Abstract
Background
The SARS-CoV-2 pandemic is a worldwide challenge. The CRIT-CoV-U pilot study generated a urinary proteomic biomarker consisting of 50 peptides (COV50), which predicted death and disease progression from SARS-CoV-2. After the interim analysis presented for the German Government, here, we aimed to analyse the full dataset to consolidate the findings and propose potential clinical applications of this biomarker.
Methods
CRIT-CoV-U was a prospective multicentre cohort study. In eight European countries (Austria, France, Germany, Greece, North Macedonia, Poland, Spain, and Sweden), 1012 adults with PCR-confirmed COVID-19 were followed up for death and progression along the 8-point WHO scale. Capillary electrophoresis coupled with mass spectrometry was used for urinary proteomic profiling. Statistical methods included logistic regression and receiver operating characteristic curve analysis with a comparison of the area under curve (AUC) between nested models. Hospitalisation costs were derived from the care facility corresponding with the Markov chain probability of reaching WHO scores ranging from 3 to 8 and flat-rate hospitalisation costs adjusted for the gross per capita domestic product of each country.
Findings
From June 30 to Nov 19, 2020, 228 participants were recruited, and from April 30, 2020, to April 14, 2021, 784 participants were recruited, resulting in a total of 1012 participants. The entry WHO scores were 1–3 in 445 (44%) participants, 4–5 in 529 (52%) participants, and 6 in 38 (4%) participants; and of all participants, 119 died and 271 had disease progression. The odds ratio (OR) associated with COV50 in all 1012 participants for death was 2·44 (95% CI 2·05–2·92) unadjusted and 1·67 (1·34–2·07) when adjusted for sex, age, BMI, comorbidities, and baseline WHO score; and for disease progression, the OR was 1·79 (1·60–2·01) when unadjusted and 1·63 (1·41–1·91) when adjusted (p<0·0001 for all). The predictive accuracy of the optimised COV50 thresholds was 74·4% (71·6–77·1%) for mortality (threshold 0·47) and 67·4% (64·4–70·3%) for disease progression (threshold 0·04). When adjusted for covariables and the baseline WHO score, these thresholds improved AUCs from 0·835 to 0·853 (p=0·033) for death and from 0·697 to 0·730 (p=0·0008) for progression. Of 196 participants who received ambulatory care, 194 (99%) did not reach the 0·04 threshold. The cost reductions associated with 1 day less hospitalisation per 1000 participants were million Euro (M€) 0·887 (5–95% percentile interval 0·730–1·039) in participants at a low risk (COV50 <0·04) and M€2·098 (1·839-2·365) in participants at a high risk (COV50 ≥0·04).
Interpretation
The urinary proteomic COV50 marker might be predictive of adverse COVID-19 outcomes. Even in people with mild-to-moderate PCR-confirmed infections (WHO scores 1–4), the 0·04 COV50 threshold justifies earlier drug treatment, thereby potentially reducing the number of days in hospital and associated costs.
Funding
German Federal Ministry of Health.
Introduction
The SARS-CoV-2 pandemic is a challenge for health care worldwide. Globally, from Nov 15 to 21, 2021, nearly 3·6 million new cases and more than 51 000 deaths were reported, reflecting continual increases in both metrics compared with the preceding weeks.1 Despite the roll-out of vaccines, the pandemic continues to burden health care, given the emergence in 2022 of the omicron variant, which has higher transmissibility and potentially more resistance against immunological responses to vaccines or a previous infection than previous variants.2, 3 Patients with COVID-19 who are admitted to hospital are usually stratified for risk on the basis of age, obesity, other comorbidities, and several disease severity scales.4
Research in context.
Evidence before this study
The literature and guidelines were reviewed with the objective of assessing the efficacy of interventions in people with COVID-19 relative to the disease stage at presentation. We searched PubMed from Jan 1, 2020, to Dec 31, 2021, using the terms: (COVID-19 OR SARS-CoV-2) AND (clinical trials OR randomised trials OR randomized trials OR RCTs), which identified 52 articles published in English in 2020 and 2021, and which were all read and summarised. 11 studies enrolled patients into ambulatory care with mild-to-moderate disease, whereas all other studies recruited patients admitted to hospital with moderate-to-severe disease. The median number of days from symptom onset to intervention varied from 1 to 13 days. Corticosteroids, antiviral drugs, anti-inflammatory drugs, antiviral monoclonal antibodies, and fluvoxamine reduced the viral load and disease progression, whereas all other tested drugs and convalescent plasma did not modify the disease course. All studies applied clinical criteria, risk factors, comorbidities, or disease-severity scales to stratify for risk. No study implemented a predictive biomarker to triage patients for ambulatory versus hospital care or to assess the need for early intervention. Among the directives for the management of people with COVID-19, only the Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften Leitlinie guidelines mentioned the use of COV50, a urinary proteomic profile biomarker.
Added value of this study
This study is the first to include a COVID-19-specific biomarker to guide early intervention. COV50 consists of 50 differentially regulated urinary peptides and is able to predict death and disease progression in adults with mild-to-moderate PCR-confirmed COVID-19 infection. The predictive accuracy of the optimised COV50 thresholds was 74·4% for mortality and 67·4% for disease progression. When adjusted for covariables and then the baseline WHO score, the continuously distributed urinary marker and its optimised thresholds improved the area under the curves from 0·835 to 0·854 to 0·853 for death and from 0·697 to 0·740 to 0·730 for disease progression. Using the 0·04 threshold to differentiate low COVID-19-associated risk from high COVID-19-associated risk would allow selecting patients with mild disease at presentation for earlier drug treatment, thereby decreasing the risk of worsening disease and death and reducing hospitalisation costs.
Implications of all the available evidence
The crucial question emerging from the COVID-19 pandemic, and from the omicron variant's becoming dominant with high transmissibility, is how to prevent deterioration to critical illness in people who are infected. A COV50 score of 0·04 or higher predicts disease progression in addition to clinical criteria. Even in patients with mild-to-moderate disease (WHO stages 1–4), a high-risk COV50 score is an indication for early in-hospital treatment, thereby valorising the results of the 2020–21 trials and reducing the burden on health care. COV50 testing can also be applied for the selection of patients in randomised clinical trials of innovative COVID-19 treatment methods, in which risk at presentation is an issue in the choice between ambulatory versus hospitalised care or in the treatment method to be tested. COV50 is registered in Germany and available for clinical use in the EU.
Patients with COVID-19 have a prothrombotic or thrombophilic state, with elevations of several biomarkers reflecting thrombosis, fibrinolysis, and inflammation, which are associated with disease severity and prognosis.5 However, none of these biomarkers are specific for COVID-19 and most have not had a prospective validation of the action threshold that defines the need for intervention.5 In contrast, urinary proteomic profiling (UPP) generates classifiers that are representative of pathogenic molecular mechanisms, which in the case of SARS-CoV-2 infection are generally independent of the virus strain and might inform treatment, in particular with pharmacological agents not specifically directed against the variable S-protein domains of mutated SARS-CoV-2 variants. After a request from the German Government, an interim analysis of the CRIT-CoV-U study described the discovery, replication, and internal and external validation of COV50.6 This novel UPP biomarker consists of 50 dysregulated urinary peptides (appendix pp 8–10) and predicts death and progression across the COVID-19 WHO stages beyond risk factors and comorbidities.6 The objectives of the current study were to consolidate the interim findings in the full CRIT-CoV-U study sample and to propose the potential applications of the COV50 marker in clinical practice and trial design.
Methods
Study design and participants
The CRIT-CoV-U project complied with the Helsinki declaration. The Ethics Committee of the German-Saxonian Board of Physicians (Dresden, Germany; number EKBR88/20·1) and the Institutional Review Boards of the recruiting sites provided ethical approval. The protocol was deposited at the German Register for Clinical Studies (number DRKS00022495), which is linked to the WHO International Clinical Trial Registry Platform. The English version of the protocol is available for download from: https://crit-cov.de/files/Crit-Cov/Crit-Cov-U-study-protocol.pdf.
CRIT-CoV-U was a prospective multicentre cohort study.6 Eligible participants were non-anuric adults (≥18 years), capable of giving written informed consent, with PCR-confirmed SARS-CoV-2 infection diagnosed in ambulatory care or on the first day of admission to hospital. All participants meeting the eligibility criteria were enrolled without any exclusion in two phases: 228 participants were recruited from June 30 to Nov 19, 2020, and were included in the interim report;6 the recruitment of a further 784 participants was done from April 30, 2020, to April 14, 2021, so that the full study cohort comprised 1012 individuals. Five hospitals participated in the initial enrolment of participants and an additional 12 in the continued recruitment. Two sites were located in Innsbruck and Vienna, Austria (65 participants enrolled), one in Paris, France (49 participants), seven in Bayreuth, Berlin, Düsseldorf, Hamburg, Leipzig, München, and Stuttgart, Germany (458 participants), one in Athens, Greece (30 participants), one in Skopje, North Macedonia (137 participants), four in Gdańsk, Katowice, Kraków, and Wrocław, Poland (149 participants), one in Sevilla, Spain (23 participants), and two in Skövde and Umea, Sweden (101 participants).
Procedures
All participants were followed up until recovery, hospital discharge, or death. On days 0–3, 4–7, and 10–21 after diagnosis, participants who were alive were staged according to the 8-point WHO Clinical Progression Scale.7 Electronic case report forms (MARVIN EDC; XClinical, Munich, Germany) were used for data compilation. For UPP, 10-mL urine samples were collected in borated test tubes (ExactoBac-U; Sarstedt, Nümbrecht, Germany) and kept at –20°C or less until assayed. The methods for capillary electrophoresis coupled with mass spectrometry, for peptide sequencing, and for the evaluation, calibration, and quality control of the mass spectrometric data are described in detail in the appendix (pp 2–5). For identification of the urinary biomarker, 196 urine samples were randomly selected from those available from participants at days 4–7 and days 10–21 after diagnosis, excluding participants at WHO stages 4–5, allowing for a comparison of the UPP profiles at WHO stages 1–3 (n=116) and 6–8 (n=80).6 The disease-specific classifier was developed by support vector machine modelling and cross-validated by a take-one-out procedure with significance adjusted for the false-discovery rate set at 0·05.6 The derivation cohort included 228 participants, and the validation cohort included 99 participants (appendix p 11). Finally, to investigate the applicability of the COV50 biomarker, from Feb 7 to March 16, 2022, a further 62 participants consecutively admitted to hospital with PCR-confirmed COVID-19 infection without exclusion were enrolled at the Department of Infectious Diseases and Tropical Medicine at the Nephrology and Kuratorium für Dialyse und Nierentransplantation Renal Unit and Rheumatology at St Georg Hospital (Leipzig, Germany) and followed up until death or discharge. These 62 participants underwent the same measurements and were statistically analysed by the same methods as in the main study. SARS-CoV-2 variants were established using next-generation sequencing of the whole genome of SARS-CoV-2 strains.
Statistical analysis and outcomes
The initial sample size calculations, informed by a proof-of-concept study,8 required 212 participants with life-threatening COVID-19 (WHO stage ≥6) to be compared with 271 participants with mild symptoms (WHO stage <4) to identify and validate a UPP biomarker with 75% sensitivity and 80% specificity. Given the 33% progression rate from mild to severe disease in the pilot study,8 and accounting for a 15% rate of missing data, the sample size for the full study was initially set at 645 participants. On the basis of the interim study,6 in which the mortality rate was 10% (23/228), the sample size for the full study was revised to 1000 participants.
For database management and statistical analysis, SAS software (version 9·4) was used. Significance was a two-tailed p value of 0·05 or less. Means were compared using the large-sample z test or ANOVA, and proportions were compared using Fisher's exact test. The predefined endpoints were mortality and progression measured using the 8-point WHO scale of disease severity.6 The 95% CIs of rates were computed as R ± 1·96 × √ (R × [100 – R] /T), where R is the rate and T is the number of participants at risk of developing an adverse outcome. The risk of incident endpoints was derived from the baseline COV50 score by logistic regression, unadjusted or adjusted for sex, age, the entry WHO scale, and comorbidities including hypertension, heart failure, diabetes, and cancer. These covariables were selected because they were in line with known clinical risk factors for COVID-19-related mortality,9 and because they had also been applied in the interim report,6 thereby maintaining consistency. In participants admitted to hospital, serum creatinine was measured, allowing the calculation of the glomerular filtration rate using the formula published by the Chronic Kidney Disease Epidemiology Collaboration,10 and further adjustment of the logistic models for the estimated glomerular filtration rate was done. Correlations between categorical variables were computed using Fisher's z transformation. The differences in the COV50 odds ratios (ORs) between initial and continued recruitment were tested by introduction of the interaction between the study phase and baseline COV50 in the logistic models. Performance of COV50 in risk stratification was assessed by the area under the receiver operating characteristic curve (AUC-ROC) and the Delong approach to compare the area under the curves (AUCs) between nested models. The COV50 thresholds optimised by the Youden index were 0·47 for mortality and 0·04 for worsening WHO score.6 To evaluate the usefulness of the optimised COV50 thresholds in clinical decision making, multivariable logistic models were run, adjusted for sex, age categorised in tertiles (<55·0, 55·0–74·9, and ≥75·0 years), the entry WHO score, the presence versus absence of obesity (BMI ≥30 kg/m2), and comorbidities (hypertension, heart failure, diabetes, and cancer). From these models, in which participants with an entry WHO score of 6 were excluded, each patient's probability of an endpoint was exported and compared with the reference category by computing least square means in generalised linear models. In this analysis the lowest level of each categorised risk factor was used as reference.
Using SAS interactive matrix language (version 9.4), a Markov chain simulation11 was bootstrapped 1000 times to generate the transition probabilities from the entry WHO score to the maximum WHO score during follow-up (appendix p 16). Transition probabilities were computed for the whole cohort and for various risk strata, which were defined by the entry COV50 score and age (<65 vs ≥65 years). The transition probabilities allowed extrapolating the number of participants reaching follow-up WHO scores of 3–4, 5, and 6–8, and therefore requiring regular (score 3–4), intermediate (score 5), or intensive (score 6–8) care. Point estimates and uncertainty limits were derived from the median and from the 5th to the 95th percentile interval of the bootstrapped distributions. Next, the expected hospitalisation costs were computed in three steps. First, the daily hospitalisation costs were adjusted for the gross per capita domestic product of each country12 averaged over 10 years (2011–21) using as a benchmark the diagnosis-related per-day hospitalisation costs applicable in Germany in 2021: €540 for regular care, €1590 for intermediate care, and €1770 for intensive care. Next, the derived hospitalisation costs, the observed number of days in hospital, and the simulated number of participants allowed for the computation of the point estimates and uncertainty intervals of the expected costs. Finally, the cost estimates for the whole cohort and the risk strata were expressed in million Euro (M€) per 1000 participants hospitalised for 1 week. Hospitalisation costs were balanced against the cost of the COV50 test (€850 per test).
Role of the funding source
The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or the writing of the report.
Results
From June 30 to Nov 19, 2020, 228 participants were recruited, and from April 30, 2020, to April 14, 2021, 784 participants were recruited. The 1012 participants making up the full dataset were on average aged 62·3 years, included 447 (44%) women, 557 (55%) participants with hypertension, 154 (15%) with heart failure, 257 (25%) with diabetes, and 106 (11%) participants with cancer (appendix p 22). The WHO score at enrolment (table 1 ) was 1–3 in 445 (44%) participants, 4–5 in 529 (52%) participants, and 6 in 38 (4%) participants. The mean (IQR) COV50 score at baseline was –0·23 (–1·27 to 0·80; appendix p 23). Compared with the initially enrolled participants, those recruited later scored lower on the WHO scale (table 1; p<0·0001), included more participants with a history of cancer (6% with the initial phase vs 12% with the continued phase; p=0·012), but fewer participants on inhibitors of the renin-angiotensin system (54% with the initial phase vs 39% with the continued phase; p<0·0001). Otherwise, participants recruited initially and later had similar characteristics (table 1), particularly a similar entry COV50 score (–0·19 vs –0·24; p=0·59). In the whole study population (appendix p 12), the proportion of women and the mean values of diastolic blood pressure decreased across the four increasing quartiles of COV50 score distribution at baseline, whereas age, heart rate, and the rates of hypertension, heart failure, diabetes, and cancer increased. Among 816 participants admitted to hospital, the glomerular filtration rate averaged 85·6 (SD 37·6) mL per min per 1·73 m2 (table 1), but the glomerular filtration rate was not measured in 186 ambulatory participants.
Table 1.
Baseline characteristics
|
Recruitment phase cohort |
Full cohort (N=1012) | ||||
|---|---|---|---|---|---|
| Initial (N=228) | Continued (N=784) | p value | |||
| WHO score | |||||
| 1–3 | 90 (39%) | 355 (45%) | <0·0001 | 445 (44%) | |
| 4–5 | 107 (47%) | 422 (54%) | .. | 529 (52%) | |
| 6 | 31 (14%) | 7 (1%) | .. | 38 (4%) | |
| COV50 score | −0·19 (1·52) | −0·24 (1·36) | 0·59 | −0·23 (1·40) | |
| Ethnicity | |||||
| White ethnicity | 205 (90%) | 685 (87%) | 0·30 | 890 (88%) | |
| All other ethnicities* | 23 (10%) | 99 (13%) | .. | 122 (12%) | |
| Sex | |||||
| Women | 94 (41%) | 353 (45%) | 0·31 | 447 (44%) | |
| Men | 134 (59%) | 431 (55%) | .. | 565 (56%) | |
| Hypertension | 137 (60%) | 420 (54%) | 0·082 | 557 (55%) | |
| Heart failure | 30 (13%) | 124 (16%) | 0·33 | 154 (15%) | |
| BMI ≥30 kg/m2 | 59 (26%) | 192 (24%) | 0·67 | 251 (25%) | |
| Diabetes | 65 (28%) | 192 (24%) | 0·22 | 257 (25%) | |
| Cancer | 13 (6%) | 93 (12%) | 0·012 | 106 (11%) | |
| Use of RAS blockers | 122 (54%) | 305 (39%) | <0·0001 | 427 (42%) | |
| Age | 63·1 (17·1) | 62·1 (18·0) | 0·46 | 62·3 (17·8) | |
| Systolic blood pressure (mm Hg) | 129·8 (23·2) | 127·7 (19·0) | 0·16 | 128·2 (20·1) | |
| Diastolic blood pressure (mm Hg) | 75·9 (13·5) | 76·2 (11·7) | 0·74 | 76·2 (12·2) | |
| Heart rate (beats per min) | 83·4 (15·1) | 81·9 (15·6) | 0·21 | 82·2 (15·5) | |
| BMI (kg/m2) | 28·0 (5·4) | 27·5 (5·2) | 0·23 | 27·6 (5·2) | |
| Glomerular filtration rate (mL per min per 1·73 m2)† | 93·4 (51·0) | 83·2 (32·1) | 0·0095 | 85·6 (37·6) | |
Data presented as n (%) or mean (SD). COV50 score is the ratio of the actual value to the standard run against each sample. RAS blockers indicate blocker of the renin-angiotensin system, including angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers. Systolic and diastolic blood pressure and heart rate were missing in two initially recruited participants and 29 participants recruited later. The p value refers to the differences in the patients' characteristics between initial recruitment (June 30 to Nov 19, 2020) and continued recruitment (April 30, 2020, to April 14, 2021). RAS=renin-angiotensin system.
All other ethnicites include Asian ethnicity (9 [1%]), Black ethnicity (14 [1%]), and not recorded (99 [10%]).
Glomerular filtration rate estimated from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration formula was measured in 191 patients admitted to hospital in the initial phase, 625 patients in the continued recruitment phase, and 816 patients overall.
No patient was lost to follow-up. Median follow-up was 10 days (5th to 95th percentile interval, 1–34) for mortality (number of deaths, 119) and 10 days (2–26) for worsening WHO score (number of participants with endpoint, 271). In all 1012 participants, the correlation coefficient of the baseline COV50 score with the baseline WHO score were 0·663 (95% CI 0·627–0·696) and with the maximal WHO score during follow-up were 0·663 (0·627–0·697); and when adjusted for the glomerular filtration rate in 816 participants admitted to hospital, these estimates were 0·442 (0·403–0·482) with the baseline WHO score and 0·458 (0·378–0·539) with the maximal WHO score during follow-up. The baseline COV50 distribution shifted upward significantly when plotted against the highest WHO score attained during follow-up (p<0·0001; appendix p 24). In the whole study population (table 2 ), the relative risk of death expressed per 1-SD increment in COV50 score at baseline was 2·44 (95% CI 2·05–2·92) unadjusted and 1·67 (1·34–2·07) when fully adjusted for sex, age, BMI, the presence of comorbidities, and the baseline WHO score; for progression in the WHO score (p<0·0001; table 2), the corresponding ORs were 1·79 (1·60–2·01) unadjusted and 1·63 (1·41–1·91) when fully adjusted. In analyses dichotomised by study phase, the risk associated with COV50 was similar for both endpoints, irrespective of adjustment (table 2). The unadjusted ORs for mortality were 2·45 (1·69–3·54) for the initial recruitment group versus 2·47 (2·02–3·03) for the continued recruitment group (interaction p value 0·96) and for worsening WHO score, 1·95 (1·52–2·51) for the initial recruitment group versus 1·77 (1·56–2·02) for the continued recruitment group (interaction p value 0·52). The fully adjusted estimates (table 2) for mortality were 2·27 (1·34–3·83) for the initial recruitment group versus 1·55 (1·21–1·98) for the continued recruitment group (interaction p value 0·94); and for worsening WHO score, 2·32 (1·55–3·48) for the initial recruitment group versus 1·51 (1·27–1·78) for the continued recruitment group (interaction p value 0·16).
Table 2.
Odds ratios relating outcome to COV50 by recruitment phase
|
Initial phase cohort |
Continued phase cohort |
Full cohort |
||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Mortality | ||||||
| Number of deaths/number at risk (%) | 25/228 (11%) | .. | 94/784 (12%) | .. | 119/1012 (12%) | .. |
| Unadjusted | 2·45 (1·69–3·54) | <0·0001 | 2·47 (2·02–3·03) | <0·0001 | 2·44 (2·05–2·92) | <0·0001 |
| Adjusted for sex and age | 2·30 (1·57–3·37) | <0·0001 | 1·88 (1·50–2·35) | <0·0001 | 2·04 (1·68–2·47) | <0·0001 |
| Adjusted for sex, age, and baseline WHO score | 2·18 (1·30–3·64) | 0·0030 | 1·54 (1·21–1·96) | 0·0005 | 1·65 (1·34–2·05) | <0·0001 |
| Adjusted for sex, age, BMI, comorbidities, and baseline WHO score | 2·27 (1·34–3·83) | 0·0023 | 1·55 (1·21–1·98) | 0·0005 | 1·67 (1·34–2·07) | <0·0001 |
| Progressing WHO score | ||||||
| Number of endpoints or events/number at risk (%) | 50/228 (22%) | .. | 221/784 (28%) | .. | 271/1012 (27%) | .. |
| Unadjusted | 1·95 (1·52–2·51) | <0·0001 | 1·77 (1·56–2·02) | <0·0001 | 1·79 (1·60–2·01) | <0·0001 |
| Adjusted for sex and age | 1·81 (1·38–2·35) | <0·0001 | 1·50 (1·29–1·73) | <0·0001 | 1·56 (1·38–1·77) | <0·0001 |
| Adjusted for sex, age, and baseline WHO score | 2·32 (1·56–3·46) | <0·0001 | 1·52 (1·29–1·79) | <0·0001 | 1·65 (1·42–1·92) | <0·0001 |
| Adjusted for sex, age, BMI, comorbidities, and baseline WHO score | 2·32 (1·55–3·48) | <0·0001 | 1·51 (1·27–1·78) | <0·0001 | 1·63 (1·41–1·91) | <0·0001 |
Odds ratios given with 95% CIs express the risk for 1SD increment increases in COV50 score. Initial recruitment lasted from June 30, to Nov 19, 2020, and continued recruitment from April 30, 2020, to April 14, 2021. Comorbidities include hypertension, heart failure, diabetes, and cancer. OR=odds ratio.
In unadjusted analyses of the whole study population, the AUC of the continuously distributed COV50 urinary marker was 0·81 (95% CI 0·77–0·85) for mortality and 0·72 (0·68–0·75) for worsening WHO score (appendix p 13). In the whole study population, the incidence proportion of mortality among participants with a COV50 score less than the optimised threshold (0·47) was 30 (4·32%; 95% CI 2·81–5·84%) of 694 versus 89 (28·0%; 23·1–33·0%) of 318 with a COV50 score equal to or higher than the optimised threshold (p<0·0001); the corresponding incidence proportions of a worsening WHO score, analysed using an optimised threshold of 0·04, were 88 (15·0%; 12·1–17·9%) of 587 for a COV50 score less than the optimised threshold versus 183 (43·1%; 38·4–47·8%) of 425 for a COV50 score equal to or higher than the optimised threshold (p<0·0001). The optimised 0·47 threshold for mortality resulted in 74·8% (66·0–82·3%) sensitivity, 74·4% (71·4–77·2%) specificity, and 74·4% (71·6–77·1%) accuracy; for worsening WHO score, the optimised 0·04 threshold generated 67·5% (61·6–73·1) sensitivity, 67·3% (63·8–70·7%) specificity, and 67·4% (64·4–70·3%) accuracy (appendix p 13). For both endpoints, these estimates were consistent in the early and continued recruitment phases (appendix p 13).
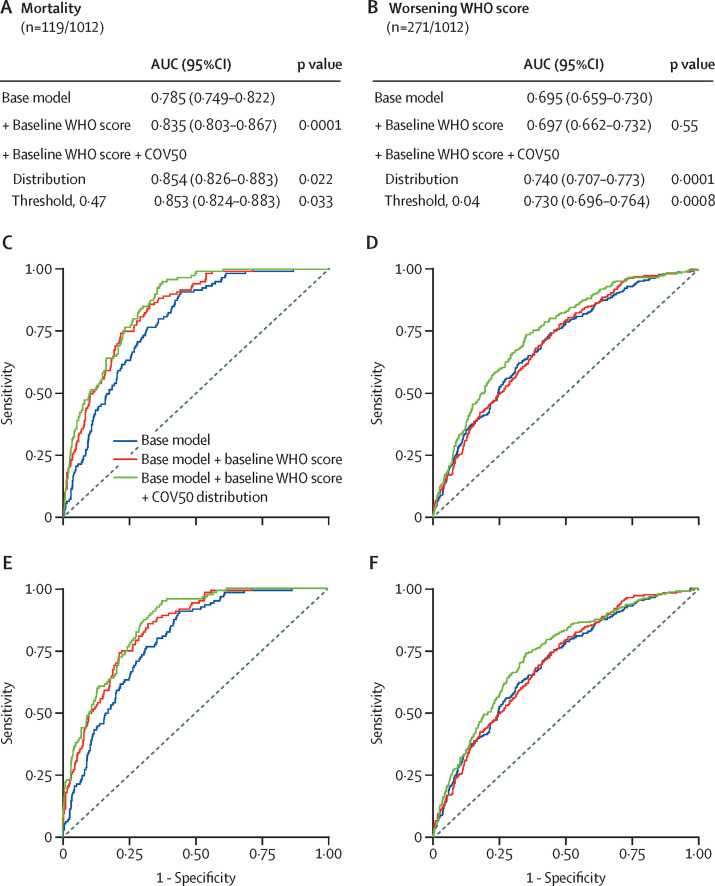
The ORs and discriminatory performance of single risk factors are summarised by recruitment phase in the appendix (p 14). In the whole study population, when adjusted for sex, age, BMI, comorbidities, and the baseline WHO score, COV50 analysed as a continuously distributed variable and categorised per threshold significantly improved the AUC (figure ). For mortality, accounting for the continuously distributed COV50 marker resulted in an increase of the AUC from 0·835 (95% CI 0·803–0·867) to 0·854 (0·826–0·883; p=0·022) and for the 0·47 COV50 threshold, to 0·853 (0·824–0·883; p=0·033). For worsening WHO score, the AUC increased from 0·697 (0·662–0·732) to 0·740 (0·707–0·773; p=0·0001) for the continuously distributed marker and to 0·730 (0·696–0·764; p=0·0008) for the 0·04 threshold. Additional adjustment for the glomerular filtration rate in 816 participants admitted to hospital produced confirmatory results (appendix pp 14, 25). In multivariable logistic models from which participants with an entry WHO score of 6 were excluded (table 3 ), the probability of death increased 3·6 times (p<0·0001) from 6·2% (95% CI 5·8–6·6%) to 22·3% (21·7–23·0%) if the 0·47 threshold was reached. Furthermore, the probability of progression of the WHO score increased 2·97 times (p<0·0001) from 14·4% (14·2–14·7%) to 42·7% (42·4–42·9%) if the 0·04 threshold was attained. Age and the entry WHO score were the only other significant risk factors, with the times increase between the low-risk and high-risk strata ranging up to 2·14.
Figure.
COV50 performance adjusted for baseline risk factors in the full dataset for mortality and worsening WHO score
Figure shows the sensitivity and specificity of the urinary marker COV50 for mortality versus survival (panels A–C) and for progression versus non-progression in the baseline WHO score (panels D–F) during follow-up. The base model included sex, age, BMI, and the presence of comorbidities (hypertension, heart failure, diabetes, or cancer). In subsequent steps, the baseline WHO score was added and then COV50 score as a continuously distributed variable (panels B and E) or as a categorised variable based on an optimised threshold of 0·47 for mortality (panel C) or 0·04 for a worsening WHO score (panel F). At each step, the p values are for the comparison with the preceding model. AUC=area under the curve.
Table 3.
Probability of reaching an endpoint by risk factor
|
Mortality (108/974 [11%]) |
Worsening WHO score (259/974 [27%]) |
|||||
|---|---|---|---|---|---|---|
| Probability of endpoint | Times difference | p value | Probability of endpoint | Times difference | p value | |
| Sex | ||||||
| Women | 13·2% (12·6–13·7%) | .. | .. | 27·4% (27·1–27·6%) | .. | .. |
| Men | 15·5% (14·9–15·9%) | 1·17 | 0·15 | 32·8% (32·5–33·0%) | 1·20 | 0·057 |
| Age | ||||||
| <55 years | 9·8% (9·1–10·4%) | .. | .. | 23·6% (23·3–23·9%) | .. | .. |
| 55–74 years | 12·1% (11·5–12·6%) | 1·23 | .. | 27·7% (27·4–27·9%) | 1·17 | .. |
| ≥75 years | 21·0% (20·3–21·6%) | 2·14 | <0·0001 | 38·9% (38·5–39·2%) | 1·64 | 0·0007 |
| Entry WHO score | ||||||
| 1–3 | 10·8% (10·3–11·4%) | .. | .. | 35·0% (34·7–35·3%) | .. | .. |
| 4–5 | 17·7% (17·2–18·2%) | 1·64 | 0·0002 | 25·1% (24·9–25·3%) | 0·72 | 0·0019 |
| Obesity | ||||||
| Absent | 14·6% (14·3–15·0%) | .. | .. | 30·0% (29·9–30·2%) | .. | .. |
| Present | 13·9% (13·2–14·5%) | 0·95 | 0·84 | 30·5% (29·7–30·4%) | 1·02 | 0·99 |
| Comorbidities | ||||||
| Absent | 13·4% (12·8–13·9%) | .. | .. | 26·6% (26·3–26·8%) | .. | .. |
| Present | 15·1% (14·6–15·7%) | 1·13 | 0·27 | 33·3% (33·0–33·5%) | 1·25 | 0·029 |
| COV50 score | ||||||
| Less than threshold | 6·2% (5·8–6·6%) | .. | .. | 14·4% (14·2–14·7%) | .. | .. |
| Threshold or more | 22·3% (21·7–23·0%) | 3·60 | <0·0001 | 42·7% (42·4–42·9%) | 2·97 | <0·0001 |
974 was the number of patients when patients with an entry WHO score of 6 were excluded. Data presented as probability, % (95% CI). The probabilities of reaching an endpoint were derived from logistic models, in which all risk factors were categorised and mutually adjusted. For each risk factor, the lowest risk category was the reference in computing the times difference with higher categories. Obesity was a BMI of at least 30 kg/m2. The COV50 threshold was 0·47 for mortality and 0·04 for worsening WHO score. For both endpoints, the number of events and patients at risk are given. The significance of each risk factor was derived from the multivariable logistic models.
Of 1012 participants, 196 (19%) received only ambulatory care, of whom 194 (99%) had a baseline COV50 score of less than 0·04; the other 816 (81%) participants were admitted to hospital and carried forward in the computation of predicted hospitalisation costs, on the basis of the Markov-chain transition probabilities (appendix p 16) and the simulated number of participants reaching follow-up WHO scores ranging from 3 to 8 (appendix p 17). The predicted hospitalisation costs are described in detail in table 4 and summarised by risk category in the appendix (p 18). In participants at a low risk (COV50 <0·04), the predicted hospitalisation costs standardised on the basis of each country's gross per capita domestic product to 1000 participants hospitalised for 1 week in regular care and intermediate care were greater than intensive care (appendix p 18): M€4·617 (5–95% percentile interval 4·137–5·103) versus M€1·591 (0·973–2·170). Among participants at a high risk (COV50 ≥0·04), the hospitalisation costs showed an opposite structure, with lower costs in regular plus intermediate versus intensive care: M€3·740 (3·289–4·257) versus M€10·946 (9·596–12·296). Measures of treatment efficacy, extracted from the literature review and exemplary trials (appendix p 19), showed reductions in the number of hospitalisation days or fewer hospitalisation days until recovery. The cost reductions associated with 1 day less hospitalisation per 1000 participants (table 4; appendix p 18) were M€0·887 (0·730–1·039) in participants at a low-risk (COV50 <0·04) and M€2·098 (1·839–2·365) in the high-risk stratum (COV50 ≥0·04).
Table 4.
Simulated hospitalisation costs by baseline COV50 score, age class, and the hospital facility at entry
|
Follow-up WHO score |
Cost reduction associated with 1 day less in hospital per 1000 patients | ||||
|---|---|---|---|---|---|
| 3–4 | 5 | 6–8 | All scores (3–8) | ||
| COV50 score (range −3·26 to 3·39; 19–96 years) | |||||
| Days in regular care | 8 (4–13) | 14 (8–20) | 14 (6–24) | .. | .. |
| Cost of regular care, M€ | 2·198 (2·094–2·302) | 0·684 (0·608–0·759) | 1·942 (1·723–2·174) | .. | .. |
| Days in intermediate care | .. | 11 (5–17) | 12 (5–23) | .. | .. |
| Cost of intermediate care, M€ | .. | 1·048 (0·931–1·164) | 2·311 (2·050–2·587) | .. | .. |
| Days in intensive care | .. | .. | 6 (4–17) | .. | .. |
| Cost of intensive care, M€ | .. | .. | 2·183 (1·937–2·444) | .. | .. |
| Days in all care facilities | .. | .. | .. | 9 (4–15) | .. |
| Cost of all care, M€ | .. | .. | .. | 10·366 (9·343–11·430) | 1·481 (1·335–1·633) |
| COV50 score <0·04 (19–96 years) | |||||
| Days in regular care | 7 (4–12) | 12 (6–16) | 20 (10–29) | .. | .. |
| Cost of regular care, M€ | 2·732 (2·574–2·897) | 0·897 (0·744–1·050) | 0·494 (0·315–0·674) | .. | .. |
| Days in intermediate care | .. | 8 (5–13) | 38 (18–58) | .. | .. |
| Cost of intermediate care, M€ | .. | 0·988 (0·819–1·156) | 0·592 (0·337–0·807) | .. | .. |
| Days in intensive care | .. | .. | 6 (4–11) | .. | .. |
| Cost of intensive care, M€ | .. | .. | 0·505 (0·321–0·689) | .. | .. |
| Days in all care facilities | .. | .. | .. | 8 (4–13) | .. |
| Cost of all care, M€ | .. | .. | .. | 6·208 (5·110–7·273) | 0·887 (0·730–1·039) |
| COV50 score ≥0·04, (19–96 years) | |||||
| Days in regular care | 10 (5–14) | 16 (10–20) | 12 (5–23) | .. | .. |
| Cost of regular care, M€ | 1·696 (1·548–1·860) | 0·855 (0·723–1·003) | 3·338 (2·926–3·749) | .. | .. |
| Days in intermediate care | .. | 14 (8–21) | 12 (5–20) | .. | .. |
| Cost of intermediate care, M€ | .. | 1·189 (1·005–1·394) | 3·911 (3·429–4·394) | .. | .. |
| Days in intensive care | .. | .. | 6 (4–18) | .. | .. |
| Cost of intensive care, M€ | .. | .. | 3·697 (3·241–4·153) | .. | .. |
| Days in all care facilities | .. | .. | .. | 11 (5–17) | .. |
| Cost of all care, M€ | .. | .. | .. | 14·686 (12·872–16·553) | 2·098 (1·839–2·365) |
| COV50 score ≥0·04, <65 years | |||||
| Days in regular care | 7 (4–16) | 17 (14–20) | 30 (24–36) | .. | .. |
| Cost of regular care, M€ | 1·481 (1·257–1·704) | 1·700 (1·373–1·962) | 3·235 (2·654–3·898) | .. | .. |
| Days in intermediate care | .. | 12 (6–15) | 15 (6–19) | .. | .. |
| Cost of intermediate care, M€ | .. | 2·001 (1·616–2·309) | 3·415 (2·802–4·116) | .. | .. |
| Days in intensive care | .. | .. | 5 (4–14) | .. | .. |
| Cost of intensive care, M€ | .. | .. | 3·200 (2·626–3·856) | .. | .. |
| Days in all care facilities | .. | .. | .. | 11 (5–17) | .. |
| Cost of all care, M€ | .. | .. | .. | 15·032 (12·328–17·845) | 2·147 (1·761–2·549) |
| COV50 score ≥0·04, ≥65 years | |||||
| Days in regular care | 10 (5–14) | 12 (10–20) | 11 (4–26) | .. | .. |
| Cost of regular care, M€ | 1·785 (1·581–1·977) | 0·139 (0·109–0·169) | 1·194 (1·027–1·362) | .. | .. |
| Days in intermediate care | .. | 16 (8–24) | 12 (4–25) | .. | .. |
| Cost of intermediate care, M€ | .. | 0·881 (0·688–1·074) | 4·130 (3·551–4·709) | .. | .. |
| Days in intensive care | .. | .. | 12 (6–20) | .. | .. |
| Cost of intensive care, M€ | .. | .. | 3·972 (3·415–4·529) | .. | .. |
| Days in all care facilities | .. | .. | .. | 11 (5–17) | .. |
| Cost of all care, M€ | .. | .. | .. | 12·101 (10·371–13·820) | 1·729 (1·482–1·974) |
Data shown as the median number of days (IQR) as observed in the CRIT-Cov-U cohort; and hospitalisation costs per 1000 patients hospitalised for 1 week per care facility (median and 5–95% percentile interval) were extrapolated from the distributions of patients (expected by the Markov chain simulation) reaching follow-up WHO scores of 3–4, 5, and 6–8 and the care facility corresponding with disease severity (ie, regular care for score 3–4, intermediate care for score 5, and intensive care for score 6–8). Cost estimates in intermediate and intensive care facilities also include the costs of lower care facilities to which patients were admitted before or after they reached their maximal WHO score during follow-up. M€=million Euro.
The 62 participants enrolled in the 2022 substudy (appendix p 20) were on average aged 64·9 (19·8) years and included 26 women (42%). These participants had a high-risk profile, as evidenced by the prevalence of obesity (n=12 [19%]), hypertension (n=38 [61%]), diabetes (n=25 [40%]), cancer (n=12 [19%]), chronic obstructive lung disease (n=7 [11%]), or the use of immunosuppressants (n=13 [21%]). Moreover, 22 (35%) had a history of chronic kidney disease. One patient was infected by the delta variant and 61 (98%) by the omicron strain. The number of participants vaccinated was 44 (71%; appendix p 20). Median (5–95th percentile interval) follow-up was 5 days (1–24), during which six participants (10%) had a worsening of their baseline WHO score. Two participants died. The correlation coefficient of the baseline COV50 score during follow-up with the baseline WHO score was 0·527 (0·319–0·686) and for the maximal WHO score, the correlation coefficient was 0·626 (0·446–0·757); adjusted for the glomerular filtration rate, these estimates were 0·455 (0·305–0·605) for the baseline WHO score and 0·566 (0·354–0·778) for the maximal WHO score. The ORs expressing the risk of a worsening WHO score associated with a 1-SD increment in COV50 were 4·87 (95% CI 1·06–22·4 [p=0·042]) unadjusted; 6·81 (1·25–37·0 [p=0·026]) adjusted for the baseline WHO score, and 7·14 (1·28–39·8 [p=0·025]) additionally adjusted for age.
Discussion
COV50 is a novel urinary biomarker, consisting of 50 dysregulated urinary peptides (appendix pp 8–10). COV50 predicts death and disease progression when adjusting for clinical risk factors, comorbidities, and the WHO score at presentation. COV50 analysed as a single continuously distributed risk factor generated AUCs for mortality and disease progression substantially greater than a 10-year age increment or a 1-point increase in the baseline WHO score (appendix p 14). The optimised COV50 thresholds had 74·4% predictive accuracy for mortality and 67·4% for disease progression (appendix p 13). Both COV50 thresholds and COV50 as continuously distributed variable significantly improved the AUC (figure). Additional adjustment for the glomerular filtration rate confirmed the predictive accuracy of COV50 (appendix p 25).
Specificity, sensitivity, accuracy, and AUC are notable statistical variables that define the performance of a novel biomarker. However, when faced with individual participants, clinicians rarely base treatment strategies on these metrics, but instead commonly rely on risk-carrying action thresholds. However, standard COVID-19-related risk factors, such as sex, age, obesity, and the presence of comorbidities, are generic in the sense that they predict worse outcomes for various diseases. The COV50 biomarker was associated with increased mortality and progression to more severe disease, even with cumulative adjustment for these risk factors. In analyses from which participants hospitalised in intensive care units were excluded (entry WHO score 6), the probability of death or disease progression increased around 3 times if the optimised COV50 thresholds were exceeded. Age and the entry WHO score were other significant risk factors; however, with no more than an approximately 2-times increase between the low-risk strata and high-risk strata. This approach, in which clinical judgement and experience are key, is likely to become the strategy for rolling out the COV50 biomarker in the risk stratification and the care of people infected with COVID-19. A high-risk test outcome should move participants across the action threshold, allowing the early administration of effective treatments, either in ambulatory or hospitalised care. A few exemplary trials selected from an extensive literature review8 were summarised in the appendix (p 19). The COV50 test is registered in Germany and available for clinical application and research purposes throughout the EU.
The participants enrolled in the CRIT-CoV-U study were unvaccinated and infected by the contemporary virus strains in the interval from June 30, 2020, to April 14, 2021. In the substudy, 61 of 62 participants (98%) had been infected by the omicron variant and 44 of 62 participants (71%) had been vaccinated at least once (appendix p 20). In keeping with the observations in the full CRIT-CoV-U cohort, the correlation coefficients of COV50 with the baseline and maximal follow-up WHO scores were significant, irrespective of the adjustment for glomerular filtration rate. The unadjusted and adjusted ORs expressing the risk of a worsening WHO score associated with a 1-SD increment in COV50 (6 events [10%]) were significant. Nevertheless, these ORs are only presented for information purposes, given that only six participants of 62 participants had worsening WHO scores, resulting in wide CIs.
Urine specimens contain more than 20 000 peptides, of which approximately 5000 are typically detectable in a single urine sample. These peptides provide a molecular signature of progressing SARS-CoV-2 infection independent of the virus strain. A comprehensive multilevel proteomic study13 profiled the interactome of SARS-CoV-2 and its influence on transcriptome, proteome, ubiquitinome, and phosphoproteome of a lung-derived human cell line. This study revealed that SARS-CoV-2 infection dysregulates the transcription of the growth factor β pathway, known for its involvement in tissue fibrosis and epidermal growth factor receptor-mediated signalling, which downstream modulates cell survival and motility and the innate immune responses. The activation of the transcription growth factor β pathway is in keeping with the most prominent characteristic of the COV50 UPP (appendix pp 8–10), which is the shift in collagen fragments, in particular collagen α1.2 On infection, the reactive inflammatory cascade activates fibroblasts,14 leading to excessive extracellular matrix deposition in response to injury. The COV50 UPP signature also points to enhanced α1-antitrypsin degradation in line with reports showing that α1-antitrypsin deficiency is associated with life-threatening COVID-19.15 Another hallmark of the COV50 UPP is the reduction in urinary peptides derived from CD99.16 This observation reflects the loss of endothelial integrity, interference with the transendothelial migration of monocytes, neutrophils, and T cells,16 and damage of the endothelial tight junctions. The resulting exposure of collagen to the circulating blood triggers the thrombotic complications specific for COVID-19.17 As is also observed in chronic obstructive lung disease,18 with increasing COVID-19 severity, the UPP reveals downregulation of the polymeric immunoglobulin receptor,16 which is highly expressed in the trachea and the lung and mediates IgA transcytosis.18 Thus, the urinary peptide fragments included in the multidimensional COV50 biomarker are compatible with the established molecular pathogenic mechanisms activated by SARS-CoV-2 infection. In addition to these molecular mechanisms, reduced disease tolerance (as associated with chronological ageing), accelerated biological ageing,19 comorbidities, and frailty drive disease progression.20 Of note, the key results shown in the figure were adjusted for sex, age, BMI, and the WHO stage at enrolment. Furthermore, socioeconomic deprivation, low educational attainment, insufficient disease awareness, misinformation, and inadequate access to health care or vaccination are non-biomolecular factors underlying adverse health outcomes in people with COVID-19.21
Cost-effectiveness balances health-care costs against non-monetary units, such as quality-adjusted life-years (QALYs).22 The QALY-based value proposition is well established in the UK, Sweden, Belgium, the Netherlands, Luxembourg, and some eastern European countries, but health-care insurers in Germany and France prefer assessing changes in clinical outcomes instead.22 CRIT-CoV-U was not designed to address health-economic issues. The administration of quality-of-life questionnaires, the instruments to turn QALY's into metrics, was impossible in an emergency care setting. Ethics approvals allowing access to claims databases were not requested. However, the simulations in the current Article (table 4; appendix p 18) provide some information on the balance between the costs of administering the COV50 test (M€0·850 per 1000 participants) against potential health-care savings associated with earlier intervention; for instance on account of the reduction in the number of hospitalisation days, reported to be 5–10 days in three trials.23, 24, 25 Because a high UPP risk profile justifies an earlier intervention rather than later treatment guided by clinical deterioration, presumably applying the test will not affect drug costs. One possible limitation of the Markov chain simulation is that the WHO scores at presentation of the participants in CRIT-CoV-U were used as the initial distribution vector, so the medians of the simulated patient distributions, as presented in the appendix (p 17), closely reflect the baseline distribution vector. However, the main objective of running the Markov chain analysis was to generate uncertainty intervals as captured by the 5th and 95th percentiles of the simulated number of participants with possible outcomes.
Among the strong points of our study is the high consistency in the discriminatory performance of COV50 among participants recruited both initially and later (table 2; appendix pp 13–15), which shows a high degree of cohesion in the results within the CRIT-CoV-U cohort. The calibration of the UPP profile (appendix p 3) accounts for interindividual differences in renal function and urinary flow. Furthermore, additional adjustment for the glomerular filtration rate in participants admitted to hospital strengthened the main analysis in the full cohort and showed that the calibration had met its objective (appendix pp 15, 25). Nevertheless, the current results should also be interpreted within the context of obvious limitations. First, CRIT-CoV-U is an observational cohort study. Randomised clinical trials are the optimal strategy for applying treatments guided by COV50 risk profiling. Second, as outlined above, future research should address the health-economic implications of the timing and choice of therapeutic interventions in participants with a low-risk COV50 score versus a high-risk COV50 score. Third, CRIT-CoV-U enrolled adults, who were predominantly white Europeans. How ethnicity might affect the UPP is currently under investigation in the Urinary Proteomics Combined with Home Blood Pressure Telemonitoring for Health Care Reform trial (NCT04299529).26 Along similar lines, the recruitment of participants into the present study involved 19 centres in eight countries and was driven by the temporal and geographical spread of the successive waves of the pandemic. Therefore, it is difficult to assess to what extent the study participants were representative of the wider population, in which the COV50 test might be used in clinical practice. Fourth, one limitation of the capillary electrophoresis combined with mass spectrometry approach is the application of ultrafiltration with the threshold set at 20 kDa, so that larger proteins escape analysis. Finally, although UPP risk profiling provides insight on the ideal timing of intervention, vaccination is by far the primordial strategy in addressing the COVID-19 pandemic, although vaccination alone cannot be sufficient to restore population health to the pre-COVID-19 era.3
In conclusion, to our knowledge COV50 is a novel biomarker predictive of death and disease progression in adults with COVID-19. Independent of clinical risk markers, the operational COV50 thresholds have a discriminatory accuracy of approximately 70%, even in participants with mild disease. A high-risk COV50 test administered within 4 days of a positive PCR test justifies earlier treatment in participants with mild-to-moderate disease (WHO scores 1–4), in whom clinical risk factors often leave the prognosis uncertain. Another potential application of COV50 is in the selection of participants to be enrolled in randomised clinical trials of novel COVID-19 therapies, in which risk is an issue in the choice between ambulatory versus hospitalised care or in which a treatment method is being tested.
Data sharing
The study protocol is available at the German Register for Clinical Studies (www.drks.de; number DRKS00022495) and the English version can be downloaded from https://crit-cov.de/files/Crit-Cov/Crit-Cov-U-study-protocol.pdf. Anonymised participants data accompanied by a data dictionary will be made available upon request directed to the corresponding author. Proposals will be reviewed and approved by the funder, investigators, and collaborators on the basis of scientific merit and submitted for approval to the Ethics Committee of the German-Saxonian Board of Physicians, Dresden, Germany (number EK BR 88/20·1). After approval of a proposal, data can be shared through a secure online platform after signing a data access and confidentiality agreement. Data will be made available for up to 3 years after a request has been received and approved.
Declaration of interests
HM is the co-founder and co-owner of Mosaiques-Diagnostiques (Hannover, Germany). JS and JR are employees of Mosaiques-Diagnostics. HDR received consulting fees and honoraria for presentations from Alexion, AstraZeneca, and Bristol-Myers-Squibb. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This study was funded by the German Federal Ministry of Health acting upon a decree from the German Federal Parliament. The Non-Profit Research Institute Alliance for the Promotion of Preventive Medicine received a non-binding grant from OMRON Healthcare (Kyoto, Japan).
Contributors
RW, HM, JM, CL, and JB conceptualised the study. HM, JS, JR, and JM did the proteomic urine analyses. JAS and LT did the statistical analysis. JAS reviewed the literature and drafted the Research in Context section. JAS, HM, and JB wrote the first draft of the manuscript. Y-LY did the graphics. All authors contributed to the recruitment and follow-up of participants and to the construction and maintenance of the database, and interpreted the results, commented on successive drafts of the manuscript, and approved the final version. All authors had full access to all the data and are guarantors of the data integrity.
Contributor Information
CRIT-CoV-U investigators:
Jan A Staessen, Ralph Wendt, Yu-Ling Yu, Sven Kalbitz, Lutgarde Thijs, Justyna Siwy, Julia Raad, Jochen Metzger, Barbara Neuhaus, Armin Papkalla, Heiko von der Leyen, Alexandre Mebazaa, Emmanuel Dudoignon, Goce Spasovski, Mimoza Milenkova, Aleksandra Canevska-Taneska, Mercedes Salgueira Lazo, Mina Psichogiou, Marek W Rajzer, Lukasz Fulawka, Magdalena Dzitkowska-Zabielska, Guenter Weiss, Torsten Feldt, Miriam Stegemann, Johan Normark, Alexander Zoufaly, Stefan Schmiedel, Michael Seilmaier, Benedikt Rumpf, Mirosław Banasik, Magdalena Krajewska, Lorenzo Catanese, Harald Rupprecht, Beata Czerwienska, Björn Peters, Åsa Nilsson, Katja Rothfuss, Christoph Lübbert, Harald Mischak, Joachim Beige, Jörg Ermisch, Nils Kellner, Lydia Peruth-Stutzmann, Stefanie Schroth, Jonathan Schmidt, Ulrike Schmidt, Daniel Breuer, Fariza Abeud, Marie-Celine Fournier, Badr Louadah, Rocio Molas, Fraile Loreto Rojas, Fabiola Alonso García, Isabel Garcia Sánchez, Ioana Cezara Hrom, Andrzej Więczek., Matthias Schwab, Kei K Asayama, Tine W Hansen, Gladys E Maestre, Dimitrios Basoulis, Georgios Karamanakos., Pawel Lis, Agnieszka Olszanecka, Rosa Bellmann-Weiler, Lucas Lanser, Alicia Edin, Matthias NE Forsell, Bernd Stegmayr, Björn-Erik Ole Jensen, Hans-Martin Orth, Sylke Borstel, Agata Mikolajewska, Manfred Hecking, Lukas Schmölz, Michał Hoffmann, Krzysztof Narkiewicz, Agnieszka Matera-Witkiewicz, Justyna Zachciał, Monika Litwin, and Patrycja Marciniak
Supplementary Material
References
- 1.WHO Weekly epidemiological update on COVID-19—23 November 2021. Nov 23, 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19-23-november-2021
- 2.Poudel S, Ishak A, Perez-Fernandez J, et al. Highly mutated SARS-CoV-2 omicron variant sparks significant concern among global experts - what is known so far? Travel Med Infect Dis. 2022;45 doi: 10.1016/j.tmaid.2021.102234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399:1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knight SR, Ho A, Pius R, et al. Risk stratification of patients admitted to hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: development and validation of the 4C Mortality Score. BMJ. 2020;370 doi: 10.1136/bmj.m3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorog DA, Storey RF, Gurbel PA, et al. Current and novel biomarkers of thrombotic risk in COVID-19: a consensus statement from the International COVID-19 Thrombosis Biomarkers Colloquium. Nat Rev Cardiol. 2022;19:475–495. doi: 10.1038/s41569-021-00665-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wendt R, Thijs L, Kalbitz S, et al. A urinary peptidomic profile predicts outcome in SARS-CoV-2-infected patients. EClinicalMedicine. 2021;36 doi: 10.1016/j.eclinm.2021.100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCafferty K, Hollowood Z, Allan M, Lockhart D, Chorlton J, Martin J. ARCADIA study protocol: a phase II, randomised, double-blind, placebo-controlled clinical trial to assess the safety and efficacy of AZD1656 in patients with diabetes hospitalised with suspected or confirmed COVID-19. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2021-049650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wendt R, Kalbitz S, Lübbert C, et al. Urinary proteomics associate with COVID-19 severity: pilot proof-of-principle data and design of multicentric diagnostic study. Proteomics. 2020;10 doi: 10.1002/pmic.202000202. [DOI] [PubMed] [Google Scholar]
- 9.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKinney G. Markov chains as a predictive analytics technique using SAS/IML. 2018. https://www.lexjansen.com/wuss/2018/128_Final_Paper_PDF.pdf
- 12.OECD Gross domestic product (GDP) 2021. https://data.oecd.org/gdp/gross-domestic-product-gdp.htm
- 13.Stukalov A, Girault V, Grass V, et al. Multilevel proteomics reveals host perturbations by SARS-CoV-2 and SARS-CoV. Nature. 2021;594:246–252. doi: 10.1038/s41586-021-03493-4. [DOI] [PubMed] [Google Scholar]
- 14.Bollong MJ, Yang B, Vergani N, et al. Small molecule-mediated inhibition of myofibroblast transdifferentiation for the treatment of fibrosis. Proc Natl Acad Sci USA. 2017;114:4679–4684. doi: 10.1073/pnas.1702750114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ortiz A, Cozzolino M, Fliser D, et al. Chronic kidney disease is a key risk factor for severe COVID-19: a call to action by the ERA-EDTA. Nephrol Dial Transplant. 2021;36:87–94. doi: 10.1093/ndt/gfaa314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siwy J, Wendt R, Albalat A, et al. CD99 and polymeric immunoglobulin receptor peptides deregulation in critical COVID-19: a potential link to molecular pathophysiology? Proteomics. 2021;21 doi: 10.1002/pmic.202100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopes RD, de Barros E Silva PGM, Furtado RHM, et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;397:2253–2263. doi: 10.1016/S0140-6736(21)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gohy ST, Detry BR, Lecocq M, et al. Polymeric immunoglobulin receptor down-regulation in chronic obstructive pulmonary disease. Persistence in the cultured epithelium and role of transforming growth factor-β. Am J Respir Crit Care Med. 2014;190:509–521. doi: 10.1164/rccm.201311-1971OC. [DOI] [PubMed] [Google Scholar]
- 19.Martens DS, Thijs L, Latosinska A, et al. Urinary peptidomics to address age-related disabilities: a prospective population study. Lancet Healthy Longev. 2021;2:e690–e703. doi: 10.1016/S2666-7568(21)00226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wanhella KJ, Fernandez-Patron C. Biomarkers of ageing and frailty may predict COVID-19 severity. Ageing Res Rev. 2022;73 doi: 10.1016/j.arr.2021.101513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.COVID-19 Forecasting Team Variation in the COVID-19 infection-fatality ratio by age, time, and geography during the pre-vaccine era: a systematic analysis. Lancet. 2022;399:1469–1488. doi: 10.1016/S0140-6736(21)02867-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johannesson M. The relationship between cost-effectiveness analysis and cost-benefit analysis. Soc Sci Med. 1995;41:483–489. doi: 10.1016/0277-9536(94)00353-u. [DOI] [PubMed] [Google Scholar]
- 23.Hung IFN, Lung KC, Tso EYK, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID-19 - final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thijs L, Asayama K, Maestre GE, et al. Urinary proteomics combined with home blood pressure telemonitoring for health care reform trial: rational and protocol. Blood Press. 2021;30:269–281. doi: 10.1080/08037051.2021.1952061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study protocol is available at the German Register for Clinical Studies (www.drks.de; number DRKS00022495) and the English version can be downloaded from https://crit-cov.de/files/Crit-Cov/Crit-Cov-U-study-protocol.pdf. Anonymised participants data accompanied by a data dictionary will be made available upon request directed to the corresponding author. Proposals will be reviewed and approved by the funder, investigators, and collaborators on the basis of scientific merit and submitted for approval to the Ethics Committee of the German-Saxonian Board of Physicians, Dresden, Germany (number EK BR 88/20·1). After approval of a proposal, data can be shared through a secure online platform after signing a data access and confidentiality agreement. Data will be made available for up to 3 years after a request has been received and approved.