Abstract
Green chemistry seeks to design less hazardous chemicals, but many of the efforts to replace chemicals have resulted in so-called “Regrettable Substitutions”, when a chemical with an unknown or unforeseen hazard is used to replace a chemical identified as problematic. Here, we discuss the literature on regrettable substitution and focus on an oft-mentioned case, Bisphenol A, which was replaced with Bisphenol S—and the lessons that can be learned from this history. In particular, we focus on how Green Toxicology can offer a way to make better substitutions.
Keywords: Green Toxicology, regrettable substitutions, Bisphenol A, life cycle analysis, alternatives assessments
INTRODUCTION
One of the goals of “Green Chemistry” is to design less hazardous chemicals,1 often to replace existing chemicals with risks that have been deemed unacceptable by regulatory authorities, consumers, or businesses. In theory, this process should, over time, result in reduced risk of adverse effects based on chemical exposures for consumers. It should also improve product stewardship with lower attrition—the term coined for the loss of lead substances in drug development—as well as fewer surprises and reduced suffering in the regulatory testing phase.2 However, chemicals are not always replaced by more “green” chemicals. Instead, they are replaced with chemicals that simply have different or unknown hazards, as these replacements have not yet been broadly tested or only tested for the hazard which was a concern for the chemical to be replaced. Such substitutions are known as “regrettable substitutions” and represent, at some level, a failure: of chemists to design safer chemicals, of toxicologists to identify hazard, or of the regulatory authorities to keep consumers and workers safe.
Indeed, there is a public perception, shared by some working in the field of public health, that regrettable substitutions are the norm rather than the exception,3 despite the fact that within the toxicology literature such substitutions are rarely studied systematically. In fact, among toxicology journals, we discovered that the literature on regrettable substitutions is somewhat slim, and quantitative analysis of substitutions even more rare. It is hard to imagine that this reality does not weaken faith in both science and policy. Therefore, it is imperative that we begin to investigate both how widespread regrettable substitutions are and what caused such failures, and how toxicology needs to change.
Here, we set out to analyze the literature on regrettable substitutions and discuss some of the common causes of regrettable substitutions that could be improved by toxicologists. We should note that there is a broader discussion on substitutions, which may include other relevant terms (e.g., “informed substitutions”), within both the risk analysis community and the Life Cycle Analysis community. We focused on “regrettable substitutions”, as this is the term employed most often by toxicologists (e.g., “informed substitutions” returns no results in PubMed). Lastly, by focusing on one of the most commonly given examples of a regrettable substitution—Bisphenol S (BPS) for Bisphenol A (BPA)—we illustrate what kinds of tools and approaches need to be developed and what mistakes should not be repeated.
LITERATURE REVIEW
We compiled a list of regrettable substitutions by searching PubMed, the Web of Science, and Google Scholar (excluding both patents and citations; search was performed between December 12 and 24th) for the phrase “regrettable substitution”. “Regrettable substitution” first appeared in the literature in 2011 and was formally addressed within the context of the National Research Council’s (NRC) alternatives assessments report,4 although the discussion of “risk−risk trade-offs” in the literature within the risk sciences field is somewhat more established. One article5 included a list of seven chemicals commonly referred to as regrettable substitution chemicals, while our literature search found examples of six more chemicals (Table 1) that were mentioned as regrettable substitutions—although it should be noted that, ultimately, for many of these chemicals it is difficult to establish if they are truly regrettable, as the term is not defined precisely. Only two studies focused on a quantitative assessment: one which looked specifically at alterations in use patterns over time within the plasticizers in Sweden and found an overall decline in the use of Substances of Very High Concern (SVHC),6 and another which looked at trade-offs in insecticide usage.7 Most of the chemicals were repeatedly referred to in the literature (e.g., within the Google Scholar list, BPA was mentioned in 156 articles; phthalates, 118; flame retardants, 177).
Table 1.
Examples of Regrettable Substitutions in the Literaturea
| target chemical/target chemical class (function) | end point of concern for target chemical | regrettable substitute | end point of concern for regrettable substitute | ref |
|---|---|---|---|---|
| Bisphenol-A (BPA) (plasticizer) | Endocrine disruption | Bisphenol-S (BPS), Bisphenol-F (BPF) | Endocrine activity | Ticker et al. 20195 |
| Lead (additive in gasoline) | Neurotoxicity | Methyl tert-butyl ether (MBTE) | Aquatic toxicity | Ticker et al. 20195 |
| Methylene chloride (solvent carrier in adhesives) | Acute toxicity, Carcinogenicity | 1-Bromopropane (nPB) | Carcinogenicity, Neurotoxicity | Ticker et al. 20195 |
| Methylene chloride (brake cleaners) | Acute toxicity, Carcinogenicity | n-Hexane | Neurotoxicity | Ticker et al. 20195 |
| Polybrominated diphenyl ethers (PBDEs) (flame retardant) | Persistence, Neurotoxicity, Reproductive toxicity, Carcinogenicity (penta and deca) | Tris (2,3-dibromopropyl) phosphate | Carcinogenicity, Aquatic toxicity | Ticker et al. 20195 |
| Trichloroethylene (TCE) (metal degreaser) | Carcinogenicity | 1-Bromopropane (nPB) | Neurotoxicity, Carcinogenicity | Ticker et al. 20195 |
| Chlorofluorocarbons (CFCs) (refrigerant) | Ozone depletion | Hydrofluorocarbons (HFCs) | Greenhouse gas | Ticker et al. 20195 |
| γ-Hexachloro-cyclohexane (pesticide) | Neurotoxicity | Imidacloprid | Bee colony collapse | Fantke et al. 202015 |
| Phthalates (plasticizers) | Endocrine activity | DINCH | Endocrine activity | Jamarani et al. 201816 |
| Organochlorine, Carbamate, and Organophosphates (pesticides) | Neurotoxicity | Bacillus thuringiensis (Bt) pesticides produced inside GMO | Safety unclear | Latham 201617 |
| DDT (pesticide) | Reproductive toxicity, thyroid inhibition | Cholorpyrifos | Neurotoxicity, thyroid inhibition | Ubaid ur Rahman 2021;18 Demeneix 201919 |
| Atrazine (pesticide) | Persistence, ecological effects | Terbuthylazine | Persistence, ecological effects | Latham 201617 |
| Organophosphorus and N-methyl carbamate (pesticides) | Neurotoxicity | Pesticide mix dominated by neonicotinoids and pyrethroids | Bee colony collapse | DiBartolomeis 20197 |
In addition to the review article by Tickner et al., we identified 6 additional chemical substitutions that were referred to as regrettable substitutions. Overall, there was a fair amount of redundancy in the literature and few articles discussed in detail the trade-offs for such substitutions. Only two quantitative analyses that were identified: one, not in the table above as it focused on a functional class (plasticizers)6 and DiBartolomeis 20197 which focused on insecticides.
The considerably larger sweep of articles from Google Scholar versus PubMed (301 vs 4) likely reflects not only the broader search algorithm of Google compared to PubMed, but also the fact that much of the literature on regrettable substitutions is in journals not included in the traditional biomedical literature—i.e., articles in environmental law reviews, or journals specific to the chemical industry or the chemical engineering community. It should be emphasized that this is not a systematic review;8 we only sought to offer a preliminary survey of the scientific landscape surrounding regrettable substitutions and hope that this prompts a more precise assessment going forward.
Many of the articles focused on alternatives assessments and their use in substitutions. Alternatives assessments can help guide the selection of safer substitutes; however, many alternatives assessments tend to focus on hazard.4 While hazard assessment is arguably one of the most important tenets of an alternatives assessment, a hazard-based approach overlooks other basic considerations, such as functionality, feasibility, or even economics. In addition, hazard-based alternatives assessments to analyze novel chemistries as substitutes may be limited by a lack of data, but some frameworks are designed to capture data gaps (e.g., Clean Production Action’s GreenScreen for Safer Chemicals) (Clean Production Action 2018 https://www.greenscreenchemicals.org/images/ee_images/uploads/resources/GreenScreen_Guidance_v1_4_2018_01_Final.pdf), but others simply flag data gaps and work with the data that is available to characterize overall chemical hazard. In addition, these softwares and services can be very costly, though with CHEMforward a non-profit solution is also available.9
Although alternative assessments are robust, this, in turn, means that they are often labor intensive. This type of approach is not always practical for companies aiming to “green” their larger ingredient palettes, but this is where a list-based approach can be useful: screening chemicals against lists of substances of concern can capture extremely hazardous chemicals from the beginning of the substitution process, and it can generally be completed in a quick and cost-effective manner. The trade-off with the “list of lists” approach is that, although many chemicals can be screened relatively quickly, chemicals that are novel chemistries with yet unknown hazards or those that are moderately hazardous may go unflagged.10 Notably, a recent assessment of 22 chemical inventories from 19 countries and regions identified over 350 00011 chemicals and mixtures of chemicals that have been registered for production and use. This compares to about 23 000 chemicals registered under the Registration, Evaluation, Authorisation, and Restriction of Chemicals (REACH)12 regulation and is not even available as a machine-readable database.13 This gives an idea how much toxicity information is not produced or at least not publicly available. It also illustrates that we need pragmatic ways of read-across to fill data-gaps by intra- and extrapolating from what we know to chemicals with no information.
There is also an ever-increasing number of alternatives assessment frameworks. For example, Jacobs et al.14 carried out an analysis of 20 alternatives assessment frameworks, and as this does not include the many in-house alternative assessments, there are certainly more than 20 frameworks in use. The authors found that there is often overlap with the publicly available frameworks; however, each also has unique attributes. As a consequence, it is possible that different results may be produced depending on the paradigm used, which can lead to a less desirable substitute.
What lessons can be learned about how toxicology can improve to avoid regrettable substitutions? Our analysis indicated a handful of common reasons for regrettable substitutions, summarized in Figure 1 and detailed below:
Figure 1.
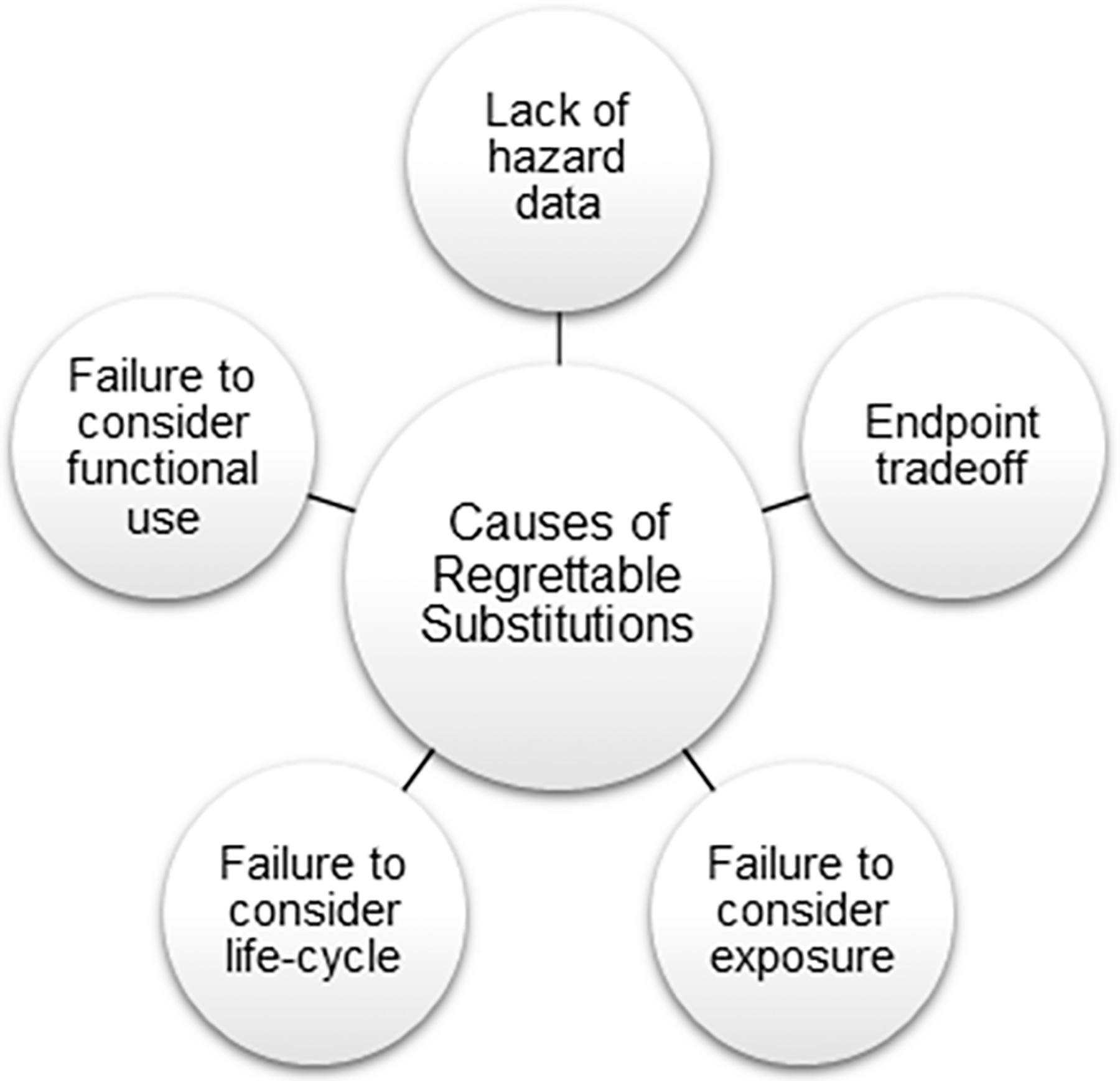
Causes of regrettable substitutions. While this list is certainly not exhaustive, regrettable substitutions can largely be attributed to a handful of issues: (1) lack of hazard data on the replacement chemical; (2) trading off one hazard end point for another; (3) failure to consider exposure; (4) failure to consider life-cycle concerns; and (5) failure to consider functional use.
Lack of Data on Hazard.
One of the most commonly cited causes of regrettable substitutions is inadequate data on the proposed replacement chemical, which does indeed give the impression that regulatory authorities are inappropriately vetting chemicals before allowing their use and, upon discovering hazard or risk, merely replacing them with different chemicals of equally unknown risk—hence the description of the process as “playing whack-a-mole”.20 In some instances, however, the hazard was simply unknowable initially—few could have foreseen the endocrine disrupting effects of phthalates when di(2-ethylhexyl)phthalate (DEHP) was first manufactured in 1931.
However, the process of chemical substitution may inadvertently encourage chemicals with no data compared to existing chemicals. Much of the push to substitute BPA came from consumers, and the easiest way to make a product “BPA-Free” was to swap a similar chemical, BPS, which at the time had no data. One might think that consumers would eschew a chemical with uncharacterized hazard, but remarkably, the only study in our sample that focused on consumer perception found the opposite—consumers preferred chemicals with no established hazard to chemicals with known hazards.21
Ultimately, the combination of consumer preference, stakeholder concerns with efficiency and economy, and regulatory hurdles for new chemicals compared to chemicals which have been grandfathered in the US Toxic Substances Control Act (TSCA)—which includes 83 000 chemicals with data for 3%22—causes a selection that, perversely, favors chemicals with no data.
One of the key reasons for a lack of data is the time and expense of traditional toxicology testing23—and the fact that the testing required for regulatory regimes may not actually reveal the relevant hazard, as is often the case for endocrine disruptors, which will be discussed below in the context of BPA. While our understanding of the ways in which cellular perturbations can lead to adverse outcomes has grown exponentially over the past decades, many of the commonly used regulatory toxicology tests were developed in an era before most receptors were even characterized. This data gap, therefore, cannot be remedied simply by calling for more testing—it can only be filled if testing is radically revamped in a way that makes it quick, economical, and molecularly comprehensive. This is at the heart of Green Toxicology, which seeks to develop tools that are based on either in silico or in vitro assays that offer a quick read-out of hazards.24
Trading off One End Point or Population for Another.
One clear example of such burden shifting is the substitution of chlorinated solvents predominantly because of ecotoxicity concerns with the functionally equivalent n-hexane, which is neurotoxic and leads to nerve damage in humans. For this to be avoided, toxicology has to be able to make accurate predictions about functionally similar chemicals—this can only be accomplished if we improve our Quantitative Structure Activity Relationship (QSAR) models and automated read-across25,26 with both enough data and enough sophistication to disambiguate toxicity within similar classes of chemicals (for example, solvents). As yet, most QSARs are focused on fairly straightforward end points, such as skin sensitization, or mutagenicity, for which there is a large data set and a fairly sophisticated understanding of structure activity relationships. For end points that commonly occur in regrettable substitutions (endocrine disruption, neurotoxicity, developmental toxicity), QSARs are much more rudimentary and unlikely to provide sufficient discriminatory power.
Failure to Consider Exposure.
Ultimately, we care about risk—which is a function of hazard and exposure—yet many screening-level assessments and regulations focus on hazard alone, perhaps because it is less complicated and the ultimate goal is to engineer away hazard. Yet, that may not always be possible, and failure to take into consideration exposure means that several list-based approaches include relatively innocuous chemicals such as zinc, based on the hazard of zinc fumes—an unlikely exposure scenario for many uses. Therefore, a regrettable substitution can happen if hazard is emphasized at the expense of exposure—as it was argued happened when copper was “blacklisted” for use as an exterior building material and replaced with asphalt or poly(vinyl chloride) based material, largely because of human health data based on copper salts or fumes, and not relevant to exposures likely to result from use in roofing material.27 In point of fact, regulation or prioritization based on hazard alone is likely an imprecise tool, as ultimately everything will have some level of hazard.
On the other hand, failure to accurately predict exposures can lead to regrettable substitutions—one justification for the replacement of brominated flame retardants with organophosphorus flame retardants (OPFRs) was the presumed lower exposure based on the shorter persistence of the latter. Owing in part to different patterns of use and off-gassing, however, exposure turned out to be quite high for OPFRs.28 Notably, the concept of thresholds of toxicological concern (TTC) can help in a very pragmatic way with exposure considerations,29 which is being expanded to more and more hazards such as reproductive toxicity30 with the availability of larger chemical toxicity databases.
Failure to Consider Life-Cycle Concerns.
Life-cycle aspects are often neglected as a component in assessing chemical alternatives, but they are essential to unveil trade-offs between different impacts as well as predicting potential exposures. Many plastics are not intrinsically toxic, but heating as well as UV exposure can cause considerable leaching of monomers and therefore higher than predicted exposures.31 Hazard end points are typically one of the key inputs for Life Cycle Analysis. However, in many instances a binary end point is used (i.e., mutagenicity or skin sensitization as positive or negative) when potency might be more useful,32 but data are typically lacking. More broadly still, there is no method to weigh different end points—is a chemical with high potency for skin sensitization to be preferred to a chemical with a low, but not zero, potential for reproductive and developmental effects? Because of this, Life Cycle Analysis is trying to move away from potency-based metrics to severity-based metrics (i.e., disability-adjusted life years, DALY) or a combination approach.33 An example, how environmental burden of disease (EBoD) disability estimates can be translated into DALY for a comprehensive assessment over different hazards was given by Gohlke et al., 201134). A sufficiently nuanced understanding of the population-level effects of using a chemical—especially if both extraction and disposal are considered—is difficult to derive from the standard data produced by toxicology as now practiced and would require a move to Pathway of Toxicity/Adverse Outcome Pathway approach combined with exposomics.35
Focusing Only on Eliminating Chemicals of Concern Fails to Consider the “Functional Use” of the Chemical.
Overly focusing on the toxicity profile of a chemical of concern encourages incremental change and drop-in approaches—and usually the most friction-free way of eliminating a chemical of concern is substituting it by a closely related chemical, likely with poorly characterized hazard, for the chemical of concern. A better alternative is to focus on functionality and look for opportunities for more drastic change—perhaps by identifying nonchemical or process options to fulfill the function or determine that the function is not even necessary.5,36
Interestingly, we found only two articles that focused specifically on the impacts of substitutions on a class of chemicals in a quantitative manner to determine if overall toxicity had been reduced or increased. DiBartolomeis et al.7 looked at the total mass of insecticides used combined with persistence to estimate exposure to honeybees, using oral acute toxicity (LD50) as a reference value for arthropod toxicity. They concluded that the switch from n-methyl carbamate and organophosphate compounds to neonicotinoids and pyrethroids did result in a lower volume of pesticide application; however, owing in part to the greater persistence and toxicity of neonicotinoids and higher exposures to bees in some scenarios, overall, the toxicity of pesticides had increased. Sackmann et al.6 looked at regulatory impacts on the overall toxicity of plasticizers using C&L hazard statements as proxy for toxicity and an unregulated chemical class as a control. According to this analysis, overall, the chemical hazard burden was decreased for both human health and aquatic toxicology, despite the fact that some instances of “regrettable substitutions” had taken place. Both articles were forced to make some simplifying assumptions about toxicity—the paper on pesticides focused narrowly on arthropod oral LD50 as the only relevant hazard end point, while the plasticizers used C&L warnings as a proxy for hazard and focused on tonnage vs exposures. Despite these limitations, both papers offer examples of the sort of larger-scale analysis of the consequences of shifting uses in industrial chemicals, and offer a glimpse of how we could answer, in the future, the question of whether substitutions are regrettable or not. Going forward, such approaches would likely benefit from more precise estimates of toxicity—again, focusing on the need for better hazard data—as well as better metrics of exposure.
BPA—POSTER CHILD FOR REGRETTABLE SUBSTITUTION
BPA (and the replacement with BPS) was the most commonly mentioned discrete chemical in our search, and here we discuss it in-depth, as it both illustrates many of the issues above, and calls into focus the ways in which toxicology needs to change to avoid such substitutions going forward.
BPA has received an extraordinary amount of attention: it has the most studies associated with it of any industrial chemical in the Comparative Toxicogenomics Database (CTD),37 which shows BPA as associated with 202 diseases using curated data only (i.e., an explicit association between the chemical and disease studied) and an astounding 11 380 diseases inferred through patterns of genetic perturbation, the large number of the latter likely due to the fact that CTD shows a total of 2257 papers indicating that BPA effects some 6617 human genes—or nearly 25% of the known human genes typically studied in an RNASeq experiment. PubChem has over 6000 papers with BPA as a keyword. A simple text search of “Bisphenol A” and “Hazard” in Google Scholar returns over 41 000 results.
One would think that such a high level of scrutiny would definitively settle BPA’s mechanism of action, hazards, and an appropriate regulatory response. In fact, that has not happened, and it is likely that the mountain of data has merely made the debate more contentious. The debate resulted in the Consortium Linking Academic and Regulatory Insights on BPA Toxicity (BPA-CLARITY) Study, a multiyear, multi-institution study of BPA which would combine standard guideline compliant toxicology with 14 different academic investigations of more subtle end points—all with appropriate blinding, and the data locked before analysis—yet this, too, failed to definitively answer the question.38 How can toxicology, in the 21st century, not be able to answer such a question? Here, we propose a few reasons, as each suggests areas where toxicology can improve.
To begin with, BPA was known early on as an estrogenic compound, but only weakly so, and it was initially assumed that such low-affinity binding was unlikely to be of much relevance owing to the presence of other endogenous and dietary compounds that would likely outcompete BPA in vivo. For example, the naturally occurring bisphenol F has similar estrogenic properties and is found in mustard up to 8 mg/kg.39 However, this turned out to be either overly simplified or wrong—BPA (and other similar bisphenols, including presumably BPS) has a high binding efficiency to the orphan receptor ERRγ, as well as membrane-bound ER.40,41 In point of fact, the exact mechanism(s) are still debated—but much of the early confusion could have been avoided had hazard assessment not looked under the lamppost for the keys—assuming the estrogen receptor was the one and only mode of action—but instead taken a more systems-level, data-driven approach to look for Pathways of Toxicity.42 Public pressure resulted in BPA being replaced with Bisphenol S—and while the latter did indeed have lower estrogen receptor binding, it binds with a higher affinity to the other receptors BPA is thought to bind to,43 which demonstrates the perils of chemical substitution in the absence of a clear mechanism.
One reason BPA has proven a challenge is because of a non-monotonic dose−response curve, or low dose effects. These are actually slightly different, but related, issues. “Low-dose effects” are typically used to describe effects seen at a dose lower than an established no-observed-adverse-effect-level (NOAEL) or lowest-observed-adverse-effect-level (LOAEL) or alternatively at doses that are commonly seen in the human population, but as such, it is not a precisely defined term, and in point of fact, a recent review indicated that “low-dose effects” was used to describe doses of 8−12 orders of magnitude difference, and often very far from presumed human exposures.44
On the other hand, a non-monotonic dose−response (NMDR) curve is described mathematically as a change in sign of the slope of the dose−response curve. Either way, both represent a challenge for regulatory toxicology, which typically uses too few doses to accurately detect an NMDR, and relatively high doses that often result in frank toxicity (e.g., liver pathology) vs lower doses which result in more subtle end points (e.g., increased prostate weight). As such, the typical tests mandated for product registration and used as the basis for exposure limits will not identify an NMDR and may miss effects at low doses, calling into question the point-of-departure. While this blind spot has proven most acute for BPA, it is of equal concern for any chemical purported to be an endocrine disruptor—including phthalates, plasticizers, and brominated flame retardants. That low dose effects occur is noncontroversial, but they tend to be isolated to one species or one dose point, and therefore, there is some debate about the extent to which such findings should inform regulations when larger studies are negative.45 It is also agreed that NMDRs are common among hormones and therefore not unlikely in endocrine disrupting chemicals, and there are several mechanisms that can result in an NMDR (e.g., agonist vs antagonist at low vs high doses, or when the effects of one receptor stimulated at a low dose is less obvious when higher doses stimulate multiple mechanisms). However, finding statistically robust evidence of a non-monotonic dose−response curve is difficult using in vitro studies, such as transcriptomics, owing to the intrinsic noise of such studies and the use of models that presume some linearity in gene response to interpret them, and equally challenging in in vivo studies owing to the expense of using enough dose−response points and the overall variability of animals. This difficulty is illustrated by two recent systematic reviews of in vivo, in vitro, and epidemiological evidence for NMDRs which found that the overwhelming number of articles had relatively weak evidence for NMDRs (i.e., only one outlying dose, insufficient number of doses to establish an NMDR).46,47 In fact, while 14% of the data sets concerned BPA, this was not one of the data sets that actually demonstrated an NMDR.46 This is not to say that NMDRs do not exist, and perhaps are more common than traditional toxicology would indicate—our own research indicates different mechanisms of BPA at a low point on the dose−response curve vs the higher dose48—but it does point to a need to rethink how we approach such questions. Ideally, given the many chemicals of concern which are believed to be endocrine disruptors and the numbers required to look for subtle end points at multiple points on the dose− response curve, it requires a method that is more efficient than animal testing.
An additional issue is the lack of concordance between academic and regulatory toxicology: Regulatory toxicology focuses on reproducibility, is not hypothesis-driven, and is usually done to very careful good laboratory practice (GLP) standards in contract research facilities with a specific aim of establishing a dose that can be used as a point-of-departure for hazard assessment. Academic toxicology tends to focus less on reproducibility and more on exploring hypothetical mechanisms, and often is done in a more ad hoc manner by nonspecialists (usually graduate students). The problem arises when the latter is used for hazard or risk evaluation in a regulatory environment. In a recent evaluation by FDA for studies done on BPA to clarify mechanisms or dose, the overwhelming amount of research could not be used either for hazard assessment or for risk analysis—121 of 142 studies were rejected, with the most common reasons summarized in Table 2. This would appear to indicate that a vast amount of the research done in academic laboratories has its usefulness unnecessarily limited by a failure to have adequate statistical power, randomization, or blinding.49 Our own analysis of reproductive and developmental studies found that most studies were not useful for systematic review for similar reasons.50 This points to a clear need to improve the quality of academic studies, especially when animals are used.
Table 2.
Study Quality Issues Associated with Studies on BPAa
| study type | number of studies reviewed | studies deemed “useful” for hazard identification and/or risk assessment | study quality issues | |
|---|---|---|---|---|
| Pharmacokinetic | 34 | 3 useful for hazard identification and risk assessment | Study focus was limited Small sample size Inconsistency between study concentrations and those existing in the literature (e.g., controlled human absorption, distribution, metabolism, and excretion (ADME), animal pharmacokinetic (PK), and physiologically based pharmacokinetic (PBPK) modeling) Potential contamination with BPA monomer |
|
| Neurotoxicology | 36 | 1 useful for hazard identification and risk assessment; 6 useful for hazard identification | Not designed to establish NOAELs Routes of exposure that were not relevant to human exposure (e.g., subcutaneous, intraperitoneal) No dose confirmation Control issues (e.g., varying degrees of control for environmental exposure; controls were sometimes not reported) Did not account for lactation exposure (i.e., dose in dams may not have been high enough to ensure adequate transfer to pups via lactation) Doses ranged widely Less than 3 doses used No positive control Exposure was not confirmed Human exposures were estimated through single urine samples Did not account for litter effects Overinterpretation of marginally significant results Inappropriate extrapolation of findings to humans |
|
| Reproductive and Developmental | 5 | 2 useful for hazard identification | Routes of exposure that were not relevant to human exposure (e.g., subcutaneous, implanted osmotic pumps) Not guideline compliant Reproductive parameters were not evaluated in both sexes Less than 3 doses used Lack of complete reporting in study design and procedures Vehicle used was oil; some of these oils have been shown to have estrogenic constituents |
|
| Carcinogenesis | 5 | None | Routes of exposure that were not relevant to human exposure (e.g., subcutaneous, implanted osmotic pumps) Vehicle used was oil; some of these oils have been shown to have estrogenic constituents Less than 3 doses used Large fold-change in doses used Study design limitations |
|
| Other End Points (Systemic effects) | 13 | 2 useful for hazard identification | Routes of exposure that were not relevant to human exposure (e.g., subcutaneous, intraperitoneal) Vehicle used was oil; some of these oils have been shown to have estrogenic constituents Diets contained phytoestrogens Litter effects were not accounted for No positive control Rodent housing information was not provided Exposure was not confirmed BPA in housing materials was not assessed Dosing solutions were not certified |
|
| Epidemiology | 48 | 7 useful for hazard identification | Did not account for extraneous contamination of BPA samples by the collecting device Used blood or cord-blood measurements; however, these have been demonstrated to be unreliable metrics, as BPA has a short half-life in plasma Plasma BPA cannot adequately discriminate between true signal and noise Single time points (i.e., mostly cross-sectional studies) Associations were reported for transformed but not original data Use of plastic sample containers but potential for BPA leaching was not assessed Small sample size Single exposure measure Not controlling for confounders Not controlling for multiple hypothesis testing Poor description of sample collection timing No clinical significance for measured outcome Highly variable outcome used as target end point Arbitrary definition of clinical outcome Selective reporting of positive results No dose–response relationship Inconsistent inter- or intrastudy results Lack of generalizability Reverse causality |
|
Although 142 studies were carried out on BPA, only a select few (21) were deemed “useful” for hazard identification and/or risk assessment. The remaining 121 studies had a number of associated quality issues which prevented them from being included in a hazard or risk assessment.
A final issue clouding the debate about BPA is the perception of partisanship in science and/or conflict of interest. Ultimately, these debates concern complex issues and often ambiguous data, but can turn into personalized disagreements with mutual accusations of bias and conflicts of interest, as has happened with BPA,51 as well as atrazine and other topics in environmental health.52 It goes without saying that conflicts of interest (as well as data suppression) are very real and concerning and are especially problematic in the case of industry-funded studies. Recent systematic reviews of industry-funded studies in medical devices and drugs found they consistently reported more positive effects—despite having similar (or, in the case of blinding, improved) risk-of-bias protocols53 compared to non-industry-funded studies. However, bias is not exclusive to one side: both “white-hat” bias (being biased toward conclusions that further a public health goal)54 and publication bias55 can distort results. While funding should always be considered, categorically disqualifying studies ultimately makes science about something other than the data itself.
Ultimately, a better way forward is by making data as transparent and reproducible as possible: this includes making all original data available and preferably locked down, requiring that all data produced from animal studies be made available, even if the results are negative or ambiguous, to avoid bias by failure to publish; adopting GLP and good cell culture practice (GCCP)56 practices; careful attention in the materials and methods sections as Good Reporting Practices;57 and consistent use of both randomization and blinding. While the BPA-CLARITY study may not have settled the debate about BPA, it has at least moved the debate from the data to the interpretation of the data—which is a small, but necessary, step.
DISCUSSION
What are the lessons for toxicology going forward? One clear need is both better quality and reporting of data as well as better types of data to clarify hazard. This includes first of all larger data sets with more chemicals, including making more legacy data machine-readable—while we have begun this process with the ECHA\REACH data sets,58 there remains a great deal of data that is not useful for models.
Moreover, we need higher dimensional data sets for many more chemicals—our analysis of the publications per chemical in the CTD database indicated that a handful of chemicals (BPA, benzo(a)pyrene, valproic acid, and dioxin) are responsible for a disproportionately large number of mechanistic studies in toxicology—the top 10 chemicals each have more than 1000 papers annotated, while over 5000 chemicals have less than 5 papers. Moreover, the marginal value of doing one more small study on BPA is at this point somewhat limited—as it is not data we lack but the ability to make sense of the data—and those resources would likely be better spent on extending our understanding of more chemicals. Our own work has demonstrated that large, high-dimensional data sets can answer both fundamental questions about gene function as well as illuminate the mechanisms of toxicity.59,60 Larger, more chemically diverse data sets, will help improve the in silico approaches needed to steer chemical design toward less hazardous substitutes.
Since it is impossible to foresee all hazards, we must also develop methodologies that can continuously monitor and identify unintended health risks of anthropogenic chemicals. As it is, once a chemical is in widespread consumer use, it is de facto being tested on humans, but we have no methodologies in place to systematically monitor potential health effects. Recently, several weak, but significant, associations between common medicines and adverse outcomes—as an example, anticholinergics and dementia61—have come to light thanks to mining of electronic health records or other large population studies. It is unlikely that such a small association for a disease that lacks adequate animal models would have been detected without such human data, as it includes exposures over a larger time period and as well as mixtures (e.g., multiple types of anticholinergic medicines). At the same time, we are exposed to thousands of anthropogenic chemicals, yet we lack the methods and data infrastructure to link exposures to outcomes in a similar way. This points to a need for toxicology to extend to a human, exposome-based approach35 to tackle such associations.
An additional take-away is the need to incorporate the data needs of regulatory toxicology as a part of graduate education in environmental health departments. More broadly, this includes the importance of robust statistical methods, proper randomization and blinding for in vitro and in vivo studies, and full data availability. While many may view this as overly burdensome for studies aimed at publication rather than a regulatory submission, the reality is that such considerations are crucial to reproducibility and therefore should be central to all science.
It is also important to improve systems level thinking for both toxicologists and chemists—this will likely mean a new emphasis on interdisciplinary approaches, integration of disparate qualitative and quantitative data, and methods for analyzing cumulative and interactive effects in different populations. When new chemical substitutions are proposed, both toxicologists and chemists need to think not just about the chemical itself but about the possible implications both upstream of chemical manufacturing and downstream during consumer use. Many of the environmental challenges of the future will likely not involve simplistic solutions, but instead a complicated weighing of trade-offs that requires an understanding of complicated, connected systems.
Finally, it is worth keeping in mind that the history of regrettable substitutions actually contains many victories. DDT is often given as an example of a chemical that was a “regrettable substitution”, but the reality is DDT replaced such chemicals as lead arsenate—a far more toxic pesticide—and was responsible for saving perhaps more lives than any other chemical by wiping out malaria in many countries.62 At the same time, it had many unforeseen consequences, and its persistence and widespread, indiscriminate use meant that we are still dealing with those consequences today. There is, in fact, still a scientific debate about the trade-offs. Science, therefore, is doing what it should: it offered a better solution to an existing hazard, discovered that this entailed problems, and is slowly working to improve again.
Funding
Emily Golden was supported by NIEHS training grant (T32 ES007141).
Footnotes
The authors declare no competing financial interest.
Complete contact information is available at: https://pubs.acs.org/10.1021/acssuschemeng.0c09435
Contributor Information
Alexandra Maertens, Center for Alternatives to Animal Testing (CAAT), Johns Hopkins Bloomberg School of Public Health, Department of Environmental Health and Engineering, Baltimore, Maryland 21205, United States.
Emily Golden, Center for Alternatives to Animal Testing (CAAT), Johns Hopkins Bloomberg School of Public Health, Department of Environmental Health and Engineering, Baltimore, Maryland 21205, United States.
Thomas Hartung, Center for Alternatives to Animal Testing (CAAT), Johns Hopkins Bloomberg School of Public Health, Department of Environmental Health and Engineering, Baltimore, Maryland 21205, United States; CAAT-Europe, University of Konstanz, 78464 Konstanz, Germany.
REFERENCES
- (1).Zimmerman JB; Anastas PT Chemistry. Toward Substitution with No Regrets. Science 2015, 347 (6227), 1198–1199. [DOI] [PubMed] [Google Scholar]
- (2).Maertens A; Anastas N; Spencer PJ; Stephens M; Goldberg A; Hartung T Green Toxicology. ALTEX 2014, 31 (3), 243–249. [DOI] [PubMed] [Google Scholar]
- (3).Opinion: Most chemical substitutions are regrettable; https://www.foodpackagingforum.org/news/opinion-most-chemical-substitutions-are-regrettable (accessed Dec 27, 2020).
- (4).Committee on the Design and Evaluation of Safer Chemical Substitutions: A Framework to Inform Government and Industry Decision; Board on Chemical Sciences and Technology; Board on Environmental Studies and Toxicology; Division on Earth and Life Studies; National Research Council. A Framework to Guide Selection of Chemical Alternatives; National Academies Press (US): Washington, DC, 2014. DOI: 10.17226/18872. [DOI] [PubMed] [Google Scholar]
- (5).Tickner J; Jacobs MM; Mack NB Alternatives Assessment and Informed Substitution: A Global Landscape Assessment of Drivers, Methods, Policies and Needs. Sustainable Chemistry and Pharmacy 2019, 13, 100161. [Google Scholar]
- (6).Sackmann K; Reemtsma T; Rahmberg M; Bunke D Impact of European Chemicals Regulation on the Industrial Use of Plasticizers and Patterns of Substitution in Scandinavia. Environ. Int 2018, 119, 346–352. [DOI] [PubMed] [Google Scholar]
- (7).DiBartolomeis M; Kegley S; Mineau P; Radford R; Klein K An Assessment of Acute Insecticide Toxicity Loading (AITL) of Chemical Pesticides Used on Agricultural Land in the United States. PLoS One 2019, 14 (8), No. e0220029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Hoffmann S; de Vries RBM; Stephens ML; Beck NB; Dirven HAAM; Fowle JR 3rd; Goodman JE; Hartung T; Kimber I; Lalu MM; Thayer K; Whaley P; Wikoff D; Tsaioun K A Primer on Systematic Reviews in Toxicology. Arch. Toxicol 2017, 91 (7), 2551–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).ChemFORWARD; https://www.chemforward.org (accessed Dec 31, 2020).
- (10).Maertens A; Plugge H Better Metrics for “Sustainable by Design”—Towards an in Silico Green Toxicology for Green (er) Chemistry. ACS Sustainable Chem. Eng 2017, 6, 1999. [Google Scholar]
- (11).Wang Z; Walker GW; Muir DCG; Nagatani-Yoshida K Toward a Global Understanding of Chemical Pollution: A First Comprehensive Analysis of National and Regional Chemical Inventories. Environ. Sci. Technol 2020, 54 (5), 2575–2584. [DOI] [PubMed] [Google Scholar]
- (12).Meigs L; Smirnova L; Rovida C; Leist M Animal Testing and Its alternatives−The Most Important Omics Is Economics. ALTEX 2018, 35, 275. [DOI] [PubMed] [Google Scholar]
- (13).Hartung T Making Big Sense from Big Data in Toxicology by Read-Across. ALTEX 2016, 33 (2), 83–93. [DOI] [PubMed] [Google Scholar]
- (14).Jacobs MM; Malloy TF; Tickner JA; Edwards S Alternatives Assessment Frameworks: Research Needs for the Informed Substitution of Hazardous Chemicals. Environ. Health Perspect 2016, 124 (3), 265–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Fantke P; Huang L; Overcash M; Griffing E; Jolliet O Life Cycle Based Alternatives Assessment (LCAA) for Chemical Substitution. Green Chem. 2020, 22 (18), 6008–6024. [Google Scholar]
- (16).Jamarani R; Erythropel HC; Nicell JA; Leask RL; Marić M How Green Is Your Plasticizer? Polymers 2018, 10 (8), 834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Latham J Unsafe at any dose? Diagnosing chemical safety failures, from DDT to BPA; https://truthout.org/articles/unsafe-at-any-dose-diagnosing-chemical-safety-failures-from-ddt-to-bpa/ (accessed Dec 31, 2020).
- (18).Ubaid ur Rahman H; Asghar W; Nazir W; Sandhu MA; Ahmed A; Khalid N A Comprehensive Review on Chlorpyrifos Toxicity with Special Reference to Endocrine Disruption: Evidence of Mechanisms, Exposures and Mitigation Strategies. Sci. Total Environ 2021, 755, 142649. [DOI] [PubMed] [Google Scholar]
- (19).Demeneix BA Evidence for Prenatal Exposure to Thyroid Disruptors and Adverse Effects on Brain Development. Eur. Thyroid J 2020, 8 (6), 283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Allen J Stop Playing Whack-a-Mole with Hazardous Chemicals. The Washington Post; December 16, 2016. [Google Scholar]
- (21).Scherer LD; Maynard A; Dolinoy DC; Fagerlin A; Zikmund-Fisher BJ The Psychology of “regrettable Substitutions”: Examining Consumer Judgements of Bisphenol A and Its Alternatives. Health Risk Soc. 2014, 16 (7−8), 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Crawford SE; Hartung T; Hollert H; Mathes B; van Ravenzwaay B; Steger-Hartmann T; Studer C; Krug HF Green Toxicology: A Strategy for Sustainable Chemical and Material Development. Environ. Sci. Eur 2017, 29 (1), 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Hartung T Food for Thought⋯ on Alternative Methods for Chemical Safety Testing. ALTEX. 2010, 3–14. [DOI] [PubMed] [Google Scholar]
- (24).Maertens A; Hartung T Green Toxicology-Know Early About and Avoid Toxic Product Liabilities. Toxicol. Sci 2018, 161 (2), 285–289. [DOI] [PubMed] [Google Scholar]
- (25).Helman G; Shah I; Williams AJ; Edwards J; Dunne J; Patlewicz G Generalized Read-Across (GenRA): A Workflow Implemented into the EPA CompTox Chemicals Dashboard. ALTEX 2019, 36 (3), 462–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Ball N; Madden J; Paini A; Mathea M; Palmer AD; Sperber S; Hartung T; van Ravenzwaay B Key Read across Framework Components and Biology Based Improvements. Mutat. Res., Genet. Toxicol. Environ. Mutagen 2020, 853, 503172. [DOI] [PubMed] [Google Scholar]
- (27).Meyer JS; Claytor CA; Gorsuch JW; Dwyer RL Misapplication of Generic Hazard-Classification Schemes for Versatile, Sustainable Building Materials: Copper as an Example. Hum. Ecol. Risk Assess 2017, 23 (7), 1703–1730. [Google Scholar]
- (28).Blum A; Behl M; Birnbaum L; Diamond ML; Phillips A; Singla V; Sipes NS; Stapleton HM; Venier M Organo-phosphate Ester Flame Retardants: Are They a Regrettable Substitution for Polybrominated Diphenyl Ethers? Environ. Sci. Technol. Lett 2019, 6 (11), 638–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Hartung T Thresholds of Toxicological Concern − Setting a Threshold for Testing below Which There Is Little Concern. ALTEX. 2017, 331–351. [DOI] [PubMed] [Google Scholar]
- (30).van Ravenzwaay B; Jiang X; Luechtefeld T; Hartung T The Threshold of Toxicological Concern for Prenatal Developmental Toxicity in Rats and Rabbits. Regul. Toxicol. Pharmacol 2017, 88, 157–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Teuten EL; Saquing JM; Knappe DR; Barlaz MA; Jonsson S; Björn A; Rowland SJ; Thompson RC; Galloway TS; Yamashita R; Ochi D; Watanuki Y; Moore C; Viet PH; Tana TS; Prudente M; Boonyatumanond R; Zakaria MP; Akkhavong K; Ogata Y; Hirai H; Iwasa S; Mizukawa K; Hagino Y; Imamura A; Saha M; Takada H Transport and Release of Chemicals from Plastics to the Environment and to Wildlife. Philos. Trans. R. Soc., B 2009, 364 (1526), 2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Heflich RH; Johnson GE; Zeller A; Marchetti F; Douglas GR; Witt KL; Gollapudi BB; White PA Mutation as a Toxicological Endpoint for Regulatory Decision-Making. Environ. Mol. Mutagen 2020, 61 (1), 34–41. [DOI] [PubMed] [Google Scholar]
- (33).Krewitt W; Pennington DW; Olsen SI; Crettaz P; Jolliet O;et al. Indicators for Human Toxicity in Life Cycle Impact Assessment. In Toward Best Available Practice for Life Cycle Impact Assessment; 2002; p 123. [Google Scholar]
- (34).Gohlke JM; Thomas R; Woodward A; Campbell-Lendrum D; Prüss-Üstün A; Hales S; Portier CJ Estimating the Global Public Health Implications of Electricity and Coal Consumption. Environ. Health Perspect 2011, 119 (6), 821–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Sillé F; Karakitsios S; Kleensang A; Koehler K; Maertens A; Miller GW; Prasse C; Quiros-Alcala L; Ramachandran G; Hartung T The Exposome: A New Approach for Risk Assessment. ALTEX 2020, 37 (1), 3–23. [DOI] [PubMed] [Google Scholar]
- (36).Tickner JA; Schifano JN; Blake A; Rudisill C; Mulvihill MJ Advancing Safer Alternatives through Functional Substitution. Environ. Sci. Technol 2015, 49 (2), 742–749. [DOI] [PubMed] [Google Scholar]
- (37).Davis AP; Grondin CJ; Johnson RJ; Sciaky D; King BL; McMorran R; Wiegers J; Wiegers TC; Mattingly CJ The Comparative Toxicogenomics Database: Update 2017. Nucleic Acids Res. 2017, 45 (D1), D972–D978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Davis E Academics clash with FDA over CLARITY-BPA results; https://chemicalwatch.com/70439/academics-clash-with-fda-over-clarity-bpa-results (accessed Dec 27, 2020).
- (39).Zoller O; Brüschweiler BJ; Magnin R; Reinhard H; Rhyn P; Rupp H; Zeltner S; Felleisen R Natural Occurrence of Bisphenol F in Mustard. Food Addit. Contam., Part A 2015, 33 (1), 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).MacKay H; Abizaid A A Plurality of Molecular Targets: The Receptor Ecosystem for Bisphenol-A (BPA). Horm. Behav 2018, 101, 59–67. [DOI] [PubMed] [Google Scholar]
- (41).Okada H; Tokunaga T; Liu X; Takayanagi S; Matsushima A; Shimohigashi Y Direct Evidence Revealing Structural Elements Essential for the High Binding Ability of Bisphenol A to Human Estrogen-Related Receptor-Gamma. Environ. Health Perspect 2008, 116 (1), 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Bouhifd M; Andersen ME; Baghdikian C; Boekelheide K; Crofton KM; Fornace AJ Jr; Kleensang A; Li H; Livi C; Maertens A; McMullen PD; Rosenberg M; Thomas R; Vantangoli M; Yager JD; Zhao L; Hartung T The Human Toxome Project. ALTEX 2015, 32 (2), 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Rochester JR; Bolden AL Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ. Health Perspect 2015, 123, 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Teeguarden JG; Hanson-Drury S A Systematic Review of Bisphenol A “Low Dose” Studies in the Context of Human Exposure: A Case for Establishing Standards for Reporting “Low-Dose” Effects of Chemicals. Food Chem. Toxicol 2013, 62, 935–948. [DOI] [PubMed] [Google Scholar]
- (45).Melnick R; Lucier G; Wolfe M; Hall R; Stancel G; Prins G; Gallo M; Reuhl K; Ho S-M; Brown T; Moore J; Leakey J; Haseman J; Kohn M Summary of the National Toxicology Program’s report of the endocrine disruptors low-dose peer review Environ. Health Perspect 2002110427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Beausoleil C; Beronius A; Bodin L; Bokkers BGH; Boon PE; Burger M; Cao Y; De Wit L; Fischer A; Hanberg A; Leander K; Litens-Karlsson S; Rousselle C; Slob W; Varret C; Wolterink G; Zilliacus J Review of Non-monotonic Dose-responses of Substances for Human Risk Assessment. EFS3 2016, 13 (5), 1. [Google Scholar]
- (47).Varret C; Beronius A; Bodin L; Bokkers BGH; Boon PE; Burger M; De Wit-Bos L; Fischer A; Hanberg A; Litens-Karlsson S; Slob W; Wolterink G; Zilliacus J; Beausoleil C; Rousselle C Evaluating the Evidence for Non-Monotonic Dose-Response Relationships: A Systematic Literature Review and (re-)analysis of in Vivo Toxicity Data in the Area of Food Safety. Toxicol. Appl. Pharmacol 2018, 339, 10–23. [DOI] [PubMed] [Google Scholar]
- (48).Maertens A; Tran V; Kleensang A; Hartung T Weighted Gene Correlation Network Analysis (WGCNA) Reveals Novel Transcription Factors Associated with Bisphenol A Dose-Respons. Front. Genet 2018, 9, 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Percie du Sert N; Hurst V; Ahluwalia A; Alam S; Avey MT; Baker M; Browne WJ; Clark A; Cuthill IC; Dirnagl U; Emerson M; Garner P; Holgate ST; Howells DW; Karp NA; Lazic SE; Lidster K; MacCallum CJ; Macleod M; Pearl EJ; Petersen OH; Rawle F; Reynolds P; Rooney K; Sena ES; Silberberg SD; Steckler T; Würbel H The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18 (7), No. e3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Stephens ML; Akgün-Ölmez SG; Hoffmann S; De Vries R; Flick B; Hartung T; Lalu M; Maertens A; Witters H; Wright R; et al. Adaptation of the Systematic Review Framework to the Assessment of Toxicological Test Methods: Challenges and Lessons Learned With the Zebrafish Embryotoxicity Test. Toxicol. Sci 2019, 171 (1), 56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Willyard C Allegations of Bias Cloud Conflicting Reports on Bisphenol A’s Effects. Nat. Med 2007, 13 (9), 1002. [DOI] [PubMed] [Google Scholar]
- (52).Mebane CA; Sumpter JP; Fairbrother A; Augspurger TP; Canfield TJ; Goodfellow WL; Guiney PD; LeHuray A; Maltby L; Mayfield DB; McLaughlin MJ; Ortego LS; Schlekat T; Scroggins RP; Verslycke TA Scientific Integrity Issues in Environmental Toxicology and Chemistry: Improving Research Reproducibility, Credibility, and Transparency. Integr. Environ. Assess. Manage 2019, 15 (3), 320–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Lundh A; Lexchin J; Mintzes B; Schroll JB; Bero L Industry Sponsorship and Research Outcome. Cochrane Database of Systematic Reviews. 2017, DOI: 10.1002/14651858.MR000033.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Cope MB; Allison DB White Hat Bias: Examples of Its Presence in Obesity Research and a Call for Renewed Commitment to Faithfulness in Research Reporting. Int. J. Obes 2010, 34 (1), 84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Young NS; Ioannidis JPA; Al-Ubaydli O Why Current Publication Practices May Distort Science. PLoS Med. 2008, 5 (10), e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Pamies D Good Cell and Tissue Culture Practice 2.0 (GCCP 2.0) − Draft for Stakeholder Discussion and Call for Action. ALTEX. 2020, 490–492. [DOI] [PubMed] [Google Scholar]
- (57).Coecke S; Balls M; Bowe G; Davis J; Gstraunthaler G; Hartung T; Hay R; Merten O-W; Price A; Schechtman L; Stacey G; Stokes W Guidance on Good Cell Culture Practice. ATLA, Altern. Lab. Anim 2005, 33, 261–287. [DOI] [PubMed] [Google Scholar]
- (58).Luechtefeld T; Maertens A; Russo DP; Rovida C; Zhu H; Hartung T Global Analysis of Publicly Available Safety Data for 9,801 Substances Registered under REACH from 2008−2014. ALTEX 2016, 33 (2), 95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Maertens A; Luechtefeld T; Kleensang A; Hartung T MPTP’s Pathway of Toxicity Indicates Central Role of Transcription Factor SP1. Arch. Toxicol 2015, 89 (5), 743–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Maertens A; Tran VP; Maertens M; Kleensang A; Luechtefeld TH; Hartung T; Paller CJ Author Correction: Functionally Enigmatic Genes in Cancer: Using TCGA Data to Map the Limitations of Annotations. Sci. Rep 2020, 10 (1), 9718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Coupland CAC; Hill T; Dening T; Morriss R; Moore M; Hippisley-Cox J Anticholinergic Drug Exposure and the Risk of Dementia: A Nested Case-Control Study. JAMA Int. Med 2019, 179 (8), 1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Rosenthal PJ; John CC; Rabinovich NR Malaria: How Are We Doing and How Can We Do Better? Am. J. Trop. Med. Hyg 2019, 100 (2), 239–241. [DOI] [PMC free article] [PubMed] [Google Scholar]


