Abstract
In Huntington’s Disease (HD), the output of striatal indirect pathway medium-sized spiny neurons (MSNs) is altered in its target region, the external globus pallidus (GPe). In a previous study we demonstrated that selective optogenetic stimulation of indirect pathway MSNs induced prolonged decay time of GABA responses in GPe neurons. Here we identified the mechanism underlying this alteration. Electrophysiological recordings in slices from symptomatic R6/2 and wildtype (WT) mice were used to evaluate, primarily, the effects of GABA transporter (GAT) antagonists on responses evoked by optogenetic activation of indirect pathway MSNs. In addition, immunohistochemistry (IHC) and Western blots (WBs) were used to examine GAT-3 expression in HD and wildtype (WT) mice. A GAT-3 blocker (SNAP5114) increased decay time of GABA responses in WT and HD GPe neurons, but the effect was significantly greater in WT neurons. In contrast, a GAT-1 antagonist (NO-711) or a GABAB receptor antagonist (CGP 54626) produced small increases in decay time but no differential effects between genotypes. IHC and WBs showed reduction of GAT-3 expression in the GPe of HD mice. Thus, reduced expression or dysfunction of GAT-3 could underlie alterations of GPe responses to GABA inputs from striatum and could be a target for therapeutic intervention.
Keywords: Huntington’s disease, External Globus Pallidus, GABA Transporters, Western blots, Electrophysiology
Introduction
The histopathological hallmark of Huntington’s disease (HD) is the loss of striatal medium-sized spiny neurons (MSNs) (Vonsattel and DiFiglia 1998; Waldvogel et al. 2015). As HD progresses, there is a natural sequence of cell loss with indirect pathway neurons (iMSNs) projecting to the external globus pallidus (GPe) degenerating first, followed by direct pathway neurons (dMSNs) (Albin et al. 1992; Reiner et al. 1988). Reduced GPe inhibitory control leads to involuntary choreiform movements (Reiner 2004).
Rodent models of HD replicate some symptoms of HD. However, cell loss is not prominent. Instead, synaptic dysfunction predates neurodegeneration and probably underlies much of the phenotype (Levine et al. 2004). While MSN excitability is increased due to higher input resistance, alterations in the output of these neurons is unknown because of elevated GABAergic tone promoted by enhanced interneuron firing (Cepeda et al. 2004; Holley et al. 2019). GABA transmission changes affect iMSNs primarily (Cepeda et al. 2013), which will alter their output to the GPe. However, compensatory postsynaptic mechanisms may occur in the GPe. Human and animal studies have reported changes in GABA receptor subunits (Allen et al. 2009; Du et al. 2016; Garret et al. 2018; Waldvogel et al. 2015). It has been postulated that the upregulation of both GABAA and GABAB receptors in HD increases the capacity of GPe neurons to compensate for the reduced GABA release after loss of iMSNs (Allen et al. 2009).
Previously, we demonstrated enhanced GABAergic responses in GPe neurons of mouse models of HD (Barry et al. 2018). While the amplitude of the response did not change significantly, the decay time was significantly prolonged. In contrast, another study reported no changes in decay time but significant changes in amplitude of GABA responses (Perez-Rosello et al. 2019). In order to clarify the underlying mechanisms, here we examined the role of GABA transporters and GABAB receptors using electrophysiological recordings in combination with optogenetics, immunohistochemistry (IHC) and Western blotting (WB).
Materials and Methods
Mice:
Experimental procedures were performed in accordance with the US Public Health Service Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of California Los Angeles. Adenosine type 2A receptor (A2A)-Cre mice were crossed with wildtype (WT) female C57BL/6xCBA mice transplanted with R6/2 ovaries to generate R6/2 and WT littermates expressing A2A-Cre. CAG repeat lengths for R6/2 mice averaged 155±4. Mice were >60 days old (78±2 days vs. 67±1 days for WT vs. R6/2 respectively), well into the symptomatic stage for the R6/2 line. Mice were housed in a cage (≤5 mice per cage) with physical enrichment (nestlets) in a 12 h light/ 12 h dark cycle with controlled room temperature (69-72°C) and humidity (35-60%) and ad libitum access to food and water. Male and female mice were used and data were pooled.
Electrophysiology:
Detailed electrophysiological methods have been published (Barry et al. 2018; Cepeda et al. 2013; Holley et al. 2015). Briefly, mice (WT: 10 M, 6 F; R6/2: 10 M, 9 F) were anesthetized with isoflurane and sacrificed. The brain was removed and placed in ice-cold N-Methyl-D-glucamine (NMDG) solution containing (in mM): 102 NMDG, 3 KCl, 1.25 NaH2PO4, 30 NaHCO3, 20 HEPES, 0.5 CaCl2, 10 MgSO4, 85 HCl and 40 glucose). Sagittal slices (300 μm) were transferred to an incubating chamber with artificial cerebrospinal fluid (ACSF) containing (in mM): 130 NaCl, 3 KCl, 1.25 NaH2PO4, 26 NaHCO3, 2 CaCl2, 2 MgCl2, and 10 glucose, oxygenated with 95% O2-5% CO2 (pH 7.2-7.4, 290-310 mOsm). Whole-cell patch clamp recordings in voltage clamp mode were obtained with a MultiClamp 700B Amplifier and pClamp software (RRID:SCR_011323). Patch pipette (3-5 MΩ) internal solution contained (in mM): 130 Cs-methanesulfonate, 10 CsCl, 4 NaCl, 1 MgCl2, 5 MgATP, 5 EGTA, 10 HEPES, 5 GTP, 10 phosphocreatine, and 0.1 leupeptin (pH 7.2, 270 mOsm).
Optogenetics and drug treatments:
We used optical stimulation to activate iMSNs selectively. Channelrhodopsin-2 (ChR2) and its reporter gene enhanced yellow fluorescent protein (EYFP) were inserted in a double-floxed inverted open reading frame viral vector and stereotaxically injected into the striatum of 1 month-old WT and R6/2 mice crossed with A2A-Cre mice (1.0 mm anterior and 2.0 mm lateral to Bregma, at a depth of 3.1 mm from the dura). After Cre recombination, ChR2-EYFP was selectively expressed in A2A-Cre neurons. To examine evoked GABA responses in GPe neurons the following protocol was used: a slow ramp voltage command (3 s duration) from a holding voltage of −70 mV to +10 mV was first applied. Then the cell was held at the new voltage for 2 s, after which the optical stimulation (470 nm, 1 mW, 0.5 ms) was delivered. After 2 additional s at +10 mV the membrane potential was brought back to the original holding potential. This protocol was repeated 3 times (interval between sweeps was 30 s) and the responses to light stimulation were averaged. SNAP5114 (GAT-3 antagonist, 20 μM), NO711 (GAT-1 antagonist, 10μM), or CGP 54626 (GABAB receptor antagonist, 1μM) (all from Tocris, Minneapolis, MN) were bath applied (10-20 min) to examine alterations in decay time (90%-10%) of the evoked GABA response measured with Clampfit v10.7 (Molecular Devices, Sunnyvale, CA; RRID:SCR_011323).
IHC:
Mice (WT: 2M, 1F; R6/2: 3M) were anaesthetized and transcardially perfused with phosphate buffered saline (PBS, pH = 7.4) and 4% paraformaldehyde (PFA) in PBS. Brains were extracted and post-fixed in PFA for 48 h at 4°C, then transferred into 20% sucrose in PBS. Coronal slices (30 μm thick) were cut, thawed in 1x Tris-phosphate buffer solution (TBS), rinsed, permeabilized with 5% normal goat serum, 2% bovine serum albumin (BSA), 0.3% Triton-X in TBS, and then incubated with rabbit-polyclonal GAT-3 antibody (1:2000 in serum block) (Abcam, Cambridge, MA; Cat # ab181783; host rabbit; immunogen rat GAT-3 C terminal (15 amino acids) (RRID:AB_2858195) overnight at 4°C. Slices were treated with Vectastain® Elite® ABC Kit, then incubated with 3,3’- diaminobenzidine (DAB) and 3% hydrogen peroxide for 5-10 min, mounted on Superfrost Plus slides, dehydrated, cleared, and coverslipped. Images of the GPe and surrounding striatum in coronal slices were captured on a Zeiss Apotome Confocal microscope with bright field illumination and a 5x lens, using the same capture settings for all images. GAT-3 intensity was measured using image analysis software imageJ (RRID:SCR_003070). GAT-3 intensity was measured in regions of interest (ROI) (striatum and GPe) in coronal slices either with the primary GAT-3 antibody added (samples) or without GAT-3 antibody (background). The ROI background was subtracted from the ROI samples to calculate GAT-3 intensity in both the striatum and GPe. GAT-3 intensity was expressed as arbitrary units (a.u.).
GAT-3 Protein Expression:
GAT-3 protein expression was assessed in both GPe and striatum whole-tissue extracts and membrane-enriched synaptoneurosomes (Chang et al. 2012) by WB analysis (Sarafian et al. 2017). For whole tissue studies, GPe and striatum tissues were dissected from both wild type (WT, n=2) and R6/2 mice (n=3) and quickly homogenized using a hand-held Teflon homogenizer in 1.5 ml microcentrifuge tubes for 30 sec on ice. Radioimmunoprecipitation assay buffer (RIPA) buffer supplemented with 1μg/ml soybean trypsin inhibitor, 1 μg/ml aprotinin, and 10 μM phenylmethylsulfonyl fluoride (PMSF) was used for homogenization. Aliquots of the homogenate were taken for protein measurement using the Bio-Rad coomassie G250 assay kit with BSA for standard. Synaptoneurosome fractions were prepared from GPe and striatum (WT: 2 M, 3F and R6/2: 4 M, 1 F) using a previously described method (Chang et al. 2012; Johnson et al. 1997). Briefly, 1 mm slices from WT and R6/2 mouse brains were homogenized in a modified Krebs-Henseleit buffer. The final eluent was centrifuged at 3,500 rpm for 15 min at 4oC to generate tripartite synaptoneurosome pellets. Pellets were resuspended and 5 μl aliquots were used to measure protein concentration as described above for whole tissue extracts. Five or 10 μg of each sample were boiled in SDS sample buffer and loaded onto acrylamide gels. Samples were transferred onto Polyvinylidene Fluoride membranes. After blocking with 5% milk in TBS/Tween, membranes were exposed to antibodies to GAT-3 and to mouse-monoclonal glyceraldehyde 3 phosphate dehydrogenase (GAPDH) (1:2,000 each) (EMD Millipore; Burlington, MA; Cat# MAB374; host mouse; immunogen GADPH from rabbit muscle; RRID AB_2107445) overnight at 4°C. After washing, membranes were stained with secondary antibodies IRDye 800CW (abcam ab216773) anti rabbit for GAT-3 and IRDye 800CW (abcam ab216772) anti mouse for GAPDH (1:2,500 each). Staining intensities were quantified using ImageJ software and GAT-3 levels were normalized to GAPDH housekeeping protein levels.
Data Analysis and Statistics:
Analysis of data was done blindly to gender and genotype. Values are reported as mean±SEM. Differences between group means were assessed with appropriate paired or unpaired Student’s t-tests. Differences were considered statistically significant when p<0.05.
Results
GABA Transporters:
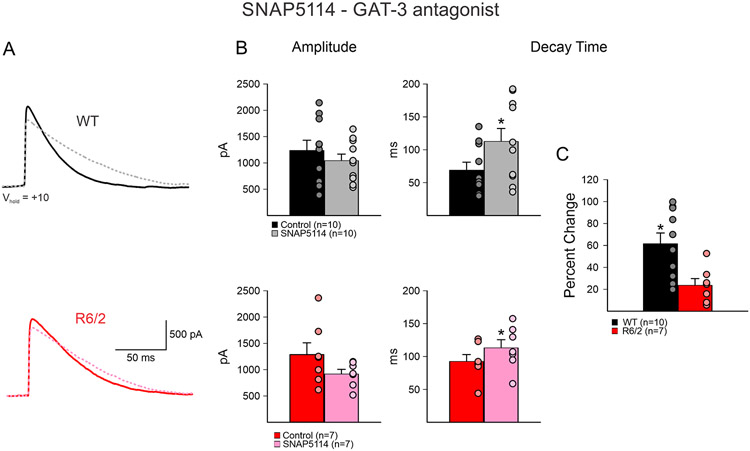
Confirming previous findings (Barry et al. 2018), GPe neurons from symptomatic R6/2 mice, regardless of pharmacological treatment groups, showed a significant increase in evoked GABA responses mean decay times (55.3±5.5 ms in WT, n=29 cells vs. 84.3±7.7 ms in R6/2, n=23 cells, p=0.003). In contrast, the mean amplitudes were not different (1219.7±121.2 pA in WT vs. 1410.5±186.0 pA in R6/2, p=0.38). Bath application of the GAT-3 inhibitor, SNAP5114 (20 μM) produced a significant increase of mean decay times in both WT and R6/2 GPe neurons (WT n=10; before 69.2±12.1 ms vs. after 112.9±19.6 ms, p=0.0008: R6/2 n=7; before 92.6±10.4 ms vs. after 113.2±12.4 ms, p=0.01) (Fig. 1A, B). However, the mean percent change was significantly greater in WT compared to R6/2 neurons (61.6±9.8% WT vs. 23.7±6.1% R6/2, p=0.009) (Fig. 1C). In contrast, the change in response amplitude was not significantly different (WT; 1238.4±192.7 pA vs. 1043.0±124.8 pA, p=0.40: R6/2; 1286.0±224.2 vs. 916.5±88.7 pA, p=0.17) (Fig. 1B).
Fig. 1.
A. SNAP5114 (GAT-3 antagonist, 20 μM) (dashed lines) caused an increase in decay time of evoked GABA responses in GPe neurons due to activation of iMSNs in both WT and R6/2 mice. B. SNAP5114 bath application caused no significant change in average amplitude in either WT or R6/2 GPe neurons. However, a significant increase in decay time was observed in both groups. Each dot represent one individual cell. C. The percent change of decay time of WT neurons was significantly larger than in R6/2 neurons after application of SNAP5114.
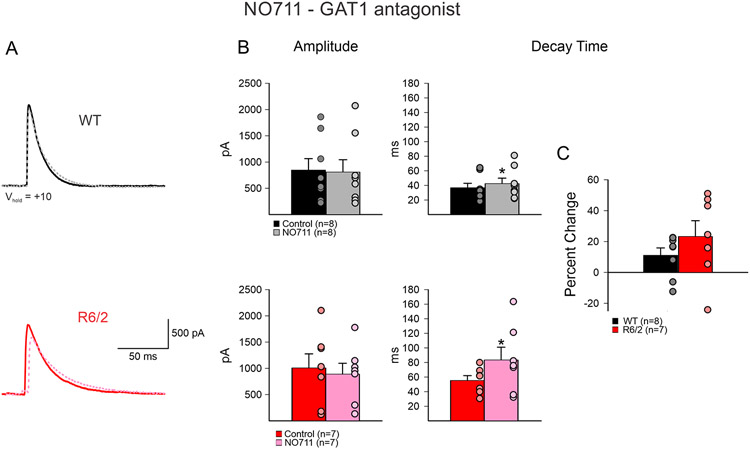
Bath application of NO711 (GAT-1 antagonist, 10 μM) also led to a significant increase of decay time in both WT and R6/2 neurons (WT n=8; before 37.0±6.0 ms vs. after 42.6±7.5 ms, p=0.04: R6/2 n=7; before 55.5±6.6 ms vs. after 83.6±17.5 ms, p=0.03) (Fig. 2). However, the mean percentage increase, while slightly greater in R6/2 compared to WT neurons, was not significantly different (11.1±4.8 % WT vs. 23.2±10.2 % R6/2, p=0.28). There were no significant differences in mean amplitudes (WT; before 849.3±218.5 pA vs. after 812.3±234.6 pA, p=0.91: R6/2 before 1010.4±267.0 vs. after 891.5±206.9 pA, p=0.73).
Fig. 2.
A. NO711 (GAT-1 antagonist, 10 μM) (dashed lines) caused a small increase in decay time of evoked GABA responses from iMSNs to GPe neurons in both WT and R6/2 mice. B. NO711 bath application caused no change in amplitude of responses in either WT or R6/2 neurons. However, it caused significant increase in decay time in both WT and R6/2 neurons. C. The percent change of decay time of WT neurons was not significantly different from R6/2 neurons after application of NO711.
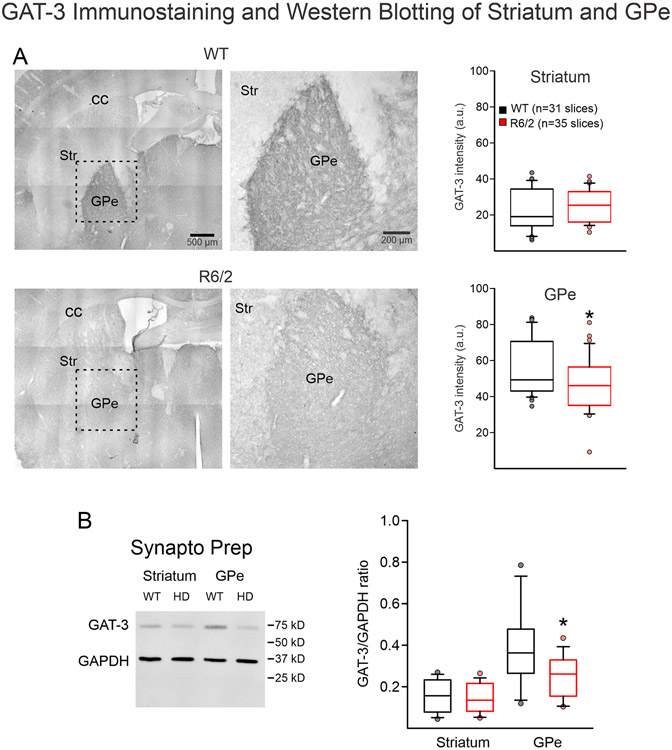
We then used IHC to measure GAT-3 transporter expression in the GPe and striatum. Overall, GAT-3 expression in GPe was greater than in striatum, confirming previous reports (Galvan et al. 2005; Ikegaki et al. 1994). As GAT-3 is mainly localized in astrocytes (Jin et al. 2011), We observed that GAT-3 expression was significantly reduced in the GPe of R6/2 mice compared to WT littermates (56.2±2.7 WT vs. 46.1±2.5 R6/2, p=0.01), while there was no difference in GAT-3 expression in the striatum (21.8±2.1 WT vs. 24.3±9.6 R6/2, p=0.35; n=3 WT mice, 31 samples and n=3 R6/2 mice, 35 samples) (Fig 3A).
Fig. 3.
A. Bright field tiled and magnified images (left) [dashed line box shows magnified region on the right] of GAT-3 immunostaining of striatum (Str) and GPe in WT vs. R6/2 mice showed no change in Str GAT-3 expression levels, but a significant decrease in GAT-3 expression in the GPe. Bar graphs (right) show quantitative data. B. Synaptoneurosome preparations of Str or GPe from WT vs. R6/2 mice showed no change in GAT-3 expression in the Str, but a statistically significant decrease of GAT-3 expression in the GPe.
To confirm results observed with IHC, we used WB with the same GAT-3 antibody and GAPDH as control. We measured GAT-3 protein levels in both whole-tissue lysate and in synaptoneurosome preparations of GPe and striatum. The whole-tissue GPe lysate showed no difference in the mean ratios of GAT-3/GADPH expression in WT vs. R6/2 mice (0.31±0.08 WT vs. 0.39±0.06 R6/2, p=0.48; n=2 WT and n=3 R6/2 mice, 1 sample per mouse). Initial negative results using the crude whole-tissue preparation, led us to change methodology. As GAT-3 is present predominantly in astrocytes, we reasoned that the synaptoneurosome preparation, which isolates tripartite synapses including nerve terminals, postsynaptic membranes, and astrocytes (see Chang et al., 2012) would be more idoneous for our study. When synaptoneurosome preparations were examined, a significant decrease in the mean GAT-3/GADPH ratio was observed in R6/2 mice compared to WT littermates (0.39±0.05 WT vs. 0.25±0.03 R6/2, p=0.01, n=5 WT and n=5 R6/2 samples) (Fig. 3B). Neither whole-tissue lysate (0.08±0.03 WT vs. 0.10±0.04 R6/2, p=0.62) nor synaptoneurosome preparations (0.15±0.08 WT vs. 0.14±0.07 R6/2, p=0.74) (Fig. 3B) of the striatum showed significant differences in GAT-3 expression.
GABAB Receptors:
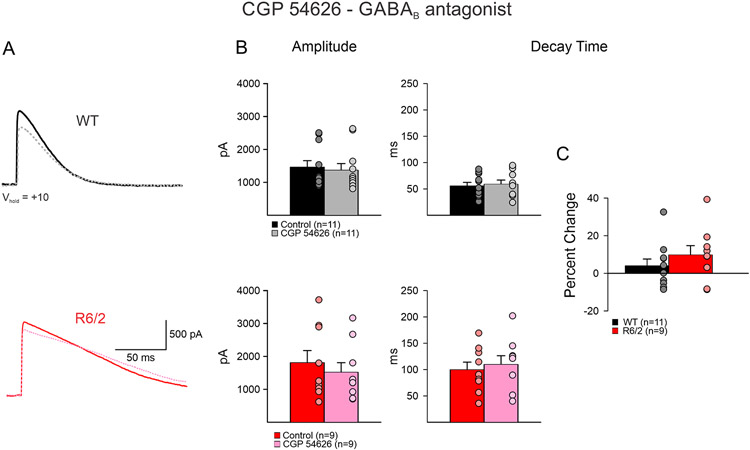
As in HD GABAB receptor subunits are upregulated (Allen et al. 2009), we also examined whether alterations in the optically-evoked GABA responses decay times could be due to changes in GABAB receptors. Bath application of CGP 54626 (GABAB antagonist, 1 μM) led to marginal increases in decay time in both WT and R6/2 neurons (WT n=11; before 56.0 ±6.4 ms vs. after 59.2±7.7 ms, p=0.202: R6/2 n=9; before 100.3±14.2 ms vs. after 110.3±16.4 ms, p=0.09) (Fig. 4A, B). The mean percentage increase, while slightly greater in R6/2 compared to WT neurons, was not significantly different (4.1±3.5 % WT vs. 9.9±4.9 % R6/2 p=0.34) (Fig. 4C). There were no significant differences in mean amplitudes (WT; before 1472.0±193.2 pA vs. after 1377.6±199.5 pA, p=0.30: R6/2 before 1818.5±366.4 vs. after 1527.1±289.1 pA, p=0.07) (Fig. 4B).
Fig. 4.
A. CGP 54626 (GABAB receptor antagonist, 1μM) (dashed lines) caused no differences in decay time of evoked GABA responses from iMSNs to GPe neurons in both WT and R6/2 mice. B. CGP 54626 bath application caused no change in amplitude of response in either WT or R6/2 neurons, nor did it cause a change in decay time in both WT and R6/2 neurons. C. The percent change of decay time of WT neurons was not significantly different than R6/2 after application of CGP 54626.
Discussion
The main finding of this study is that the increased decay time of GABA responses evoked in GPe neurons by stimulation of iMSNs is probably caused by alterations in expression or function of GAT-3. Thus, while the GAT-3 inhibitor, SNAP5114 caused a large increase in decay time of GABA responses in WT neurons, the effect in R6/2 neurons was significantly reduced. In addition, NO711, a GAT-1 antagonist also caused a significant increase of decay time in both WT and R6/2 neurons, but the difference in percentage increase was not statistically significant. GAT-3 immunostaining showed no changes in the striatum of WT vs. R6/2 mice, but a significant decrease occurred in the GPe of R6/2 mice compared to WT littermates. Finally, while whole-tissue lysate showed no difference in GAT-3 expression, synaptoneurosome preparations showed a significantly higher amount of GAT-3 in WT vs. R6/2 preparations. This result could be due to improper trafficking of GAT-3 to cell membrane surface.
Two possible mechanisms could be involved in slower decay times of GABA responses in R6/2 mice; changes in GABAA receptor subunits, and/or changes in GABA transporters. Changes in receptor subunits have already been reported (Du et al. 2016; Garret et al. 2018), whereas the role of GABA transporters remained unknown. GAT-1 is enriched throughout the brain whereas GAT-3 is localized to specific regions including the GPe (Galvan et al. 2005; Ikegaki et al. 1994). As GAT-3 is mainly localized in astrocytes (Jin et al. 2011), the present results predict abnormal function of GPe astrocytes, as shown in striatum of HD mice (Tong et al. 2014).
In contrast to our previous study (Barry et al. 2018), another study (Perez-Rosello et al. 2019) reported no changes in decay time but significant changes in amplitude of GABA responses. However, the potential mechanisms were not clarified as both pre- and postsynaptic mechanisms were invoked. While methodological differences could explain the discrepancy, both studies coincided on the outcome, i.e., an increase in GABA current charge. Interestingly, in that study it was shown that optogenetic stimulation of iMSNs inhibited GPe neuron firing for longer periods of time in Q175 HD mice compared to WTs, suggesting that the inhibitory neurotransmitter may remain functional in the synaptic cleft longer.
The current model to explain abnormal movements in HD, specifically chorea, postulates that initial loss of iMSNs disinhibits GPe activity (Reiner 2004). In contrast, our finding of increased inhibition of GPe neurons seems at odds with this prediction. However, we have to consider the dynamic nature of the disease. The model applies to the early stages of the disease and mainly to adult-onset HD. The late stage of HD and the juvenile form of the disease are characterized by rigidity and akinesia, more akin to Parkinson’s disease (PD). R6/2 model mice do not display chorea and better replicate juvenile HD. In that sense, our findings are not entirely unexpected. Increased inhibition of GPe neurons, along with reduced inhibition of direct pathway MSNs (Barry et al. 2018), could underlie PD-like symptoms. Interestingly, in a rodent model of PD produced by dopamine depletion, a reduction in GAT-3 expression in the GPe was observed (Chazalon et al. 2018). Notably, in HD models there is a significant loss of dopamine release in the symptomatic stage (Cepeda et al. 2014).
From a clinical perspective, the present results could help design specific drugs targeting GABA transporters in the GPe as a new treatment for HD symptoms in human patients. However, to date no pharmacological agonist to enhance GAT-3 function is available (Chazalon et al. 2018). Further, in order to produce efficient upregulation of GAT-3 function, provided an agonist becomes available, administration would have to be local, e.g., specific to GPe. This could be better achieved by increasing GAT-3 protein in GPe chronically, e.g., using local viral expression. Alternatively, improving astrocyte function in the GPe could provide another strategy, as already shown in striatum (Tong et al. 2014).
Significance Statement.
Synaptic communication is significantly altered in Huntington’s disease (HD) model mice. In a previous study, we demonstrated increased duration of GABA responses evoked by optogenetic stimulation of striatal terminals in the external segment of the globus pallidus, an area of the basal ganglia critically involved in movement control. Here we examined the mechanism underlying this increase in GABA responses and demonstrate that it is partly due to dysfunction of a GABA transporter, GAT-3. Restoring the function of this transporter could help improve motor symptoms of HD.
Acknowledgments
This study received funding from NIH grants NS41574, NS096994 (MSL) and F32NS093813 (JB). We would like to thank Dr. Sandra Holley for insightful comments on the manuscript.
Footnotes
Conflict of Interest and Financial Disclosures
The authors declare they do not have any conflicts of interest of financial disclosures.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Albin RL, Reiner A, Anderson KD, Dure LSt, Handelin B, Balfour R, Whetsell WO Jr., Penney JB, Young AB. 1992. Preferential loss of striato-external pallidal projection neurons in presymptomatic Huntington's disease. Ann Neurol 31(4):425–430. [DOI] [PubMed] [Google Scholar]
- Allen KL, Waldvogel HJ, Glass M, Faull RL. 2009. Cannabinoid (CB(1)), GABA(A) and GABA(B) receptor subunit changes in the globus pallidus in Huntington's disease. J Chem Neuroanat 37(4):266–281. [DOI] [PubMed] [Google Scholar]
- Barry J, Akopian G, Cepeda C, Levine MS. 2018. Striatal Direct and Indirect Pathway Output Structures Are Differentially Altered in Mouse Models of Huntington's Disease. J Neurosci 38(20):4678–4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Galvan L, Holley SM, Rao SP, Andre VM, Botelho EP, Chen JY, Watson JB, Deisseroth K, Levine MS. 2013. Multiple sources of striatal inhibition are differentially affected in Huntington's disease mouse models. J Neurosci 33(17):7393–7406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Murphy KP, Parent M, Levine MS. 2014. The role of dopamine in huntington's disease. Prog Brain Res 211:235–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Starling AJ, Wu N, Nguyen OK, Uzgil B, Soda T, Andre VM, Ariano MA, Levine MS. 2004. Increased GABAergic function in mouse models of Huntington's disease: reversal by BDNF. J Neurosci Res 78(6):855–867. [DOI] [PubMed] [Google Scholar]
- Chang JW, Arnold MM, Rozenbaum A, Caputo A, Schweizer FE, Huynh M, Mathern GW, Sarafian TA, Watson JB. 2012. Synaptoneurosome micromethod for fractionation of mouse and human brain, and primary neuronal cultures. J Neurosci Methods 211(2):289–295. [DOI] [PubMed] [Google Scholar]
- Chazalon M, Paredes-Rodriguez E, Morin S, Martinez A, Cristovao-Ferreira S, Vaz S, Sebastiao A, Panatier A, Boue-Grabot E, Miguelez C, Baufreton J. 2018. GAT-3 Dysfunction Generates Tonic Inhibition in External Globus Pallidus Neurons in Parkinsonian Rodents. Cell reports 23(6):1678–1690. [DOI] [PubMed] [Google Scholar]
- Du Z, Chazalon M, Bestaven E, Leste-Lasserre T, Baufreton J, Cazalets JR, Cho YH, Garret M. 2016. Early GABAergic transmission defects in the external globus pallidus and rest/activity rhythm alteration in a mouse model of Huntington's disease. Neuroscience 329:363–379. [DOI] [PubMed] [Google Scholar]
- Galvan A, Villalba RM, West SM, Maidment NT, Ackerson LC, Smith Y, Wichmann T. 2005. GABAergic modulation of the activity of globus pallidus neurons in primates: in vivo analysis of the functions of GABA receptors and GABA transporters. J Neurophysiol 94(2):990–1000. [DOI] [PubMed] [Google Scholar]
- Garret M, Du Z, Chazalon M, Cho YH, Baufreton J. 2018. Alteration of GABAergic neurotransmission in Huntington's disease. CNS Neurosci Ther 24(4):292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley SM, Galvan L, Kamdjou T, Dong A, Levine MS, Cepeda C. 2019. Major Contribution of Somatostatin-Expressing Interneurons and Cannabinoid Receptors to Increased GABA Synaptic Activity in the Striatum of Huntington's Disease Mice. Frontiers in synaptic neuroscience 11:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley SM, Joshi PR, Parievsky A, Galvan L, Chen JY, Fisher YE, Huynh MN, Cepeda C, Levine MS. 2015. Enhanced GABAergic Inputs Contribute to Functional Alterations of Cholinergic Interneurons in the R6/2 Mouse Model of Huntington's Disease. ENEURO:0008–0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegaki N, Saito N, Hashima M, Tanaka C. 1994. Production of specific antibodies against GABA transporter subtypes (GAT1, GAT2, GAT3) and their application to immunocytochemistry. Brain Res Mol Brain Res 26(1-2):47–54. [DOI] [PubMed] [Google Scholar]
- Jin XT, Galvan A, Wichmann T, Smith Y. 2011. Localization and Function of GABA Transporters GAT-1 and GAT-3 in the Basal Ganglia. Front Syst Neurosci 5:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Chotiner JK, Watson JB. 1997. Isolation and characterization of synaptoneurosomes from single rat hippocampal slices. J Neurosci Methods 77(2):151–156. [DOI] [PubMed] [Google Scholar]
- Levine MS, Cepeda C, Hickey MA, Fleming SM, Chesselet MF. 2004. Genetic mouse models of Huntington's and Parkinson's diseases: illuminating but imperfect. Trends Neurosci 27(11):691–697. [DOI] [PubMed] [Google Scholar]
- Perez-Rosello T, Gelman S, Tombaugh G, Cachope R, Beaumont V, Surmeier DJ. 2019. Enhanced striatopallidal gamma-aminobutyric acid (GABA)A receptor transmission in mouse models of huntington's disease. Mov Disord 34(5):684–696. [DOI] [PubMed] [Google Scholar]
- Reiner A. 2004. Can lesions of GPe correct HD deficits? Exp Neurol 186(1):1–5. [DOI] [PubMed] [Google Scholar]
- Reiner A, Albin RL, Anderson KD, D'Amato CJ, Penney JB, Young AB. 1988. Differential loss of striatal projection neurons in Huntington disease. Proc Natl Acad Sci U S A 85(15):5733–5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafian TA, Littlejohn K, Yuan S, Fernandez C, Cilluffo M, Koo BK, Whitelegge JP, Watson JB. 2017. Stimulation of synaptoneurosome glutamate release by monomeric and fibrillated alpha-synuclein. J Neurosci Res 95(9):1871–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X, Ao Y, Faas GC, Nwaobi SE, Xu J, Haustein MD, Anderson MA, Mody I, Olsen ML, Sofroniew MV, Khakh BS. 2014. Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington's disease model mice. Nat Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonsattel JP, DiFiglia M. 1998. Huntington disease. J Neuropathol Exp Neurol 57(5):369–384. [DOI] [PubMed] [Google Scholar]
- Waldvogel HJ, Kim EH, Tippett LJ, Vonsattel JP, Faull RL. 2015. The Neuropathology of Huntington's Disease. Current topics in behavioral neurosciences 22:33–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.