Abstract
Gene 3 of bacteriophage T4 participates at a late stage in the T4 tail assembly pathway, but the hypothetical protein product, gp3, has never been identified in extracts of infected cells or in any tail assembly intermediate. In order to overcome this difficulty, we expressed gp3 in a high-efficiency plasmid expression vector and subsequently purified it for further analysis. The N-terminal sequence of the purified protein showed that the initial methionine had been removed. Variant C-terminal amino acid sequences were resolved by determining the cysteine content of the protein. The molecular mass of 20.6 kDa for the pure protein was confirmed by Western blotting, using a specific anti-gp3 serum for which the purified protein was the immunogen. We also demonstrated, for the first time, the physical presence of gp3 in the mature T4 phage particle and localized it to the tail tube. By finding a nonleaky, nonpermissive host for a gene 3 mutant, we could clearly demonstrate a new phenotype: the slow, aberrant elongation of the tail tube in the absence of gp3.
The history of T4 gene 3 and its product, gp3, is long and rather murky. gp3 is a minor T4 tail protein that until this report had not been detected in phage particles or in infected cells. Morphological analysis of mutant lysates by Epstein et al. (14) showed that functional gene 3 (like genes 2 and 4) was required for the joining of heads and tails. King (24, 25) subsequently found that the gene 3 product appeared to act at the top of the tail tube, to stabilize the tail sheath and prepare the tail for addition of the terminal capping protein, gp15.
All attempts to identify gp3 on sodium dodecyl sulfate (SDS)-polyacrylamide gels failed (10, 26, 27). Despite this, King and Mykolajewycz (27) made a brilliant suggestion on the role of gp3 which would “…form a terminal annulus very similar to the P19 annuli and have a molecular weight very close to that of P19. …and would be very difficult to detect.” Eventually, a molecular mass of 29 kDa was attributed to gp3 by Kikuchi and King (Fig. 1 of reference 23), and that (incorrect) value persisted in the literature (3). Subsequently, Lipinska et al. (32) published a molecular mass for gp3 of 20.6 kDa based on DNA sequence data and SDS-polyacrylamide gels of specifically radiolabeled gp3. The DNA sequence data of Koch et al. (28) predicted a stop codon 7 codons upstream of the first prediction, and a new estimated molecular mass of 19.7 kDa was supported by SDS-polyacrylamide gels of recombinant gp3. In this paper, we describe the expression and purification of recombinant gp3 in Escherichia coli and show that the predicted N- and C-terminal sequences are consistent with the nucleotide sequencing data of Lipinska et al. (32) and a mature molecular mass of 20,156 Da. A review by Coombs and Arisaka (7) cites unpublished data (mostly those presented here) that basically confirm that gp3 and gp19 comigrate as suggested.
A specific immune serum raised against gp3 was used to demonstrate that gp3 is expressed late in infection. The same serum was used to demonstrate for the first time that gp3 is indeed present in complete phage particles, as well as in isolated tail tubes. Finally, we show that defective gp3 production can lead to longer-than-normal tails under certain conditions. We conclude that gp3 is an integral part of the tail, probably localized at the proximal tip of the tube, to fulfill its role in preventing abnormal extension of the tail tube during assembly.
MATERIALS AND METHODS
Bacteria, phages, and plasmids.
Bacteria, phages, and plasmids are listed in Table 1.
TABLE 1.
Bacterial strains, phages, and plasmids
| Strain, phage, or plasmid | Genotype or characteristic(s) | Reference or source |
|---|---|---|
| E. coli K-12 strains | ||
| CR63 | supD60 (Su+) | Lab strain (F.A.E.) |
| CAJ70 | supUGA (Su+) | 40 |
| K-12(λ)/s | sup0 (Su−) | 11 |
| E. coli B strains | ||
| Bb | sup0 (Su−) | Lab strain (E.B.G.) |
| Be | sup0 (Su−) | Lab strain (F.A.E.) |
| B40Su− | argF40(Am) sup0 (Su−) | Lab strain (F.A.E.) |
| B40SuI+ | argF40(Am) supD60 (Su+) | Lab strain (F.A.E.) |
| B40SuII+ | argF40(Am) supE44 (Su+) | Lab strain (F.A.E.) |
| B40SuIII+ | argF40(Am) supF66 (Su+) | Lab strain (F.A.E.) |
| HB101 | F−hds20(rB− mB−) recA13 ara-14 ΔproA3 lacY1 galK2 rpsL20(Smr) xyl-5 mtl-1 supE44 leuB6 | 4 |
| BL21(λDE3) | F−hsdS(rB− mB−) gal (int::PlacUV5-T7 gene 1 imm 21 min5) | 44;1; |
| Phage strains | ||
| T4D+ | Wild type | Lab strain |
| T4 3− NG418 | Mutation in gene 3 | Lab strain (F.A.E.) |
| T4 3− NG131 | Mutation in gene 3 | Lab strain (F.A.E.) |
| T4 19opC95 | Opal mutation in gene 19 | W. B. Wood |
| T4 10amB255 | Amber mutation in gene 10 | Lab strain (F.A.E.) |
| T4 3+/15−/23−/34− 15amN133/23amH11/34amB25 | Defective in head formation (gene 23amH), tail fiber formation (gene 34amB25), and tail completion (gene 15amN133) | Lab construct (11) |
| T4 3−/15−/23−/34− NG418/15amN133/23amH11/34amB25 | Defective in head formation (gene 23amH11), tail fiber formation (gene 34amB25), and tail completion (gene 15amN133 and gene 3amNG418) | This work |
| M13mp7 | M13 derivative with polylinker containing an inverted repeat | 33 |
| Plasmids | ||
| pTFP2110 | pBR322 derivative carrying in the PstI site a 2.8-kb insert containing T4 phage genes 3 to 53 | 49 |
| pET9 | pBR322 derivative used for gene expression; Kanr | 45 |
| pLysS | pACYC184 derivative carrying T7 gene 3.5 (coding for T7 lysozyme); Cmr | 43 |
| pAVgp3 (pAV9 · 3 · 1) | pET9 derivative expressing T4 gene 3; Kanr | This work |
(i) Bacteria.
E. coli HB101, a nonrestricting, recA strain, obtained from A. Torriani (Massachusetts Institute of Technology, Cambridge), was routinely used for plasmid construction. E. coli BL21(DE3), carrying a defective λ with the gene for T7 RNA polymerase under the control of the lacUV5 promoter, and E. coli BL21(DE3)/pLysS (with a plasmid expressing T7 lysozyme) were provided by F. W. Studier (Brookhaven National Laboratories, Upton, N.Y.). These strains were used to express gp3 from the plasmid pAVgp3. K-12(λ)/s was used to grow extracts of the gene 3 amNG418 mutant since it was much less leaky than B strains.
(ii) Phage.
T4D wild type used for determining the kinetics of gp3 expression in the infected cell was from E. B. Goldberg's lab. T4D wild type used in all other experiments described was from F. A. Eiserling's lab. The T4 gene 10 mutant amB255 was obtained from W. B. Wood (University of Colorado, Boulder). The T4 gene 3 mutant amNG418 was from F. A. Eiserling's lab. M13mp7 was obtained from GIBCO BRL, Gaithersburg, Md.
(iii) Plasmids.
Plasmid pTFP2110, which includes genes 3 to 53 on a 2.8-kb insert, was obtained from J. Abelson (California Institute of Technology, Pasadena). Plasmid pET9, used for gene 3 expression, was a gift from F. W. Studier.
(iv) Construction of a plasmid expressing gp3.
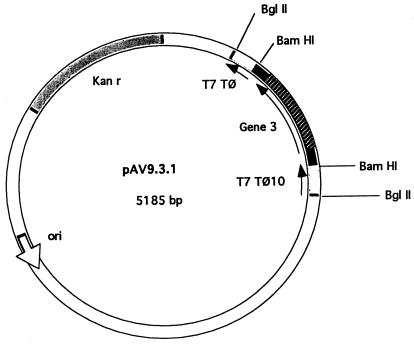
All methods were essentially as described in the work of Sambrook et al. (39). A 0.9-kb SspI-PstI DNA fragment, containing gene 3, was isolated from pTFP2110. After the ends of the purified fragment were filled in with Klenow fragment, BamHI linkers were ligated to the blunt ends and the mixture was digested with BamHI. The 0.9-kb fragment with BamHI ends was purified from a low-melting-point agarose gel and cloned into the BamHI site of the pET9 vector (44). The resulting plasmid containing gene 3 in the correct orientation was called pAV9 · 3 · 1 (Fig. 1). This plasmid, referred to as pAVgp3 for simplicity, was then introduced into E. coli BL21(DE3) (using Kanr selection) and E. coli BL21(DE3)/pLysS (using Kanr Camr selection) to allow IPTG (isopropyl-β-d-thiogalactopyranoside)-regulated expression of gp3 from the T7 promoter.
FIG. 1.
Construction of pAVgp3 (pAV9 · 3 · 1). See Materials and Methods for details.
Media.
L broth is a 1% Bacto Tryptone, 0.5% yeast extract, and 0.5% NaCl adjusted to pH 7.0 with 1 N NaOH. M9 is 0.3% KH2PO4, 0.6% Na2HPO4, 0.1% NH4Cl, and 0.4% glucose, to which MgSO4 at 1 mM final concentration was added. ZB medium is 1% N-Z-amine A and 0.5% NaCl. ZB-M9 includes all components of both ZB and M9. T2 plates contain 1% Bacto Tryptone, 0.8% NaCl, 0.2% Na citrate, 0.1% glucose, and 1.2% agar neutralized with 1 N NaOH. T2 top agar is of the same composition as T2 plates but with 0.6% agar. Antibiotics were typically used at the concentrations of 100 μg of ampicillin per ml, 30 μg of kanamycin per ml, and 25 μg of tetracycline and chloramphenicol per ml. IPTG was used at 0.4 mM concentration for induction.
Buffers.
Cracking buffer is 60 mM Tris-HCl (pH 6.8) and 2% SDS. Ellman reaction buffer is 0.1 M sodium phosphate (pH 8.0) with or without 6 M guanidinium chloride. TED buffer is 50 mM Tris-HCl (pH 8.0), 2 mM EDTA, and 0.1 mM dithiothreitol. Lysis buffer is TED buffer to which 0.1 M NaCl, 5% glycerol, and 200 mg of lysozyme/ml have been added. NET is 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, and 5 mM EDTA. TEGD buffer is TED plus 5% glycerol. Sonication buffer is 50 mM Tris-HCl (pH 8.0), 2 mM EDTA, 0.1 mM dithiothreitol, 0.1 M NaCl, 5% glycerol, and 100 mg of lysozyme per ml. BU is 13.3 g of Na2HPO4 · 7H2O, 4 g of NaCl, and 3 g of KH2PO4 per liter. TNA is 20 mM Tris-HCl (pH 8.0), 225 mM NaCl, and 0.1% bovine serum albumin (BSA).
Chemicals and enzymes.
Enzymes were obtained from GIBCO BRL, New England Biolabs (Beverly, Mass.), IBI (New Haven, Conn.), and Boehringer Mannheim Biochemicals (Mannheim, Germany). Complete and incomplete Freund's adjuvant, Coomassie brilliant blue R-250, anti-rabbit immunoglobulin G (IgG)-alkaline phosphatase conjugate, nitrocellulose membranes, molecular mass standards, and DEAE–Sephadex A-50 were purchased from Sigma Chemical Co. (St. Louis, Mo.). The polyvinylidene difluoride membrane was from Millipore Co. (Bedford, Mass.). Protogel 30% (wt/vol) acrylamide and 0.8% (wt/vol) bisacrylamide stock solution was purchased from National Diagnostics (Manville, N.J.). Ellman's reagent, 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB), was from Pierce Chemical Co. (Rockford, Ill.).
Overexpression and purification of gp3.
E. coli BL21(DE3)/pAVgp3 was grown at 37°C in ZB-M9 with 50 μg of kanamycin per ml to an optical density at 600 nm (OD600) of 0.5 to 1.0. IPTG was added to induce gp3 synthesis, after which the cells were collected and frozen at −70°C. The cells were resuspended in sonication buffer and sonicated on ice for 2 min. The suspension was clarified by centrifugation for 20 min at 17,000 × g at 4°C. (NH4)2SO4 to 35% saturation was added to the supernatant fluid, containing most of the gp3, and the precipitate was dissolved in 0.1 volume of TEGD containing 0.1 M NaCl. The partially purified gp3 was loaded onto a DEAE–Sephadex A-50 column (9 by 130 mm) equilibrated with the same buffer. After washing, the column was eluted with a linear gradient of 0.1 to 0.5 M NaCl in TEGD buffer at 10 ml per h. Fractions of 1.7 ml were collected and assayed for absorption at 280 nm, and a molecular weight profile of the proteins was run on SDS–12% polyacrylamide gels. Protein bands were visualized with Coomassie brilliant blue R-250. Fractions with significant gp3 were pooled and concentrated by 50% saturation with ammonium sulfate. The precipitate was dissolved in 0.3 M ammonium acetate (pH 6.0)–5% glycerol and fractionated on a Superdex 75 gel filtration column (16 by 600 mm; Pharmacia) at a flow rate of 0.7 ml/min. Pooled fractions containing gp3 were concentrated by dialysis against 30% glycerol in TEGD buffer and stored at −20°C.
Induction of cell culture and analysis of cellular proteins.
An overnight culture of E. coli BL21(DE3)/pAVgp3 grown in ZB medium was used to inoculate fresh ZB-M9 medium containing 1 mM MgSO4 and 0.4% glucose. At an OD600 of 0.7, IPTG was added to induce gp3 synthesis. At 30-min intervals, 25-ml aliquots of cells were harvested by centrifugation, resuspended in lysis buffer, and intermittently sonicated in an ice bath for 45 s, in order to reduce the viscosity. The samples were run on SDS–12% polyacrylamide gels and stained with Coomassie blue, and the gel was scanned on an LKB Ultroscan XL scanner (Pharmacia LKB) to determine the gp3/total protein ratio which was synthesized upon induction. Then the proteins were electroblotted onto nitrocellulose or a polyvinylidene difluoride membrane for Western blot analysis (47). To detect gp3, rabbit anti-gp3 and alkaline phosphatase-conjugated goat anti-rabbit IgG were used.
Determination of protein concentration.
Protein concentration was determined by the Bio-Rad protein assay (5) or by UV absorption (280-nm/260-nm ratio) during the steps of protein purification. BSA was used as the standard.
NH2-terminal protein sequencing.
Purified gp3 (see above) was dialyzed against 0.85% sodium chloride to remove any Tris and glycerol in the sample which might interfere with sequencing. The amino-terminal sequence of the dialyzed gp3 was determined by automated Edman degradation (Applied Biosystems model 447A).
Determination of cysteine in gp3.
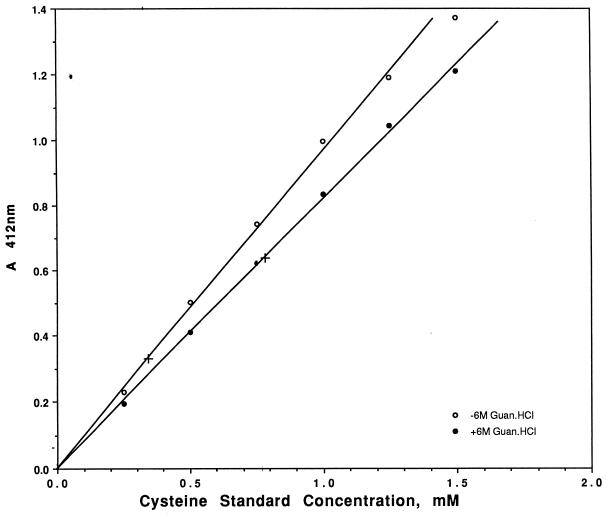
Ellman's reagent (DTNB), was used essentially as described by the manufacturer to quantitate free sulfhydryl groups in purified gp3. Purified gp3 (5.7 ml; 334 μg/ml) was dialyzed overnight against 0.85% sodium chloride saline, frozen in liquid nitrogen, and evaporated in a rotary unit below 25°C until dry. The gp3 protein sample was dissolved in 250 μl of water to a final concentration of 7.6 mg/ml (0.38 mM). A fresh 3 mM cysteine standard solution was prepared with Ellman reaction buffer immediately before use, and dilutions were prepared for standard curves. Twenty-five-microgram aliquots of a cysteine standard or of a gp3 sample were added to the reaction tubes containing 5 μl of 4 mg of DTNB per ml and 0.25 ml of the reaction buffer. The tubes were mixed and incubated at room temperature for 15 min, and the absorbance at 412 nm was determined. The values obtained for gp3, with or without guanidinium chloride, were compared to standard cysteine concentrations in a similar condition.
Preparation of anti-gp3 antiserum.
The proteins in the crude extract were separated by SDS-polyacrylamide gel electrophoresis (PAGE). After electrophoresis, the gel was washed with deionized water, lightly stained with 0.05% Coomassie brilliant blue R-250 in water, and washed again with water. The weakly stained gp3 band was excised with a scalpel and fragmented by passing it back and forth through two syringes connected by a syringe adapter. A gel mixture containing Freund's complete adjuvant for the primary injection and with incomplete Freund's adjuvant for the three subsequent boosters was injected intradermally into a female New Zealand White rabbit, at 1-week intervals. A final intravenous booster was made with 300 μg of column-purified gp3 dialyzed into saline. The rabbit was bled, and serum was prepared 6 weeks after the primary immunization.
Kinetics of gp3 production in T4 phage-infected cells.
The overnight culture of E. coli B was diluted 100 fold in L broth with 0.2% glucose, grown at 37°C with aeration to an OD600 of 0.5, and infected with T4 phage at a multiplicity of infection of 8. Aliquots of 10 ml were taken at intervals from 0 to 90 min, chilled with frozen L broth, and stored on ice. Samples of these cultures were sedimented, and the cell pellets were resuspended in cracking buffer, heated at 95°C, sonicated to reduce viscosity, run on SDS-polyacrylamide gels, and transferred onto nitrocellulose membranes for Western blot analysis.
Detection of gp3 in phage particles and tail structures.
T4D+ phage particles, sheathed tails, tube baseplates, and tubes were purified by the method of Duda et al. (method B in reference 12). Samples of each were loaded onto two identical acrylamide gels and run simultaneously. One gel was stained with Coomassie brilliant blue R-250; proteins on the other gel were electroblotted onto a nitrocellulose membrane (47). The membrane was blocked with 1% BSA and then incubated with anti-gp3 antiserum (prepared as described above). gp3 was visualized by alkaline phosphatase immunoblotting (Protoblot; Promega Corp., Madison, Wis.).
Immunoelectron microscopy of purified tail structures.
Tails and tube baseplates were purified as described in reference 12. Structures were treated with anti-gp3 antiserum (as described above) and colloidal gold-conjugated secondary antibodies as follows. One drop of a diluted sample of purified tail structures was adsorbed to a carbon-coated Parlodion film on a grid for 2 min, rinsed with 5 drops of BU, blocked with 5 mg of BSA per ml for 5 min, and rinsed with 5 drops of TNA. The sample was then incubated with 1 drop of diluted anti-gp3 for 5 min, rinsed with 5 drops of TNA, incubated with diluted (1:10 into TNA) 10-nm-colloidal-gold-conjugated anti-rabbit IgG secondary antibody (Ted Pella, Inc., Redding, Calif.) for 5 min, and rinsed with 3 drops each of TNA and then distilled water. The sample was then stained with 3 drops of 0.5% uranyl acetate for 5 to 10 s each as described in reference 13.
Immunoprecipitation of purified tail structures.
One milliliter of structures or purified protein was incubated with 1 μl of antiserum in 100 μl of NET with 0.5% Tween for 1 h on ice. Twenty-five microliters of a 10% suspension of heated and fixed Staphylococcus aureus cells (Immunoprecipitin; GIBCO BRL) was added to the antibody-antigen mixture. After 30 min on ice, the resulting complexes were pelleted. The supernatant was saved for SDS-PAGE analysis, and the pellet was washed two times with 150 μl of NET buffer with 0.5% Tween. The pellet and the supernatant were each mixed with 50 μl of SDS sample buffer and heated to 100°C for 5 min. Samples were run on SDS-polyacrylamide gels, prepared as described for method B in reference 12, and transferred to nitrocellulose (47). Proteins were detected by alkaline phosphatase immunodetection (Protoblot) with specific antisera for gp3, gp29 (1), or T4 tail tubes (12).
Elongation of tail tubes in the absence of gp3.
T4 3−/15−/23−/34− and 3+/15−/23−/34− phage were grown on the strongly restrictive host, K-12(λ)/s, on which T4 gene 3 mutants are not leaky. Lysates were prepared from each infection and sedimented into sucrose gradients, and bands containing tails were isolated as described in reference 12. Samples were prepared for electron microscopy, and images were recorded by the methods described in reference 13.
RESULTS
Expression and purification of gp3.
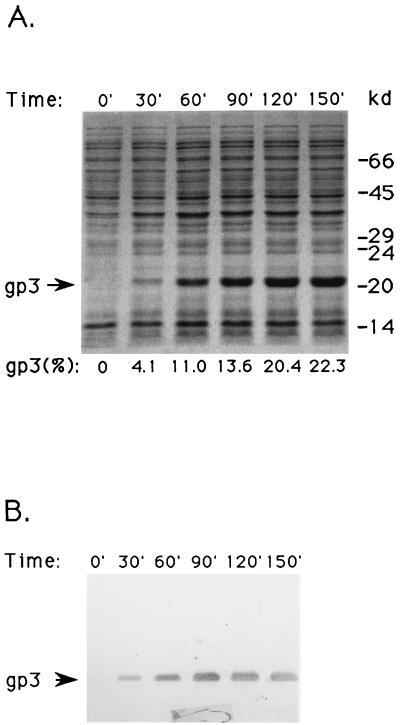
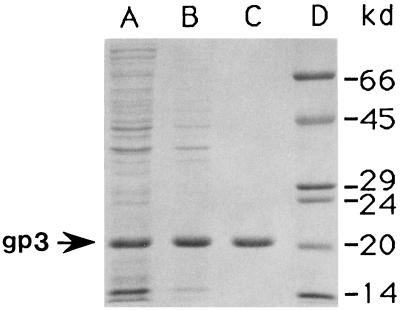
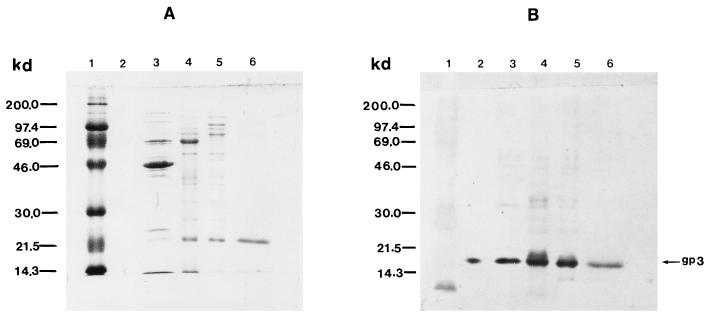
Gene 3 was cloned into an efficient expression vector (Fig. 1) to facilitate purification and analysis. Figure 2 shows that production of gp3 reaches 22% of the total protein in E. coli BL21(DE3)/pAVgp3 by 150 min after induction. Figure 3 (lane A) shows that most of the gp3 is found in the supernatant fluid of the crude extract, indicating a cytoplasmic localization. The lack of formation of intracellular inclusion bodies, despite the massive synthesis of gp3, may reflect its hydrophilic and highly acidic nature (pI = 4.22). After chromatography on DEAE–Sephadex A-50, only one minor contaminating protein, of 80.2 kDa, was visible on Coomassie blue-stained gels (data not shown). This contaminant was removed by chromatography on the Superdex 75 column (lane C). The purified protein was used for studies of the protein structure and for the production of anti-gp3 antiserum.
FIG. 2.
Induction kinetics of gp3. Proteins from E. coli BL21(DE3)/pAVgp3 cell lysates were separated by SDS-PAGE. (A) Coomassie blue-stained gel of samples taken at 30-min intervals after addition of IPTG to the culture. gp3 as a percentage of total protein is given below each lane. (B) Western blot of the same samples as shown in panel A except that fivefold-less protein was used.
FIG. 3.
Purification of gp3. SDS-polyacrylamide gels of gp3 at various stages of purification are shown. Lane A, supernatant of the cell lysate after sedimentation; lane B, ammonium sulfate precipitate; lane C, purified gp3 after DEAE–Sephadex A-50 and Superdex-75 column chromatographies; lane D, molecular mass markers. The arrow denotes the 55-kDa band of gp3.
N-terminal sequencing.
The availability of pure gp3 enabled us to determine the N-terminal sequence of the protein by Edman degradation. The sequence of the first 11 amino acids, Ser-Gln-Ala-Leu-Gln-Gln-Ile-Phe-Asn-Gln-Ala, is identical to that predicted for residues 2 to 12. The initial methionine is presumably removed posttranslationally, as expected (2, 9, 17).
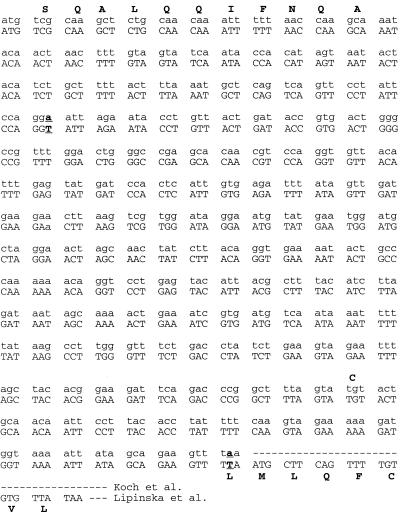
Cysteine content of gp3 supports the longer putative protein sequence.
There are two discrepancies in the DNA sequences of gene 3 (Fig. 4). A minor one occurs at bp 123, where both an A (32) and a T (28) were found, but both encode Gly41. A more important difference occurred at bp 530, where again both an A (6, 28) and a T (32) were found, the former at the center of the stop codon, TAA, and the latter at the center of the Leu177 codon, TTA. The latter would continue to add the eight C-terminal codons, LMLQFCVL, before the next in-frame TAA stop codon. Thus, the shorter protein should have only one Cys whereas the longer protein should have two. The resulting small difference in lengths between the two putative gp3 proteins makes their resolution by SDS-PAGE unlikely. Instead, we determined the number of cysteines. Figure 5 shows that 0.33 mM Cys was detected in a sample of 0.38 mM gp3. Thus, native gp3 has about one accessible Cys per molecule (actually, 0.87) which reacts with DTNB. Figure 5 also shows that after denaturation in 6 M guanidinium chloride, 0.77 mM Cys was detected in a sample of 0.38 mM gp3. This result demonstrates that an additional Cys is rendered reactive with DTNB after denaturation to yield a total of two Cys residues per molecule (i.e., 0.77 mM Cys/0.38 mM gp3 = 2.03). This result supports the C-terminal sequence (LMLQFCVL) of the gp3 open reading frame (32).
FIG. 4.
Comparison of two sequences of T4 phage gene 3. Amino acids are indicated by boldface capital letters at top and bottom; variant nucleotides are indicated by boldface and underlining.
FIG. 5.
Sulfhydryl content of gp3. Open circles, standard curve for the reaction of cysteine with Ellman's reagent. Solid circles, the same but in the presence of 6 M guanidinium chloride. +, reactivity of a 0.38 mM gp3 sample in Ellman's reagent with and without 6 M guanidinium chloride.
Although this conclusion depends on the accuracy of our protein concentration measurement, it seems likely to be correct because (i) the N-terminal sequence is correct, and the molecular weight fits only the open reading frame illustrated and jibes with the extended C terminus; and (ii) the number of available Cys residues in native gp3 is nearly one per molecule and in denatured gp3 is almost exactly two per molecule, thus demonstrating the accuracy of the ratio of the measurements which fit the most likely interpretation. Therefore, we think it most likely that gp3 is a protein of 183 residues (without the N-terminal Met) with a molecular mass of 20,516 Da.
Kinetics of gp3 production of T4-infected cells.
Figure 6 shows that gene 3 translation can be detected in T4-infected cells by anti-gp3 antibody on an immunoblot from 15 through 90 min after infection at 37°C. This is consistent with the finding of Broida and Abelson (6), who showed that gp3 transcription starts 8 min after infection (at 30°C). Translation of gp3 appears to start before 15 min postinfection at 37°C and continues until lysis, as is the case for most other late proteins.
FIG. 6.
Kinetics of gp3 production of T4-infected cells. Infected cells were sampled at 15-min intervals after infection and lysed. They were run on SDS-polyacrylamide gels, and a Western blot probed with anti-gp3 rabbit serum was prepared. Lanes a through g correspond to 0, 15, 30, 45, 60, 75, and 90 min after infection, respectively. Lane h is purified gp3.
Detection of gp3 in phage particles and tail structures.
In order to determine if gp3 is a component of the phage particle, we performed an immunoblot analysis of an SDS-polyacrylamide gel of T4 phage and several tail substructures with the anti-gp3 antibody preparation. Figure 7A shows a Coomassie blue-stained gel, while Fig. 7B shows the immunoblot of an identical gel run in parallel. Figure 7B (lane 3) shows that gp3 is indeed present in T4 phage. Purified tails (lane 4), tube baseplates (lane 5), and tubes from which the baseplates had been removed (lane 6) were also found to contain gp3. Thus, the specific anti-gp3 antiserum enabled us to determine, for the first time, that gp3 is present in the T4 phage particle, and in particular in tube baseplates and purified tubes.
FIG. 7.
Content of gp3 in various phage structures. Shown are a Coomassie blue-stained polyacrylamide gel (A) and a Western blot of an identical gel probed with anti-gp3 antiserum (B). Quantities of the samples were adjusted to show a clear band on the Western blot. Lanes 1, molecular mass markers; lanes 2, purified gp3; lanes 3, T4D+ phage particles; lanes 4, tails; lanes 5, tube baseplates; and lanes 6, tubes.
Antiserum to denatured gp3 fails to recognize gp3 in native tail structures.
In order to localize gp3 more precisely in the native tail structure (and substructures), we attempted to immunolabel tubes and tube baseplates in situ with anti-gp3 antibodies and colloidal gold after they had been adsorbed to electron microscope grids. Even though the tail structures were positive for gp3 by immunoblotting after denaturation and separation by SDS-PAGE (Fig. 7), gp3 present in the intact structures did not react with the anti-gp3 antiserum (data not shown).
We also attempted to immunoprecipitate intact tube baseplates from solution by incubating them with anti-gp3 and then by secondary binding to S. aureus followed by sedimentation. Immunoblots of SDS-polyacrylamide gels of the denatured pellets and supernatants were probed with anti-gp29 serum. Anti-gp29 serum was used as a probe, rather than anti-gp3, to ensure that the structures being detected were intact tube baseplates and not simply soluble gp3 associated with tail structures (gp29 is a tail tube-associated protein). The immunoblot of the precipitate showed no evidence of gp29, which implies that no tail structures were immunoprecipitated with anti-gp3 (data not shown). The supernatants were positive for gp29, showing that the tube baseplates indeed remained in the supernatant (data not shown).
Strain-dependent, suppressor-independent growth of the T4 gene 3 amber mutant.
King was originally unable to determine the sequence of interactions among genes 18, 3, and 15 in in vitro complementation experiments (24) because of the high background of phage and gene 3 activity produced in E. coli B. Later, King (25) ordered the steps of assembly by adding an additional amber to the complementation recipient and growing the gene 3 amber at a higher temperature, but the background was still high. When gene 3 ambers are plated on E. coli B at a high titer to measure reversion frequency, a background of thousands of small plaques surrounds the large plaques of revertants. This is referred to as a leaky phenotype (Table 2). The high background in King's experiments and the leaky plating phenotype are related and indicate that some active gp3 is produced when a gene 3 amber grows on E. coli B. We were surprised to discover that gene 3 ambers do not grow well on B40SuI+. Indeed, the gene 3 amber mutants do not grow well on any of our isogenic B strains: supD, supE, supF, or sup0 (Table 2). Limited experiments to explore the pattern of suppression identified one K-12 strain, K-12(λ)/s (11), that failed to grow the gene 3 mutants (Table 2). Mutations with a similar unusual differential growth phenotype, between B and K strains, have been previously reported for T4 genes 8, 53 (15), and 60 (52). This presented an opportunity to reexamine the role of gene 3 in T4 tail assembly under stringent conditions that had not been previously available.
TABLE 2.
Efficiency of plating of gene 3 amber mutant NG418 on various E. coli strains
| E. coli host strain | Relevant genotype | Nonsense mutation suppressed | Phage plateda
|
|||
|---|---|---|---|---|---|---|
| Gene 3 amber NG418×b | Gene 10 amber B255 | Gene 19 opal C95 | Wild type T4D+ | |||
| K-12 strains | ||||||
| CR63 | supD60e | Amber | 1 | 1 | 10−5 | 0.8 |
| CAJ70 | supUGA | Opal | 0.9 | 3 × 10−7 | 1 | 0.9 |
| K-12(λ)/s | sup0 | None | 1.5 × 10−6 | 5 × 10−6 | 8 × 10−7 | 0.8 |
| B strainsc | ||||||
| Be | sup0 | None | ∼10−5 (leaky)d | ∼10−5 | <10−6 | 1 |
| B40Su0 | sup0 | None | ∼10−5 (leaky) | ∼10−5 | <10−6 | 1 |
| B40SuI | supD60e | Amber | ∼10−5 (leaky) | ∼1 | <10−6 | 1.1 |
| B40SuII | supE44 | Amber | ∼10−5 (leaky) | ∼1 | <10−6 | 1 |
| B40SuIII | supF66 | Amber | ∼10−5 (leaky) | ∼1 | <10−6 | 0.9 |
Plating efficiency on nonpermissive hosts is expressed relative to growth on the suppressing strain (defined as 1).
NG418 was crossed to rI48 and then to wild type to remove extraneous mutations and renamed amNG418×.
The B40 strains are all isogenic except for the suppressor mutations listed.
Leaky indicates that on plates showing 100 to 200 small- to medium-sized plaques, 2 × 103 to >40 × 103 minute plaques were seen.
The supD60 allele in B40SuI+ is derived from CR63.
Elongation of tail tubes in the absence of gp3.
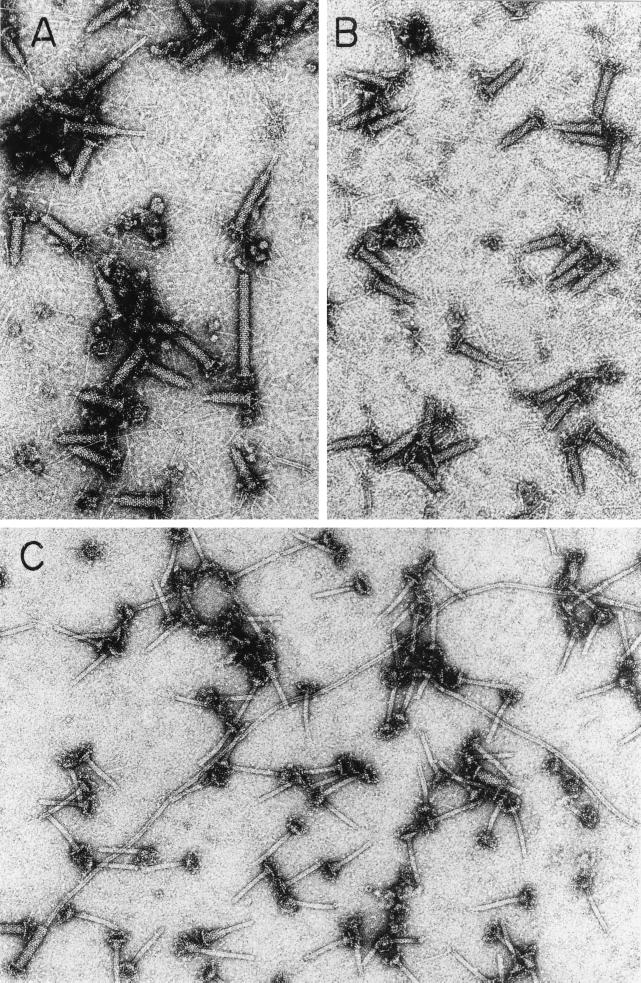
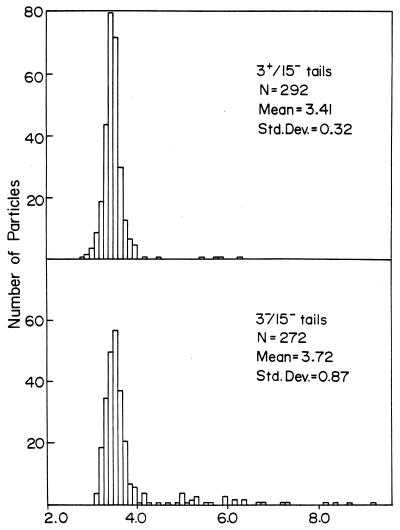
The gene 3 amNG418 mutation was added to the multiple mutant 15−/23−/34−, which is routinely used for producing tube baseplates (12). We then compared the structures produced by 3+/15−/23−/34− phage (in the presence of gp3) and 3−/15−/23−/34− (in the absence of gp3), both grown on the sup0, gene 3 mutant-restricting host, K-12(λ)/s. We found a noticeable fraction of elongated tail structures present both in the gene 3-defective lysates (Fig. 8A and 9, bottom) and in preparations of gene 3-defective purified tails stored for a week at 4°C (Fig. 8C). The 3+ lysate (Fig. 8B and 9, top) shows only tail tubes of normal length, and many of these tails are only partially sheathed. The elongated tubes which we observed in 3− lysates, after a week at 4°C (Fig. 8C), were often 10 to 15 times longer than normal. These results suggest that the function of gp3 is to terminate the polymerization of the tail tube at the correct length.
FIG. 8.
T4 gp3 prevents elongation of tails beyond the correct length. (A and B) Electron micrographs of negatively stained lysates of cells infected with tail-producing mutants that have either inactive gp3 (A) or active gp3 (B). Notice the long tails in panel A (the tails are considered long whether or not elongated tubes are completely covered with sheath). (C) Electron micrograph of the extra-long tubes found in a preparation of purified 3−/15− tails after storage for a week at 4°C. The extra-long tubes apparently form in vitro when subunits depolymerize from a fraction of the tubes and repolymerize onto tubes which are not properly terminated at the correct length by gp3. The mutants T43−/15−/23−/34− (A and C) and T43+/15−/23−/34− (B) are described in Table 1.
FIG. 9.
Length distribution of tails made with or without gp3. The lengths of tails present in electron micrographs made from lysates of mutant infected cells (Fig. 8) were measured in arbitrary units as described previously (12). The top panel is from a 3+/15−/23−/34− lysate (as in Fig. 8B), and the bottom panel is from a 3−/15−/23−/34− lysate (as in Fig. 8A). The means and standard deviations of the measurements and the numbers of particles counted (N) are shown in each panel. The values along the x axis are tail lengths in millimeters, measured directly on electron micrograph negatives at ×24,000 magnification.
DISCUSSION
In this work, we have shown that gp3 is an integral part of the tail tube and has a role in preventing aberrant elongation of tail tubes. The successful detection of gp3 in tail structures by immunoblotting shows that gp3 is incorporated into tails prior to the addition of gp15 (the tail terminator), since the structures used were isolated from cells infected with a gene 15 amber mutant. It has been established previously that gp15 is the final protein, probably located at the top of the tail (25), which is added to an otherwise complete tail (27) that has both a tube (of gp19) and a sheath (of gp18). It has also been shown that 3− tails can bind gp15 only after gene 3 acts upon tube baseplates (25). These results strongly suggests that gp3 acts as a bridging layer since it binds to the tubes (probably at the end) and is needed to bind gp15. King and Mykolajewycz (27) speculated that a hexamer of gp3, resembling a hexameric gp19 annulus, binds to the terminal gp19 annulus. Indeed, gp3 may be hexameric, since its reported sedimentation coefficient of 6S (25) is consistent with that size of oligomer. We were unable to directly visualize the location of gp3 in intact tail structures by immunoelectron microscopy, but the data discussed above suggest it is at the end of the tail tube. Failure to detect gp3 in the intact structures by microscopy or immunoprecipitation may be due to the specificity of the anti-gp3 antiserum (which was made using a denatured gp3 immunogen and therefore might not recognize the native, assembled protein) or to the inaccessibility of recognizable gp3 epitopes, possibly covered by other tail proteins.
Though King (24) saw no increase in the length of 3− tails, we showed that a significant fraction of 3− tail tubes is longer than normal. We attribute the discrepancy to the small but significant production of gp3 in gene 3 amber mutants grown on the conventional nonpermissive strain B (24, 25). The greater stringency of E. coli K-12(λ)/s for the growth of gene 3 amNG418 mutant phage enabled us to reduce the expression of active gp3 to a level where a new phenotype of 3− mutants could be observed. T4 tails that have both gp3 and gp15 are very stable in 0.1 M phosphate buffer, but elongated tubes can be found in purified T4 tails after storage at a high concentration at a low ionic strength (48). Subunit depolymerization and repolymerization (probably after the loss of gp3 and gp15) are a likely explanation for such elongation. The lysates used for the preparations for Fig. 8A, 8B, and 9 were only 1 day old when samples were prepared for electron microscopy. We think that the appearance of the small fraction of elongated tail tubes in the gene 3-defective lysate is significant and suggests that the gene 3 product suppresses abnormal elongation past the length set by the tail length ruler, gp29 (1). The very long tail tubes on the tube baseplates found in purified preparations of gene 3-defective tail structures stored for a week (Fig. 8C) are a new phenotype. This phenotype is partly analogous to that of gpU of phage λ, where the lack of gpU results in unregulated elongation of λ tails, but only after pausing at the normal length for a short time (20, 21, 22). Although a tape-measure-type mechanism of tail length determination exists both in T4 (1) and in λ (see references 16 and 21 for reviews), there is apparently a terminator protein that blocks the slower polymerization of the tail tube subunits that can occur even after the length of the tube has matched that of the ruler protein. Termination mutations, which lead to production of extra-long, defective tails in vivo, have also been identified in phages P2 (31) and SP01 (36), which suggests a common mechanism shared by many phages. In λ phage, gpU is located at the head-proximal end of the tail and was also found to be a hexamer (in solution in the presence of Mg2+) (21, 22). By analogy, gp3 would cap the upper gp19 annulus and thereby prevent the aberrant, slower addition of gp19 beyond that regulated by gp29.
Bacteriophage T4 tail length determination appears to involve the separate but coupled processes of elongation, governed by the ruler protein gp29, and termination, effected by gp3. Tubes of gp19 have not been observed to form in vivo in the absence of baseplates in any mutant background (7), and so it appears that gp19 requires a baseplate to initiate polymerization in vivo (25). The protein gp54, which adds to the baseplate immediately before tube initiation (25), is a likely candidate for the actual initiation factor (7). The mechanism by which gp19 subunits add is not known, but the mechanism of growth is possibly regulated by a conformational change in the subunits that occurs as each layer is completed. Once initiated, tube growth is thought to be limited by the length of gp29 but may be limited only by the supply of unassembled gp19 under conditions where the ruler mechanism is bypassed. Temperature-sensitive missense mutations in gene 19 produce stable short tail tubes of variable length in vivo when mutants are grown at a semipermissive temperature. This suggests that defective gp19 subunits can block elongation (25) or, alternatively, that premature termination may occur by defective interaction with gp29 or gp3.
Since there is good physical evidence that the gp29 ruler protein extends the full length of the interior of the tail tube in completed tails, it is attractive to speculate that termination of tube elongation at the normal length involves the influence of the ends of the internal gp29 molecules. In one scenario, a domain of gp29 that resides at the growing end of the tube facilitates the polymerization of tube subunits until it becomes sequestered by the completed tube and gp3 binds to the topmost gp19 annulus to prevent further elongation. In the absence of gp3, new annuli of gp19 could continue to be added, but only slowly, without the cooperative assistance of gp29. In an alternative model, a domain of gp29 that protrudes at the growing end of the tube inhibits gp3 binding until it is sequestered by the elongating tube. In this model, gene 3 mutants should rapidly produce very long tails, but this does not occur. In fact, 3− tubes elongate very slowly. Similar models have been proposed and discussed in detail by Katsura (21) for regulation of λ tail length, and still others can be proposed. With this report of abnormal tail elongation in T4 gene 3 mutants, we confirm the general principle that bacteriophage tail length is primarily governed by a tape-measuring protein (gp29) but is also assisted by a termination factor (gp3) that recognizes the correct length.
ACKNOWLEDGMENTS
We thank D. Coombs, P. Ferguson, and Paul Hyman for suggestions to improve the paper.
E.B.G. acknowledges the financial assistance of the NIH. for grant GM13511, NSF grant 9834603, ONR grant N00014-98-1-0784, and the dean's office of the Tufts School of Medicine for a special grant. F.A.E. acknowledges the financial assistance of the NSF for grant DMB 8705427.
A.V., G.R.W., M.G., and R.L.D. contributed equally to this paper.
REFERENCES
- 1.Abuladze N K, Gingery M, Tsai J, Eiserling F A. Tail length determination in bacteriophage T4. Virology. 1994;199:301–310. doi: 10.1006/viro.1994.1128. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Bassat A, Bauer K. Amino-terminal processing of proteins. Nature. 1987;326:315. [Google Scholar]
- 3.Berget P B, King J. T4 tail morphogenesis. In: Mathews C K, Kutter E M, Mosig G, Berget P B, editors. Bacteriophage T4. Washington, D.C.: American Society for Microbiology; 1983. pp. 246–258. [Google Scholar]
- 4.Boyer H W, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969;41:459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- 5.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Broida J, Abelson J. Sequence organization and control of transcription in the bacteriophage T4 tRNA region. J Mol Biol. 1985;185:545–563. doi: 10.1016/0022-2836(85)90071-3. [DOI] [PubMed] [Google Scholar]
- 7.Coombs D, Arisaka F. T4 tail structure and function. In: Karam J D, Drake J W, Kreuzer K N, Mosig G, Hall D H, Eiserling F A, Black L W, Spicer E K, Kutter E, Carlson K, Miller E S, editors. Molecular biology of bacteriophage T4. Washington, D.C.: American Society for Microbiology; 1994. pp. 259–281. [Google Scholar]
- 8.Cummings D J. A remeasurement of the molecular weights of T-even bacteriophage substructural proteins. J Virol. 1972;59:123–128. doi: 10.1128/jvi.9.3.547-550.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalbøge H, Bayne S, Pedersen J. In vivo processing of N-terminal methionine in E. coli. FEBS Lett. 1990;266:1–3. doi: 10.1016/0014-5793(90)90001-b. [DOI] [PubMed] [Google Scholar]
- 10.Dickson R C. Protein composition of the contracted sheath of bacteriophage T4. Virology. 1974;59:123–138. doi: 10.1016/0042-6822(74)90210-4. [DOI] [PubMed] [Google Scholar]
- 11.Doermann A H, Boehner L. The identification of complex genotypes in bacteriophage T4. I. Methods Genet. 1970;66:417–428. doi: 10.1093/genetics/66.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duda R L, Gingery M, Eiserling F A. Potential length determiner and DNA injection protein is extruded from bacteriophage T4 tail tubes in vitro. Virology. 1986;151:296–314. doi: 10.1016/0042-6822(86)90051-6. [DOI] [PubMed] [Google Scholar]
- 13.Duda R L, Gingery M, Ishimoto L K, Eiserling F A. Expression of plasmid-encoded structural proteins permits engineering of bacteriophage T4 assembly. Virology. 1990;179:728–737. doi: 10.1016/0042-6822(90)90140-m. [DOI] [PubMed] [Google Scholar]
- 14.Epstein R H, Bolle A, Steinberg C M, Kellenberger E, Boy de la Tour E, Chevalley R, Edgar R S, Susman M, Denhardt G H, Lielausis A. Physiological studies of conditional lethal mutants of bacteriophage T4D. Cold Spring Harbor Symp Quant Biol. 1963;28:375–392. [Google Scholar]
- 15.Georgopoulos C, Georgiou M, Selzer G, Eisen H. Bacteriophage T4 mutants which will propagate on E. coli K12 but not on E. coli B. Experientia. 1977;33:1157–1158. doi: 10.1007/BF01922300. [DOI] [PubMed] [Google Scholar]
- 16.Hendrix R W. The length determination in double-stranded DNA bacteriophages. Curr Top Microbiol Immunol. 1988;136:21–29. doi: 10.1007/978-3-642-73115-0_2. [DOI] [PubMed] [Google Scholar]
- 17.Hirel P-H, Schmitter J-M, Dessen P, Fayet G, Blanquet S. Extent of N-terminal methionine excision from Escherichia coli proteins is governed by the side-chain length of the penultimate amino acid. Proc Natl Acad Sci USA. 1989;86:8247–8251. doi: 10.1073/pnas.86.21.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishimoto L K, Ishimoto K S, Cascino A, Cipollaro M, Eiserling F A. The structure of three bacteriophage T4 genes required for tail-tube assembly. Virology. 1988;164:81–90. doi: 10.1016/0042-6822(88)90622-8. [DOI] [PubMed] [Google Scholar]
- 19.Ishimoto L K, Elisha J, Eiserling F A. Expression and regulation of genes coding for three bacteriophage T4 tube associated proteins. Virology. 1990;175:586–590. doi: 10.1016/0042-6822(90)90446-x. [DOI] [PubMed] [Google Scholar]
- 20.Katsura I. Morphogenesis of bacteriophage lambda tail. Polymorphism in the assembly of the major tail protein. J Mol Biol. 1976;107:307–326. doi: 10.1016/s0022-2836(76)80007-1. [DOI] [PubMed] [Google Scholar]
- 21.Katsura I. Mechanism of length determination in bacteriophage lambda tails. Adv Biophys. 1990;26:1–18. doi: 10.1016/0065-227x(90)90004-d. [DOI] [PubMed] [Google Scholar]
- 22.Katsura I, Tsugita A. Purification and characterization of the major protein and the terminator protein of the bacteriophage λ tail. Virology. 1977;76:129–145. doi: 10.1016/0042-6822(77)90290-2. [DOI] [PubMed] [Google Scholar]
- 23.Kikuchi Y, King J. Genetic control of bacteriophage T4 baseplate morphogenesis. I. Sequential assembly of the major precursor, in vivo and in vitro. J Mol Biol. 1975;99:645–672. doi: 10.1016/s0022-2836(75)80178-1. [DOI] [PubMed] [Google Scholar]
- 24.King J. Assembly of the tail of bacteriophage T4. J Mol Biol. 1968;32:231–262. doi: 10.1016/0022-2836(68)90007-7. [DOI] [PubMed] [Google Scholar]
- 25.King J. Bacteriophage T4 tail assembly: four steps in core formation. J Mol Biol. 1971;58:693–709. doi: 10.1016/0022-2836(71)90034-9. [DOI] [PubMed] [Google Scholar]
- 26.King J, Laemmli U. Bacteriophage T4 tail assembly: structural proteins and their genetic identification. J Mol Biol. 1973;75:315–337. doi: 10.1016/0022-2836(73)90024-7. [DOI] [PubMed] [Google Scholar]
- 27.King J, Mykolajewycz N. Bacteriophage T4 tail assembly: proteins of the sheath, core and baseplate. J Mol Biol. 1973;75:339–358. doi: 10.1016/0022-2836(73)90025-9. [DOI] [PubMed] [Google Scholar]
- 28.Koch T, Lamm N, Rüger W. Sequencing, cloning and overexpression of genes of bacteriophage T4 between map positions 74.325 and 77.184. Nucleic Acids Res. 1989;17:4392. doi: 10.1093/nar/17.11.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kutter E, Stidham T, Guttman B, Kutter E, Batts D, Peterson S, Djavakhishvili T, Arisaka F, Mesyanzhinov V, Rüger W, Mosig G. Genomic map of bacteriophage T4. In: Karam J D, Drake J W, Kreuzer K N, Mosig G, Hall D H, Eiserling F A, Black L W, Spicer E K, Kutter E, Carlson K, Miller E S, editors. Molecular biology of bacteriophage T4. Washington, D.C.: American Society for Microbiology; 1994. pp. 491–519. [Google Scholar]
- 30.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 31.Lengyel J A, Goldstein R N, Marsh M, Calendar R. Structure of the bacteriophage P2 tail. Virology. 1974;62:161–174. doi: 10.1016/0042-6822(74)90312-2. [DOI] [PubMed] [Google Scholar]
- 32.Lipinska B, Krishna Rao A S M, Bolten B M, Balakrishnan R, Goldberg E B. Cloning and identification of bacteriophage T4 gene 2 product gp2 and action of gp2 on infecting DNA in vivo. J Bacteriol. 1989;171:488–497. doi: 10.1128/jb.171.1.488-497.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Messing J, Crea R, Seeburg P H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981;9:309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moody M F. Application of optical diffraction to helical structures in the bacteriophage tail. Phil Trans R Soc Lond B. 1971;261:181–195. doi: 10.1098/rstb.1971.0049. [DOI] [PubMed] [Google Scholar]
- 35.Mosig G, Eiserling F A. Phage T4 structure and metabolism. In: Calendar R, editor. The bacteriophages. Vol. 2. New York, N.Y: Plenum Publishing Corp.; 1988. pp. 521–605. [Google Scholar]
- 36.Parker M L, Eiserling F A. Bacteriophage SP01 structure and morphogenesis. I. Tail structure and length regulation. J Virol. 1983;46:239–249. doi: 10.1128/jvi.46.1.239-249.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poglazov B F. Self-assembly of T-even bacteriophages in morphogenesis. FEBS Symp. 1970;21:181–193. [Google Scholar]
- 38.Poglazov B F, Nikolskaya T I. Self-assembly of the protein of bacteriophage T2 tail cores. J Mol Biol. 1969;43:231–233. doi: 10.1016/0022-2836(69)90094-1. [DOI] [PubMed] [Google Scholar]
- 39.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 40.Sambrook J F, Fan D P, Brenner S. A strong suppressor specific for UGA. Nature. 1967;214:452–453. doi: 10.1038/214452a0. [DOI] [PubMed] [Google Scholar]
- 41.Smith M H. Molecular weights of proteins and some other materials including sedimentation, diffusion and frictional coefficients and partial specific volumes. In: Sober H A, editor. Handbook of biochemistry. Selected data for molecular biology. Cleveland, Ohio: CRC Press, Inc.; 1975. pp. C3–C35. [Google Scholar]
- 42.Strigini P, Gorini L. Ribosomal mutations affecting efficiency of amber suppression. J Mol Biol. 1970;47:517–530. doi: 10.1016/0022-2836(70)90319-0. [DOI] [PubMed] [Google Scholar]
- 43.Studier F W. Use of bacteriophage T7 lysozyme to improve an inducible T7 expression system. J Mol Biol. 1991;219:37–44. doi: 10.1016/0022-2836(91)90855-z. [DOI] [PubMed] [Google Scholar]
- 44.Studier F W, Moffatt B A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 45.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 46.To C M, Kellenberger E, Eisenstark A. Disassembly of T-even bacteriophage into structural parts and subunits. J Mol Biol. 1969;46:493–511. doi: 10.1016/0022-2836(69)90192-2. [DOI] [PubMed] [Google Scholar]
- 47.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tschopp J F, Smith P R. Extra-long T4 tails produced in in vitro conditions. J Mol Biol. 1978;114:281–286. doi: 10.1016/0022-2836(77)90211-x. [DOI] [PubMed] [Google Scholar]
- 49.Velten J, Abelson J. The generation and analysis of clones containing bacteriophage T4 DNA fragments. J Mol Biol. 1980;137:235–248. doi: 10.1016/0022-2836(80)90327-7. [DOI] [PubMed] [Google Scholar]
- 50.Wagenknecht T, Bloomfield V A. In vitro polymerization of bacteriophage T4D tail core subunits. J Mol Biol. 1977;116:347–359. doi: 10.1016/0022-2836(77)90074-2. [DOI] [PubMed] [Google Scholar]
- 51.Wang G R, Vianelli A, Goldberg E B. Bacteriophage T4 self-assembly: in vitro reconstitution of recombinant gp2 into infectious phage. J Bacteriol. 2000;182:672–679. doi: 10.1128/jb.182.3.672-679.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yegian C D, Mueller M, Selzer G, Russo V, Stahl F W. Properties of DNA-delay mutants of bacteriophage T4. Virology. 1971;37:615–623. doi: 10.1016/0042-6822(71)90090-0. [DOI] [PubMed] [Google Scholar]