Abstract
To dissect genetically the regulation of NorA, a multidrug transporter of Staphylococcus aureus, we analyzed the differential expression of the norA promoter using a transcriptional fusion with a β-lactamase reporter gene. Expression studies with an arlS mutant revealed that the norA promoter is ArlS dependent. The arlR-arlS locus was shown to code for a two-component regulatory system. The protein ArlR has strong similarity to response regulators, and ArlS has strong similarity to protein histidine kinases. We have also analyzed the 350-bp region upstream of the Shine-Dalgarno sequence of norA by gel mobility shift experiments. It was shown that only the 115-bp region upstream of the promoter was necessary for multiple binding of an 18-kDa protein. From transcriptional fusions, we have localized four different putative boxes of 6 bp, which appear to play a role in the binding of the 18-kDa protein and in the up-regulation of norA expression in the presence of the arlS mutation. Furthermore, the gel mobility shift of the 18-kDa protein was modified in the presence of the arlS mutation, and the arlS mutation altered the growth-phase regulation of NorA. These results indicate that expression of norA is modified by a two-component regulatory system.
For many years, antibiotics have been effective in the treatment of many infectious diseases caused by a range of pathogens, including Staphylococcus aureus. The occurrence of antibiotic resistance, however, has transformed some previously treatable diseases into a new threat to public health. One of the mechanisms underlying antibiotic resistance involves the extrusion of the compounds by an efflux pump or carrier (29). The most intriguing mechanisms of drug extrusion are those that include a wide variety of structurally unrelated compounds as substrates for multidrug resistance (MDR) transporters. On the basis of bioenergetic and structural criteria, the known transporters are subdivided into (i) ATP-binding cassette-type transporters and (ii) secondary transporters. The secondary transporters use the electrochemical proton gradient or proton motive force across the cytoplasmic membrane to extrude drugs, whereas the first group utilizes the free energy of ATP hydrolysis (4). The secondary transporters comprise the largest group of known drug extrusion systems in bacteria. They have been subdivided into three different groups: the major facilitator superfamily (MFS), the resistance nodulation and cell division family, and the family of small multidrug resistance (Smr). The MFS family is characterized by the presence of either 12 or 14 putative transmembrane segments. In S. aureus, an MDR pump named NorA was previously sequenced and characterized (14, 17, 23, 24, 38). NorA belongs to the MFS family frequently found in bacteria (4). NorA protects the cell from a number of lipophilic and monocationic compounds such as ethidium bromide, cetrimide, benzalkonium chloride, tetraphenylphosphonium bromide, and acriflavine, as well as some hydrophilic quinolones (14, 17, 23, 24).
The regulation and the physiological function of NorA, however, is not known. Efflux pumps, such as Bmr of Bacillus subtilis, which has similarity to NorA, possess a regulatory gene downstream or upstream of the structural gene. bmrR, which is downstream of bmr, is responsible for the regulation of expression of Bmr (1). For NorA, the protein encoded by the open reading frame found upstream of norA on the chromosome lacks similarity to any known regulator (data not shown).
In order to elucidate the regulation of norA, we analyzed the differential expression of the norA promoter using a transcriptional fusion with a β-lactamase reporter gene. We found that norA expression is affected by ArlS, a member of a newly described two-component regulatory system (B. Fournier and D. C. Hooper, unpublished data). We also performed gel mobility shift experiments on fragments containing the norA promoter: it was shown that only the 115-bp region upstream of the promoter was necessary for multiple bindings of an 18-kDa protein and that the binding of this protein was modified in the arlS mutant.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacterial strains and plasmids used in this study are listed in Table 1. Staphylococci were cultivated in Trypticase soy broth (TSB) at 37°C unless otherwise stated. Escherichia coli cells were grown in Luria-Bertani medium.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype or relevant characteristic(s)a | Source or reference |
|---|---|---|
| Strains | ||
| S. aureus | ||
| ISP794 | 8325 pig-131 | 34 |
| MT23142 | 8325 pig-131 flqB | 24 |
| BF15 | 8325 pig-131 flqB arlS::Tn917LTV1 | This study |
| RN4220 | 8325-4 r− | 18 |
| KLE820 | RN4220 norA::cat | 14 |
| RN6390 | 8325-4 Hla+ Prt+ | 26 |
| RN6911 | RN6390 agr::tetM | 27 |
| ALC136 | RN6390 sar::Tn917LTV1 | 8 |
| ALC135 | RN6390 agr::tetM sar::Tn917LTV1 | 7 |
| E. coli DH5α | F-φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 phoA hsdR17 (rK− mK−) supE44O λ− thi-1 gyrA96 relA1 | Gibco BRL |
| Plasmids | ||
| pGEM3-zf(+) | 2.9-kb E. coli cloning vector, Apr | Promega |
| pGB2 | 4.3-kb E. coli cloning vector, Smr | 9 |
| pWN2018 | 10.5-kb S. aureus promoter-probe vector, Cmr | 39 |
| pE194 | 3.7-kb S. aureus cloning vector, Emr | 33 |
| pSK265 | 3-kb S. aureus cloning vector, Cmr | 32 |
| pBF8-30 | 315 bp containing the entire promoter of norA cloned upstream of the blaZ gene of pWN2018 | This study |
| pBF3-50 | 250 bp containing a truncated promoter of norA cloned upstream of the blaZ gene of pWN2018 | This study |
| pBF4-3 | 130 bp containing a truncated promoter of norA cloned upstream of the blaZ gene of pWN2018 | This study |
| pBF15-5 | 110 bp containing a truncated promoter of norA cloned upstream of the blaZ gene of pWN2018 | This study |
| pBF16-4 | 2.4-kb PCR product containing the arlR-arlS locus cloned into pGB2 + pE194 | This study |
| pBF17 | 2.4-kb PCR product containing the arlR-arlS locus cloned into pGB2 + pSK265 | This study |
Resistance determinants: Ap, ampicillin; Sm, streptomycin; Cm, chloramphenicol; Em, erythromycin.
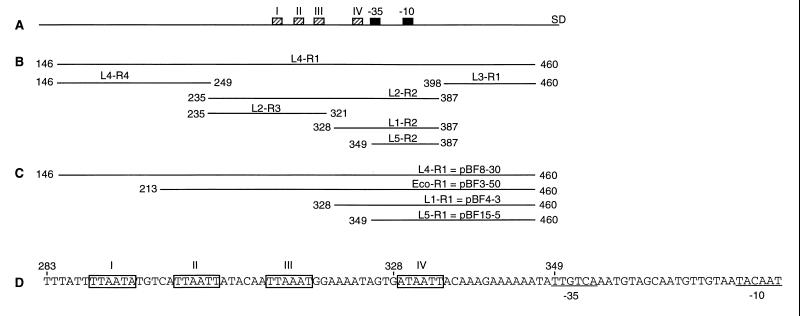
To construct transcriptional fusions of norA with the β-lactamase gene blaZ (norA::blaZ), PCR-generated DNA fragments, L4-R1, L1-R1, and L5-R1, located upstream of the Shine-Dalgarno sequence of norA (Fig. 1C) were cloned in pGEM3-zf(+), introduced into E. coli DH5α, and sequenced. The DNA fragments were then subcloned into the promoter-probe vector pWN2018 using KpnI and PstI sites to generate plasmids pBF8-30 (L4-R1), pBF4-3 (L1-R1), and pBF15-5 (L5-R1). To construct pBF4-3, an EcoRV site present 132 bp upstream of the Shine-Dalgarno sequence and the SmaI site of the vector were used to remove 67 bp of pBF8-30.
FIG. 1.
Maps of the different DNA segments of the norA promoter examined in this study. The numbers indicate the nucleotide positions according to Yoshida et al. (38). (A) Schematic map of the norA promoter. The −35 and −10 consensus sequences are indicated by black boxes, and the Shine-Dalgarno site is marked SD. The repeated sequences, shown in panel D, are indicated by hatched boxes. (B) PCR fragments used in band shift experiments. (C) Schematic map showing the DNA cloned upstream of the β-lactamase gene in transcriptional fusions. (D) Sequence of the region upstream of the −35 consensus sequence. Repeated sequences are boxed, and the −35 and −10 sequences are underlined.
To construct a plasmid containing the arlR-arlS locus, a 2.4-kb product containing arlR and arlS was amplified by PCR using Vent DNA polymerase (New England Biolabs), chromosomal DNA of ISP794, and two primers containing the BamHI site. The PCR product contained about 300 bp upstream and 100 bp downstream of the arlR-arlS locus. PCR products were digested by BamHI and ligated into the BamHI site of pGB2 (9). The resulting plasmid containing the arlR-arlS locus in pGB2 was cut with PstI and introduced into the PstI site of plasmid pSK265, which has the S. aureus replicon of pC194 to give pBF17, or into the PstI site of plasmid pE194 to give pBF16-4 (Table 1).
These plasmids were introduced into S. aureus RN4220, a restriction-deficient strain, by electroporation before being introduced into the derivatives of strain ISP794.
DNA manipulations.
Plasmid DNA isolation was performed using the Qiagen midiprep kit. S. aureus was transformed with plasmid DNA by electroporation (11). Chromosomal DNA from S. aureus was prepared as described previously (34).
Enzyme assays.
In order to measure norA promoter activity, cells containing different plasmids in which the norA promoter controls β-lactamase expression were grown in TSB at 37°C to an OD600 of 0.9. The whole culture was assayed for β-lactamase activity using nitrocefin as a substrate as described by Ji et al. (16), except that incubation was done at room temperature. β-Lactamase activities are expressed in micromoles of nitrocefin hydrolyzed per hour per gram of cell protein. Assays of chloramphenicol acetyltransferase (CAT) activity were used as a control for the copy number of the fusion plasmids. Crude extracts were prepared by lysis with lysostaphin (80 μg/ml) for 30 min at 37°C, and CAT activity was determined as previously described (26). Protein concentrations were determined by the Bradford method (Bio-Rad).
Preparation of cell-free extracts.
Cell-free extracts were prepared as previously described with some modifications (22). Cells (OD600 = 0.9) were washed once in buffer A (20 mM Tris-HCl, 50 mM MgCl2, 1 mM dithiothreitol, 0.1 mM EDTA, 5% glycerol) and frozen at −70°C overnight. The pellet was suspended in 10 ml of buffer A, containing 0.1 mg of lysostaphin per ml, and incubated 3.5 h on ice. The suspension was frozen overnight at −70°C. After thawing on ice, 6 ml of buffer A containing 1.3 M KCl was added and incubated on ice for 30 min. The bacterial lysate was left 30 min at room temperature before centrifugation at 40,000 × g for 30 min to remove debris. The supernatant was dialyzed 3 h against water and frozen at −70°C.
Gel mobility shift analysis.
A gel electrophoresis DNA mobility shift assay was used to identify DNA-binding proteins. DNA fragments were synthesized by PCR (Fig. 1B). One of the primers in each reaction was labeled with [γ-32P]ATP using polynucleotide kinase.
Radiolabeled DNA fragments (20,000 counts/min/reaction) were incubated with the indicated amount of protein extract from S. aureus in 10 μl of binding buffer (10 mM HEPES [pH 8.0], 60 mM KCl, 4 mM MgCl2, 0.1 mM EDTA [pH 8.0], 0.1 mg of bovine serum albumin (BSA) per ml, 0.25 mM dithiothreitol) containing 1 μg of poly(dI-dC), 200 ng of sheared herring sperm DNA, and 10% glycerol as previously described (13). In the case of the purified protein, 100 ng of poly(dI-dC) and 5% glycerol were used in addition to the binding buffer. The reaction mixture was incubated 15 min at room temperature and analyzed by 5% nondenaturing polyacrylamide electrophoresis.
Purification of the protein binding to the norA promoter.
DNA fragment L2-R2 (150-bp) (Fig. 1B) was generated by PCR using a purified biotinylated primer (Gibco BRL), separated by agarose gel electrophoresis, and after cutting out the band, purified by QiaQuick (Qiagen). Nine micrograms of DNA was immobilized on 2 mg of magnetic beads with covalently coupled streptavidin (Dynabeads M-280; Dynal) according to the manufacturer's protocol. As previously described (22), DNA bound to beads was incubated with 200 μg of protein extract in 800 μl of binding buffer containing 600 μg of herring sperm DNA per ml for 15 min at room temperature. Beads were washed once with binding buffer containing 5 mg of herring sperm DNA per ml without BSA and twice with binding buffer without BSA. Proteins were eluted in 100 μl of binding buffer containing 0.5 M NaCl. Two different elutions were pooled, dialyzed against water for 1 h, and concentrated in a Speed-Vac evaporator. The samples were separated on a sodium dodecyl sulfate (SDS)–11% polyacrylamide gel. Proteins were detected by silver staining (Bio-Rad).
RESULTS
Effect of inactivation of arlS on norA expression.
In order to find loci involved in the regulation of norA, we used a library of Tn917LTV1 insertions in the chromosome of strain MT23142 using selection for higher and lower levels of resistance to tetraphenylphosphonium bromide, a substrate of NorA. MT23142 carries the flqB mutation. flqB, a cis-acting mutation of norA, is localized downstream of the initiation start site of norA and overexpresses norA (24). The mutant BF15 showed a slight increase of resistance to tetraphenylphosphonium bromide and contained a Tn917LTV1 insertion in the arlS gene from the arlR-arlS locus (Fournier and Hooper, unpublished). The arlR-arlS locus codes for a two-component regulatory system. The protein ArlR has strong similarity to response regulators from the PhoB-OmpR family, and ArlS has similarity to protein histidine kinases (Fournier and Hooper, unpublished).
To determine the effect of chromosomal arlS and flqB mutations on norA expression, plasmid pBF8-30 carrying the full promoter region of norA fused to blaZ was introduced into strains ISP794 (wild type), MT23142 (flqB), and BF15 (arlS). β-Lactamase activity was increased 2.6-fold in BF15 relative to ISP794 (Table 2), but there was no difference in activity between MT23142 and ISP794 (data not shown). The difference of 2.6-fold of norA expression between ISP794 and BF15 (Table 2) was obtained with a culture grown until an OD600 of 0.9. When the culture was grown until an OD600 of 1.5, the β-lactamase activity was 5,900 U of β-lactamase per g of proteins for ISP794 and 36,600 U of β-lactamase per g of proteins for BF15, indicating that for the arlS mutant (BF15) norA expression was sixfold higher than that of the wild-type strain (ISP794) during early stationary phase. In order to verify that the β-lactamase activity increase was not due to differences in plasmid copy number, CAT activity of the fusion plasmid was determined, and no differences were observed in the three strains (data not shown). Introduction of plasmid pBF16-4 carrying the arlR-arlS locus into BF15 (pBF8-30) decreased the expression of norA seen in BF15 (Table 2), whereas introduction of the same plasmid into ISP794 (pBF8-30) did not modify norA expression (Table 2). Thus, the flqB mutation does not affect norA expression in trans, but disruption of arlS itself contributes to increased norA expression.
TABLE 2.
Effect of the arlS deletion and truncated norA promoters on norA expression
| Plasmid | Strain
|
|||
|---|---|---|---|---|
| ISP794
|
BF15
|
|||
| β-Lactamase activitya | Ratiob | β-Lactamase activitya | Ratiob | |
| pWN2018 | ≤2,000 | ≤2,000 | ||
| pBF8-30 | 9,400 ± 330 | 1.0 | 24,300 ± 180 | 1.0 |
| pBF8-30 plus pBF16-4 | 9,200 ± 146 | 1.0 | 16,700 ± 1430 | 0.7 |
| pBF3-50 | 11,700 ± 390 | 1.2 | 25,500 ± 850 | 1.0 |
| pBF4-3 | 9,510 ± 510 | 1.0 | 7,770 ± 420 | 0.3 |
| pBF15-5 | 9,230 ± 810 | 1.0 | 10,700 ± 1540 | 0.4 |
Results are expressed in units of β-lactamase per gram of proteins. All determinations were performed in triplicate.
Ratios of β-lactamase activities with respect to that of the wild-type promoter in both strains.
An 18-kDa protein binds to the norA promoter.
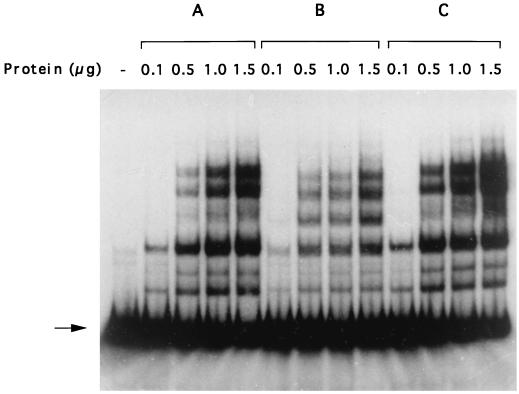
In order to determine how the arlR-arlS locus might control norA expression, we analyzed the protein(s) that binds to the norA promoter by gel mobility shifts using different DNA fragments. As seen in Fig. 2A, the first fragment L4-R1 (Fig. 1B) of 315 bp, containing the entire norA promoter from the Shine-Dalgarno sequence extending 200 bp upstream, exhibits several shifts with the protein extract from the wild-type strain ISP794. With increasing concentrations of protein, the intensity of the bands increased. Band shifts were reduced by increasing amounts of the unlabeled L4-R1 DNA but were not affected by nonspecific DNA, indicating that the protein(s) bound was specific to L4-R1 DNA. We then tested three separate DNA fragments, L4-R4, L2-R2, and L3-R1, which constituted separate domains of L4-R1 (Fig. 1B). The cell extract (2 μg of proteins) mixed with fragments L4-R4 and L3-R1 produced no band shifts (data not shown). In contrast, the cell extract mixed with fragment L2-R2 produced a band shift pattern identical to that of L4-R1 (Fig. 2B), indicating that the protein(s) binds to this region. The multiple bands seen in mobility shift assays suggested that L2-R2 DNA bound different numbers of protein molecules. Competition experiments with unlabeled specific and nonspecific DNA also confirmed that binding to L2-R2 was specific (Fig. 2B).
FIG. 2.
Gel mobility shift analysis of the interaction of protein extracts from the wild-type strain ISP794 with different fragments of the norA promoter and the effect of unlabeled DNA. The radiolabeled fragment (arrow) was incubated with increasing amounts of protein extracts. The labeled fragments used in these experiments are L4-R1 (315 bp) (A), L2-R2 (153 bp) (B), L2-R3 (87 bp) (C), L1-R2 (60 bp) (D), and L5-R2 (39 bp) (E). The protein(s) binds to the tested fragment and retards its mobility (a different gel was used for each fragment). An unlabeled fragment of 350 bp amplified by PCR from a Klebsiella oxytoca promoter and an unlabeled fragment of the tested fragment serve as specificity control (NSPE DNA and SPE DNA, respectively). Protein and DNA concentrations and ratios of unlabeled fragments to labeled fragments used in this assay are indicated in the tables above the figures.
To localize further the site of protein binding, fragment L2-R2 was divided in two smaller fragments, L2-R3 and L1-R2 (Fig. 1B). Each of these fragments showed only two band shifts (Fig. 2C and D), in contrast to L2-R2, which exhibited at least five band shifts. These results indicate that either several different proteins bind to the fragment L2-R2 or the same protein binds in multiples to L2-R2. The specificity of the binding was again demonstrated by competition experiments (Fig. 2C and D). The last fragment tested, L5-R2, a smaller fragment of L1-R2 (Fig. 1B), did not show any shift when mixed with 2 μg of protein extracts (Fig. 2E). Higher concentrations of protein extracts were needed to generate a shift of fragment L1-R2, in comparison to the fragment L2-R3, indicating weaker binding (Fig. 2C and D). Together, these results indicate that the protein binding site on L1-R2 is located between positions 328 and 349 (Fig. 1B).
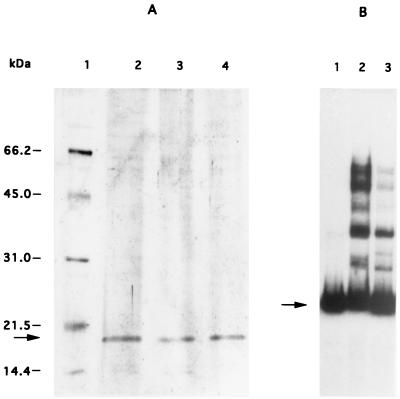
In order to determine if the mobility shift was due to one or several proteins, we used magnetic beads coupled to the L2-R2 DNA fragment to isolate the bound protein(s). The crude extract of the wild-type strain ISP794 was adsorbed to these beads, and the bound protein(s) were eluted and separated by SDS-polyacrylamide gel electrophoresis (PAGE). A single 18-kDa protein was identified (Fig. 3A, lane 1). To confirm that only one protein species bound to the fragment L2-R2, we then performed the same experiment to capture protein bound by the two smaller fragments L2-R3 and L1-R2, and the same protein was found (Fig. 3A, lanes 2 and 3). To verify that this single protein was responsible for the multiple shifts observed for the fragment L2-R2, a band shift experiment was done using the eluted protein obtained from the fragment L2-R2. The same pattern of multiple band shifts was seen with the eluted protein as with the crude extract (Fig. 3B), indicating that this single protein was sufficient to generate the multiple band shifts.
FIG. 3.
Isolation of the protein from the wild-type strain ISP794 binding to different fragments of the norA promoter. Different fragments of DNA were immobilized on magnetic beads. Proteins binding to these fragments were then used for different analyses. (A) SDS-PAGE analysis of protein released from DNA affinity magnetic beads. Lane 1, standard proteins (in kilodaltons); lane 2, fragment L2-R2; lane 3, fragment L1-R2; lane 4, fragment L2-R3. The 18-kDa protein is indicated by an arrow on the left. (B) Gel mobility shift analysis of fragment L2-R2 with affinity-purified extracts from strain ISP794. Lane 1, control DNA without protein; lane 2, purified protein; lane 3, 0.5 μg of protein from crude extracts of ISP794. Free DNA is indicated by an arrow.
The previous experiment suggested that several molecules of the same protein bound to multiple or at least two binding sites. In order to find the repeated sequence to which the 18-kDa protein bound, we analyzed the sequence between nucleotides 235 and 349 and found four repeated sequences (boxes) (Fig. 1D). The consensus sequence of these boxes was TTAATT. The fourth putative box (ATAATT) was present between nucleotides 328 and 349 (Fig. 1D), consistent with our data indicating that a binding site was located between 328 and 349. Fragment L2-R3 contained three putative boxes, a finding that correlates with the stronger binding of the 18-kDa protein to this fragment relative to fragment L1-R2, which contains only one box (Fig. 2C and D). Footprinting experiments will be necessary to confirm that these boxes are the binding sites of the 18-kDa protein.
In order to understand further the role of upstream DNA sequences shown to be involved in binding of the 18-kDa protein in norA expression, we constructed transcriptional fusions encompassing varying sequences of the upstream region of the norA promoter (Fig. 1C). These plasmids were introduced into ISP794 (wild type) and BF15 (arlS). In ISP794, little or no difference in β-lactamase expression was observed between the different truncated promoters (pBF3-50, pBF4-3, and pBF15-5) and the complete promoter (pBF8-30) (Table 2). In contrast, in the arlS mutant BF15 expression of β-lactamase from plasmids pBF4-3 and pBF15-5 was reduced by 70 and 60%, respectively (Table 2). The truncated promoter of pBF4-3 corresponds to the fragment L1-R2 used for the band shift experiments (Fig. 2D). Thus, the increase in norA expression in the arlS mutant is dependent on sequences between nucleotides 213 and 328 (Fig. 1C). Since binding of the 18-kDa protein to L2-R3 sequences contributes to full binding pattern of the larger L2-R2 DNA fragment and removal of the L2-R3 sequence reduces norA expression, it is possible that binding of this protein modulates norA expression.
Binding of the 18-kDa protein is modified in the arlS mutant.
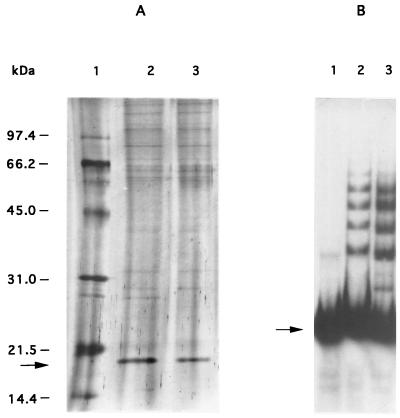
To study further the effect of arlR-arlS on norA expression, gel mobility shift experiments were done with crude extracts of the arlS mutant (BF15). As seen in Fig. 4B and 5B, extracts from the arlS mutant gave a band shift pattern different from that of the wild-type strain ISP794 (Fig. 4A). The first band was identical to that of the wild type, whereas the three other bands migrated slightly differently. When we complemented the arlS mutant with the plasmid pBF17 encoding arlR-arlS, the band shift pattern became identical to that of the wild type (Fig. 4C). In addition, the amount of shifted bands was consistently lower with extracts containing identical amounts of total protein from BF15 in comparison to BF15 (pBF17) and ISP794 (Fig. 4). Using magnetic beads to which the L2-R2 fragment (Fig. 1B) was coupled, we isolated the 18-kDa protein from the wild-type strain and from the arlS mutant (Fig. 5A). A band shift experiment with the protein eluted from the arlS mutant gave a pattern similar to that of the crude extract (Fig. 5B), indicating that the 18-kDa protein is present in the wild-type strain and in the arlS mutant. However, the pattern and extent of binding of this protein to the norA promoter is modified in the arlS mutant.
FIG. 4.
Gel mobility shift analysis of the interaction of the protein extracts from different strains with the complete norA promoter. The radiolabeled fragment L2-R2 (arrow) was incubated with increasing amounts of protein extracts. The protein(s) binds to the tested fragment and retards its mobility. Lanes: A, strain ISP794; B, arlS mutant BF15; C, arlS mutant BF15 containing pBF17.
FIG. 5.
Isolation of the protein from the mutant BF15 binding to the fragment L2-R2. (A) SDS-PAGE analysis of protein released from affinity-purified extracts from different strains. Lane 1, standard proteins (in kilodaltons); lane 2, purified protein from ISP794; lane 3, purified protein from BF15. The 18-kDa protein is indicated by an arrow on the left. (B) Gel mobility shift analysis of fragment L2-R2 with affinity-purified protein extracts from BF15 and fragment L2-R2. Lane 1, control DNA without protein; lane 2, purified protein; lane 3, 0.5 μg of protein from crude extracts of BF15. Free DNA is indicated by an arrow.
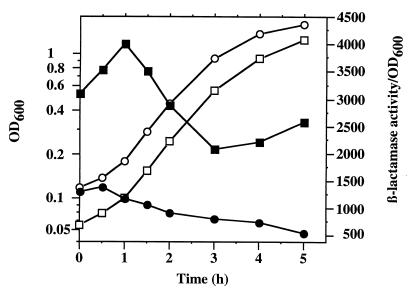
The arlR-arlS locus alters the growth-phase regulation of NorA.
In S. aureus, regulation of many proteins is affected by growth phase (30). To analyze the effect of growth phase on norA expression, an overnight culture was diluted 1/50 in TSB, and every half hour, OD600 and β-lactamase activity were determined. The ratio of β-lactamase activity/OD600 as an estimate of specific activity was then calculated. For the parental strain, β-lactamase-specific activity decreased throughout the logarithmic phase (Fig. 6). For the arlS mutant, the β-lactamase-specific activity was over twofold higher than that of the parental strain and also decreased during logarithmic phase (Fig. 6). In contrast to the parental strain, the arlS mutant exhibited a plateau and slight rebound in β-lactamase-specific activity as early stationary phase was entered. Thus, growth-phase regulation of norA expression is also altered in the arlS mutant.
FIG. 6.
Effect of the arlS mutation on NorA regulation during the growth. The parent strain MT23142 (circles) and the arlS mutant BF15 (squares) containing the plasmid pBF8-30 were grown at 37°C. The ratio of β-lactamase/OD600 was calculated as an estimate of specific activity. Open symbols indicate OD600, and solid symbols indicate the ratio of β-lactamase activity/OD600.
Because growth-phase regulation of protein expression is mediated by the agr and sar loci (30), we evaluated the effects of mutations in these loci on norA expression. We introduced the plasmid pBF8-30 in wild-type strain RN6930, agr (RN6911), sar (ALC136), and agr sar (ALC135) isogenic mutants. β-Lactamase activity of mutant cells was similar to that of the wild-type strain (data not shown).
DISCUSSION
Here we have shown that the arlR-arlS locus encoding an apparent two-component regulatory system is involved in the expression of the multidrug efflux pump NorA and in the binding of an 18-kDa protein to the norA promoter region.
The 18-kDa protein binding to the norA promoter does not appear to have any effect on norA promoter expression under normal growth conditions in the wild-type strain, whereas a modified pattern of its binding is associated with increased promoter expression when arlS is disrupted (Table 2). In the wild-type strain, the 18-kDa protein might function as a regulator that is activated in the presence of increased concentrations of a putative inducer. In the arlS mutant, several hypotheses could be considered to explain increased expression of norA. First, the arlR-arlS locus might directly control NorA. Because the 18-kDa protein modified its binding when arlS was disrupted, the protein that binds to the norA promoter could be ArlR, the response regulator of the arlR-arlS locus. ArlR-ArlS appears to constitute a two-component regulatory system (Fournier and Hooper, unpublished), such as those that mediate adaptative responses of bacteria to their environment. These systems are composed of a transmembrane sensor (histidine protein kinase) and its associated response regulator (35). In general, the transmembrane protein binds a specific ligand, the signal, and autophosphorylates at a conserved histidine residue. The phosphorylated sensor then relays the phosphate to aspartic residues in the response regulator (28). The response regulator can in turn stimulate or repress target genes at the level of transcription. The protein ArlR belongs to the PhoB-OmpR group. These regulators are known to bind a region upstream of the promoter of their target genes and to modify gene expression (e.g., PhoB in the phosphate regulon [21] or OmpR in the porin regulon [19, 37] of E. coli). The pattern of band shift from the purified 18-kDa protein from the wild-type strain and the arlS mutant was similar to that from the crude extract from each. Although we cannot rule out the purification of a complex protein inducer, this suggests that the difference in the pattern of band shift was due to the protein itself and not to another component present in the crude extract. As a protein histidine kinase, ArlS likely phosphorylates the response regulator ArlR. This phosphorylation might modify the binding of the regulator (2). In the case of OmpR, phosphorylation by EnvZ (31) modifies OmpR binding to the promoter of its target gene ompF (37). Moreover, response regulators such as PhoB or OmpR often have one or several consensus sequences that function as regulator-binding sites upstream of the promoter (20, 35, 37), such as the putative TTAATT boxes associated with binding of the 18-kDa protein. We can speculate that, in the absence of ArlS, ArlR is not phosphorylated and that its binding to the norA promoter as well as norA expression is modified. In such a case, removal of the binding sites of the regulator protein would also modify norA expression (Table 2). Together, these studies suggest that the protein binding to the norA promoter could be the response regulator ArlR. However, response regulators such as ArlR are dephosphorylated due to an autophosphatase activity. The half-lives of hydrolysis of phosphoaspartate groups in regulator proteins at neutral pH and ambient temperature range from only a few seconds to several hours, with most exhibiting intermediate values of several minutes (36). Therefore, it seems unlikely that ArlR remained phosphorylated throughout the complete DNA affinity purification, which lasted at least 3 h. Furthermore, the size of ArlR predicted from its amino acid sequence is 25.5 kDa (Fournier and Hooper, unpublished), rather than the 18 kDa observed for the protein binding to the norA promoter. If ArlR is itself directly involved in modulation of norA expression by binding to the promoter region, then additional processing of ArlR must have occurred.
A second possibility is that the 18-kDa protein is phosphorylated by ArlS. Cross talk may result from cross-specificities in which sensors of similar sequence phosphorylate nonpartner regulators (40). For example, the histidine kinase CheA can phosphorylate the Ntr transcription factor NR1 (25). We can speculate that the 18-kDa protein not derived from ArlR is directly phosphorylated by ArlS.
Finally, the arlR-arlS locus might affect norA expression indirectly by modifying another gene affecting the activity of the 18-kDa protein (for example, the gene producing the physiological inducer of NorA). In such a case, the 18-kDa protein would bind differently to the norA promoter in the presence and in the absence of the inducer. For the related efflux pump Bmr, its regulator BmrR binds to the bmr promoter and enhances expression in the presence of some inducing substrates. It has been shown that the Bmr substrates that induce Bmr expression interact directly with BmrR (1). The binding of inducers to its C-terminal domain converts BmrR into an activator of transcription from the bmr promoter. This activation is likely to occur through untwisting of the spacer region of the promoter, which serves as the BmrR-binding site. This untwisting leads to proper positioning of the promoter motifs binding RNA polymerase and thus initiates transcription (41). Recently, it has been shown in B. subtilis that the two MDR pumps, Bmr and Blt, that have high similarity with NorA, are regulated by a global transcriptional activator, Mta, a member of the Mer family of bacterial regulatory proteins. Thus, these pumps are controlled by specific transcriptional activators, BmrR and BltR, and by a global regulator, Mta. The individually expressed N-terminal DNA-binding domain of Mta interacts directly with the promoters of bmr and blt and induces transcription of these genes (3). Since no regulator gene was found around norA, we can speculate that norA is controlled only by the 18-kDa protein that could be a global regulator. Moreover, we found another mutant, MT1222, which also modifies norA expression and for which no modification was found in the arlR-arlS locus, indicating that an additional locus is also involved in the norA regulation (Fournier and Hooper, unpublished). Thus, the mutant locus of MT1222 could represent the gene encoding the protein binding to the norA promoter. The identity of the 18-kDa protein that binds to the norA promoter will be further studied.
The expression of norA is affected by the growth phase (Fig. 6). norA expression appears to increase during early logarithmic phase followed by a decrease during late logarithmic and early stationary phases. Further decrease in expression occurs in stationary phase since the supernatant culture medium from an overnight culture (mixed 50% with TSB) decreases β-lactamase activity of ISP794 (pBF8-30) twofold compared to that of late logarithmic phase (data not shown). Thus, a component secreted by S. aureus in the medium acts directly or indirectly to reduce norA expression in different phases of growth.
Because the arlR-arlS locus, which affects norA expression, is involved in autolysis of S. aureus (Fournier and Hooper, unpublished), we can speculate that NorA is perhaps also involved in autolysis and protects the cell by removing autolysins or products from autolysis, which would be toxic if allowed to accumulate. In S. aureus, another two-component regulatory system, lytS-lytR, is also involved in autolysis (5) and regulates a gene, lrgA, encoding a protein showing characteristics in common with the bacteriophage murein hydrolase transporter family of proteins known as holins (6). As some murein hydrolases lack N-terminal signal sequences, it has been speculated that holin-like proteins might be involved in the export of bacterial murein hydrolases (10). However, Triton X-100- or penicillin-induced autolysis does not stimulate norA expression in the wild-type strain (data not shown), and the norA mutant KLE820 had a rate of Triton X-100-induced autolysis similar to that of its parent strain RN4220 (data not shown). A third two-component regulatory system agrC-agrA and another related locus, sar, are also involved in autolysis in S. aureus (12). The agr and sar loci regulate other cellular functions: synthesis of extracellular toxins and enzymes (i.e., alpha-toxin, beta-hemolysin, enterotoxins, lipases, proteases, etc.) and synthesis of cell-surface proteins (protein A, fibronectin-binding protein, capsular polysaccharide type 5, and coagulase) (15, 27, 30), indicating the multiplicity of functions of the two-component regulatory systems. norA expression in agr and/or sar mutants was similar to that in the wild-type strain, indicating that neither agr and sar loci nor cellular functions controlled by these loci modified norA expression. Thus, we can speculate that the arlR-arlS locus might regulate other physiological functions that affect the native substrate of NorA.
Our findings identify for the first time several components likely involved in the complex regulation of norA expression, including a two-component regulatory system, ArlR-ArlS, and specific binding of an 18-kDa protein to the norA promoter. Substances accumulating in the medium of stationary-phase cells may act through these and other regulatory elements. Further studies will be required, however, to identify the 18-kDa protein, which binds to the norA promoter. Nevertheless, our findings of norA regulation by a two-component regulatory system open a new avenue for investigation of the molecular mechanisms of the multidrug efflux pumps, their regulation, and their physiological role.
ACKNOWLEDGMENTS
We thank Xiamei Zhang for technical assistance, Annie Gravel for helpful discussions, Ambrose Cheung for the gift of strains RN6930, RN6911, ALC135, and ALC136, and Kim Lewis for providing strain KLE820. We also thank Steven J. Projan and Georges Rapoport for critical review of this manuscript.
This work was supported by a U.S. Public Health Service grant AI23988 (to D.C.H.) from the National Institutes of Health.
REFERENCES
- 1.Ahmed M, Borsch C M, Taylor S S, Vazquez-Laslop N, Neyfakh A A. A protein that activates expression of a multidrug efflux transporter upon binding the transporter substrates. J Biol Chem. 1994;269:28506–28513. [PubMed] [Google Scholar]
- 2.Aiba H, Nakasai F, Mizushima S, Mizuno T. Phosphorylation of a bacterial activator protein, OmpR, by a protein kinase, EnvZ, results in stimulation of its DNA-binding ability. J Biochem. 1989;106:5–7. doi: 10.1093/oxfordjournals.jbchem.a122817. [DOI] [PubMed] [Google Scholar]
- 3.Baranova N N, Danchin A, Neyfakh A A. Mta, a global regulator of the Bacillus subtilis multidrug-efflux transporters. Mol Microbiol. 1999;31:1549–1559. doi: 10.1046/j.1365-2958.1999.01301.x. [DOI] [PubMed] [Google Scholar]
- 4.Bolhuis H, van Veen H W, Poolman B, Driessen A J M, Konings W N. Mechanisms of multidrug transporters. FEMS Microbiol Rev. 1997;21:55–84. doi: 10.1111/j.1574-6976.1997.tb00345.x. [DOI] [PubMed] [Google Scholar]
- 5.Brunskill E W, Bayles K W. Identification and molecular characterization of a putative regulatory locus that affects autolysis in Staphylococcus aureus. J Bacteriol. 1996;178:611–618. doi: 10.1128/jb.178.3.611-618.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunskill E W, Bayles K W. Identification of LytSR-regulated genes from Staphylococcus aureus. J Bacteriol. 1996;178:5810–5812. doi: 10.1128/jb.178.19.5810-5812.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung A L, Eberhardt K J, Chung E, Yeaman M R, Sullman P M, Ramos M, Bayer A S. Diminished virulence of a sar−/agr− mutant of Staphylococcus aureus in the rabbit model of endocarditis. J Clin Investig. 1994;94:1815–1822. doi: 10.1172/JCI117530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung A L, Projan S J. Cloning and sequencing of sarA of Staphylococcus aureus, a gene required for the expression of agr. J Bacteriol. 1994;176:4168–4172. doi: 10.1128/jb.176.13.4168-4172.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Churchward G, Belin D, Nagamine Y. A pSC101-derived plasmid which shows no sequence homology to other commonly used cloning vectors. Gene. 1984;31:165–171. doi: 10.1016/0378-1119(84)90207-5. [DOI] [PubMed] [Google Scholar]
- 10.del Mar Lleò M, Fontana R, Solioz M. Identification of a gene (arpU) controlling muramidase-2 export in Enterococcus hirae. J Bacteriol. 1995;177:5912–5917. doi: 10.1128/jb.177.20.5912-5917.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fournier B, Hooper D C. Mutations in topoisomerase IV and DNA gyrase of Staphylococcus aureus: novel pleiotropic effects on quinolone and coumarin activity. Antimicrob Agents Chemother. 1998;42:121–128. doi: 10.1128/aac.42.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujimoto D F, Bayles K W. Opposing roles of the Staphylococcus aureus virulence regulators, Agr and Sar, in Triton X-100- and penicillin-induced autolysis. J Bacteriol. 1998;180:3724–3726. doi: 10.1128/jb.180.14.3724-3726.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gravel A, Fournier B, Roy P H. Complexes obtained with the integron integrase IntI1 at the attI1 site. Nucleic Acids Res. 1998;26:4347–4355. doi: 10.1093/nar/26.19.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsieh P-H, Siegel S A, Rogers S A, Davis D, Lewis K. Bacteria lacking a multidrug pump: a sensitive tool for drug discovery. Proc Natl Acad Sci USA. 1998;95:6602–6606. doi: 10.1073/pnas.95.12.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janzon L, Arvidson S. The role of the delta-lysin (hld) in the regulation of virulence genes by the accessory gene regulator (agr) in Staphylococcus aureus. EMBO J. 1990;9:1391–1399. doi: 10.1002/j.1460-2075.1990.tb08254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji G, Beavis R C, Novick R P. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc Natl Acad Sci USA. 1995;92:12055–12059. doi: 10.1073/pnas.92.26.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaatz G W, Seo S M. Inducible NorA-mediated multidrug resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1995;39:2650–2655. doi: 10.1128/aac.39.12.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreiswirth B N, Lofdalh S, Betley M J, O'Reilly M, Schlievert P M, Bergdoll M S, Novick R P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature (London) 1983;305:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- 19.Maeda S, Mizuno T. Evidence for multiple OmpR-binding sites in the upstream activation sequence of the ompC promoter in Escherichia coli: a single OmpR-binding site is capable of activating the promoter. J Bacteriol. 1990;172:501–503. doi: 10.1128/jb.172.1.501-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makino K, Shinagawa H, Amemura M, Nakata A. Nucleotide sequence of the phoB gene, the positive regulatory gene for the phosphate regulon of Escherichia coli K-12. J Mol Biol. 1986;190:37–44. doi: 10.1016/0022-2836(86)90073-2. [DOI] [PubMed] [Google Scholar]
- 21.Makino K, Shinagawa H, Amemura M, Kinura S, Nakata A, Ishihama A. Regulation of the phosphate regulon of Escherichia coli. Activation of pstS transcription by PhoB protein in vitro. J Mol Biol. 1988;203:85–95. doi: 10.1016/0022-2836(88)90093-9. [DOI] [PubMed] [Google Scholar]
- 22.Morfeldt E, Tegmark K, Arvidson S. Transcriptional control of the agr-dependent virulence gene regulator, RNAIII, in Staphylococcus aureus. Mol Microbiol. 1996;21:1227–1237. doi: 10.1046/j.1365-2958.1996.751447.x. [DOI] [PubMed] [Google Scholar]
- 23.Neyfakh A A, Borsch C M, Kaatz G W. Fluoroquinolone resistance protein NorA of Staphylococcus aureus is a multidrug efflux transporter. Antimicrob Agents Chemother. 1993;37:128–129. doi: 10.1128/aac.37.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng E Y, Trucksis M, Hooper D C. Quinolone resistance mediated by NorA: physiologic characterization and relationship to flqB, a quinolone resistance locus on the Staphylococcus aureus chromosome. Antimicrob Agents Chemother. 1994;38:1345–1355. doi: 10.1128/aac.38.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ninfa A J, Ninfa E G, Lupas A N, Stock A, Magasanik B, Stock J. Crosstalk between bacterial chemotaxis signal transduction proteins and regulators of transcription of the Ntr regulon: evidence that nitrogen assimilation and chemotaxis are controlled by a common phosphotransfer mechanism. Proc Natl Acad Sci USA. 1988;85:5492–5496. doi: 10.1073/pnas.85.15.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Novick R P. Genetic systems in staphylococci. Methods Enzymol. 1991;204:587–636. doi: 10.1016/0076-6879(91)04029-n. [DOI] [PubMed] [Google Scholar]
- 27.Novick R P, Ross H F, Projan S T, Kornblum J, Kreiswirth B, Moghazeh S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 1993;12:3967–3975. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parkinson J S. Signal transduction schemes of bacteria. Cell. 1993;73:857–871. doi: 10.1016/0092-8674(93)90267-t. [DOI] [PubMed] [Google Scholar]
- 29.Paulsen I T, Brown M H, Skurray R A. Proton-dependent multidrug efflux systems. Microbiol Rev. 1996;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Projan S J, Novick R P. The molecular basis of pathogenicity. In: Crossley K B, Archer G L, editors. The staphylococci in human disease. New York, N.Y: Churchill Livingstone; 1997. pp. 55–81. [Google Scholar]
- 31.Rampersaud A, Harlockers S L, Inouye M. The OmpR protein of Escherichia coli binds to sites in the ompF promoter region in a hierarchical manner determined by its degree of phosphorylation. J Biol Chem. 1994;269:12559–12566. [PubMed] [Google Scholar]
- 32.Ranelli D M, Jones C L, Johns M B, Mussey G J, Khan S A. Molecular cloning of staphylococcal enterotoxin B gene in Escherichia coli and Staphylococcus aureus. Proc Natl Acad Sci USA. 1985;82:5850–5854. doi: 10.1073/pnas.82.17.5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shivakumar A G, Gryczan T J, Kuzlov Y J, Dubnau D. Organization of the pE194 genome. Mol Gen Genet. 1980;174:241–252. doi: 10.1007/BF00425450. [DOI] [PubMed] [Google Scholar]
- 34.Stahl M L, Pattee P A. Confirmation of protoplast fusion-derived linkages in Staphylococcus aureus by transformation with protoplast DNA. J Bacteriol. 1983;154:406–412. doi: 10.1128/jb.154.1.406-412.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stock J B, Ninfa A J, Stock A M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989;53:450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stock J B, Surette M G, Levit M, Park P. Two-component signal transduction signal transduction systems: structure-function relationships and mechanisms of catalysis. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C.: American Society for Microbiology; 1995. pp. 25–51. [Google Scholar]
- 37.Tsung K, Brissette R E, Inouye M. Identification of the DNA-binding domain of the OmpR protein required for transcriptional activation of the ompF and ompC genes of Escherichia coli by in vivo DNA footprinting. J Biol Chem. 1989;264:10104–10109. [PubMed] [Google Scholar]
- 38.Yoshida H, Bogaki M, Nakamura S, Ubukata K, Konno M. Nucleotide sequence and characterization of the Staphylococcus aureus norA gene, which confers resistance to quinolones. J Bacteriol. 1990;172:6942–6949. doi: 10.1128/jb.172.12.6942-6949.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang P-H, Projan S J, Leason K R, Novick R P. Translational fusion with a secretory enzyme as an indicator. J Bacteriol. 1987;169:3082–3087. doi: 10.1128/jb.169.7.3082-3087.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wanner B L. Signal transduction and cross regulation in the Escherichia coli phosphate regulon by PhoR, CreC, and acetyl phosphate. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C.: American Society for Microbiology; 1995. pp. 203–221. [Google Scholar]
- 41.Zheleznova E E, Markham P N, Neyfakh A A, Brennan R G. Structural basis of multidrug recognition by BmrR, a transcription activator of a multidrug transporter. Cell. 1999;96:353–362. doi: 10.1016/s0092-8674(00)80548-6. [DOI] [PubMed] [Google Scholar]