Abstract
Expression of the Bacillus subtilis glpD gene, which encodes glycerol-3-phosphate (G3P) dehydrogenase, is controlled by termination or antitermination of transcription. The untranslated leader sequence of glpD contains an inverted repeat that gives rise to a transcription terminator. In the presence of G3P, the antiterminator protein GlpP binds to glpD leader mRNA and promotes readthrough of the terminator. Certain mutations in the inverted repeat of the glpD leader result in GlpP-independent, temperature-sensitive (TS) expression of glpD. The TS phenotype is due to temperature-dependent degradation of the glpD mRNA. In the presence of GlpP, the glpD mRNA is stabilized. glpD leader-lacZ fusions were integrated into the chromosomes of B. subtilis and Escherichia coli. Determination of steady-state levels of fusion mRNA in B. subtilis showed that the stability of the fusion mRNA is determined by the glpD leader part. Comparison of steady-state levels and half-lives of glpD leader-lacZ fusion mRNA in B. subtilis and E. coli revealed significant differences. A glpD leader-lacZ fusion transcript that was unstable in B. subtilis was considerably more stable in E. coli. GlpP, which stabilizes the transcript in B. subtilis, did not affect its stability in E. coli. Primer extension analysis showed that the glpD leader-lacZ fusion transcript is processed differently in B. subtilis and in E. coli. The dominating cleavage site in E. coli was barely detectable in B. subtilis. This site was shown to be a target of E. coli RNase III.
The steady-state level of mRNA in a cell is a function of the rate of mRNA synthesis and the rate of its decay. For bacteria, there is a wealth of information on the regulation of mRNA synthesis (see, e.g., reference 27), while much less is known about mechanisms of mRNA decay (5, 6, 35).
Most of our knowledge about bacterial mRNA decay is based on studies of Escherichia coli (26, 34). In a simple model, an initial endoribonucleolytic attack at the 5′ end of an mRNA opens up the molecule for internal downstream cleavages and the fragments generated are subsequently degraded by exoribonucleases (12). The initial cleavage is performed by one of two endoribonucleases, RNase E, encoded by the rne gene (7), or RNase III, encoded by the rnc gene (3, 8). The endonucleolytic activity of RNase E is localized to the N-terminal half of the protein, which, unlike the C-terminal half, is essential for E. coli viability (29, 31). RNase III is primarily involved in maturation of stable RNA but also in degradation of some mRNA species. The hydrolytic exoribonuclease RNase II and the phosphorolytic exoribonuclease polynucleotide phosphorylase (PNPase) are important for the final (3′-to-5′) degradation of an mRNA to mono- and oligonucleotides. For a few E. coli mRNA species, binding of specific proteins has been found to have a decisive influence on mRNA half-life (28, 43).
Much less is known about mRNA degradation in Bacillus subtilis. In several bacterial species, but not B. subtilis, sequence homologues to the N-terminal part of RNase E have been found (24, 25). E. coli RNase III has a homologue in B. subtilis called Bs-RNase III which has been shown to cleave rRNA in an E. coli Rnc mutant (45). However, E. coli RNase III cannot cleave a B. subtilis phage SP82 mRNA species at a site which is cleaved by Bs-RNase III (33). RNase III may be an essential enzyme in B. subtilis (36), but it is not in E. coli (3). PNPase accounts for more than 90% of the exoribonuclease activity in B. subtilis cell extracts. It is unclear if the cells also contain an enzyme related to RNase II (10). However, the gene for PNPase can be deleted in B. subtilis with little effect on overall cell physiology or the half-life of bulk mRNA (44).
Transcription of the B. subtilis glpD gene (and other glp genes) is controlled by termination or antitermination of transcription at an inverted repeat in the 5′ untranslated leader of glpD mRNA (21, 22, 38). We have isolated a number of mutants carrying mutations in the glpD leader which allow increased transcription through the inverted-repeat region. These mutants have enhanced levels of the glpD gene product and grow on glycerol as a sole carbon and energy source in the absence of an activated form of the antiterminator protein GlpP. Some of the corresponding mutants are temperature sensitive (TS) for growth on glycerol. This phenotype has been shown to be due to an increased, temperature-dependent rate of degradation of glpD mRNA. The TS phenotype is suppressed by the GlpP protein in the presence of glycerol-3-phosphate (G3P), which is the inducer of the glp regulon (17). It is possible that the wild-type glpD transcript is also TS in the absence of GlpP and G3P. However, we have not yet succeeded in producing sufficient amounts of wild-type glpD transcript under noninducing conditions to test this possibility. With this caveat in mind, we will refer to the class of glpD leader mutations described above as TS.
In the present work we have studied the decay of B. subtilis wild-type and TS glpD leader transcripts in B. subtilis and E. coli. Fusions were constructed between B. subtilis wild-type and TS glpD leaders and the E. coli lacZ gene, and the fusions were integrated into the chromosomes of B. subtilis and E. coli. In B. subtilis we have found that the stability of the fusion transcript is determined by the glpD leader sequence, i.e., in the absence of GlpP, a TS leader causes rapid and temperature-dependent degradation of the fusion transcript. In E. coli the TS fusion transcript is much more stable and decays at the same rate as the wild-type fusion transcript. Additionally, GlpP does not influence the decay of the fusion transcripts in E. coli although it does function as a specific antiterminator protein in the species (16). Finally, we show that the cleavage patterns at the 5′ ends of the fusion transcripts are distinctly different in E. coli and B. subtilis. The most striking difference is that the major cleavage product in E. coli is barely detectable in B. subtilis. This cleavage product is missing in an E. coli Rnc mutant.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this work are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Characteristics | Source or reference |
|---|---|---|
| B. subtilis | ||
| BR95 | trpC2 pheA1 ilvC1 | Our collection |
| LUR252 | trpC2 ilvC1 glpP12 glpD52 (insertion of an extra GC in the glpD leader) | 17 |
| LUZ9595 | BR95 with insertion of a wild-type glpD leader-lacZ fusion into amyE; Kmr | 18 |
| LUZ1212 | LUR252 with insertion of a LUR252 glpD leader-lacZ fusion into amyE; Kmr | This work |
| E. coli | ||
| MC4100 | F−araD139 Δ(argF-lac)U169 rpsL150 relA1 flbB5301 deoC1 ptsF25 rbsR | 14 |
| MC4100D1 | MC4100 [λφ(wild-type glpD leader-lacZ fusion)] | 16 |
| MC4100D2 | MC4100 [λφ(LUR252 glpD leader-lacZ fusion)] | This work |
| BL321 | F−thi-1 argH1 gal-6 lacY1 mtl-2 xyl-7 malA1 ara-13 str-9 tonA2 lambdar supE44 rnc-105 | 40 |
| BL322 | As BL321 but rnc+ | 40 |
| Plasmids | ||
| pHP13 | Cmr Emr | 19 |
| pPHis1 | Derivative of pHP13 carrying a glpP gene coding for GlpP with six extra carboxy-terminal histidine residues | 18 |
| pMD433 | ΔamyE::′lacZ; Kmr Apr | 9 |
| pLUM1041 | Derivative of pMD433; ΔamyE::wild-type glpD leader-lacZ fusion | 18 |
| pLUM1043 | Derivative of pMD433; ΔamyE::LUR252 glpD leader-lacZ fusion | This work |
Growth of bacteria for extraction of RNA.
B. subtilis was grown in minimal salts (1) with 0.5% casein hydrolysate and required amino acids (40 mg liter−1) with shaking at 200 rpm. The bacteria were grown at different temperatures to an optical density at 600 nm (OD600) of 0.5. The cultures were then induced with glycerol (1.5 g liter−1) for 15 min. E. coli was grown in Luria broth containing 40 mM G3P with shaking at 200 rpm. The bacteria were grown at different temperatures to an OD600 of 0.5.
Samples were taken for RNA extraction, or the cells were incubated with rifampin (B. subtilis, 100 mg liter−1; E. coli, 500 mg liter−1) and nalidixic acid (E. coli, 20 mg liter−1) for various times before samples were taken. The 0-min samples were taken 2 min after addition of the antibiotics.
Construction of strains.
B. subtilis LUZ1212 was obtained by transforming B. subtilis LUR252 with pLUM1043 and isolating a kanamycin-resistant, amylase-negative transformant according to the protocol for the isolation of B. subtilis LUZ9595 (18). Plasmid pLUM1043 was constructed in the same way as pLUM1041 with chromosomal DNA from LUR252 as a template for PCR (18).
E. coli MC4100D2 was constructed in analogy with E. coli MC4100D1 as described by Glatz et al. (16), with chromosomal DNA from B. subtilis LUR252 as a template for PCR.
DNA and RNA techniques.
PCR and DNA cloning techniques were applied according to standard protocols (39). Total RNA from B. subtilis was extracted as described by Resnekov et al. (37). Total RNA from E. coli was extracted as described by Emory et al. (13). Electrophoresis of RNA for Northern blots was done as described by Thomas (42), and the RNA was then blotted onto Hybond-N filters (Amersham). A single-stranded (ss) DNA probe for Northern blots was generated by ssPCR with primer GlpDBamII (18), cold d(A, G, T)TP, and [α32P]dCTP (Amersham). To generate the template for the ssPCR, a fragment was amplified by PCR from B. subtilis LUR252 with primers GlpDBamI (18) and GlpDBamII. The PCR fragment was cleaved with AvaII, and a 215-bp fragment containing part of the glpD leader together with the first 33 codons of glpD was isolated for use as a template. After hybridization, the radioactivities of the bands were quantitated with a PhosphorImager (Molecular Dynamics). Primer extension analysis was performed according to the method of Ayer and Dynan (2). The primer used was complementary to positions +111 to +130 of the glpD leader (5′-ATTGATGATTCATCATTACG-3′).
RESULTS
The glpD leader is a stability determinant of glpD leader-lacZ fusion mRNA.
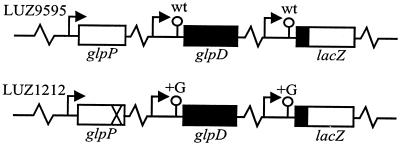
We have previously shown that the 5′ untranslated leader of the B. subtilis glpD transcript affects its half-life (17). In order to determine whether interactions between the leader and other parts of the glpD transcript are important for stability, the following experiments were done. Gene fusions were made in which a DNA fragment of about 400 bp containing the 5′ part of the glpD region, including the promoter, the leader sequence, and the first 33 codons, was coupled in frame to E. coli lacZ. Two fusions were made, one with the wild-type glpD leader sequence from B. subtilis BR95 and the other with the mutant glpD leader sequence from B. subtilis LUR252. The mutant leader sequence has an extra GC pair in the inverted repeat, i.e., the leader RNA has an extra G, which leads to increased constitutive (GlpP-independent) expression of the glpD gene. The glpD transcripts produced in the absence of GlpP are TS. The fusions were inserted in single copies into the amyE gene of B. subtilis, the wild-type glpD leader fusion was inserted into BR95, and the mutant glpD leader fusion was inserted into LUR252. A schematic description of the resulting strains, LUZ9595 and LUZ1212, is given in Fig. 1.
FIG. 1.
Schematic representation of B. subtilis LUZ9595 and LUZ1212. The glpD promoter and leader from BR95 and LUR252 were amplified by PCR and cloned in frame with lacZ in pMD433. The glpD leader-lacZ fusions were integrated into the chromosome at the amyE locus in the cognate strain.  , promoter; ○, glpD leader with inverted repeat; X, glpP12 mutation; wt, wild type; +G, insertion of an extra GC pair in the inverted repeat;
, promoter; ○, glpD leader with inverted repeat; X, glpP12 mutation; wt, wild type; +G, insertion of an extra GC pair in the inverted repeat;  , intervening chromosomal DNA.
, intervening chromosomal DNA.
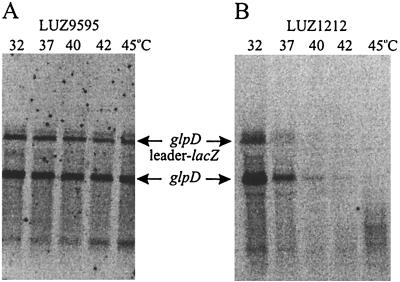
The steady-state levels of glpD mRNA and glpD leader-lacZ fusion mRNA in LUZ9595 and LUZ1212 were measured under inducing conditions and at different temperatures ranging from 32 to 45°C. The mRNA was analyzed in Northern blots with a probe specific for the glpD leader. As can be seen in Fig. 2, both a glpD and a glpD leader-lacZ fusion transcript are detected at all temperatures in induced LUZ9595, and the amounts are similar at all temperatures. The smaller band, which increases in intensity with temperature, represents a truncated fusion transcript that also hybridizes with a lacZ-specific probe (data not shown). In LUZ1212, the steady-state levels of glpD and glpD leader-lacZ mRNA rapidly decrease with increasing growth temperature and transcripts are not detectable above 40°C. After the membranes had been probed with the glpD probe, they were stripped and reprobed with a DNA fragment specific for the sdhC gene (32). The steady-state levels of sdhC mRNA were essentially the same at all temperatures in both strains (data not shown). The β-galactosidase and G3P dehydrogenase (GlpD) activities of LUZ9595 and LUZ1212 measured under inducing conditions at 32 and 45°C correlated well with the corresponding mRNA levels (15). From these results we conclude that the glpD leader is a major stability determinant for both the glpD and the glpD leader-lacZ fusion transcripts in B. subtilis.
FIG. 2.
Northern blots showing steady-state levels of glpD and glpD leader-lacZ mRNA in B. subtilis LUZ9595 (wild-type glpD leader) (A) and B. subtilis LUZ1212 (mutant glpD leader) (B). Total RNA was extracted from cells grown and induced at the temperatures indicated. The lanes contained 5 μg of LUZ9595 RNA and 20 μg of LUZ1212 RNA.
A B. subtilis glpD leader transcript is more stable in E. coli than in B. subtilis.
More is known about mRNA degradation in E. coli than in any other bacterium, and mutants affected in different components of the mRNA degradation machinery are available (26). We next wanted to take advantage of E. coli to further analyze the decay of B. subtilis wild-type and TS glpD leader-lacZ fusion transcripts. It should be emphasized that GlpP also promotes antitermination of transcription at the glpD leader in E. coli (16). The inverted repeat in the glpD leader sequence is, however, a less efficient stop signal in E. coli than in B. subtilis, as evidenced by a high background of expression of glpD leader-lacZ fusions in E. coli. This difference makes possible an analysis of wild-type glpD leader-lacZ fusion transcripts in E. coli in the absence of GlpP. In vitro runoff transcriptional analysis has shown that E. coli sigma-70 RNA polymerase passes through the inverted repeat unaided, whereas no readthrough was detected with B. subtilis sigma-A RNA polymerase holoenzyme (15).
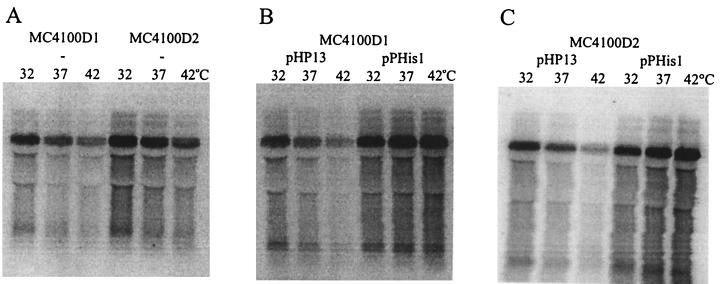
In a previous report (16), the wild-type glpD leader-lacZ fusion was integrated into the chromosome of E. coli to give strain MC4100D1, and similarly, the mutant glpD leader-lacZ fusion was now integrated to give strain MC4100D2. Plasmids pHP13 and pPHis1 were then introduced into MC4100D1 and MC4100D2. pHP13 is a B. subtilis-E. coli shuttle plasmid, and pPHis1 is a derivative which carries a gene coding for a His-tagged and biologically active derivative of GlpP (18). The relative steady-state levels of glpD leader-lacZ mRNA were measured in MC4100D1 and MC4100D2 grown at 32, 37, and 42°C and in the presence (pPHis1) or absence (pHP13) of GlpP. The resulting Northern blots are shown in Fig. 3. The relative steady-state level of the fusion transcript at 32°C was assigned an arbitrary value of 1 for each strain. The steady-state levels at the other temperatures were then calculated relative to the value at 32°C. By dividing the values for the MC4100D1 strains with the values for the MC4100D2 strains at each temperature, we obtained a comparative measure of the temperature stability of the two fusion transcripts (Table 2). These experiments demonstrate that there is no temperature-dependent difference between the steady-state amounts of the glpD leader-lacZ fusion transcripts in MC4100D1 and MC4100D2. The steady-state amounts of the transcripts increase in the presence of GlpP. Since it is shown below that GlpP does not increase the relative stability of the transcripts, this increase should be due to the antitermination effect of GlpP (16). For unknown reasons, this effect is more pronounced at higher temperatures.
FIG. 3.
Northern blots showing steady-state levels of glpD leader-lacZ mRNA in E. coli MC4100D1 (wild-type glpD leader) and MC4100D2 (mutant glpD leader) carrying no plasmid (−), pHP13, or pPHis1. Total RNA was extracted from cells grown at the temperatures indicated. The lanes contained 20 μg of RNA, except the MC4100D1 plus pPHis lanes, which contained 10 μg of RNA.
TABLE 2.
Comparison of steady-state levels of wild-type and mutant glpD leader-lacZ mRNA in E. coli MC4100D1 and MC4100D2 at different temperaturesa
| Plasmid | MC4100D1/MC4100D2 ratio
|
||
|---|---|---|---|
| 32°C | 37°C | 42°C | |
| None | 1 | 1.0 | 1.1 |
| pHP13 | 1 | 1.0 | 1.1 |
| pPHis1 | 1 | 1.1 | 0.8 |
Relative amounts of mRNA were calculated from Fig. 3.
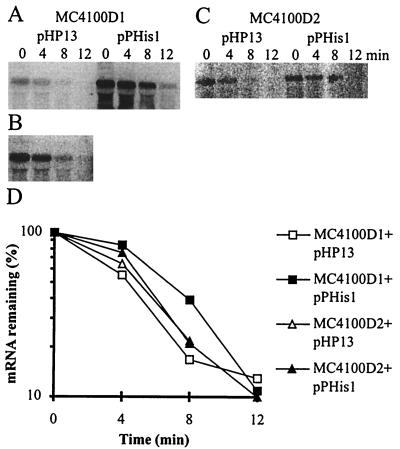
To confirm the above-mentioned results, the half-lives of the two fusion transcripts were measured at 42°C in the presence and absence of GlpP (Fig. 4). Linear regression analysis gave a half-life of 3 to 4 min in all cases. Importantly, the experiments show that the mutant glpD leader-lacZ fusion transcript decays much more slowly in E. coli than in B. subtilis, where a transcript from the mutant glpD leader has a half-life of about 1 min at 32°C and less than 20 s at 45°C (17). Furthermore, the presence of GlpP does not increase the stability of the transcript as it does in B. subtilis.
FIG. 4.
Northern blots showing degradation of wild-type glpD leader-lacZ mRNA in E. coli MC4100D1 carrying pHP13 or pPHis1 (A), wild-type glpD leader-lacZ mRNA in E. coli MC4100D1 carrying pHP13 (overexposed film) (B), or mutant glpD leader-lacZ mRNA in E. coli MC4100D2 carrying pHP13 or pPHis1 (C). (D) Half-life plots. The cells were grown at 42°C, and total RNA was extracted at 0, 4, 8, and 12 min after the addition of rifampin and nalidixic acid. The lanes contained the following amounts of RNA: MC4100D1 plus pHP13, 20 μg; MC4100D1 plus pPHis1, 10 μg; MC4100D2 plus pHP13, 40 μg; MC4100D2 plus pPHis1, 5 μg.
A glpD leader transcript is differently processed in B. subtilis and E. coli.
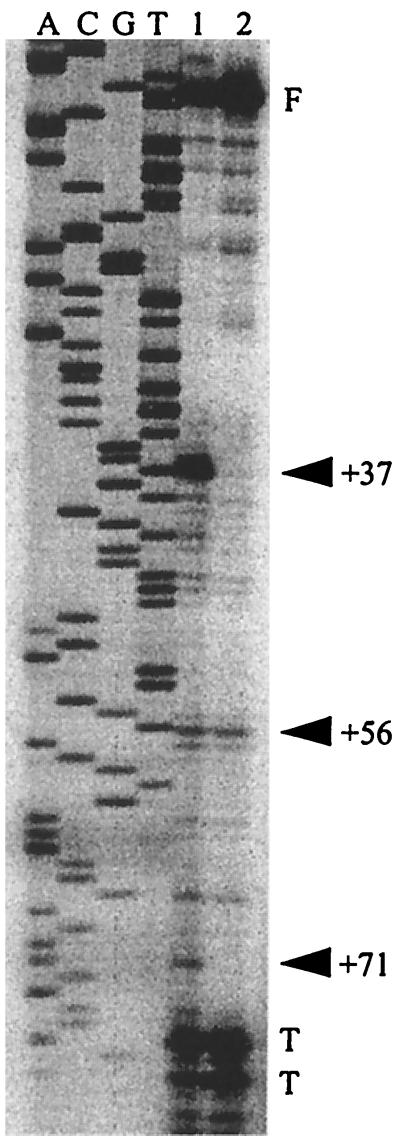
The previous experiments showed that a glpD leader transcript which is TS in B. subtilis is much more stable in E. coli. The following experiments were done to investigate whether this reflects different processing of the 5′ region of the transcript in the two bacteria. RNA was extracted from B. subtilis LUZ1212 and E. coli MC4100D2, both having in their chromosomes the mutant glpD leader-lacZ fusion and carrying pHP13 or pPHis1. B. subtilis was grown at 45°C and E. coli at 42°C. RNA samples were taken immediately before the addition of rifampin (B. subtilis) or rifampin and nalidixic acid (E. coli) and at various times thereafter. Primer extension products obtained with RNA from each sample were then characterized. The primer used is complementary to a region just downstream of the inverted repeat of the glpD leader. Very different patterns of primer extension products were obtained from the two bacteria (Fig. 5A and B). The most prominent band in E. coli (MC4100D2) is at position +37. This band is barely detectable in B. subtilis (LUZ1212 plus pPHis1). It was not possible to identify breakdown products from B. subtilis in the absence of GlpP (LUZ1212 plus pHP13) due to the small amounts of fusion mRNA obtained. The bands obtained with E. coli have higher intensities in the presence of GlpP (MC4100D2 plus pPHis1), but otherwise the pattern is not different from that seen in the absence of GlpP (MC4100D2 plus pHP13). Besides the +37 band, many less prominent bands are seen in E. coli, while only a few are seen in B. subtilis. Two bands, +53 and +57, are clearly seen in both bacteria. Figure 5C shows the results obtained with wild-type glpD leader-lacZ mRNA from E. coli MC4100D1 and MC4100 carrying pLUM1041, which contains a wild-type glpD leader-lacZ fusion. The patterns of primer extension products, including the +37 band, are similar to those of mutant glpD leader mRNA from E. coli MC4100D2. However, the +53 band is not obtained with wild-type glpD leader mRNA in E. coli. We will return to this in the discussion. It should be noted that the +56 and +71 bands in the wild-type glpD leader mRNA correspond to the +57 and +72 bands in the mutant glpD leader mRNA due to the extra G in the latter. In Fig. 5C, it is also seen that, similar to what was found with mutant glpD leader mRNA, the cleavage pattern of wild-type glpD leader mRNA in E. coli is not affected by GlpP. Figure 6 shows the predicted secondary structures of wild-type and mutant glpD leader mRNAs, with cleavage sites +37, +53, and +56-57 indicated.
FIG. 5.
(A) Primer extension analysis of 5′ end points in glpD leader mRNA in E. coli MC4100D2 and B. subtilis LUZ1212 carrying pHP13 or pPHis1. E. coli was grown at 42°C, and B. subtilis was grown and induced at 45°C. Total RNA was extracted from samples taken immediately before the addition of rifampin (B. subtilis) or rifampin and nalidixic acid (E. coli) (st) and at various times thereafter. The 0-min (0′) samples were taken 2 min after the addition of the antibiotics. The solid arrowheads indicate possible endonucleolytic cleavage sites in E. coli, and the open arrowheads indicate cleavage sites in B. subtilis. F indicates full-length transcripts, and T indicates fragments caused by primer extension termination at secondary structures in the base of the terminator. (B) A longer exposure of the lane containing steady-state RNA from LUZ1212 plus pPHis1. (C) Primer extension analysis of 5′ end points in glpD leader mRNA in E. coli. Lane 1, MC4100 carrying pLUM1041 (contains a wild-type glpD leader-lacZ fusion); lane 2, MC4100D1 carrying pHP13; lane 3, MC4100D1 carrying pPHis1. Total RNA was extracted from cells grown at 42°C.
FIG. 6.
(A) Computer-predicted folding of the first 84 nucleotides (46) of wild-type glpD leader mRNA. The arrowheads indicate the +37 and +56 cleavage sites in E. coli. T indicates sites of primer extension termination caused by secondary structures. (B) Predicted folding of loop III of mutant glpD leader mRNA. The arrowheads indicate the +53 and +57 cleavage sites, which are seen in both B. subtilis and E. coli.
In a control experiment, glpD leader mRNA produced in vitro was used as a template for primer extension. The major bands found were a full-length transcript and some shorter products representing a stop at the 3′ end of the stem-loop (data not shown). Thus, we can conclude that degradation intermediates from the 5′ end of the glpD leader-lacZ fusion transcript are very different in B. subtilis and in E. coli.
To examine the possibility that the +37 fragment in E. coli is produced from a promoter downstream of the glpD promoter, we made a deletion starting at the 5′ end of the DNA fragment containing the glpD promoter and leader sequence and ending at position −6. The deletion caused the fusion transcript to disappear, indicating that no additional promoter is present downstream of the glpD promoter.
The +37 cleavage product is missing in an E. coli Rnc mutant.
Next, we investigated whether either of the two major endoribonucleases of E. coli is responsible for cleaving at +37 in glpD leader mRNA. Plasmid pLUM1041 was introduced into an E. coli TS Rne mutant and an E. coli Rnc mutant. The Rne mutant was grown at 32°C and shifted to 45°C for 30 min; the Rnc mutant was grown at 37°C. Total RNA was extracted, and primer extension products were characterized. The result with the Rnc mutant is shown in Fig. 7, where it is seen that the +37 band is missing, which implies that this band is generated by the action of RNase III. A band of much lower intensity at +71 in the wild type is also missing in the mutant. The RNase E-deficient mutant gave the same pattern of primer extension products as the wild type (data not shown).
FIG. 7.
Primer extension analysis of 5′ end points in wild-type glpD leader mRNA in E. coli carrying pLUM1041 (containing a wild-type glpD leader-lacZ fusion). Lane 1, BL322 (wild type); lane 2, BL321 (RNase III deficient). Total RNA was extracted from cells grown at 42°C. The symbols are defined in the legend to Fig. 5.
DISCUSSION
There exists considerable experimental evidence that the 5′ end of an mRNA molecule is an important stability determinant in both B. subtilis and E. coli (4, 11, 13, 20, 30, 32, 41). However, the structures or conditions at the 5′ end which influence mRNA stability may not always be the same in the two bacteria. For example, ribosome-binding sites, whether coupled to translation or not, can stabilize a B. subtilis transcript but not an E. coli transcript (23). The present experiments demonstrate that the glpD leader sequence determines the steady-state amounts of a TS glpD leader-lacZ fusion transcript in B. subtilis. Thus, the B. subtilis glpD leader contains the major stability determinant for the corresponding mRNA. The TS fusion transcript is about 10-fold more stable in E. coli than in B. subtilis, indicating different degradation pathways in the two bacteria.
Different processing of the glpD leader-lacZ fusion transcripts was reflected in the cleavage patterns obtained from the 5′ ends of the transcripts. Most striking is the fact that the major cleavage product at +37 in E. coli was barely seen in B. subtilis. The +37 fragment was absent in an E. coli Rnc mutant, implying that it results from cleavage by RNase III. When the patterns of primer extension products are further compared, some additional points can be made. GlpP has no apparent effect on the patterns in E. coli (Fig. 5A and C). A comparison of the cleavage pattern of the mutant glpD leader (Fig. 5A) with that of the wild-type glpD leader in E. coli (Fig. 5C) shows that the +56-57 band is present in both while the +53 band is missing in the latter. We recall that +53 and +57 bands in the mutant leader correspond to +52 and +56 bands in the wild-type leader. Also, in B. subtilis, the mutant leader gives rise to +53 and +57 bands (Fig. 5A and B) whereas only the +56 band is obtained with the wild-type leader (data not shown). We suggest that expansion of loop III due to the G insertion in the mutant leader (Fig. 6) increases the probability for endoribonuclease cleavage between U and A at +53 in the first part of the loop. Loop III thus seems to be a target for endoribonucleases of similar specificities in the two bacteria. It has been shown for a B. subtilis phage SP82 transcript that Bs-RNase III will cleavage in a bulge containing the sequence CAUG (33). We note that the same sequence is found at the cleavage site +57 in the loop of glpD leader mRNA.
Our knowledge of mRNA turnover in B. subtilis and of RNases as well as other proteins involved is quite limited. The fact that E. coli is often taken as the paradigm for mRNA decay in bacteria mainly reflects a lack of data from other species. We therefore thought that a comparison of the decay of an mRNA in B. subtilis and E. coli should provide valuable information. Our data on the stability and processing of glpD leader-lacZ fusion transcripts point to important differences in the mechanisms of mRNA decay in the two bacteria. That such differences can exist should be taken into account in comparative studies of gene control in different bacteria. We find it particularly interesting that RNase III appears to generate the major cleavage product in E. coli, a product which can hardly be detected in B. subtilis. This raises questions about the roles and substrate specificities of RNase III and its homologue in B. subtilis.
ACKNOWLEDGMENTS
We thank Lars Rutberg for valuable discussions, Lars Hederstedt, Charles Kurland, and Lars Rutberg for critically reading the manuscript, and Bernt Eric Uhlin for sending the RNase-deficient E. coli mutants.
This project was supported by grants from the Swedish Medical Research Council and the Emil and Wera Cornell Foundation.
REFERENCES
- 1.Anagnostopoulos C, Spizizen J. Requirements for transformation in Bacillus subtilis. J Bacteriol. 1961;81:741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayer D E, Dynan W S. Simian virus 40 major late promoter: a novel tripartite structure that includes intragenic sequences. Mol Cell Biol. 1988;8:2021–2033. doi: 10.1128/mcb.8.5.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babitske P, Granger L, Kushner S R. Analysis of mRNA decay and rRNA processing in Escherichia coli multiple mutants carrying a deletion in RNase III. J Bacteriol. 1993;175:229–239. doi: 10.1128/jb.175.1.229-239.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bechhofer D. 5′ mRNA stabilizers. In: Belasco J G, Brawerman G, editors. Control of messenger RNA stability. New York, N.Y: Academic Press; 1993. pp. 31–52. [Google Scholar]
- 5.Carpousis A J, Vanzo N F, Raynal L C. mRNA degradation, a tale of polyA and multiprotein machines. Trends Genet. 1999;15:24–28. doi: 10.1016/s0168-9525(98)01627-8. [DOI] [PubMed] [Google Scholar]
- 6.Coburn G A, Mackie G A. Degradation of mRNA in Escherichia coli: an old problem with some new twists. Prog Nucleic Acid Res Mol Biol. 1999;62:55–108. doi: 10.1016/s0079-6603(08)60505-x. [DOI] [PubMed] [Google Scholar]
- 7.Cohen S N, McDowall K J. RNase E: still a wonderfully mysterious enzyme. Mol Microbiol. 1997;32:1099–1106. doi: 10.1111/j.1365-2958.1997.tb02593.x. [DOI] [PubMed] [Google Scholar]
- 8.Court D. RNA processing and degradation by RNase III. In: Belasco J G, Brawerman G, editors. Control of messenger RNA stability. New York, N.Y: Academic Press; 1993. pp. 71–116. [Google Scholar]
- 9.Dahl M K, Meinhof C-G. A series of integrative plasmids for Bacillus subtilis containing unique cloning sites in all three open reading frames for translational lacZ fusions. Gene. 1994;145:151–152. doi: 10.1016/0378-1119(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 10.Deutscher M P, Reuven N B. Enzymatic basis for hydrolytic versus phosphorolytic mRNA degradation in Escherichia coli and Bacillus subtilis. Proc Natl Acad Sci USA. 1991;88:3277–3280. doi: 10.1073/pnas.88.8.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiMari J F, Bechhofer D H. Initiation of mRNA decay in Bacillus subtilis. Mol Microbiol. 1993;7:705–717. doi: 10.1111/j.1365-2958.1993.tb01161.x. [DOI] [PubMed] [Google Scholar]
- 12.Donovan W P, Kushner S R. Polynucleotide phosphorylase and ribonuclease II are required for cell viability and mRNA turnover in Escherichia coli K12. Proc Natl Acad Sci USA. 1986;83:120–124. doi: 10.1073/pnas.83.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emory S A, Bouvet P, Belasco J G. A 5′ terminal stem-loop structure can stabilize mRNA in Escherichia coli. Genes Dev. 1992;6:135–148. doi: 10.1101/gad.6.1.135. [DOI] [PubMed] [Google Scholar]
- 14.Farewell A, Diez A A, DiRusso C C, Nyström T. Role of Escherichia coli FadR regulator in stasis survival and growth phase-dependent expression of the uspA, fad, and fab genes. J Bacteriol. 1996;178:6443–6450. doi: 10.1128/jb.178.22.6443-6450.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glatz E. Ph.D. thesis. Lund, Sweden: Lund University; 1998. [Google Scholar]
- 16.Glatz E, Farewell A, Rutberg B. The Bacillus subtilis glpD leader and antitermination protein GlpP provide a target for glucose repression in Escherichia coli. FEMS Microbiol Lett. 1998;162:93–96. doi: 10.1111/j.1574-6968.1998.tb12983.x. [DOI] [PubMed] [Google Scholar]
- 17.Glatz E, Nilsson R-P, Rutberg L, Rutberg B. A dual role for the Bacillus subtilis glpD leader and the GlpP protein in the regulated expression of glpD: antitermination and control of mRNA stability. Mol Microbiol. 1996;19:319–328. doi: 10.1046/j.1365-2958.1996.376903.x. [DOI] [PubMed] [Google Scholar]
- 18.Glatz E, Persson M, Rutberg B. Antiterminator protein GlpP of Bacillus subtilis binds to glpD leader mRNA. Microbiology. 1998;144:449–456. doi: 10.1099/00221287-144-2-449. [DOI] [PubMed] [Google Scholar]
- 19.Haima P, Bron S, Venema G. The effect of restriction on shotgun cloning in Bacillus subtilis Marburg. Mol Gen Genet. 1987;209:335–342. doi: 10.1007/BF00329663. [DOI] [PubMed] [Google Scholar]
- 20.Hansen M J, Chen L-H, Fejzo L S, Belasco J G. The ompA 5′ untranslated region impedes a major pathway for mRNA degradation in Escherichia coli. Mol Microbiol. 1994;12:707–716. doi: 10.1111/j.1365-2958.1994.tb01058.x. [DOI] [PubMed] [Google Scholar]
- 21.Holmberg C, Rutberg B. Expression of the gene encoding glycerol-3-phosphate dehydrogenase (glpD) in Bacillus subtilis is controlled by antitermination. Mol Microbiol. 1991;5:2891–2900. doi: 10.1111/j.1365-2958.1991.tb01849.x. [DOI] [PubMed] [Google Scholar]
- 22.Holmberg C, Rutberg L. An inverted repeat preceding the Bacillus subtilis glpD gene is a conditional terminator of transcription. Mol Microbiol. 1992;6:2931–2938. doi: 10.1111/j.1365-2958.1992.tb01752.x. [DOI] [PubMed] [Google Scholar]
- 23.Joyce S A, Dreyfus M. In the absence of translation, RNase E can bypass 5′ mRNA stabilizers in Escherichia coli. J Mol Biol. 1998;282:241–254. doi: 10.1006/jmbi.1998.2027. [DOI] [PubMed] [Google Scholar]
- 24.Kaberdin V R, Miczak A, Jacobsen J S, Lin-Chao S, McDowall K J, von Gabain A. The endoribonucleolytic N-terminal half of Escherichia coli RNase E is evolutionarily conserved in Synechocystis sp. and other bacteria but not the C-terminal half, which is sufficient for degradasome assembly. Proc Natl Acad Sci USA. 1998;95:11637–11642. doi: 10.1073/pnas.95.20.11637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunst F, Ogasawara N, Moszer I, et al. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 26.Kushner S R. mRNA decay. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C.: ASM Press; 1996. pp. 849–860. [Google Scholar]
- 27.Lin E C C, Lynch A S, editors. Regulation of gene expression in Escherichia coli. New York, N.Y: Chapman and Hall; 1996. [Google Scholar]
- 28.Liu M Y, Romeo T. The global regulator CsrA of Escherichia coli is a specific mRNA binding protein. J Bacteriol. 1997;179:4639–4642. doi: 10.1128/jb.179.14.4639-4642.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez P J, Marchand I, Joyce S A, Dreyfus M. The C-terminal half of RNase E which organizes the Escherichia coli degradasome participates in mRNA degradation but not rRNA processing in vivo. Mol Microbiol. 1999;33:188–199. doi: 10.1046/j.1365-2958.1999.01465.x. [DOI] [PubMed] [Google Scholar]
- 30.Mackie G A, Generaux J L, Masterman S K. Modulation of the activity of RNase E in vitro by RNA sequences and secondary structure 5′ to cleavage sites. J Biol Chem. 1997;272:609–616. [PubMed] [Google Scholar]
- 31.McDowall K J, Cohen S N. The N-terminal domain of the rne gene product has RNase E activity and is non-overlapping with the arginine-rich RNA-binding site. J Mol Biol. 1996;255:349–355. doi: 10.1006/jmbi.1996.0027. [DOI] [PubMed] [Google Scholar]
- 32.Melin L, Fridén H, Dehlin E, Rutberg L, von Gabain A. The importance of the 5′-region in regulating the stability of sdh mRNA in Bacillus subtilis. Mol Microbiol. 1990;4:1881–1889. doi: 10.1111/j.1365-2958.1990.tb02037.x. [DOI] [PubMed] [Google Scholar]
- 33.Mitra S, Bechhofer D H. Substrate specificity of an RNase III-like activity from Bacillus subtilis. J Biol Chem. 1994;269:31450–31456. [PubMed] [Google Scholar]
- 34.Nicholson A W. Escherichia coli ribonucleases: paradigms for understanding cellular RNA metabolism and regulation. In: D'Alessio G, Riordan J F, editors. Ribonucleases, structures and functions. New York, N.Y: Academic Press; 1997. pp. 1–49. [Google Scholar]
- 35.Nierlich D P, Murakawa G J. The decay of bacterial messenger RNA. Prog Nucleic Acid Res Mol Biol. 1996;52:153–217. doi: 10.1016/s0079-6603(08)60967-8. [DOI] [PubMed] [Google Scholar]
- 36.Oguro A, Kakeshita H, Nakamura K, Yamane K, Wang W, Bechhofer D H. Bacillus subtilis RNase III cleaves both 5′- and 3′-sites of the small cytoplasmic RNA precursor. J Biol Chem. 1998;273:19542–19547. doi: 10.1074/jbc.273.31.19542. [DOI] [PubMed] [Google Scholar]
- 37.Resnekov O, Rutberg L, von Gabain A. Changes in the stability of specific mRNA species in response to growth stage in Bacillus subtilis. Proc Natl Acad Sci USA. 1990;87:8355–8359. doi: 10.1073/pnas.87.21.8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rutberg B. Antitermination of transcription of catabolic operons. Mol Microbiol. 1997;23:413–421. doi: 10.1046/j.1365-2958.1997.d01-1867.x. [DOI] [PubMed] [Google Scholar]
- 39.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 40.Studier F W. Genetic mapping of a mutation that causes ribonuclease III deficiency in Escherichia coli. J Bacteriol. 1975;124:307–316. doi: 10.1128/jb.124.1.307-316.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sue K K, Cohen S D, Bechhofer D H. A polypurine sequence that acts as a 5′ mRNA stabilizer in Bacillus subtilis. J Bacteriol. 1995;177:3465–3471. doi: 10.1128/jb.177.12.3465-3471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas P S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci USA. 1980;77:5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vytvytska O, Jacobsen J S, Balcuanite G, Andersen J S, Baccarini M, von Gabain A. Host factor I, Hfq, binds to Escherichia coli ompA mRNA in a growth rate-dependent fashion and regulates its stability. Proc Natl Acad Sci USA. 1998;95:14118–14123. doi: 10.1073/pnas.95.24.14118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang W, Bechhofer D H. Properties of a Bacillus subtilis polynucleotide phosphorylase deletion strain. J Bacteriol. 1996;178:2375–2382. doi: 10.1128/jb.178.8.2375-2382.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang W, Bechhofer D H. Bacillus subtilis RNase III gene: cloning, function of the gene in Escherichia coli and construction of Bacillus subtilis strains with altered rnc loci. J Bacteriol. 1997;179:7379–7385. doi: 10.1128/jb.179.23.7379-7385.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zuker M. On finding of all suboptimal foldings of an RNA molecule. Science. 1989;244:48–52. doi: 10.1126/science.2468181. [DOI] [PubMed] [Google Scholar]