:131–135. doi: 10.1016/0378-1119(94)90643-2. [DOI] [PubMed] [Google Scholar]
- 39.Spanos A, Hübscher U. Recovery of functional proteins in sodium dodecyl sulfate gels. Methods Enzymol. 1983;91:263–277. doi: 10.1016/s0076-6879(83)91024-8. [DOI] [PubMed] [Google Scholar]
- 40.Stocki S A, Nonay R L, Reha-Krantz L J. Dynamics of bacteriophage T4 DNA polymerase function: identification of amino acid residues that affect switching between polymerase and 3′-5′ exonuclease activities. J Mol Biol. 1995;254:15–28. doi: 10.1006/jmbi.1995.0595. [DOI] [PubMed] [Google Scholar]
- 41.Truniger V, Lazaro J M, Salas M, Blanco L. A DNA binding motif coordinating synthesis and degradation in proofreading DNA polymerases. EMBO J. 1996;15:3430–3441. [PMC free article] [PubMed] [Google Scholar]
- 42.Uemori T, Ishino Y, Doi H, Kato I. The hyperthermophilic archaeon Pyrodictium occultum has two α-like DNA polymerases. J Bacteriol. 1995;177:2164–2177. doi: 10.1128/jb.177.8.2164-2177.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uemori T, Sato Y, Kato I, Doi H, Ishino Y. A novel DNA polymerase in the hyperthermophilic archaeon, Pyrococcus furiosus: gene cloning, expression, and characterization. Genes Cells. 1997;2:499–512. doi: 10.1046/j.1365-2443.1997.1380336.x. [DOI] [PubMed] [Google Scholar]
- 44.Voss S, Skerra A. Mutagenesis of a flexible loop in streptavidin leads to higher affinity for the Strep-tag II peptide and improved performance in recombinant protein purification. Protein Eng. 1997;10:975–982. doi: 10.1093/protein/10.8.975. [DOI] [PubMed] [Google Scholar]
- 45.Wang T S F, Wong S W, Korn D. Human DNA polymerase α: predicted functional domains and relationships with viral DNA polymerases. FASEB J. 1989;3:14–21. doi: 10.1096/fasebj.3.1.2642867. [DOI] [PubMed] [Google Scholar]