Abstract
Introduction
Surgical site infections (SSIs) remain a significant cause of morbidity for surgical patients worldwide and with growing rates of antibiotic resistance, the development of new nonantimicrobial techniques to target SSI reduction is crucial. This review aimed to explore available nonantibiotic intraoperative interventions to reduce the risk of SSI.
Methods
A literature search was undertaken using Medline, Web of Science, Embase, and Cochrane Library databases. Any study published from 1 January 1980 to 1 September 2021 that described any nonantibiotic intraoperative physical technique aiming to reduce SSI rates, with a primary or secondary outcome of SSI rates, was included.
Findings
A total of 45 articles were included in the final scoping review. The current nonantibiotic intraoperative interventions advised for use include chlorhexidine skin preparation with alcohol, pressurised wound irrigation, Triclosan-coated sutures for skin closure, and negative pressure wound therapy. Many other widely used surgical practices do not have the supporting evidence to validate their routine use in clinical practice to reduce SSI rates.
Conclusions
We identified several techniques that can be used in the operating theatre to provide additional opportunities to reduce SSI rates. However, strict adherence to current established SSI prevention guidelines remains the mainstay of ensuring SSI rates remain low.
Keywords: Surgical site infection, Prevention, Surgery, Intraoperative
Introduction
Surgical site infections (SSIs) are a significant cause of morbidity in the surgical population. Around one-fifth of all hospital-acquired infections are SSIs,1,2 and current SSI rates in Europe are reported between 5% and 18%,3 with even higher levels in lower income countries.4 SSIs place sizeable financial strains on healthcare systems worldwide,5 resulting in approximately 1 million additional inpatient days per year in the US alone, costing an additional US$1.6 billion.6 SSIs also have a profound impact on patient outcomes; patients with an SSI postoperatively have a risk of death between 2 and 11 times higher than those without an SSI,7–10 alongside longer hospital stays,5,11,12 increased rates of complications,13 increased rates of hospital readmission,5,14 and an overall reduction in quality of life.15
SSI prevention is perhaps an overlooked area for many practising surgeons, however a recent international Delphi survey identified that the prevention of SSIs after abdominal surgery should be placed as the highest priority topic for guideline development.16 Indeed, all aspects of the pre-, intra-, and postoperative risks can be targeted at every step of the perioperative pathway by all members of the surgical team. SSI care bundles are employed by many surgical departments to ensure a multifaceted risk factor reduction approach occurs, and these have been shown to reduce SSI rates by up to 40%,17 as well as reducing hospital costs and length of hospital stay.18
Surgeons arguably have the most influence on the intraoperative SSI reduction strategies, however outside of prophylactic antibiotics at induction,19–22 many intraoperative interventions trialled have shown limited benefit.23,24 In this scoping review, we present the current evidence around key nonantibiotic interventions available for use intraoperatively, across all surgical specialties, outwith conventional SSI bundle checklists.
Methods
To identify current literature on the main physical nonantibiotic intraoperative measures available, a literature search was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Scoping Reviews (PRISMA-ScR) guidelines.25 An agreed study plan was devised before undertaking the review. A literature search was undertaken using Medline, Web of Science, Embase, and Cochrane Library databases, with grey literature search results subsequently added using the Open Grey database. Additional studies not included in the database search were identified by searching the reference lists of retained articles.
Any study that described a nonantibiotic intraoperative mechanical technique aiming to reduce SSI rates, with a primary or secondary outcome of SSI rates, was included. We defined ‘intraoperative’ as the time between the patient being moved onto and off from the operating table. The search terms employed were based upon a previous Cochrane review on wound irrigation techniques26; the search terms were: (‘Surgical Wound Infection’ OR ‘Surgical Wound Dehiscence’ OR ‘wound complication’ OR ‘SSI’) AND (‘Prevention’ OR ‘Primary Prevention’ OR ‘Prophylaxis’) AND (‘Intra-Operative’ OR ‘Operative Techniques’ OR ‘Operative Surgical Procedures’).
The search included all articles from 1 January 1980 to 1 September 2021, with any duplicates subsequently removed. The search was performed independently by two of the authors (MB and RS), with any discrepancies resolved by discussion and consensus. All article types reporting primary data or secondary data analysis (e.g., meta-analyses) were included. Due to the descriptive nature of the review, no formal data charting process was performed. Any study reporting on nonhuman data was excluded, as were those not published in the English language.
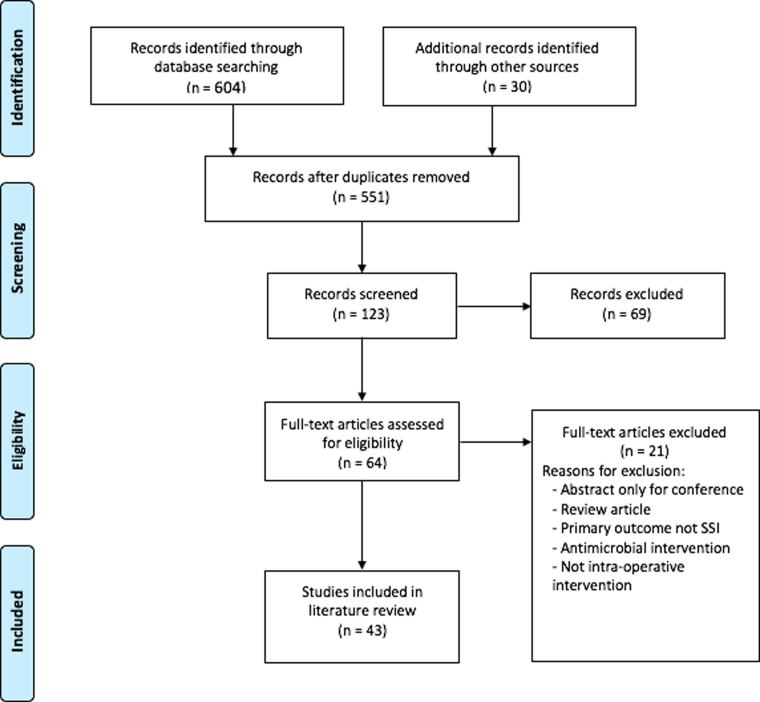
Overall, 608 articles were identified from the literature search for the scoping review, with an additional 30 records identified through other sources. Following screening, 43 articles were included in the final literature review (Figure 1). The articles identified were then categorised into subsections of Skin Preparation, Wound Irrigation, and Closure Techniques and Devices.
Figure 1 .
PRISMA flowchart. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Results
Skin preparation
Although historically performed preoperatively as routine for all surgical patients, it is now commonplace for hair removal to be performed intraoperatively, before the initial incision. Clearly, hair removal has its own benefits by reducing interference at wound closure, however its impact on SSI rates is of greater interest. High-quality systematic reviews have been performed on this topic, demonstrating minimal benefit in elective hair removal at the surgical site to improve SSI rates, both in technique (clipping relative risk (RR) 0.95 (confidence interval (CI) 0.65–1.39) versus shaving RR 1.82 (CI 1.05–3.14)) and in timing (RR 0.83, CI 0.54–1.30),27 and the overall quality of trial data available on this topic is poor.28
More sizeable benefits, however, are seen with the use of preoperative skin preparations, of which the most commonly used are chlorhexidine and povidone-iodine.29 Both skin preparations are used widely across the globe, and, whereas previous data had suggested no sizeable differences in efficacy,30 more recent evidence suggests that chlorhexidine is likely superior in its antimicrobial action, especially when used in alcohol. A large randomised controlled trial (RCT) published in the New England Journal of Medicine demonstrated that overall SSI rates were significantly lower with use of chlorhexidine than with povidone-iodine (9.5% vs 16.1%, p=0.004).31 A subsequent network meta-analysis concurred with these findings, with chlorhexidine halving the risk of infection following any clean operation compared with povidone-iodine.32
Adhesive drapes can be applied to the skin before starting the procedure, through which the initial surgical incision can then be made (eg, OpSite, Smith and Nephew). While often also impregnated with antimicrobial agents such as iodine, the use of adhesive drapes of any kind have shown no benefit in reducing overall SSI rates (RR 1.03 (CI 0.06–1.66)).33 The same is true for the use of wound protectors during the procedure; it was previously theorised that wound protectors would prevent both endogenous- and exogenous-derived pathogens coming into contact with the subcutaneous tissues during the procedure,34 and initial data were indeed promising towards their use.35–38 Yet, whereas there may be a small benefit in a select subset of patients,39 high quality data from a well-referenced RCT of 760 patients undergoing abdominal surgery has shown no benefit with their use, reporting no difference in SSI rates compared with the control groups (24.7% vs 25.4%, p=0.85).40
Wound irrigation
The presence of necrotic tissue in a wound both impedes wound closure and provides the optimal environment for pathogens to grow.41,42 Perioperative wound irrigation aims to reduce both infectious agents and necrotic debris from the wound surface,43 and is used by the vast majority of surgeons in various forms,44 povidone-iodine solution proving the most widely used by general surgeons.45 Povidone-iodine solution is known to be effective against a broad spectrum of pathogens,46 across a range of concentrations, and indeed is superior in its efficacy over saline irrigation in abdominal surgery.47 However, povidone-iodine is accompanied by the theoretical risks of local toxicity and delayed wound healing.48,49
The most recent Cochrane review on wound irrigation and SSI rates was published in 2017, amassing data from 14 studies and including over 6,000 patients, across multiple subspecialities.26 The authors concluded no discernible differences were present in the incidence of SSIs in those who receive any form of wound irrigation versus those who do not (RR 0.87, 95% CI 0.68–1.11).26 A similar systematic review, commissioned by the World Health Organisation (WHO) on the same topic and published in the same year, reported comparable results.50
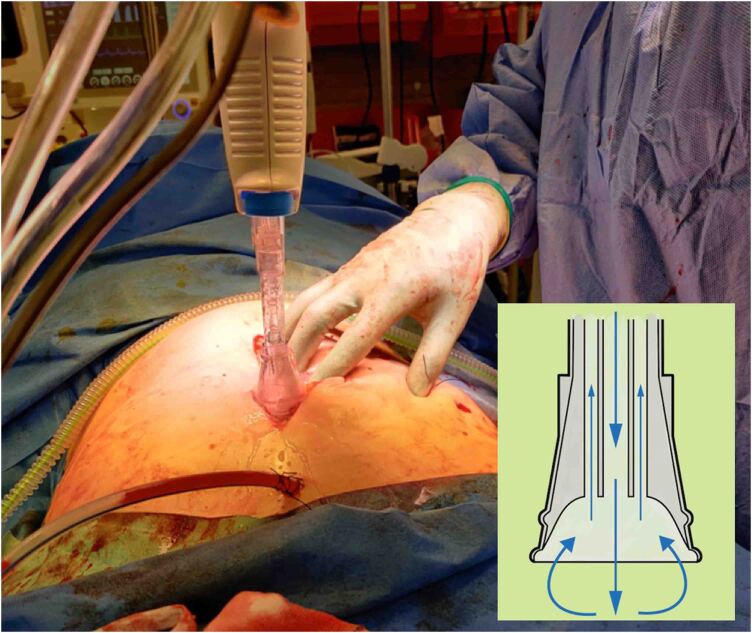
However, in the same Cochrane review, a subanalysis on the use of pressurised irrigation identified a statistically significant reduction in SSI rates. When pressurised irrigation was undertaken, an absolute risk difference of 109 fewer SSIs per 1,000 participants was observed when compared with standard irrigation methods.26 The use of pressurised irrigation, often termed ‘Pulsed Lavage’ (Figure 2), is used widely in orthopaedic surgery,51 but less so in other specialities; the use of pressurised irrigation in general surgery has shown some promise in reducing SSI rates (odds ratio 0.39 (CI 0.25–.62),52 although the majority of studies published thus far are small or retrospective.
Figure 2 .
Use of pulsed lavage at the closure of a laparotomy; inset, direction of flow for fluid irrigation.
Closure techniques and devices
Choice of suture material for closure has been shown to impact rates of SSIs. Multiple suture types are available, varying in both composition and size, for closing both the deep fascial layers and superficial adipose and skin layers. Reducing pathogens harbouring in wounds is known to reduce the risk of SSIs,53 and it is with this rationale that monofilament sutures are classically chosen for closure, providing less surface area for pathogens to survive.
However, a recent network meta-analyses has disproven this widely held dogma, showing that no specific suture type (including Polyglactin, Polydioxanone (PDS), Polypropylene and Nylon) should be considered the ‘best treatment’ for the prevention of SSI,54 with similar conclusions with the use of skin glue too.55 Nevertheless, triclosan-coated sutures (an antimicrobial agent) have begun to show promise, with a meta-analysis of RCT data performed by the National Institute for Health and Care Excellence (NICE) reporting a RR 0.71 (85% CI 0.59–0.85) for the development of a SSI with their use.56 The report suggested use of triclosan-coated sutures will save £13.62 per patient through SSI reduction alone, compared with standard absorbable sutures.56
Current evidence suggests no clear benefit of any one wound dressing type in reducing SSI risk over another,57 apart from perhaps with the use of negative pressure wound therapy (NPWT). NPWT provides a continuous delivery of negative pressure to a wound through the use of a vacuum-device, with the goal of removing excess tissue oedema and promoting granulation tissue formation.58 Although historically used solely for open wounds, the use of NPWT has been extended to include closed surgical incisions too, and its benefit in reducing SSI rates in orthopaedic, cardiothoracic and general surgical procedures has been widely described.59–62 More importantly, NPWT has been shown to be potentially cost-effective for multiple indications and is a promising area of development.63 This differs, however, to the placement of prophylactic negative pressure suction drains in the subcutaneous tissue, which have shown no benefit in reducing SSI rates in the vast majority of surgical procedures.64
Discussion
The WHO has stated that antibiotic resistance remains ‘one of the biggest threats to global health, food security, and development today’ and has become a leading cause of death around the world,65 especially in low-resource settings.66 Antibiotic resistance is as applicable to surgical spheres as to any other, and limiting the prevalence of SSIs should be seen as a priority to every surgeon worldwide. Implementation of the nonantibiotic intraoperative interventions described in this review (Table 1) should be considered by all surgeons if SSI rates are to be curtailed.
Table 1 .
Recommendations of intraoperative interventions to reduce SSI rates, with key references included
|
| Pressurised irrigation to the subcutaneous tissue at closure |
|
| Use of Triclosan-coated sutures for skin closure |
|
| Application of NPWT |
NPWT = negative pressure wound therapy; SSI = surgical site infection
Use of chlorhexidine skin preparation with alcohol, pressurised wound irrigation, Triclosan-coated sutures for skin closure and negative pressure wound therapy NPWT are nonantibiotic measures that can be feasibly incorporated into many surgeons routine surgical practice. We do not suggest that this should detract away from other aspects of the perioperative pathway, where key modifiable risk factors for SSIs, such as optimising nutrition or smoking cessation, can be targeted. Indeed, ensuring a multifaceted risk factor reduction across the perioperative period, typically through use of SSI care bundles, can produce a greater impact on SSI rates than any intraoperative intervention. However, this review provides an evidence-based assessment to practising surgeons on the current intraoperative techniques available to further reduce their SSI rates, outside of current standard SSI bundles.
There are a wide range of effective pre- and postoperative interventions that successfully influence SSI rates that are not covered in this review, as other systematic reviews have previously explored this data more extensively. Moreover, while we aimed to cover all surgical subspeciality techniques in this review, the majority of published research on this topic was in abdominal surgery. Although we did not undertake a formal cost-effectiveness analysis on the interventions reported, the key messages remain that nonantibiotic techniques to reduce SSI rates intraoperatively are available and should be adopted by all surgeons wherever feasible.
In 1958, famed Professor of Surgery and President of the American Surgical Association, Dr. William A. Altemeier, stated that ‘antibiotic therapy cannot be depended upon to prevent the development of local infection if established surgical principles or important technical details have been ignored’.67 With growing rates of antibiotic resistance worldwide, this statement remains as pertinent now as it ever did.
Author contribution
All authors were involved equally in the conception, design, process and write-up of the study.
References
- 1.Magill SS, Edwards JR, Bamberg Wet al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014; 370: 1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smyth ETM, McIlvenny G, Enstone JEet al. Four country healthcare associated infection prevalence survey 2006: overview of the results. J Hosp Infect 2008; 69: 230–248. [DOI] [PubMed] [Google Scholar]
- 3.European Centre for Disease Prevention and Control. Healthcare-associated infections: surgical site infections – Annual Epidemiological Report for 2017 [Internet]. Annual Epidemiological Report on Communicable Diseases in Europe; Accessed 26/08/2022. https://www.ecdc.europa.eu/en/publications-data/healthcare-associated-infections-surgical-site-infections-annual-1
- 4.GlobalSurg Collaborative. Surgical site infection after gastrointestinal surgery in high-income, middle-income, and low-income countries: a prospective, international, multicentre cohort study. Lancet Infect Dis 2018; 8: 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badia JM, Casey AL, Petrosillo Net al. Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries. J Hosp Infect 2017; 96: 1–15. [DOI] [PubMed] [Google Scholar]
- 6.de Lissovoy G, Fraeman K, Hutchins Vet al. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control 2009; 37: 387–397. [DOI] [PubMed] [Google Scholar]
- 7.Kirkland KB, Briggs JP, Trivette SLet al. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol 1999; 20: 725–730. [DOI] [PubMed] [Google Scholar]
- 8.Lamarsalle L, Hunt B, Schauf Met al. Evaluating the clinical and economic burden of healthcare-associated infections during hospitalization for surgery in France. Epidemiol Infect 2013; 141: 2473–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gili-Ortiz E, González-Guerrero R, Béjar-Prado Let al. Surgical site infections in patients who undergo radical cystectomy: excess mortality, stay prolongation and hospital cost overruns. Actas Urol Esp 2015; 39: 210–216. [DOI] [PubMed] [Google Scholar]
- 10.Coello R, Charlett A, Wilson Jet al. Adverse impact of surgical site infections in English hospitals. J Hosp Infect 2005; 60: 93–103. [DOI] [PubMed] [Google Scholar]
- 11.Shepard J, Ward W, Milstone Aet al. Financial impact of surgical site infections on hospitals. JAMA Surg 2013; 148: 907–914. [DOI] [PubMed] [Google Scholar]
- 12.Jenks P, Laurent M, McQuarry Set al. Clinical and economic burden of surgical site infection (SSI) and predicted financial consequences of elimination of SSI from an English hospital. J Hosp Infect 2014; 86: 24–33. [DOI] [PubMed] [Google Scholar]
- 13.Rosen MJ, Bauer JJ, Harmaty Met al. Multicenter, prospective, longitudinal study of the recurrence, surgical site infection, and quality of life after contaminated ventral hernia repair using biosynthetic absorbable mesh. Ann Surg 2017; 265: 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perencevich EN, Sands KE, Cosgrove SEet al. Health and economic impact of surgical site infections diagnosed after hospital discharge. Emerg Infect Dis 2003; 9: 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avsar P, Patton D, Ousey Ket al. The impact of surgical site infection on health-related quality of life: a systematic review. Wound manag Prev 2021; 67: 10–19. [PubMed] [Google Scholar]
- 16.National Institute for Health Research Global Research Health Unit on Global Surgery. Delphi prioritization and development of global surgery guidelines for the prevention of surgical-site infection. Br J Surg 2020; 107: 970–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zywot A, Lau CSM, Fletcher HSet al. Bundles prevent surgical site infections after colorectal surgery: meta-analysis and systematic review. J Gastrointest Surg 2017; 21: 1915–1930. [DOI] [PubMed] [Google Scholar]
- 18.Keenan JE, Speicher PJ, Thacker JKMet al. The preventive surgical site infection bundle in colorectal surgery an effective approach to surgical site infection reduction and health care cost savings. JAMA Surg 2014; 149: 1045–1052. [DOI] [PubMed] [Google Scholar]
- 19.Allen J, David M, Veerman JL. Systematic review of the cost-effectiveness of preoperative antibiotic prophylaxis in reducing surgical-site infection. BJS Open 2018; 2: 81–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchez-Manuel FJ, Lozano-García J, Seco-Gil JL. Antibiotic prophylaxis for hernia repair. Cochrane Database Syst Rev 2007; 18: CD003769. [DOI] [PubMed] [Google Scholar]
- 21.Mackeen AD, Packard RE, Ota Eet al. Timing of intravenous prophylactic antibiotics for preventing postpartum infectious morbidity in women undergoing cesarean delivery. Cochrane Database Syst Rev 2014; 5: CD009516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson RL, Gladman E, Barbateskovic M. Antimicrobial prophylaxis for colorectal surgery. Cochrane Database Syst Rev 2014; 9: CD001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swenson BR, Camp TR, Mulloy DPet al. Antimicrobial-impregnated surgical incise drapes in the prevention of mesh infection after ventral hernia repair. Surg Infect (Larchmt) 2008; 9: 23–32. [DOI] [PubMed] [Google Scholar]
- 24.Singer AJ, Quinn J V, Hollander JE. The cyanoacrylate topical skin adhesives. Am J Emerg Med 2008; 26: 490–496. [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff Jet al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: B2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norman G, Atkinson RA, Smith TAet al. Intracavity lavage and wound irrigation for prevention of surgical site infection. Cochrane Database Syst Rev 2017; 10: CD012234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanner J, Norrie P, Melen K. Preoperative hair removal to reduce surgical site infection. Cochrane Database Syst Rev 2011; 9: CD004122. [DOI] [PubMed] [Google Scholar]
- 28.Niël-Weise BS, Wille JC, van den Broek PJ. Hair removal policies in clean surgery: systematic review of randomized, controlled trials. Infect Control Hosp Epidemiol 2005 Dec; 26: 923–928. [DOI] [PubMed] [Google Scholar]
- 29.Hranjec T, Swenson BR, Sawyer RG. Surgical site infection prevention: how we do it. Surg Infect (Larchmt) 2010; 11: 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swenson BR, Hedrick TL, Metzger Ret al. Effects of preoperative skin preparation on postoperative wound infection rates A prospective study of 3 skin preparation protocols. Infect Control Hosp Epidemiol 2009; 30: 964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Darouiche RO, Wall MJ, Itani KMFet al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med 2010; 362: 18–26. [DOI] [PubMed] [Google Scholar]
- 32.Wade R, Burr N, McCauley Get al. The comparative efficacy of chlorhexidine gluconate and povidone-iodine antiseptics for the prevention of infection in clean surgery: a systematic review and network meta-analysis. Ann Surg 2020; 272. [DOI] [PubMed] [Google Scholar]
- 33.Webster J, Alghamdi A. Use of plastic adhesive drapes during surgery for preventing surgical site infection. Cochrane Database Syst Rev 2015; 2015: CD006353. [DOI] [PubMed] [Google Scholar]
- 34.Horiuchi T, Tanishima H, Tamagawa Ket al. A wound protector shields incision sites from bacterial invasion. Surg Infect (Larchmt) 2010; 11: 501–503. [DOI] [PubMed] [Google Scholar]
- 35.Sookhai S, Redmond HP, Deasy JM. Impervious wound-edge protector to reduce postoperative wound infection: A randomised, controlled trial. Lancet 1999; 353: 1585. [DOI] [PubMed] [Google Scholar]
- 36.Reid K, Pockney P, Draganic Bet al. Barrier wound protection decreases surgical site infection in open elective colorectal surgery: A randomized clinical trial. Dis Colon Rectum 2010; 53: 1374–1380. [DOI] [PubMed] [Google Scholar]
- 37.Cheng KP, Roslani AC, Sehha Net al. ALEXIS O-Ring wound retractor vs conventional wound protection for the prevention of surgical site infections in colorectal resections. Color Dis 2012; 14: e346–e351. [DOI] [PubMed] [Google Scholar]
- 38.Kang SI, Oh HK, Kim MHet al. Systematic review and meta-analysis of randomized controlled trials of the clinical effectiveness of impervious plastic wound protectors in reducing surgical site infections in patients undergoing abdominal surgery. Surgery (United States) 2018; 164: 939–945. [DOI] [PubMed] [Google Scholar]
- 39.Bressan A, Aubin J, Martel Get al. Efficacy of a dual-ring wound protector for prevention of incisional surgical site infection after pancreatoduodenectomy in patients with intrabiliary stents: a randomized controlled trial. Ann Surg 2018; 268: 35–40. [DOI] [PubMed] [Google Scholar]
- 40.Pinkney TD, Calvert M, Bartlett DCet al. Impact of wound edge protection devices on surgical site infection after laparotomy: multicentre randomized controlled trial (ROSSINI trial). BMJ 2013; 31: f4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elek SD. Experimental staphylococcal infections in the skin of man. Ann N Y Acad Sci 1956; 65: 85–90. [DOI] [PubMed] [Google Scholar]
- 42.Constantine BE, Bolton LL. A wound model for ischemic ulcers in the Guinea pig. Arch Dermatol Res 1986; 278: 429–431. [DOI] [PubMed] [Google Scholar]
- 43.Luedtke-Hoffmann KA, Schafer DS. Pulsed lavage in wound cleansing. Phys Ther 2000; 80: 292–300. [PubMed] [Google Scholar]
- 44.Diana M, Hübner M, Eisenring MCet al. Measures to prevent surgical site infections: what surgeons (should) do. World J Surg 2011; 35: 280–288. [DOI] [PubMed] [Google Scholar]
- 45.Pivot D, Tiv M, Luu Met al. Survey of intraoperative povidone-iodine application to prevent surgical site infection in a French region. J Hosp Infect 2011; 77: 363–364. [DOI] [PubMed] [Google Scholar]
- 46.Berkelman RL, Holland BW, Anderson RL. Increased bactericidal activity of dilute preparations of povidone-iodine solutions. J Clin Microbiol 1982; 15: 635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mueller TC, Loos M, Haller Bet al. Intra-operative wound irrigation to reduce surgical site infections after abdominal surgery: a systematic review and meta-analysis. Langenbeck’s Arch Surg 2015; 400: 167–181. [DOI] [PubMed] [Google Scholar]
- 48.Müller G, Kramer A. Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J Antimicrob Chemother 2008; 61: 1281–1287. [DOI] [PubMed] [Google Scholar]
- 49.Thomas GW, Rael LT, Bar-Or Ret al. Mechanisms of delayed wound healing by commonly used antiseptics. J Trauma 2009; 66: 82–90. [DOI] [PubMed] [Google Scholar]
- 50.De Jonge SW, Boldingh QJJ, Solomkin JSet al. Systematic review and meta-analysis of randomized controlled trials evaluating prophylactic intra-operative wound irrigation for the prevention of surgical site infections. Surg Infect (Larchmt) 2017; 8: 508–519. [DOI] [PubMed] [Google Scholar]
- 51.Hargrove R, Ridgeway S, Russell Ret al. Does pulse lavage reduce hip hemiarthroplasty infection rates? J Hosp Infect 2006; 62: 446–449. [DOI] [PubMed] [Google Scholar]
- 52.Bath MF, Suresh R, Davies Jet al. Does pulsed lavage reduce the risk of surgical site infection? A systematic review and meta-analysis. J Hosp Infect 2021; 118: 32–39. [DOI] [PubMed] [Google Scholar]
- 53.Murray BW, Huerta S, Dineen Set al. Surgical site infection in colorectal surgery: a review of the nonpharmacologic tools of prevention. J Am Coll Surg 2010; 211: 812–822. [DOI] [PubMed] [Google Scholar]
- 54.Zucker BE, Simillis C, Tekkis Pet al. Suture choice to reduce occurrence of surgical site infection, hernia, wound dehiscence and sinus/ fistula: a network meta-analysis. Ann R Coll Surg Engl 2019; 101: 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dumville JC, Coulthard P, Worthington HVet al. Tissue adhesives for closure of surgical incisions. Cochrane Database Syst Rev 2014: CD004287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.NICE. Plus Sutures for preventing surgical site infection [Internet]; Accessed 26/08/2022. https://www.nice.org.uk/guidance/mtg59
- 57.Dumville JC, Gray TA, Walter CJet al. Dressings for the prevention of surgical site infection. Cochrane Database Syst Rev 2016; 12: CD003091. [DOI] [PubMed] [Google Scholar]
- 58.Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997; 38: 563–577. [PubMed] [Google Scholar]
- 59.Reddix RN, Leng XI, Woodall Jet al. The effect of incisional negative pressure therapy on wound complications after acetabular fracture surgery. J Surg Orthop Adv 2010; 19: 91–97. [PubMed] [Google Scholar]
- 60.DeCarbo WT, Hyer CF. Negative-pressure wound therapy applied to high-risk surgical incisions. J Foot Ankle Surg 2010; 49: 299–300. [DOI] [PubMed] [Google Scholar]
- 61.Atkins BZ, Wooten MK, Kistler Jet al. Does negative pressure wound therapy have a role in preventing poststernotomy wound complications? Surg Innov 2009; 16: 140–146. [DOI] [PubMed] [Google Scholar]
- 62.Sahebally SM, McKevitt K, Stephens Iet al. Negative pressure wound therapy for closed laparotomy incisions in general and colorectal surgery: a systematic review and meta-analysis. JAMA Surg 2018; 153: e183467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Norman G, Goh EL, Dumville JCet al. Negative pressure wound therapy for surgical wounds healing by primary closure. Cochrane Database Syst Rev 2020; 5: CD009261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kosins AM, Scholz T, Cetinkaya M, Evans GRD. Evidence-based value of subcutaneous surgical wound drainage: The largest systematic review and meta-analysis. Plast Reconstr Surg 2013; 132: 443–450. [DOI] [PubMed] [Google Scholar]
- 65.World Health Organisation. Antibiotic Resistance [Internet]; Accessed 26/08/2022. https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance
- 66.Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022; 399: 629–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Altemeier W. The problem of postoperative wound infection and its significance. Ann Surg 1958; 147: 770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]