Abstract
Superior vena cava (SVC) obstruction, whether from benign or malignant causes, results in a variety of symptoms. It is a potential medical emergency when cerebral or laryngeal edema occurs. Endovascular therapy is the treatment of choice for patients in need of emergent relief of symptoms. This article will provide a review of SVC syndrome with a focus on endovascular treatment techniques.
Keywords: SVC syndrome, SVC stenting, stent migration, pericardial tamponade, interventional radiology
Superior Vena Cava Syndrome
Etiology
Malignancy
Malignancy is the predominant etiology of superior vena cava (SVC) syndrome. 1 Malignancy leads to SVC syndrome by compression of or, less commonly, direct tumor invasion of the SVC. SVC syndrome may be the presenting symptom in patients with previously undiagnosed malignancy. 2
Among malignant causes, lung cancer predominates, accounting for 75 to 80% of cases. 1 Approximately 4% of patients with lung cancer develop some degree of SVC syndrome during the course of their disease. It occurs in 10% of patients with small cell lung cancer (SCLC) but less than 2% of those with non-small cell lung cancer (NSCLC). 3 The tendency of SCLC to cause SVC syndrome is due to both its central location and rapid growth. 4 NSCLC, however, remains a more common overall cause of SVC syndrome because of its 9:1 case prevalence compared with SCLC. 5 Malignant mediastinal lymph nodes, whether from lymphoma or metastasis, can also lead to SVC syndrome. Among the different types of lymphoma, non-Hodgkin's lymphomas, specifically diffuse large B-cell lymphoma, lymphoblastic lymphoma, and primary mediastinal large B-cell lymphoma, are prone to SVC syndrome. Hodgkin's lymphoma rarely leads to SVC syndrome. Other malignant etiologies include thymoma and germ cell tumors.
Prognosis in patients with malignant SVC syndrome is generally poor with median survival time after the occurrence of SVC syndrome of 101 days. 6 Survival, however, is linked to tumor subtype and stage, not to the presence or absence of SVC syndrome.
Benign Lesions
The increasing utilization of intravascular devices including central venous catheters and pacer wires has led to a rising incidence of benign etiologies of SVC syndrome, now estimated to account for up to 40% of cases of SVC syndrome. 2 7 A catheter tip that is too short may predispose to SVC syndrome 8 9 ( Fig. 1 ). Other benign cases include postradiation ( Fig. 2 ) and fibrosis mediastinitis. 10 A mixed benign and malignant etiology is possible in patients with malignancy who have indwelling central venous access devices such as a port ( Fig. 3 ).
Fig. 1.
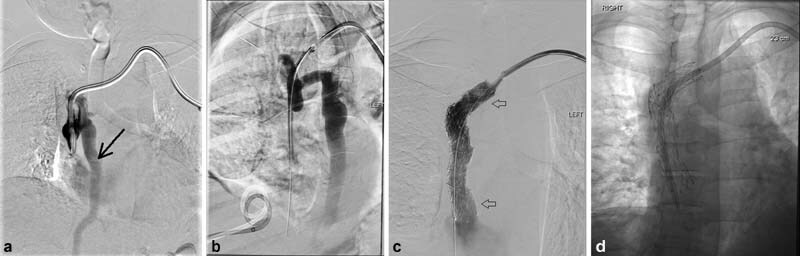
A 41-year-old man with end-stage renal disease secondary to Alport's syndrome undergoing catheter-based hemodialysis for 12 years developed acute face swelling and orbital edema. Venogram through the dialysis catheter ( a ) demonstrated the catheter tip to be in the mid- to low SVC with complete occlusion of the distal SVC and surrounding pericatheter thrombus and retrograde flow down the azygous vein (black arrow). After the occlusion was crossed ( b ), an 18 mm diameter × 80 mm length stent bare metal self-expanding Abre stent (Medtronic; Minneapolis, MN) was placed, extending into the left brachiocephalic vein (arrows, c ). Postdeployment 16-mm balloon dilation was performed. A new dialysis catheter was placed through the stent with the tip in the upper right atrium ( d ).
Fig. 2.
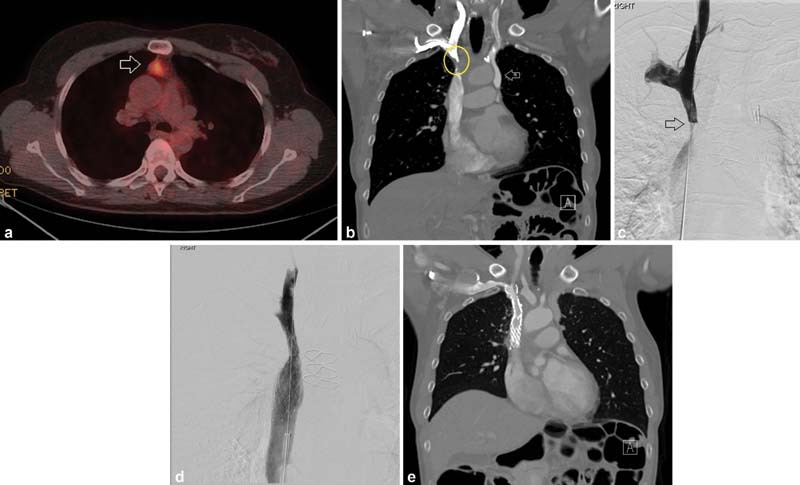
A 50-year-old woman had a prior history of thymic carcinoid (initial PET-CT, a ; arrow—PET-avid tumor) treated with 60 Gy of RT. Two years after treatment, she developed facial and RUE edema with bothersome dilation of subcutaneous collateral vessels. Contrast-enhanced CT ( b ), which showed incidental duplication of the SVC (arrow), demonstrated no evidence of residual disease but did show severe stenosis of the right-side SVC (circle) thought to be due to prior radiation therapy. The stenosis was confirmed by catheter venography (arrow, c ). A 16-mm-diameter Abre (Medtronic) stent was placed and postdilated to 10 mm with brisk flow through the previously seen stenosis ( d ). Symptoms improved by postprocedure day 1. Three-month follow-up CT showed stent patency ( e ).
Fig. 3.
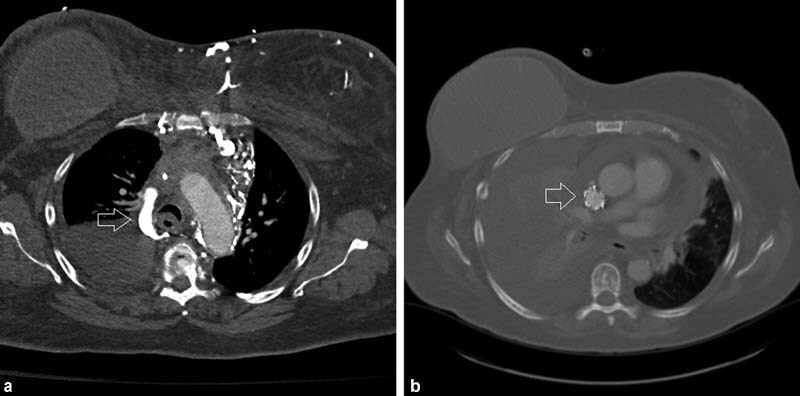
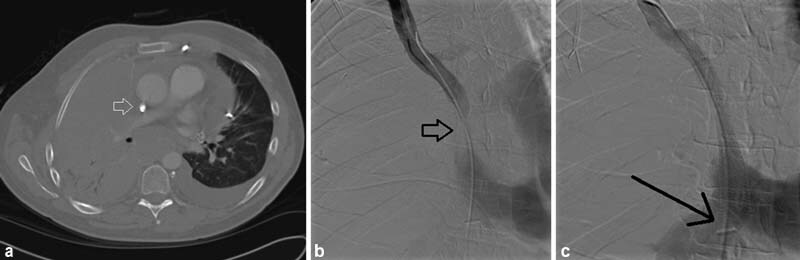
A 52-year-old woman with a history of metastatic breast cancer and indwelling port developed facial swelling and airway edema. Chest CT demonstrated complete thrombotic occlusion of the SVC around the port-a-catheter and mediastinal and chest wall collaterals including a large azygous vein (arrow, a ). The etiology of her SVC syndrome was considered mixed due to a combination of compressive mediastinal adenopathy, indwelling foreign body, and malignancy-associated hypercoagulable state. Placement of a 20-mm-diameter Cook-Z stent (Cook Medical) followed by 14-mm post-deployment balloon dilation led to prompt relief of facial swelling and airway edema. Follow-up CT 3 months later demonstrated a patent SVC stent (arrow) but progression of disease ( b ).
The etiology of SVC syndrome has changed significantly over the years. The first case report of SVC syndrome was described by Dr. William Hunter in 1757 in a patient with a syphilitic aortic aneurysm. On postmortem examination, he described the SVC as “…so much compressed by the dilated [aorta] as hardly to have anything left of [its] natural capacity and appearance.” Syphilis and other infectious etiologies, like tuberculosis, predominated until the mid-20th century when their contribution as a causative etiology of SVC syndrome dropped precipitously, coinciding with the introduction of antimicrobial therapies. 11
Anatomy
The SVC begins at the caudal confluence of the brachiocephalic veins. It carries approximately one-third of the blood flow to the right atrium. The SVC is thin walled and therefore is susceptible to external compression. The pericardial reflection occurs at the junction of the upper two-thirds and lower one-third of the SVC. 12 The azygos vein, which arches over the right main stem bronchus, enters the posterior aspect of the mid SVC. The normal adult SVC is approximately 6 to 8 cm in length and 14 to 24 mm in diameter. Left-side SVC and duplicated SVC are rare anatomic variants (see Fig. 2 ).
Collateral pathways of drainage become important in the setting of SVC obstruction. The presence of significant collaterals is correlated with the presence of clinically symptomatic SVC syndrome. When performing percutaneous biopsy or mediastinoscopy, these dilated collaterals may lead to higher risk of bleeding. 13 The azygous vein is the most important collateral drainage pathway. Obstruction across the azygous vein ostium leads to more severe symptoms. 14 Other collateral pathways include lateral thoracic, paravertebral, internal mammary, anterior chest wall veins. 15 Systemic to pulmonary vein collaterals can occur which can result in a right-to-left shunt ( Fig. 4 ).
Fig. 4.
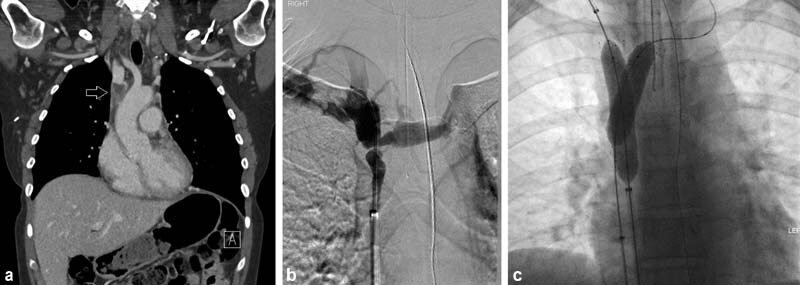
A 70-year-old woman with a new lung mass on CT presented with signs of SVC syndrome including headaches, vision changes, and facial swelling. CT ( a ) and subsequent venogram ( b ) showed focal malignant SVC occlusion (white arrow, b ) as well as systemic vein to pulmonary vein shunting (black arrow, b ). A 20 mm diameter × 10 cm long Abre (Medtronic) stent was placed extending from the left brachiocephalic vein to the SVC (arrows, c ). After dilation to 16 mm, good flow through the stent with resolution of collateral flow to the pulmonary vein ( c ). Note that more of the stent is above the stenosis than below, lessening the risk of caudal migration. The mass was subsequently biopsied and was positive for features of combined adenocarcinoma–small cell carcinoma of the lung. Unlike radiation therapy, stenting does not interfere with subsequent histologic diagnosis or treatment options. In fact, it can possibly aid with in performance of diagnostic procedures by allowing patients to lie flat to undergo biopsy and decompressing collateral vessels.
Two important systemic to portal collaterals are seen in SVC obstruction. The upper and mid esophageal veins normally drain into the azygous system. In cases of increased pressure within the azygous vein, backfilling of the esophageal venous system may occur leading to the devolvement of esophageal varices. 16 17 These varices, termed “downhill esophageal varices” by Dr. Ben Felson 18 can be treated with SVC stenting. 19 Another important systemic to portal collateral involves the drainage of dilated chest wall veins to the left portal vein via the veins of Sappey. This collateral pathway can lead to perfusional changes and globular enhancement around the falciform ligament known as the “hot quadrate sign” seen on nuclear medicine and the “CT quadrate lobe hot spot sign” on CT 20 ( Fig. 5 ). Knowing this can lead the radiologist to suggest SVC obstruction based on abdominal imaging or can help them avoid misdiagnosis of this pseudolesion as a liver mass or contrast extravasation.
Fig. 5.
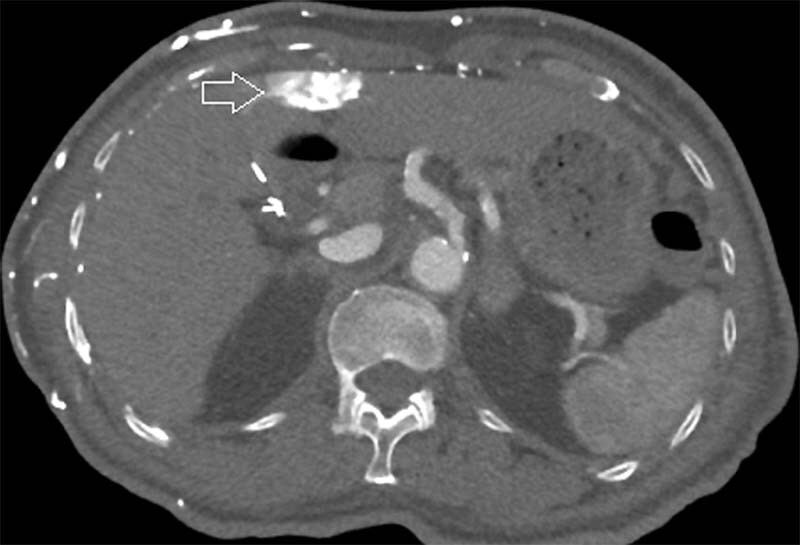
A 77-year-old woman with end-stage renal disease developed SVC occlusion due to long-standing indwelling dialysis catheter. CT abdomen demonstrates globular enhancement around the falciform ligament (arrow), the CT “hot spot sign” due to systemic to portal vein collateral flow.
Clinical Manifestations
The most common symptoms of SVC syndrome are face, neck, and arm swelling. 2 While not dangerous in and of themselves, they are a marker of potential dangerous edema elsewhere. Oropharyngeal dysphagia may lead to dyspnea, stridor, cough, or dysphagia. Cerebral edema may lead to headache, confusion, and, in the worst-case scenario, death via cerebral herniation. Symptoms are often exacerbated with lying down or bending forward. It is comforting to know that one retrospective review of nearly 2,000 patients attributed only one death directly to SVC syndrome. Therefore, while symptoms may be striking, SVC syndrome is not always the emergency it is thought to be. 21 The Kishi or the Yu scoring systems, can be used to determine the utility of stenting ( Tables 1 and 2 ).
Table 1. The Kishi scoring system 66 .
| Neurologic symptoms | Obtundation | 4 |
| Blurry vision, headaches | 3 | |
| Mental status changes | 2 | |
| Oropharyngeal symptoms | Laryngeal edema/orthopnea | 3 |
| Stridor, hoarseness, dysphagia | 2 | |
| Cough | 1 | |
| Facial symptoms | Nasal stuffiness, epistaxis, lip edema | |
| Facial swelling | 1 | |
| Vein distention | Neck and/or arm vein dilation | 1 |
| Upper extremity swelling or plethora | 1 |
Notes: A score ≥4 is considered an indication for stenting. Threshold for performance of superior vena cava stenting should be lower in patients with diagnoses on non-small cell lung cancer or mesothelioma, which are less likely to respond to systemic therapy than for chemo- and radiation-sensitive tumors such as lymphoma.
Table 2. Yu grading system indicated 22 .
| Grade | Signs/Symptoms | Incidence (estimated) | Proposed first step in definitive treatment |
|---|---|---|---|
| 0—Asymptomatic | Obstruction seen on imaging studies | 10 | None, consideration |
| 1—Mild | Subcutaneous edema in head, neck, and upper extremities. Cyanosis, plethora, venous distention | 25 | Chemo/radiation |
| 2—Moderate | Edema of head and neck with impairment including mild dysphagia, cough, eyelid movement, visual disturbance | 50 | Chemo/radiation |
| 3—Severe | Mild to moderate cerebral edema manifested by headache and dizziness Mild to moderate laryngeal edema |
10 | Stenting |
| 4—Life threatening | Severe cerebral edema manifested by confusion, acute mental status change Severe laryngeal edema manifested by stridor |
5 | Stenting |
| 5—Death | Death | <1 |
Note: While grade 1 and 2 symptoms may be visually striking, they do not necessitate emergent treatment and only rarely rapidly progress to grade 3 or 4 where urgent/emergent stenting is actually indicated.
Treatment Approaches
Initial Treatment
In cases of acute SVC syndrome with severe symptoms, initial treatment steps are important. Fortunately, less than 15% of cases of SVC obstruction present with severe symptoms. 22 23 Elevation of the head of bed and supplemental oxygen are simple steps in symptom abatement. Intubation should be performed if significant laryngeal edema is present. Steroids can reduce tumor size of lymphoma and thymoma and can be helpful if these tumor subtypes are suspected or known. There is no literature to support giving diuretics.
Chemotherapy/Radiation
In malignant cases without severe symptoms (Yu grade 1 or 2/Kishi score <4), treating the underlying tumor with chemotherapy and radiation to relieve SVC obstruction is preferred.
Prior to the mid-1990s when studies demonstrated the utility of endovascular stenting in cases of SVC syndrome, 24 25 26 patients were treated with emergent radiation therapy (RT). 27 28 While radiation remains a valuable tool in the armamentarium to treat SVC syndrome, for multiple reasons it should no longer be used as first-line therapy in patients with high-grade symptoms. First, approximately 60% of patients who have malignant SVC syndrome have no known prior diagnosis; radiation prior to biopsy obscures subsequent pathologic diagnosis. Then, there is a 3- to 30-day delay in relief of symptoms; in fact, RT may acutely exacerbate SVC obstruction due to edema. 29
In the nonemergent setting, radiation is an effective tool. By shrinking the mediastinal mass, it can relieve SVC syndrome symptoms in 75 to 80% of cases. Its effectiveness is dependent on histologic subtype; lymphoma and SCLC respond well, while radiation is less successful in NSCLC. 3 30 Radiation can induce subsequent fibrotic changes in the blood vessels of the irradiated field, a potential cause of delayed SVC obstruction 31 32 33 (see Fig. 2 ).
Chemotherapy is useful for long-term control of tumor as well and can shrink the tumor and relieve symptoms of SVC obstruction. Negative impact on quality of life from chemotherapy and radiation should be considered, especially in patients with a terminal prognosis.
Patients whose SVC obstruction and symptoms fail to respond to chemo and/or radiation or whose symptoms return after initial abatement are classified as primary failure and relapse. This occurs in approximately 10 and 20% of cases, respectively.
Endovascular Treatment
Indications
SVC stenting was first described in 1986 and currently plays a major role in the management of SVC syndrome. 34 Stenting is indicated in emergent situations, such as when the Kishi score is ≥4. Stenting is preferred over other treatment modalities, as it leads to a more rapid SVC symptoms when compared with chemotherapy and radiation. 26 35 Stenting is also indicated in cases of more mild symptoms that persist or recur after systemic treatment.
Preprocedural Evaluation
Preprocedural cross-sectional imaging, preferably with contrast-enhanced CT, is essential in both making the diagnosis and planning the procedure including expected stent length and diameter of the stent. Preprocedural imaging can also identify rare anatomic variants such as a left SVC or duplicated SVC (see case 3). If the patient is unable to lie flat, general anesthesia is indicated. In addition to a variety of possible bare metal stents and angioplasty balloons, occlusion balloons, covered stents, and drainage catheters should be immediately available for bail-out in the setting of vessel rupture or pericardial tamponade. Consent should include the risks, benefits, and alternatives to endovascular treatment.
Access
Arm, internal jugular, or femoral vein approaches are all acceptable approaches and depend on operator preference. Femoral vein approach allows for deployment and stabilization of the cranial aspect of the stent which may lessen the chances of caudal migration. Through and through access increases wire stability mitigating the chance of the stent migrating into the right ventricle.
Evaluation Prior to Stenting
Predilation should be performed only if necessary to advance the stent delivery system, as it can increase the risk of PE and vessel rupture. If performing predilation, it should be done slowly. If there is underlying thrombus, consider heparin bolus during procedure 70 U/kg.
In chronic benign occlusion, endovascular interventions are now considered first-line therapy. 36 In the setting of pacemaker-induced SVC obstruction, pacer wires were previously thought to have to be removed before recanalization and stenting to be replaced after stenting is completed. 37 However, there exist reports that stents can be safely placed adjacent to/over pacer wires without removal because pacer wires have electrical insulation and are likely covered by vessel endothelium. 38 39 Certainly, consultation with cardiology team is needed for pacer-dependent patients. Recanalization in the setting of long-standing benign occlusion can be difficult. While traditional catheter and wire crossing may be successful, sharp recanalization may be necessary ( Fig. 6 ). The back end of a hydrophilic wire, a long Chiba needle, or a radiofrequency ablation wire can be used to cross the occlusion wire. 40 Surgical SVC bypass can be considered in cases of endovascular failure. 6 While angioplasty alone may be appropriate in some cases of benign obstruction, fibrotic changes may lead to early restenosis.
Fig. 6.
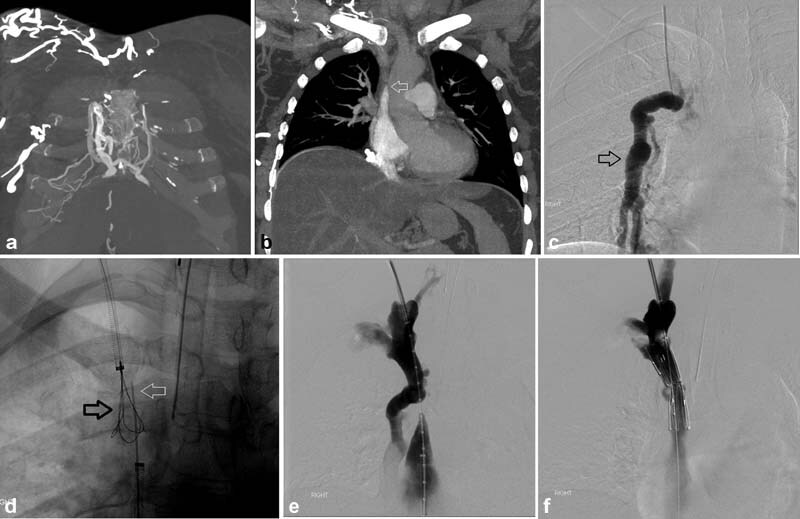
A 48-year-old woman with a history of stage II breast cancer status post mastectomy and chemotherapy developed SVC syndrome, manifesting as headaches, facial puffiness, and subjective throat swelling without evidence of active disease. The SVC occlusion was thought to be secondary to prior port placement. Coronal CT showed multiple diffuse chest wall collaterals ( a ) and SVC occlusion (arrow, b ). A venogram demonstrated SVC occlusion with a large internal mammary collateral (arrow, c ). Conventional recanalization of the SVC was unsuccessful; so, sharp recanalization was performed using a long 21-G Chiba needle (white arrow) advanced from a groin approach directed to a snare placed from a jugular approach (black arrow; ( d ). After through-and-through access was obtained ( e ), a 15-mm-diameter Z stent was placed and post dilated to 12 mm, successfully establishing in line flow to the RA without significant opacification of previously seen large internal mammary collateral vein ( f ).
Stenting
There are a wide variety of available stents ( Table 3 ). In general, bare metal self-expanding stents are preferred, as they have greater wall opposition, lower delivery profile, and are more flexible compared with other options. In most cases, a single stent is sufficient. For example, in a study published in 2017, a single stent was used in 116 of 141 patients. 1 Unilateral brachiocephalic to SVC stenting is simpler, safer, and has longer patency compared with bilateral kissing stents. 41
Table 3. Various stent choices with potential advantages and disadvantages.
| Stent type | Advantages | Disadvantages |
|---|---|---|
| Z stent (Cook Medical Inc, Bloomington, IN) |
Minimal migration due to fixation barbs No foreshortening |
Tumor in-growth due to large interstices
63
Inflexible Bulky delivery system |
| Wallstent (Boston Scientific, Natick, MA) |
Flexible Large published and clinical experience (e.g., Lanciego et al 47 ) Tight-interstices |
Stent migration (i.e., “watermelon seed”)
67
Weaker radial strength with larger diameters Foreshorten by ∼20% |
| Balloon-expandable stents | Precise deployment High radial force Can be used for staged enlargement of SVC if needed due to concerns for right heart failure |
Delayed migration if extrinsic compression is relieved Nonconformity Short lengths |
| Covered stents | Useful in cases of tumor in-growth Improved patency in benign 68 and malignant diseases 42 |
Covering of the azygous/other important collaterals Potential increased risk of migration compared with bare stents 42 Should generally not go beyond the brachiocephalic vein confluence. However, a study included 29 patients undergoing unilateral covered stent placement without development of contralateral arm swelling 69 |
While studies have shown that covered stents may have greater primary patency in both benign and malignant etiologies by precluding tumor ingrowth, they are expensive, have a larger delivery systems, generally are available in smaller sizes and have a perceived greater propensity for migration. 36 42 Cook-Z stents (Cook Medical, Bloomington, IN) have been in clinical use for more than 30 years with multiple studies spanning four decades which confirm their safety and efficacy. 43 44 45 The advantages of the Z stent include larger sizes (15–30 mm diameter) and barbs which prevent migration. However, a study published in 2020 showed that Z stents have lower efficacy in clinical success in malignant SVC obstruction due to tumor in-growth through interstices 46 ( Fig. 7 ). Other disadvantages of Z stents include the large delivery sheath (16 Fr) and rigidity of the stent. Wall stents have a long track record of safety and efficacy for SVC stenting. 47 48 Disadvantages include stent migration ( Figs. 8 and 9 ) and foreshortening. 49
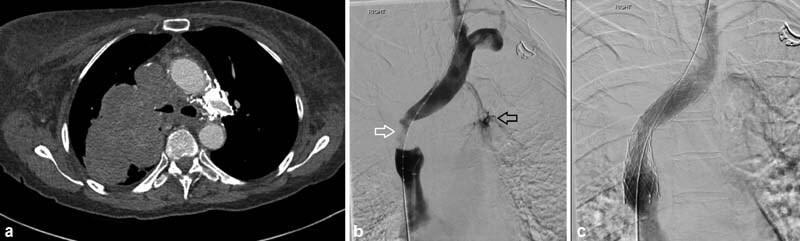
Fig. 7.
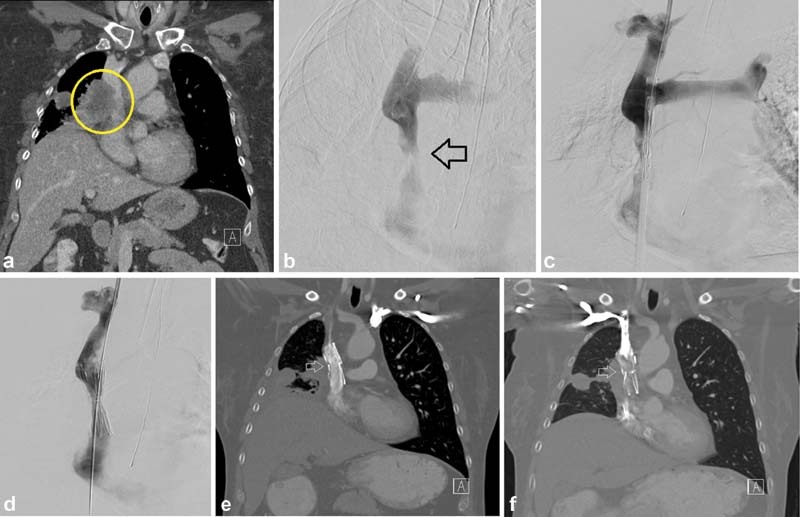
A 54-year-old woman presented with face, arm, and neck swelling as well as headaches. A CT showed large right perihilar tumor invading into the mediastinum and tumor invasion into the SVC (circle, a ), with a subsequent pathologic diagnosis of small cell lung cancer. Initial venogram and pre–stent deployment venogram ( b and c ) confirmed the SVC stenosis (arrow, b ). A 20-mm-diameter Cook-Z (Cook Medical; Bloomington, IN) stent was placed and post dilated to 10 mm ( d ). After tissue diagnosis was made, she was started on chemotherapy and radiation. CT 6 months post stenting showed a widely patent stent (arrow, e ). At 18 months, CT showed tumor invasion into the stent and SVC (arrow, f ).
Fig. 8.
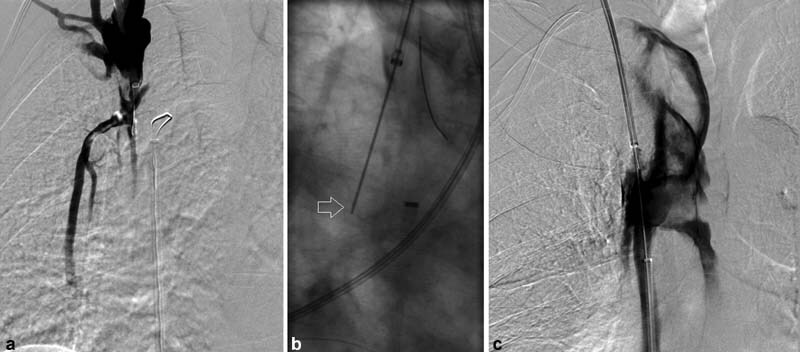
A 52-year-old woman with no significant past medical history presented with a several weeks' history of fatigue, facial congestion, cough, and dyspnea with exertion. She was found to have a large mediastinal mass ( a ), subsequently found to be poorly differentiated thymic carcinoma. A venogram revealed a long-segment SVC stenosis (arrows, b ). A 24 mm × 45 mm wallstent (Boston Scientific) was deployed (arrows, c ) which immediately migrated into the right atrium (arrows, d ). Fortunately, through-and-through access had been obtained. The stent was then retracted into the IVC using a Coda balloon (arrows, e ). A 20-mm Cook-Z stent was placed in the SVC (arrows, f ). In this case, despite the stent being appropriately oversized compared with reference vessel diameter of 18 mm, the stent migrated.
Fig. 9.
A 62-year-old man had stage 4 malignant mesothelioma complicated by SVC syndrome. CT shows complete SVC obstruction (arrow, a ). Venography revealed a low SVC obstruction (arrow, b ). A 16-mm wallstent (Boston Scientific) was placed, but the stent migrated caudally with the bottom end in the low right atrium (black arrow, c ). An 18-mm wallstent was placed within the initial stent extending into the intrahepatic IVC stabilizing the first stent and preventing its migration into the right ventricle (arrow, d ).
Stent oversizing by 10 to 20% of normal reference vessel is recommended to prevent subsequent migration, either on table or with subsequent tumor shrinkage. If possible, one should extend the stent 1 cm above and below the area of narrowing/occlusion. It is advisable to deploy more of the stent above the stenosis than below to lessen the risk of caudal migration. Too large of a stent is dangerous, as it can tear the SVC especially if postdilation is done with greater than 16-mm balloon ( Fig. 10 ); one study, which kept balloon angioplasty below 16 mm, demonstrated no cases of SVC injury or pericardial tamponade. 50 If SVC tear occurs below the pericardial reflection, pericardial tamponade may occur. 51 The potential for stent migration is not obviated with stent oversizing. Published case reports and our own institutional experience (see Fig. 7 ) suggest that stent migration exists with 24-mm-diameter stents. 52 Treatment of stent migration includes stent repositioning or bridging stent into the IVC stent, balloon-assistant snaring, and superior vena cava to inferior vena cava bridging (see Figs. 8 and 9 ). 53 54 55 Table 3 outlines potential benefits and drawbacks of various stents used for SVC stenting.
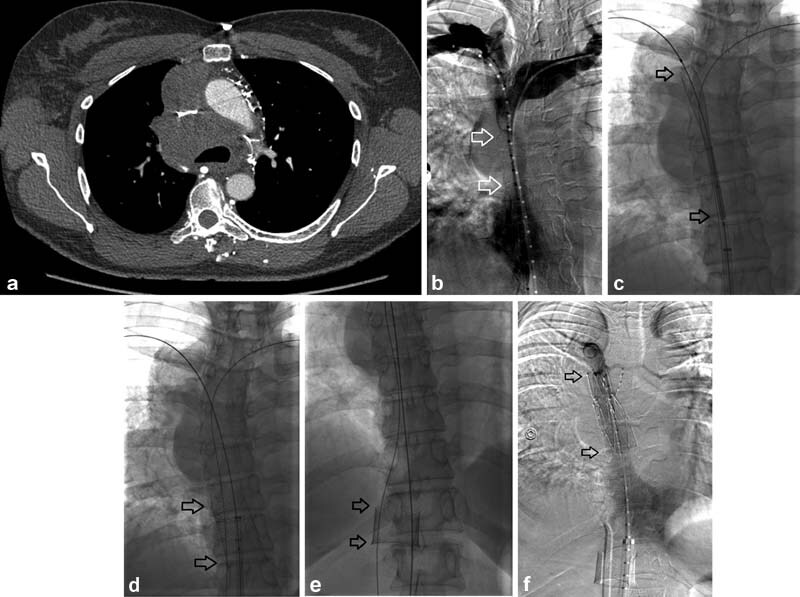
Fig. 10.
A 39-year-old woman had combined immunodeficiency syndrome and a long-standing indwelling central venous catheter for medication administration. She developed headaches, arm and facial swelling; a chest CT was performed which revealed SVC stenosis with superimposed thrombosis (arrow, a ). Venography demonstrated long-segment SVC stenosis extending into the bilateral brachiocephalic veins ( b ). Two “kissing” 14-mm Zilver (Cook Medical) brachiocephalic vein stents were deployed extending into the SVC with each dilated to 12 mm ( c ). After angioplasty she developed hypotension and hemopericardium. A pericardial drain was placed emergently (not shown) and the patient was taken to the operating room for an emergency sternotomy, pericardiotomy, and bovine pericardial patch repair of an SVC laceration in the distal SVC. SVC perforation can be avoided when post stenting balloon angioplasty is kept to 16 mm or less.
Postprocedural Management
While anticoagulation for 1 month after stent placement is common, studies have shown no greater patency with versus without anticoagulation. 50 56 57 Anticoagulation can therefore be skipped in patients with potential risks associated with anticoagulation who have good flow after stent placement.
Outcomes
Technical success is defined when there is less than 30% residual stenosis. 58 Other adjunctive intraprocedural measures of success include reduction pressure gradient to less than 3 mm Hg, rapid flow across the stent, and no filling of previously seen collaterals. Ninety-eight percent of technical success has been reported in malignant causes 1 and 88% of technical success in benign SVC syndrome. 36 In the acute emergent setting, successful stenting provides rapid relief of symptoms, typically within 24 hours. 47
Long-term patency of the stents is variable and dependent on multiple factors. Although 6-month primary patency is low, between 70 and 90%, in cases of reocclusion flow can typically be established with endovascular intervention. 6 26 59 In cases where stent occlusion has occurred once, subsequent multiple repeat interventions are sometimes necessary. 1 In cases of malignant SVC obstruction treated with bare metal stenting, tumor in-growth between stent interstices can be treated with covered stent placement ( Fig. 11 ). Patency after intervention for benign SVC obstruction is slightly worse than malignancy-related SVC syndrome, especially in patients on hemodialysis. 60 61 62
Fig. 11.
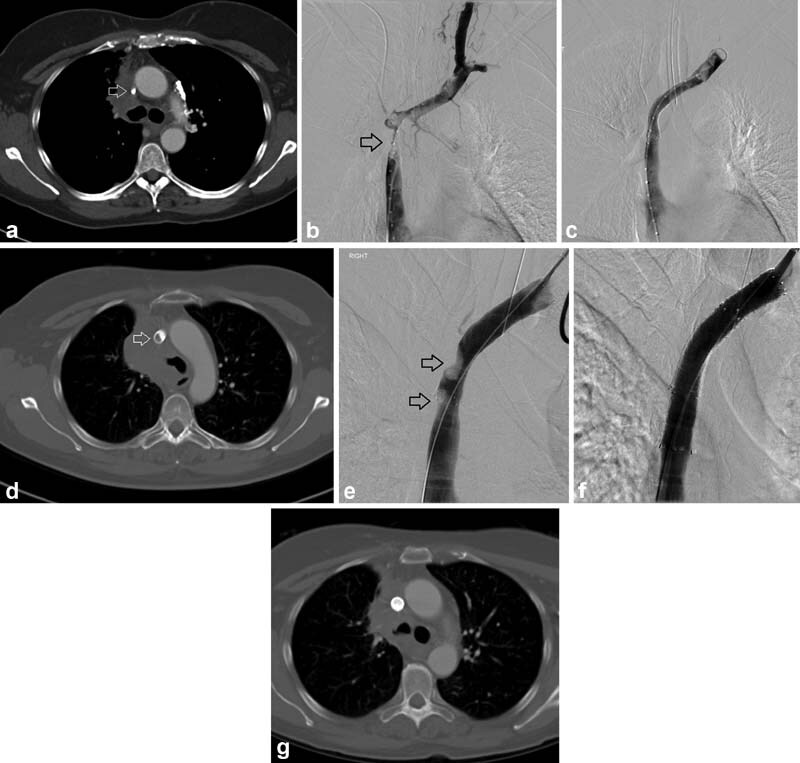
A 63-year-old woman with small cell lung cancer had facial swelling, nasal stuffiness, and orthopnea after treatment with chemotherapy and immunotherapy. Given her persistent symptoms despite systemic therapy, the decision was made to pursue endovascular therapy. CT ( a ) and venography ( b ) showed SVC occlusion (arrows) as well as right brachiocephalic vein occlusion and left brachiocephalic vein stenosis. A bare metal stent (12 mm × 60 mm Vici stent; Boston Scientific, Marlborough, MA) was placed and post–balloon dilated to 10 mm. Immediate post procedure venography showed good flow with interval non-opacification of collateral vessels ( c ). Her symptoms resolved. Six months after this bare metal stent was placed, mild symptoms recurred. CT showed tumor in-growth in the stent (arrow, d ). Venography demonstrated two areas of luminal narrowing (arrows, e ). This was treated with placement of a 12-mm-diameter covered stent across the areas of tumor in-growth ( e ). Venography ( f ) and subsequent CT ( g ) showed no residual stenosis.
Complications of Endovascular Treatment
Recent meta-analyses showed the rate of significant complications of 2% and procedure-related death of approximately 1 to 2%. 59 63 64 One of the most feared complications includes stent migration to the RA (see Figs. 8 and 9 ). Another complication is SVC perforation which can occur with overdilation of the SVC (see Fig. 10 ) or during sharp recanalization attempts ( Fig. 12 ). Aiming the needle or wire cranially (see Fig. 6 ) is often preferred, as one does not create a false track from the pressurized vessels above the occlusion.
Fig. 12.
A 71-year-old man with multiple myeloma presented with port-induced SVC occlusion ( a ). After multiple passes were made from cranial to caudal with the back end of glide wire (arrow, b ), through-and-through access was obtained. However, the patient subsequently developed hypoxia and hypotension. Contrast injection demonstrated massive extravasation into the right hemithorax ( c ). The patient suffered pulseless electrical activity arrest and died.
Another potential cause of death is acute right heart strain ( Fig. 13 ). This is especially true in a patient with concurrent pulmonary artery obstruction and in those with underlying preexisting cardiac dysfunction. 1 62 65 In patients with known concurrent pulmonary artery obstruction, pulmonary artery stenting is a potential option. In patients with suspected underlying cardiac dysfunction, preprocedural echo should be performed. Preprocedural diuresis and/or staged dilation, using a balloon-expandable stent, can be considered.
Fig. 13.
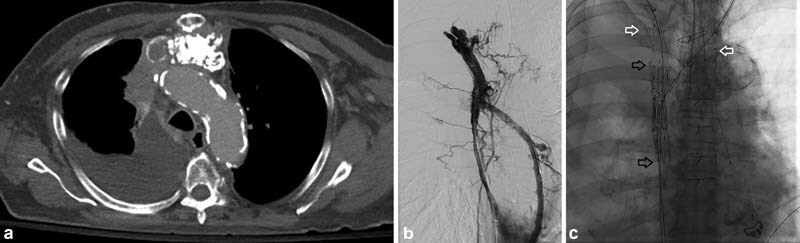
A 77-year-old man with a history of a malignant thymoma was treated with debulking surgery, chemotherapy, and radiation. He presented with progressive arm and face swelling; a CT ( a ) and venography ( b ) showed SVC occlusion with extensive collateralization. The SVC was recanalized using two overlapping 20-mm Z stents which were dilated to 16 mm. Then, kissing 12-mm covered stents (Bard Fluency; BD Bard, Franklin, NJ) were placed in the brachiocephalic veins (black arrows—Z stents; white arrows, upstream extension of fluency stents; c ). After stenting, good flow was achieved ( d ). However, oxygen saturations decreased to the <50% despite non-rebreather mask placement. A chest tube was placed and a chest radiograph showed significant pulmonary edema consistent with fluid overload and acute right heart failure ( e ). Transesophageal echo showed acute right heart failure likely due to acute increase in venous return. The patient died shortly thereafter.
Conclusion
SVC syndrome can be due to malignant or benign causes. Endovascular therapy, specifically with stenting, is the treatment of choice in severe symptoms such as oropharyngeal or cerebral edema. Stenting is also the preferred treatment in patients who do not respond to or have recurrent symptoms after chemotherapy or radiation.
Footnotes
Conflict of Interest No relevant conflict of interest or disclosures.
References
- 1.Büstgens F A, Loose R, Ficker J H, Wucherer M, Uder M, Adamus R. Stent implantation for superior vena cava syndrome of malignant cause. Röfo Fortschr Geb Röntgenstr Nuklearmed. 2017;189(05):423–430. doi: 10.1055/s-0042-122147. [DOI] [PubMed] [Google Scholar]
- 2.Rice T W, Rodriguez R M, Light R W. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore) 2006;85(01):37–42. doi: 10.1097/01.md.0000198474.99876.f0. [DOI] [PubMed] [Google Scholar]
- 3.Rowell N P, Gleeson F V. Steroids, radiotherapy, chemotherapy and stents for superior vena caval obstruction in carcinoma of the bronchus. Cochrane Database Syst Rev. 2001;(04):CD001316. doi: 10.1002/14651858.CD001316. [DOI] [PubMed] [Google Scholar]
- 4.Wan J F, Bezjak A. Superior vena cava syndrome. Hematol Oncol Clin North Am. 2010;24(03):501–513. doi: 10.1016/j.hoc.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Dela Cruz C S, Tanoue L T, Matthay R A. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med. 2011;32(04):605–644. doi: 10.1016/j.ccm.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Picquet J, Blin V, Dussaussoy C, Jousset Y, Papon X, Enon B. Surgical reconstruction of the superior vena cava system: indications and results. Surgery. 2009;145(01):93–99. doi: 10.1016/j.surg.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Schifferdecker B, Shaw J A, Piemonte T C, Eisenhauer A C. Nonmalignant superior vena cava syndrome: pathophysiology and management. Catheter Cardiovasc Interv. 2005;65(03):416–423. doi: 10.1002/ccd.20381. [DOI] [PubMed] [Google Scholar]
- 8.Caers J, Fontaine C, Vinh-Hung V. Catheter tip position as a risk factor for thrombosis associated with the use of subcutaneous infusion ports. Support Care Cancer. 2005;13(05):325–331. doi: 10.1007/s00520-004-0723-1. [DOI] [PubMed] [Google Scholar]
- 9.Seo M, Shin W J, Jun I G. Central venous catheter-related superior vena cava syndrome following renal transplantation - a case report. Korean J Anesthesiol. 2012;63(06):550–554. doi: 10.4097/kjae.2012.63.6.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deshwal H, Ghosh S, Magruder K, Bartholomew J R, Montgomery J, Mehta A C. A review of endovascular stenting for superior vena cava syndrome in fibrosing mediastinitis. Vasc Med. 2020;25(02):174–183. doi: 10.1177/1358863X19884130. [DOI] [PubMed] [Google Scholar]
- 11.McIntire F T, Sykes E M., Jr Obstruction of the superior vena cava; a review of the literature and report of two personal cases. Ann Intern Med. 1949;30(05):925–960. doi: 10.7326/0003-4819-30-5-925. [DOI] [PubMed] [Google Scholar]
- 12.Bayer O, Schummer C, Richter K, Fröber R, Schummer W. Implication of the anatomy of the pericardial reflection on positioning of central venous catheters. J Cardiothorac Vasc Anesth. 2006;20(06):777–780. doi: 10.1053/j.jvca.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Kim H J, Kim H S, Chung S H. CT diagnosis of superior vena cava syndrome: importance of collateral vessels. AJR Am J Roentgenol. 1993;161(03):539–542. doi: 10.2214/ajr.161.3.8352099. [DOI] [PubMed] [Google Scholar]
- 14.Stanford W, Jolles H, Ell S, Chiu L C. Superior vena cava obstruction: a venographic classification. AJR Am J Roentgenol. 1987;148(02):259–262. doi: 10.2214/ajr.148.2.259. [DOI] [PubMed] [Google Scholar]
- 15.Eren S, Karaman A, Okur A. The superior vena cava syndrome caused by malignant disease. Imaging with multi-detector row CT. Eur J Radiol. 2006;59(01):93–103. doi: 10.1016/j.ejrad.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Hussein F A, Mawla N, Befeler A S, Martin K J, Lentine K L. Formation of downhill esophageal varices as a rare but serious complication of hemodialysis access: a case report and comprehensive literature review. Clin Exp Nephrol. 2008;12(05):407–415. doi: 10.1007/s10157-008-0055-4. [DOI] [PubMed] [Google Scholar]
- 17.Loudin M, Anderson S, Schlansky B. Bleeding ‘downhill’ esophageal varices associated with benign superior vena cava obstruction: case report and literature review. BMC Gastroenterol. 2016;16(01):134. doi: 10.1186/s12876-016-0548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Felson B, Lessure A P. “Downhill” varices of the esophagus. Dis Chest. 1964;46:740–746. [PubMed] [Google Scholar]
- 19.Uceda P V, Peralta Rodriguez J, Vela H. Management of superior vena cava occlusion causing bleeding “downhill” esophageal varices. J Endovasc Ther. 2021;28(03):469–473. doi: 10.1177/1526602821989330. [DOI] [PubMed] [Google Scholar]
- 20.Aloufi F F, Alabdulkarim F M, Alshahrani M A. The focal hepatic hot spot (“hot quadrate”) sign. Abdom Radiol (NY) 2017;42(04):1289–1290. doi: 10.1007/s00261-016-0997-z. [DOI] [PubMed] [Google Scholar]
- 21.Ahmann F R. A reassessment of the clinical implications of the superior vena caval syndrome. J Clin Oncol. 1984;2(08):961–969. doi: 10.1200/JCO.1984.2.8.961. [DOI] [PubMed] [Google Scholar]
- 22.Yu J B, Wilson L D, Detterbeck F C. Superior vena cava syndrome – a proposed classification system and algorithm for management. J Thorac Oncol. 2008;3(08):811–814. doi: 10.1097/JTO.0b013e3181804791. [DOI] [PubMed] [Google Scholar]
- 23.Gauden S J. Superior vena cava syndrome induced by bronchogenic carcinoma: is this an oncological emergency? Australas Radiol. 1993;37(04):363–366. doi: 10.1111/j.1440-1673.1993.tb00096.x. [DOI] [PubMed] [Google Scholar]
- 24.Furui S, Sawada S, Kuramoto K. Gianturco stent placement in malignant caval obstruction: analysis of factors for predicting the outcome. Radiology. 1995;195(01):147–152. doi: 10.1148/radiology.195.1.7892457. [DOI] [PubMed] [Google Scholar]
- 25.Nicholson A A, Ettles D F, Arnold A, Greenstone M, Dyet J F. Treatment of malignant superior vena cava obstruction: metal stents or radiation therapy. J Vasc Interv Radiol. 1997;8(05):781–788. doi: 10.1016/s1051-0443(97)70660-2. [DOI] [PubMed] [Google Scholar]
- 26.Rowell N P, Gleeson F V. Steroids, radiotherapy, chemotherapy and stents for superior vena caval obstruction in carcinoma of the bronchus: a systematic review. Clin Oncol (R Coll Radiol) 2002;14(05):338–351. doi: 10.1053/clon.2002.0095. [DOI] [PubMed] [Google Scholar]
- 27.Goodman R. Superior vena cava syndrome. Clinical management. JAMA. 1975;231(01):58–61. doi: 10.1001/jama.231.1.58. [DOI] [PubMed] [Google Scholar]
- 28.Varricchio C. Clinical management of superior vena cava syndrome. Heart Lung. 1985;14(04):411–416. [PubMed] [Google Scholar]
- 29.Straka C, Ying J, Kong F M, Willey C D, Kaminski J, Kim D W. Review of evolving etiologies, implications and treatment strategies for the superior vena cava syndrome. Springerplus. 2016;5:229. doi: 10.1186/s40064-016-1900-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armstrong B A, Perez C A, Simpson J R, Hederman M A. Role of irradiation in the management of superior vena cava syndrome. Int J Radiat Oncol Biol Phys. 1987;13(04):531–539. doi: 10.1016/0360-3016(87)90068-x. [DOI] [PubMed] [Google Scholar]
- 31.Weintraub N L, Jones W K, Manka D. Understanding radiation-induced vascular disease. J Am Coll Cardiol. 2010;55(12):1237–1239. doi: 10.1016/j.jacc.2009.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Putten J W, Schlosser N J, Vujaskovic Z, Leest A H, Groen H J. Superior vena cava obstruction caused by radiation induced venous fibrosis. Thorax. 2000;55(03):245–246. doi: 10.1136/thorax.55.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castonguay M, Rodrigues G, Vincent M, Malthaner R A, Guo L R. Chemoradiation-induced superior vena cava syndrome: a case report. Can Respir J. 2008;15(08):444–446. doi: 10.1155/2008/279786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charnsangavej C, Carrasco C H, Wallace S. Stenosis of the vena cava: preliminary assessment of treatment with expandable metallic stents. Radiology. 1986;161(02):295–298. doi: 10.1148/radiology.161.2.3763891. [DOI] [PubMed] [Google Scholar]
- 35.Azizi A H, Shafi I, Zhao M. Endovascular therapy for superior vena cava syndrome: a systematic review and meta-analysis. EClinicalMedicine. 2021;37:100970. doi: 10.1016/j.eclinm.2021.100970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rizvi A Z, Kalra M, Bjarnason H, Bower T C, Schleck C, Gloviczki P. Benign superior vena cava syndrome: stenting is now the first line of treatment. J Vasc Surg. 2008;47(02):372–380. doi: 10.1016/j.jvs.2007.09.071. [DOI] [PubMed] [Google Scholar]
- 37.Klop B, Scheffer M G, McFadden E, Bracke F, van Gelder B. Treatment of pacemaker-induced superior vena cava syndrome by balloon angioplasty and stenting. Neth Heart J. 2011;19(01):41–46. doi: 10.1007/s12471-010-0052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lanciego C, Rodriguez M, Rodriguez A, Carbonell M A, García L G. Permanent pacemaker-induced superior vena cava syndrome: successful treatment by endovascular stent. Cardiovasc Intervent Radiol. 2003;26(06):576–579. doi: 10.1007/s00270-003-0070-5. [DOI] [PubMed] [Google Scholar]
- 39.Riley R F, Petersen S E, Ferguson J D, Bashir Y. Managing superior vena cava syndrome as a complication of pacemaker implantation: a pooled analysis of clinical practice. Pacing Clin Electrophysiol. 2010;33(04):420–425. doi: 10.1111/j.1540-8159.2009.02613.x. [DOI] [PubMed] [Google Scholar]
- 40.Guimaraes M, Schonholz C, Hannegan C, Anderson M B, Shi J, Selby B., Jr Radiofrequency wire for the recanalization of central vein occlusions that have failed conventional endovascular techniques. J Vasc Interv Radiol. 2012;23(08):1016–1021. doi: 10.1016/j.jvir.2012.05.049. [DOI] [PubMed] [Google Scholar]
- 41.Dinkel H P, Mettke B, Schmid F, Baumgartner I, Triller J, Do D D. Endovascular treatment of malignant superior vena cava syndrome: is bilateral wallstent placement superior to unilateral placement? J Endovasc Ther. 2003;10(04):788–797. doi: 10.1177/152660280301000416. [DOI] [PubMed] [Google Scholar]
- 42.Gwon D I, Ko G Y, Kim J H, Shin J H, Yoon H K, Sung K B. Malignant superior vena cava syndrome: a comparative cohort study of treatment with covered stents versus uncovered stents. Radiology. 2013;266(03):979–987. doi: 10.1148/radiol.12120517. [DOI] [PubMed] [Google Scholar]
- 43.Rösch J, Uchida B T, Hall L D. Gianturco-Rösch expandable Z-stents in the treatment of superior vena cava syndrome. Cardiovasc Intervent Radiol. 1992;15(05):319–327. doi: 10.1007/BF02733957. [DOI] [PubMed] [Google Scholar]
- 44.Gianturco Z-stent placement for the treatment of chronic central venous occlusive disease: implantation of 208 stents in 137 symptomatic patients. Diagn Interv Radiol. 2021;27(01):72–78. doi: 10.5152/dir.2020.19282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaines P A, Belli A M, Anderson P B, McBride K, Hemingway A P.Superior vena caval obstruction managed by the Gianturco Z Stent Clin Radiol 19944903202–206., discussion 207–208 [DOI] [PubMed] [Google Scholar]
- 46.Han T M, Bondarev S, Keller E J, Vogelzang R L, Resnick S A. Efficacy of endovascular Z-configuration stenting for malignant versus nonmalignant caval obstruction. J Vasc Surg Venous Lymphat Disord. 2020;8(06):939–944. doi: 10.1016/j.jvsv.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 47.Lanciego C, Pangua C, Chacón J I. Endovascular stenting as the first step in the overall management of malignant superior vena cava syndrome. AJR Am J Roentgenol. 2009;193(02):549–558. doi: 10.2214/AJR.08.1904. [DOI] [PubMed] [Google Scholar]
- 48.Hennequin L M, Fade O, Fays J G. Superior vena cava stent placement: results with the wallstent endoprosthesis. Radiology. 1995;196(02):353–361. doi: 10.1148/radiology.196.2.7617844. [DOI] [PubMed] [Google Scholar]
- 49.Verstandig A G, Bloom A I, Sasson T, Haviv Y S, Rubinger D. Shortening and migration of wallstents after stenting of central venous stenoses in hemodialysis patients. Cardiovasc Intervent Radiol. 2003;26(01):58–64. doi: 10.1007/s00270-002-1953-6. [DOI] [PubMed] [Google Scholar]
- 50.Fagedet D, Thony F, Timsit J F. Endovascular treatment of malignant superior vena cava syndrome: results and predictive factors of clinical efficacy. Cardiovasc Intervent Radiol. 2013;36(01):140–149. doi: 10.1007/s00270-011-0310-z. [DOI] [PubMed] [Google Scholar]
- 51.Da Ines D, Chabrot P, Motreff P. Cardiac tamponade after malignant superior vena cava stenting: two case reports and brief review of the literature. Acta Radiol. 2010;51(03):256–259. doi: 10.3109/02841850903578807. [DOI] [PubMed] [Google Scholar]
- 52.Vijayvergiya R, Kanabar K, Kaushal S, Lal A, Krishnan S. Endovascular treatment of a migrated superior vena cava stent in the right atrium. J Invasive Cardiol. 2020;32(06):E168–E169. doi: 10.25270/jic/19.00328. [DOI] [PubMed] [Google Scholar]
- 53.Taylor J D, Lehmann E D, Belli A M. Strategies for the management of SVC stent migration into the right atrium. Cardiovasc Intervent Radiol. 2007;30(05):1003–1009. doi: 10.1007/s00270-007-9109-3. [DOI] [PubMed] [Google Scholar]
- 54.Srinathan S, McCafferty I, Wilson I. Radiological management of superior vena caval stent migration and infection. Cardiovasc Intervent Radiol. 2005;28(01):127–130. doi: 10.1007/s00270-003-0183-x. [DOI] [PubMed] [Google Scholar]
- 55.Okamoto D, Takeuchi Y, Arai Y. Bridging stent placement through the superior vena cava to the inferior vena cava in a patient with malignant superior vena cava syndrome and an iodinated contrast material allergy. Jpn J Radiol. 2014;32(08):496–499. doi: 10.1007/s11604-014-0324-y. [DOI] [PubMed] [Google Scholar]
- 56.Haddad M M, Thompson S M, McPhail I R. Is long-term anticoagulation required after stent placement for benign superior vena cava syndrome? J Vasc Interv Radiol. 2018;29(12):1741–1747. doi: 10.1016/j.jvir.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 57.Thony F, Fagedet D, Michoud M, Moro-Sibilot D, Ferretti G R, Rodière M. Anticoagulation is not mandatory after stenting for malignant superior vena cava syndrome. Cardiovasc Intervent Radiol. 2014;37(05):1403–1404. doi: 10.1007/s00270-014-0902-5. [DOI] [PubMed] [Google Scholar]
- 58.Uberoi R. Quality assurance guidelines for superior vena cava stenting in malignant disease. Cardiovasc Intervent Radiol. 2006;29(03):319–322. doi: 10.1007/s00270-005-0284-9. [DOI] [PubMed] [Google Scholar]
- 59.Leon D, Rao S, Huang S.Literature review of percutaneous stenting for palliative treatment of malignant superior vena cava syndrome (SVCS)Acad Radiol2021 [DOI] [PubMed]
- 60.Majumdar S, Shoela R, Kim D J. Endovascular management of SVC syndrome due to fibrosing mediastinitis - a feasibility and safety analysis. Vasc Endovascular Surg. 2018;52(03):202–206. doi: 10.1177/1538574418757401. [DOI] [PubMed] [Google Scholar]
- 61.Kang C H, Yang S B, Lee W H. Comparison of open-cell stent and closed-cell stent for treatment of central vein stenosis or occlusion in hemodialysis patients. Iran J Radiol. 2016;13(04):e37994. doi: 10.5812/iranjradiol.37994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smayra T, Otal P, Chabbert V. Long-term results of endovascular stent placement in the superior caval venous system. Cardiovasc Intervent Radiol. 2001;24(06):388–394. doi: 10.1007/s00270-001-0055-1. [DOI] [PubMed] [Google Scholar]
- 63.Nguyen N P, Borok T L, Welsh J, Vinh-Hung V. Safety and effectiveness of vascular endoprosthesis for malignant superior vena cava syndrome. Thorax. 2009;64(02):174–178. doi: 10.1136/thx.2007.086017. [DOI] [PubMed] [Google Scholar]
- 64.Sobrinho G, Aguiar P. Stent placement for the treatment of malignant superior vena cava syndrome - a single-center series of 56 patients. Arch Bronconeumol. 2014;50(04):135–140. doi: 10.1016/j.arbres.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 65.Skovira V, Ahmed M, Genese T O. Superior vena cava syndrome in conjunction with pulmonary vasculature compromise: a case study and literature review. Am J Case Rep. 2018;19:1237–1240. doi: 10.12659/AJCR.910165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kishi K, Sonomura T, Mitsuzane K. Self-expandable metallic stent therapy for superior vena cava syndrome: clinical observations. Radiology. 1993;189(02):531–535. doi: 10.1148/radiology.189.2.8210386. [DOI] [PubMed] [Google Scholar]
- 67.Slonim S M, Dake M D, Razavi M K. Management of misplaced or migrated endovascular stents. J Vasc Interv Radiol. 1999;10(07):851–859. doi: 10.1016/s1051-0443(99)70127-2. [DOI] [PubMed] [Google Scholar]
- 68.Haddad M M, Simmons B, McPhail I R. Comparison of covered versus uncovered stents for benign superior vena cava (SVC) obstruction. Cardiovasc Intervent Radiol. 2018;41(05):712–717. doi: 10.1007/s00270-018-1906-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cho Y, Gwon D I, Ko G Y. Covered stent placement for the treatment of malignant superior vena cava syndrome: is unilateral covered stenting safe and effective? Korean J Radiol. 2014;15(01):87–94. doi: 10.3348/kjr.2014.15.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]


