Abstract
The intestinal tract is protected by epithelium-covering mucus, which is constantly renewed by goblet cells, a specialized type of epithelial cell. Mucus is largely composed of MUC2 mucin, an enormous molecule that poses a high demand on the endoplasmic reticulum (ER) for proper folding and protein assembly, creating a challenge for the secretory machinery in goblet cells. In this issue of the JCI, Grey et al. reveal that the ER resident protein and folding sensor ERN2 (also known as IRE1β) was instrumental for goblet cells to produce sufficient amounts of mucus to form a protective mucus layer. In the absence of ERN2, mucus production was reduced, impairing the mucus barrier, which allowed bacteria to penetrate and cause an epithelial cell stress response. This study emphasizes the importance of a controlled unfolded protein response (UPR) for goblet cell secretion.
Mucus-producing goblet cells
Mucus is vital for the protection of epithelial surfaces in the body. It assists in numerous functions to maintain homeostasis, acting as a selective physical barrier that traps and transports unwanted components. In the colon, mucus forms a barrier both dependent on and impenetrable to bacteria, tightly linking the mucus system with the microbiota (1, 2). At the same time, mucus provides a nutritional source for the microbiota. Diseases such as ulcerative colitis (UC), in which mucus protection fails, result in epithelial cells having increased bacterial exposure (3). In cystic fibrosis, abnormally attached mucus of the small intestine leads to bacterial overgrowth (4, 5), which is similar to the phenotype observed in many lung diseases.
Mucus is produced and secreted by the highly specialized cells of the secretory lineage, the goblet cells. These cells have been considered uninteresting and homogeneous, but recent single-cell information shows dramatic variability (6, 7). Mucins are the main structural element of mucus and they, as well as some of the other abundant mucus proteins, are large proteins that are extensively disulphide stabilized, oligomeric, and highly glycosylated. The mucins are sorted and stored in large granules before being secreted. Producing large quantities of mucins is challenging for the cell and especially the endoplasmic reticulum (ER), which requires specific molecules to cope with folding and forming correct disulphide bonds. This demand makes the goblet cells specifically vulnerable to ER overloading. Healthy cells respond to biosynthetic overload through the ER stress pathway known as the unfolded protein response (UPR), comprising three branches that (a) alleviate protein misfolding, (b) reduce protein synthesis, and (c) enhance unfolded protein degradation (8). If accumulated misfolded protein amounts are large and restoration is not achieved, the cell becomes destined for apoptosis. One of the ER stress branches, as observed in most cells including enterocytes, uses the sensor ERN1 (also called IRE1α), which signals via XBP1 to induce ER-associated degradation (ERAD) and increase expression of ER proteins assisting in folding. In addition, ERAD limits translation by degrading mRNA by regulated ERN1-dependent mRNA decay. Interestingly, all goblet cells have an additional closely related molecule, ERN2 (also called IRE1β), not found in most other cells. Its specific expression indicates a distinct function in goblet cells, likely to handle mucin protein folding. Grey et al. have previously shown that ERN2 binds ERN1 to limit the ER stress responses (9). This observation indicated that ERN2 might regulate ER function in goblet cells.
Goblet cell ER stress and inflammation
In this issue of the JCI, Grey and coauthors have continued to explore the function of ERN2 by using experimental animals (10). They nicely showed that Ern2-knockout mice had increased colon cell proliferation and elongated crypts as signs of mild spontaneous inflammation, which is similar to previous observations in Muc2-deficient mice (11). The goblet cell granule size and the number of filled goblet cells were also reduced with a diminished mucus layer (Figure 1). This finding shows that the mucus layer was less able to keep bacteria at a safe distance, triggering the epithelium to proliferate and the goblet cells to empty. The microbiota was a clear driving force for the pathology, as the germ-free Ern2–/– mice remained essentially identical to wild-type controls. The altered mucus in Ern2–/– animals also selected for less favorable microbiota that were at least partly responsible for the goblet cell and mucus phenotype observed in germ-free animals after microbiota transfer from Ern2–/– mice. Notably, this altered phenotype was reverted by wild-type microbiota, confirming the role for the microbiota in establishing a proper mucus system, as observed previously (2). As could be expected by the weaker mucus protection in the ERN2-deficient animals, these mice were more susceptible to dextran sulfate sodium–induced (DSS-induced) colitis and Citrobacter infection (12). In the absence of ERN2, the expression of Xbp1s was reduced and Xbp1–/– mice showed a phenotype similar to that of Ern2–/– mice, proving that Xbp1 splicing induced by ERN2 was required for normal goblet cell function. Effects of the microbiota on the amount of Xbp1 have also been observed in other studies (10, 13).
Figure 1. A model of ER stress response in goblet cells with and without ERN2.
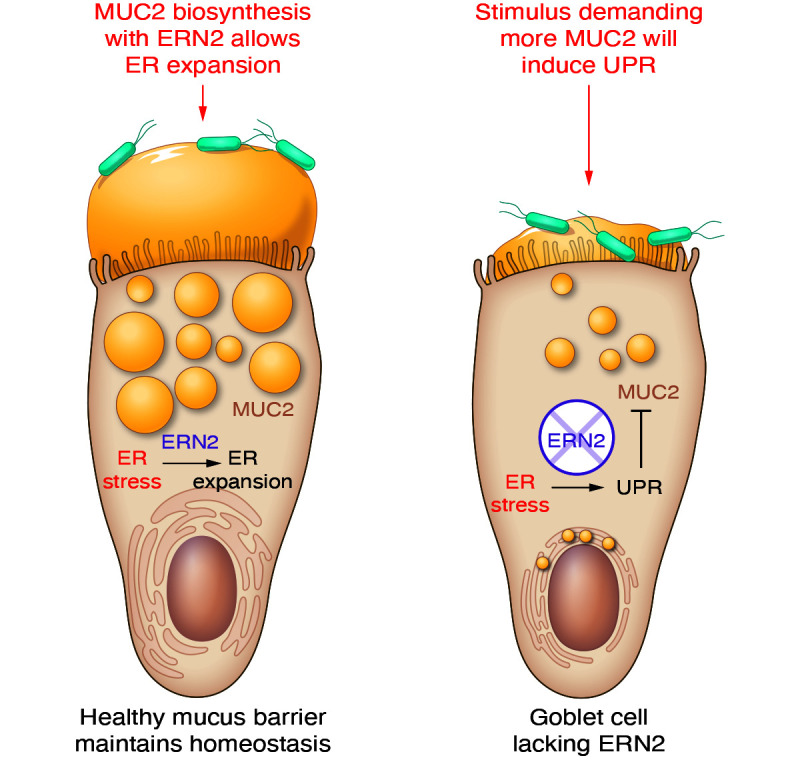
ERN2 is specifically expressed in goblet cells, where it allows for expansion of the ER, which is important for mucus production at homeostasis. In the absence of ERN2, goblet cells fail to produce normal amounts of mucins, resulting in ER mucin accumulation and poor protection of the epithelium. ERN2 controls and dampens the UPR. During enhanced mucus turnover and bacterial stimulation, as in colitis, ER stress and UPR responses are exaggerated and the mucus protection fails.
The ERN2-deficient mice resemble, in many ways, the Winnie mouse model in which misfolded MUC2 induces ER stress, as indicated by elongated crypts, fewer mucus-filled goblet cells, and increased susceptibility to induced colitis (14). Winnie mice also develop microbiota-dependent inflammation preceded by dysbiosis (15, 16). These results indicate that the mucus layer has a central role in selecting bacteria and driving epithelial and immune system responses.
UC
Clinical pathologists often refer to UC as a goblet cell–depleting condition, since there is a decrease in the number of the typical, filled goblet cells. However, this term reflects a misunderstanding, as UC results in enhanced mucus turnover with faster goblet cell emptying and increased mucus secretion, giving the visual impression of fewer goblet cells. Defective mucus generation in Ern2–/– mice and UC patients results in a faster emptying response, especially in the upper crypt, making goblet cells not clearly visible. There is also a loss of specialized sentinel goblet cells, as these cells are ejected after TLR activation (17). Increased mucus turnover places additional pressure on the goblet cells, and accumulation of MUC2 in the ER of goblet cells is often observed in patients with active UC disease (14, 18).
ER functions in goblet cells
The study by Grey et al. (10) shows that forming mature goblet cells capable of producing sufficient amounts of mucus requires a baseline level of UPR with increased levels of chaperones and ER expansion. This process was mediated by the goblet cell–specific ERN2 protein acting on Xbp1. Both ERN2 and ERN1 mediated Xbp1 splicing (10). While ERN2 and ERN1 acted in the same pathway, they were not redundant to each other, indicating that other functions must exist, a conundrum to be studied further. It is also interesting to note that, in addition to the specific and high expression of Ern2 in goblet cells, Xbp1 RNA was also more abundant in goblet cells compared with other epithelial cells, while expression of Ern1 was lower than that of Ern2 (6). The lower endonuclease activity for ERN2 compared with ERN1 and its ability to negatively regulate ERN1 clearly indicate a different and specific mechanism for this protein (9). In addition to the known mechanisms for ERN2, including autophosphorylation and multimerization, there are likely other regulatory functions and unexplored elements.
Conclusion
Goblet cells have a high demand on their biosynthesis machinery and require ERs with a high capacity for protein folding and modification. The current model suggests that goblet cells express specific proteins, including ERN2, to handle a required increased ER protein–folding capacity, which is similar to the ER stress response observed in other cell types, though without triggering the negative effects. The goblet cells require controlled ER stress to produce sufficient levels of mucus. Failure to produce and secrete mucus to form a protective barrier is detrimental, especially since impaired colon epithelial protection also alters the microbial milieu. In an attempt to resolve the imbalance, goblet cells enhance mucus turnover with rapid emptying. Without ERN2, the higher demand for mucus production results in undampened, enhanced ER stress (Figure 1). The specific features of goblet cells and their variability have long been ignored and warrant deeper studies. As ERN2 is an important molecule enriched in these cells, understanding its role in general and in the goblet cell–specific ER function is important for developing therapeutic approaches for inflammatory bowel diseases, especially UC.
Acknowledgments
The work was supported by grants from Vetenskapsrådet and the Knut and Alice Wallenberg Foundations.
Version 1. 09/01/2022
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2022, Johansson et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: J Clin Invest. 2022;132(17):e162030. https://doi.org/10.1172/JCI162030.
References
- 1.Johansson MEV, et al. The inner of the two Muc2 mucin dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A. 2008;105(39):15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johansson MEV, et al. Normalization of the host intestinal mucus systems requires long-term colonization. Cell Host Microbe. 2015;18(5):582–592. doi: 10.1016/j.chom.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johansson MEV, et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2014;213(2):281–291. doi: 10.1136/gutjnl-2012-303207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Lisle RC. Altered transit and bacterial overgrowth in the cystic fibrosis mouse small intestine. Am J Physiol Gastroint Liver Physiol. 2007;293(1):G104–G111. doi: 10.1152/ajpgi.00548.2006. [DOI] [PubMed] [Google Scholar]
- 5.Gustafsson JK, et al. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med. 2012;209(7):1263–1272. doi: 10.1084/jem.20120562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nystrom EEL, et al. An intercrypt subpopulation of goblet cells is essential for colonic mucus barrier function. Science. 2021;372(6539):257. doi: 10.1126/science.abb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burclaff J, et al. A proximal-to-distal survey of healthy adult human small intestine and colon epithelium by single-cell transcriptomics. Cell Mol Gastroenterol Hepatol. 2022;13(5):1554–1589. doi: 10.1016/j.jcmgh.2022.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma X, et al. Intestinal epithelial cell endoplasmic reticulum stress and inflammatory bowel disease pathogenesis: an update review. Front Immunol. 2017;8:1271. doi: 10.3389/fimmu.2017.01271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grey MJ, et al. IRE1b negatively regulates IRE1a signaling in response to endoplasmic reticulum stress. J Cell Biol. 2020;219(2):e201904048. doi: 10.1083/jcb.201904048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grey MJ, et al. The epithelial-specific ER stress sensor ERN2/IRE1β enables host-microbiota crosstalk to affect colon goblet cell development. J Clin Invest. 2022;132(17):e153519. doi: 10.1172/JCI153519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Velcich A, et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295(5560):1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 12.Bergstrom KSB, et al 2010. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010;6(5):e1000902. doi: 10.1371/journal.ppat.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsuru A, et al. Negative feedback by IRE1β optimizes mucin production in goblet cells. Proc Natl Acad Sci U S A. 2013;110(8):2864–2869. doi: 10.1073/pnas.1212484110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heazlewood CK, et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008;5(3):e54. doi: 10.1371/journal.pmed.0050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liso M, et al. A specific mutation in Muc2 determines early dysbiosis in colitis-prone winnie mice. Inflamm Bowel Dis. 2020;26(4):546–556. doi: 10.1093/ibd/izz279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang R, et al. Gut microbiota shape the inflammatory response in mice with an epithelial defect. Gut Microbes. 2021;13(1):1–18. doi: 10.1080/19490976.2021.1887720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Post S, et al. Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis. Gut. 2019;68(12):2142–2151. doi: 10.1136/gutjnl-2018-317571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinoda Y, et al. Immunohistochemical detection of MUC2 mucin core protein in ulcerative colitis. J Clin Lab Anal. 1998;12(3):150–153. doi: 10.1002/(SICI)1098-2825(1998)12:3<150::AID-JCLA4>3.0.CO;2-D. [DOI] [PMC free article] [PubMed] [Google Scholar]


