Abstract
Dexamethasone (DEX) was the first drug shown to save lives of critically ill coronavirus disease 2019 (COVID-19) patients suffering from respiratory distress. A hyperactivated state of neutrophils was found in COVID-19 patients compared to non-COVID pneumonia cases. Given the beneficial effects of DEX in COVID-19 patients, we investigated the effects of DEX and of other immunomodulatory drugs vitamin D3 (VD3) and retinoic acid (RA) on neutrophil function. DEX, but not VD3 or RA, significantly inhibited all tested aspects of neutrophil function, e.g., degranulation, intracellular ROS production, CXCL8 release and NETosis. Interestingly, RA displayed the opposite effect by significantly increasing both CXCL8 and NET release by neutrophils. Taken together, these data suggest that the lower COVID-19 mortality in DEX-treated patients may in part be due to the dampening effect of DEX on the inflammatory neutrophil response, which could prevent neutrophil plugs with NETS in the lungs and other inflamed organs of patients.
Keywords: Neutrophils, COVID-19, Dexamethasone, Vitamin D3, Retinoic acid
INTRODUCTION
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was officially declared a global pandemic in March 2020 by the World Health Organization (1), with currently over 353 million confirmed cases and 5.6 million associated deaths (2). While the majority of COVID-19 patients is asymptomatic or shows mild symptoms, one-fifth of patients will develop severe illness, symptoms including acute respiratory distress syndrome, sepsis and multiorgan failure (3). An elevated neutrophil-to-lymphocyte ratio (NLR) has been identified as an early risk factor for severe COVID-19 (4). Severe COVID-19 is characterized by a cytokine storm, to which pro-inflammatory monocytes and neutrophils contribute (5). Neutrophils in the lungs are both enriched and in a hyperactivated state, with upregulated IL-1β and CXCL8 expression, in COVID-19 patients compared to non-COVID pneumonia cases (5). Neutrophil plugs with NETs were notably present in the lungs and other inflamed organs such as the heart, kidney and brain of deceased COVID-19 patients, affirming an elevated activation status of neutrophils (6,7). Therefore, targeting the excessive neutrophil inflammatory response could be a crucial step in lowering the probability of progression to severe respiratory distress and eventually organ failure in COVID-19 patients.
Dexamethasone (DEX), an inexpensive and commonly applied corticosteroid, was the first drug shown to save lives of people suffering from severe COVID-19 in a large randomized, controlled trial (8,9). The effect of DEX was most pronounced in patients on ventilators amongst whom deaths were reduced by one-third (10). In contrast, no effect was observed in people without respiratory distress. Therefore, treatment guidelines recommend administration of DEX only in hospitalized patients who require supplemental oxygen. DEX is regarded as a potent general immunosuppressive drug (8), which reduces CXCL8 and TNF expression in neutrophils (11,12). How DEX affects other aspects of neutrophil function is less well-known. In addition to DEX, vitamin D3 (VD3) supplementation has been proposed as a beneficial strategy to reduce the impact of COVID (13). Furthermore, it has been suggested that retinoic acid (RA) metabolism is defective during the COVID-19 cytokine storm, which causes excessive cytokine release (14,15). Hence, RA supplementation could also be considered for treatment. However, little is known about the effects of these immunosuppressive drugs on neutrophil function.
Therefore, we investigated the effects of DEX, VD3 and RA on function of human neutrophils by determining degranulation, CXCL8 release and intracellular ROS production upon stimulation with TLR7/8 ligand Resiquimod (R848) (16) and TNF. Furthermore, we assessed the effects of these drugs on PMA-induced NETosis. We found that DEX dampens all aspects of neutrophil function assessed in this study. In contrast, VD3 did not affect function. Interestingly, RA did not alter degranulation and ROS production, but increased CXCL8 release and NETosis. Taken together, these data support a potential neutrophil dampening role for DEX, thereby providing a rationale for the use of DEX in treatment of critically ill COVID-19 patients.
MATERIALS AND METHODS
Neutrophil isolation
Blood was collected from healthy volunteer donors after informed consent. The blood collection protocol was approved by the institutional review board of the Amsterdam Medical Centre (METC 2015_074). Neutrophils were isolated using a density gradient followed by erythrocyte lysis, as previously described (17). Neutrophils were then resuspended in IMDM (Gibco; Thermo Fisher Scientific Inc., Waltham, MA, USA) supplemented with 10% heat inactivated (HI) fetal bovine serum (FBS; Hyclone; Thermo sFisher Scientific Inc.) and gentamycin (86 µg/ml; Duchefa Biochemie B.V., Haarlem, The Netherlands) and used immediately. Neutrophil purity was analyzed by flow cytometry and was always >97%.
Neutrophil culture, stimulation and flow cytometric analysis
Neutrophils were seeded at a density of 0.5×106 cells/ml in 200 µl in a flat bottom 96-well plate (Costar, Corning Inc. Corning, NY) in IMDM medium containing 10% HI-FBS and gentamycin. Subsequently, neutrophils were pretreated for 30 minutes with DEX (40 nM; #D2915 from Merck), VD3 (2.5 µM; #17936 from Sigma-Aldrich), RA (10 µM; #R2625 from Sigma-Aldrich), or controls medium, ethanol or DMSO, respectively. Then R848 (1 µg/ml; Invivogen, San Diego, CA, USA) and TNF (1 ng/ml; Miltenyi Biotec GmBH, Bergisch Gladbach, Germany) were added and neutrophils were cultured for 2 hours (degranulation), or 24 hours at 37°C. 24-hour culture supernatants were collected for the analysis of neutrophil CXCL8 release, by ELISA (Invitrogen Life Technologies, Breda, The Netherlands), as described previously (17). For assessment of ROS production, neutrophils were stimulated for 1 hour with R848 (500 ng/ml) and TNF (250 pg/ml) in the presence of 250 nM 123-dihydrorhodamine (123-DHR; Marker Gene Technologies, OR, USA), after 15 minutes pretreatment with drugs or controls. For flow cytometric analysis of CD16, CD63, and CD66b cells were washed after stimulation, stained and analyzed as previously described (17).
NETosis assay
NETosis was analyzed using an Incucyte S3 Live-Cell Analysis System (Essen BioScience, Newark, UK) and a previously described IncuCyte® NETosis assay (18). Briefly, neutrophils were seeded at a density of 1.0×105 cells/ml in 200 µl in a 96-well IncuCyte® Imagelock plate (Essen BioScience) in IMDM medium containing 10% HI-FBS and gentamycin, and incubated for 15 minutes in the presence of DEX (40 nM), VD3 (2.5 µM) or RA (10 µM) or controls medium, ethanol or DMSO, respectively. 1.5 ng/ml PMA was added after 15 minutes and neutrophils were incubated for 12 hours in presence of the cell impermeant nucleic acid binding dye YOYO™-3 Iodide (Invitrogen). Neutrophils were imaged every 15 minutes using phase contrast and red fluorescent exposure channels, using a 20× dry objective lens. Data were analyzed using the IncuCyte Basic Software (Essen BioScience), with the same parameters as previously described (17).
Statistical analysis
Data are expressed as mean ± SD or as mean. Statistical analysis was done in GraphPad Prism version 9.1.0 for Windows by using statistical tests, depending on the experimental data. The Shapiro-Wilk test was performed to test normality of data. For multiple comparisons, p-values were calculated on selected pairs (drug versus vehicle control) using a one-way ANOVA with Holm-Sidak’s post hoc correction on raw data. For single comparisons, p-values were calculated using two-tailed paired t-tests. P-values < 0.05 were considered statistically significant.
RESULTS AND DISCUSSION
Neutrophil degranulation is dampened by DEX, but not VD3 or RA
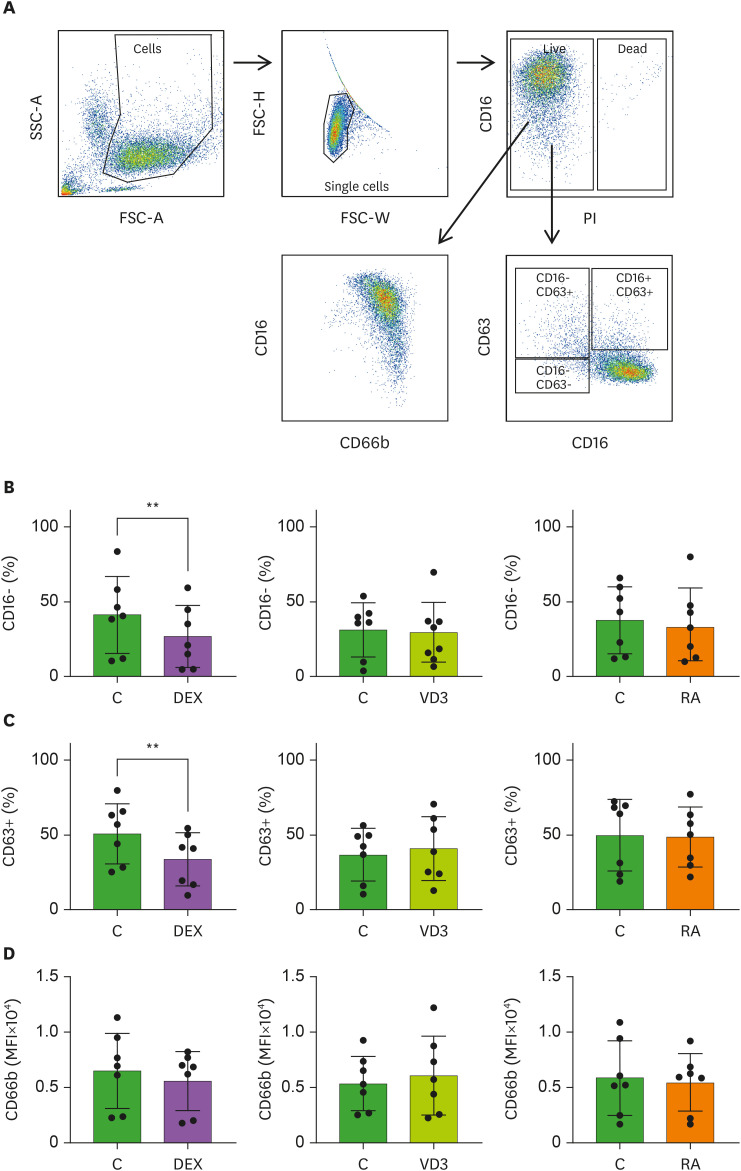
A hyperinflammatory response of neutrophils is associated with severe COVID-19 (19). Since drugs that dampen neutrophil activation may be useful in fighting SARS-CoV-2 infection, we studied whether DEX, VD3 or RA affect neutrophil degranulation by analyzing CD16 (FCγRIII), CD63 and CD66b membrane expression. Fusion of azurophilic granules with the plasma membrane increases CD63 expression, while CD66b indicates specific and gelatinase granules (20,21), and CD16 is cleaved from the surface upon the release of secretory vesicles (20,21,22,23). Neutrophils were stimulated with R848 and TNF, a mimic for viral activation, in the presence of DEX, VD3, RA or relevant controls (medium, ethanol or DMSO, respectively) and we titrated the drugs to determine the used concentration in all experiments (Supplementary Fig. 1). Data were obtained by flow cytometry and were analyzed with the gating strategy shown in Fig. 1A. Exposure of stimulated neutrophils to DEX, resulted in significant inhibition of CD16 cleavage from the membrane, while this was not affected by VD3 and RA compared to vehicle controls (Fig. 1B). Furthermore, DEX significantly decreased CD63 expression (Fig. 1C), while none of the immunomodulatory drugs affected CD66b membrane expression (Fig. 1D). Ethanol alone reduced CD16 cleavage and CD63 expression (Supplementary Fig. 1, Fig. 1B and C). These data indicate that DEX predominantly dampens degranulation of azurophilic granules (CD63) and secretory vesicles (CD16), rather than specific and gelatinase granules. Taken together, our study is the first to demonstrate that neutrophil degranulation is restricted by DEX, while VD3 an RA have no effect on degranulation. Administration of DEX to hospitalized COVID-19 patients may reduce hyperinflammatory neutrophil degranulation.
Figure 1. Neutrophil degranulation is dampened by DEX, but not VD3 or RA. Neutrophils were pretreated with DEX, VD3, RA or their respective controls medium, ethanol or DMSO (all controls abbreviated as C in figures), and cultured for 2 hours in the presence of R848 and TNF. (A) Flow cytometry plot demonstrating gating strategy to determine neutrophil degranulation. Neutrophils were gated on forward scatter (FSC-A) and side scatter (SSC-A), followed by a single cell and live gate from which the expression of CD16, CD63 and CD66b was assessed (B) Secretory vesicle degranulation as measured by percentage of CD16- neutrophils is depicted. (C) Azurophilic degranulation as measured by percentage of CD63+ neutrophils is shown. (D) Degranulation of specific and gelatinase granules is depicted as mean fluorescence intensity of CD66b. Data are representative of 7 independent experiments and are presented as mean ± SD.
**p<0.01.
CXCL8 release is dampened by DEX and strengthened by RA
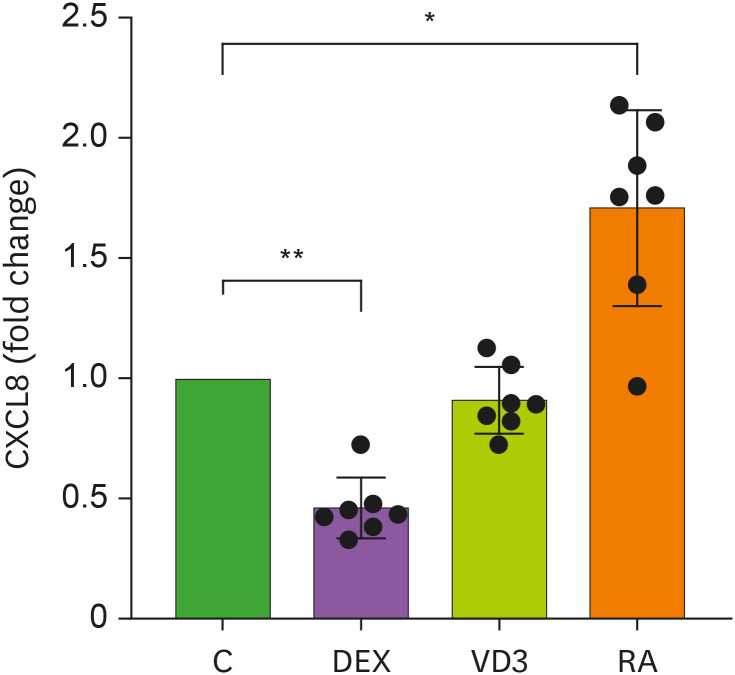
During infection neutrophils release many different mediators, including CXCL8 which is an important chemoattractant for neutrophils (22,24,25). We analyzed the release of CXCL8 after 24 hour-stimulation with R848 and TNF. In line with previous reports (11), CXCL8 release by neutrophils in presence of DEX was decreased by approximately 50%, with an average of 1.02±0.52 ng/ml (mean ± SD) CXCL8 release by DEX-treated neutrophils versus 2.24±1.14 ng/ml by medium control neutrophils (Fig. 2). Surprisingly, RA significantly increased CXCL8 release by stimulated neutrophils by 1.5-fold, whereas VD3 did not influence CXCL8 release (Fig. 2). This RA-induced effect on neutrophil function was not found for degranulation. CXCL8 is synthesized de novo upon activation and is thus regulated differently than degranulation, where granules are already pre-stored in the neutrophils and rapidly released within two hours. This could underlie the variable effects of RA on different aspects of neutrophil function. The opposite effects of RA and DEX on CXCL8 release by neutrophils could be due to opposite effects on NF-κB activity. NF-κB transcription factors are the main regulators of CXCL8 transcription (26,27). Corticosteroids, including DEX, inhibit CXCL8 transcription via repression of NF-κB activity (27). Reduced expression of NF-κB transcription factors by DEX was confirmed in human neutrophils (28). Elevated CXCL8 secretion upon RA treatment is possibly due to increased NF-κB activity, which was shown in human keratinocytes (26). However, to our knowledge, increased CXCL8 release by RA was not previously shown in neutrophils. Collectively, our data show that similar to degranulation, CXCL8 release is dampened by DEX and VD3 had no effect. Interestingly, RA increased CXCL8 secretion, whereas no effect of RA was observed on neutrophil degranulation.
Figure 2. CXCL8 release by neutrophils is affected by DEX and RA. Neutrophils were stimulated by R848 and TNF and in the presence of DEX, VD3, RA or relevant controls. CXCL8 was measured in 24-hour culture supernatants (n=7). Data are shown presented as mean ± SD relative to controls.
*p<0.05, **p< 0.01.
ROS production is reduced by DEX
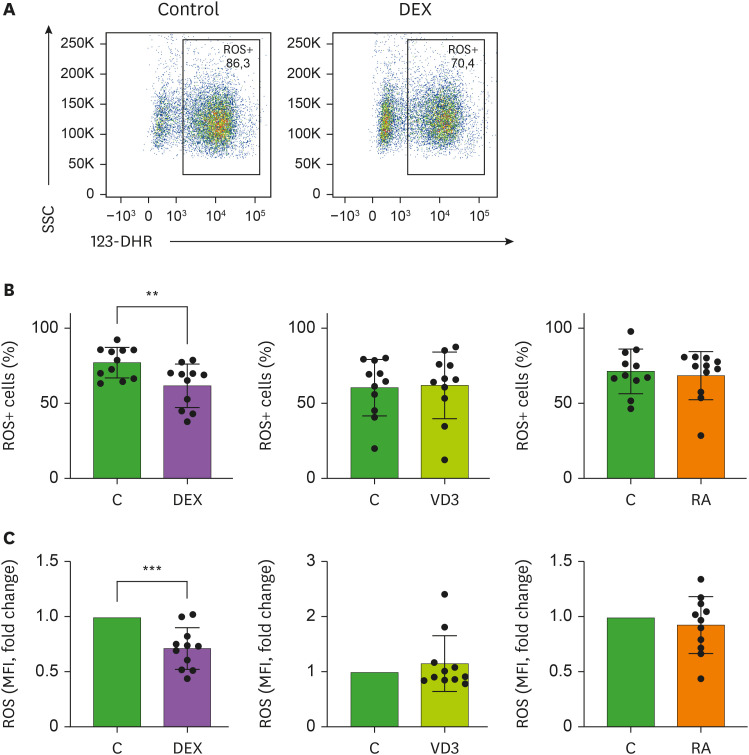
In addition to degranulation and CXCL8 secretion, neutrophil ROS production is important in the clearance of unwanted pathogens (29). However, it has been suggested that excessive ROS production by neutrophils during COVID-19 exacerbates the host immunopathological response resulting in tissue damage (30). Intracellular ROS production was determined by flow cytometry using the ROS indicator 123-DHR in R848/TNF-stimulated neutrophils in the absence or presence of DEX, VD3 or RA (Fig. 3A). Similar to neutrophil degranulation and CXCL8 release, intracellular ROS production was significantly reduced in neutrophils exposed to DEX. Accordingly, neutrophils from human volunteers injected with DEX were shown to exhibit lower extracellular ROS generation (31). In contrast, neutrophils stimulated in the presence of RA or VD3 showed no difference in ROS production compared to neutrophils stimulated with relevant controls, neither when assessing the percentage of intracellular ROS+ cells or the mean fluorescence intensity of neutrophils (Fig. 3B and C). RA was previously shown to increase N-formyl-methionyl-leucyl-fenylalanine (fMLF)-stimulated production of intracellular ROS (32), but we did not find an effect on intracellular ROS production, which could be stimulus-dependent. We used a double stimulus rather than a single stimulus for optimal neutrophil activation, which is more physiologically relevant than single stimuli given that cells encounter a plethora of pro-inflammatory cytokines and microbial or viral components (17). Our data indicate that DEX restricts ROS production in neutrophils, again demonstrating anti-inflammatory potential of DEX on neutrophil functions.
Figure 3. ROS production in neutrophils is reduced by DEX, but not VD3 or RA. Neutrophils were incubated for 1 hour in the presence of 123-DHR and activated by R848 and TNF, in the presence of DEX, VD3, RA or relevant controls. ROS production was analyzed using flow cytometry. (A) Representative flow cytometry image of neutrophils incubated with DEX or relevant control. (B-C) Effects of DEX, VD3 and RA on ROS production (n=11). (B) Intracellular ROS generation is expressed as percentage of ROS+ cells. (C) Total ROS production is expressed as mean fluorescence intensity of 123-DHR relative to controls, with mean fluorescence intensity 6,166±3,636 (mean ± SD) medium control, 4,252±3,103 ethanol control and 5,998±5,398 DMSO control. Data are presented as mean ± SD.
**p<0.01; ***p<0.005.
DEX reduces and RA increases NETosis
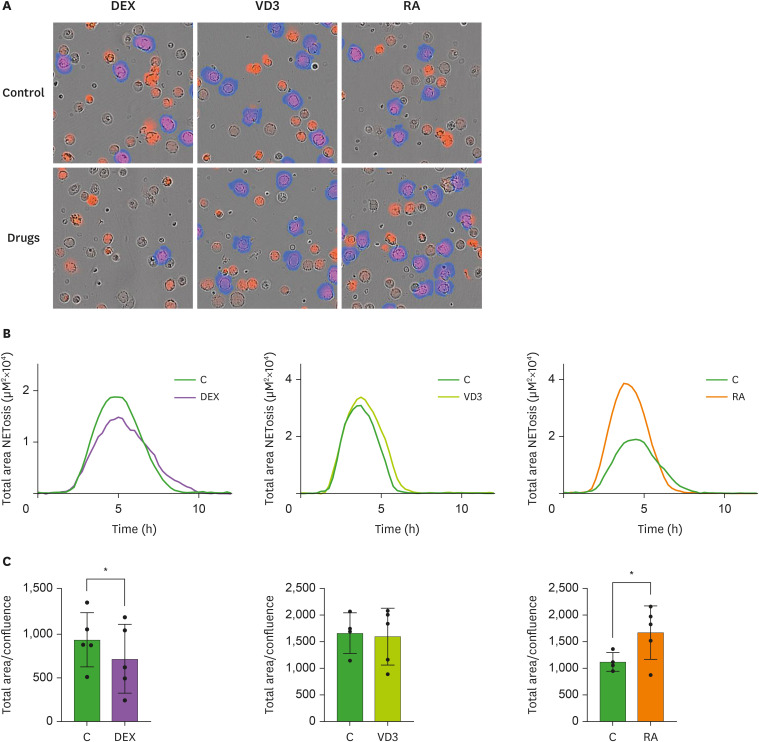
NETosis is a mechanism used by neutrophils to entrap and kill pathogens through the release of nuclear and granular content that forms a network (33). Although NETosis is important as an antimicrobial function, it requires tight regulation, since excessive NETosis can lead to severe tissue damage and exacerbation of inflammation (34,35). Neutrophil plugs with NETs were found in deceased COVID-19 patients and NET quantity correlates to disease severity (6,7,36). To examine whether DEX, VD3 or RA could dampen NETosis in neutrophils, neutrophils were incubated with 1.5 ng/ml PMA in the absence or presence of DEX, VD3 or RA. With time-lapse immunofluorescence microscopy we analyzed NETosis (Fig. 4A). Maximal NETosis was observed after 4 hours of PMA-stimulation, which was significantly reduced by DEX. VD3 did not have any effect on NETosis, while NETosis was increased by RA (Fig. 4B and C). Reduced NET release in presence of DEX was reported upon stimulation of neutrophils with Staphylococcus aureus, but not with PMA (37). However, we used a 20-fold lower dose of PMA, possibly allowing DEX to interfere with NETosis. RA was previously shown to enhance both PMA- and fMLF-induced NETosis (32). Similar to CXCL8 release, we observed opposite effects of RA and DEX on NETosis. It has been shown that inhibition of the NF-κB pathway reduces NETosis (38). Hence, the differential effects of RA and DEX on NF-κB activity could underlie their observed effects on NETosis. Moreover, peptidyl arginine deiminase 4 (PAD4) plays a critical role in the formation of NETs (39) and it has been shown that corticosteroid treatment of rheumatoid arthritis patients decreases synovial expression of PAD4 (40), indicating that DEX may affect PAD4 expression in neutrophils. In contrast, treatment of acute promyelocytic leukemia cells with RA, to differentiate them into granulocytic cells, increases PAD4 expression (41). Taken together, distinct effects of DEX and RA on NF-κB and PAD4 activity could underlie the opposing effects of these drugs on NETosis.
Figure 4. DEX reduces, while RA increases NETosis. Neutrophils were stimulated for 12 hours with PMA (1.5 ng/ml) in the presence of DEX, VD3, RA or relevant controls as well as a cell impermeable fluorescent DNA-binding dye. Fluorescence was measured by time-lapse immunofluorescence microscopy every 15 minutes to determine NETosis. (A) Overlays of phase contrast and fluorescence images showing accessible DNA in red, and extracellular DNA (>400 µm2) in blue (NETs). Images are representative of 5 independent experiments. (B) NETosis was determined as fluorescence signal area in µm2 per well shown in a 12-hour time course. Only areas larger than 400 µm2 were used for calculations (n=5). Data are presented as mean. (C) Normalized NETosis expressed as total fluorescence area divided by cell confluence after 4 hours of stimulation is depicted (n=5). Data are presented as mean ± SD.
*p<0.05.
In this study, we confirmed the well-established anti-inflammatory effect of DEX on CXCL8 release (11,12) and importantly, we show that this dampening effect of DEX extends to other aspects of neutrophil function, including intracellular ROS production, degranulation and NETosis. We observed no effects of VD3 on neutrophil function when compared to the vehicle control (ethanol), while neutrophils do express mRNA of the VD3 receptor (42). The effects of VD3 on neutrophils are rarely studied and results are contradictory, e.g. elevated versus decreased CXCL8 release by VD3 treatment (43). A limitation of our study is that we did not use (pseudo)-SARS-CoV-2 as stimulus for neutrophils. Although neutrophils may not be infected by SARS-CoV-2 (44), its components, e.g. nucleocapsid, spike proteins or ssRNA, may activate neutrophils. Purified nucleocapsid and spike proteins from SARS-CoV-2 were shown to induce NETosis, while they did not increase intracellular ROS production (45). The effect of these proteins on the release of other neutrophil derived factors, e.g., granules and CXCL8, remains to be established. Here, we used R848 in combination with TNF to activate neutrophils. Our earlier work showed that two different stimuli are needed for optimal neutrophil activation (17). R848 is a synthetic ligand that activates TLR7 and TLR8, the latter expressed by neutrophils, which recognize ssRNA (46). TNF is an important modulator of immune responses, including the response to viruses. Therefore, neutrophil stimulation with R848 and TNF may represent an attractive model to study candidate drugs for dampening neutrophil activation in COVID-19.
Taken together, our data support previous reports on a pro-inflammatory effect of RA on neutrophils and this may be of importance to treatment of neutrophil immunodeficiencies (47), while caution is warranted for potential use as a tolerogenic adjuvant in autoimmune disorders or other diseases associated with hyperactivation of neutrophils, such as COVID-19 (19,43,48). The anti-inflammatory effect of DEX on neutrophil function supports the use of DEX in hospitalized COVID-19 patients suffering from respiratory distress.
ACKNOWLEDGEMENTS
This work was supported by Amsterdam University Medical Center, University of Amsterdam and by a grant from the Dutch Arthritis Society.
Abbreviations
- 123-DHR
123-Dihydrorhodamine
- COVID-19
coronavirus disease 2019
- DEX
Dexamethasone
- fMLF
N-formyl-methionyl-leucyl-fenylalanine
- HI
heat inactivated
- NLR
neutrophil-to-lymphocyte ratio
- PAD4
peptidyl arginine deiminase 4
- RA
retinoic acid
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- VD3
vitamin D3
Footnotes
Conflicts of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Mol S, Hafkamp FMJ, De Jong EC.
- Investigation: Mol S, Hafkamp FMJ, Waqué I.
- Formal analysis: Mol S, Hafkamp FMJ.
- Supervision: De Jong EC.
- Writing - original draft: Mol S, Hafkamp FMJ.
- Writing - review: Waqué I, De Jong EC.
SUPPLEMENTARY MATERIAL
DEX dose-dependently inhibits neutrophil degranulation. Neutrophils were pretreated with DEX, VD3, RA or their respective controls medium, ethanol or DMSO and cultursed for 2 hours in the presence of R848 and TNF. (A) Secretory vesicle degranulation as measured by percentage of CD16-neutrophils is depicted, normalized to controls, mean ± SD. In the middle panel, data is normalized to medium control (35.2±21.0% CD16- neutrophils), indicated by the line at 1 and each donor is represented by a different symbol. Grey bars indicate VD3, while respective ethanol dilutions are shown in white bars. (B) Azurophilic degranulation as measured by percentage of CD63+ neutrophils is shown, normalized to controls, mean ± SD, with medium control 27.0±14.3% CD63+ neutrophils). Three independent experiments were performed.
References
- 1.Liu YC, Kuo RL, Shih SR. COVID-19: the first documented coronavirus pandemic in history. Biomed J. 2020;43:328–333. doi: 10.1016/j.bj.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO coronavirus (COVID-19) dashboard [Internet] [accessed on 26 January 2022]. https://covid19.who.int/
- 3.Li J, Huang DQ, Zou B, Yang H, Hui WZ, Rui F, Yee NT, Liu C, Nerurkar SN, Kai JC, et al. Epidemiology of COVID-19: a systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J Med Virol. 2021;93:1449–1458. doi: 10.1002/jmv.26424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang AP, Liu JP, Tao WQ, Li HM. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020;84:106504. doi: 10.1016/j.intimp.2020.106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanderbeke L, Van Mol P, Van Herck Y, De Smet F, Humblet-Baron S, Martinod K, Antoranz A, Arijs I, Boeckx B, Bosisio FM, et al. Monocyte-driven atypical cytokine storm and aberrant neutrophil activation as key mediators of COVID-19 disease severity. Nat Commun. 2021;12:4117. doi: 10.1038/s41467-021-24360-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schurink B, Roos E, Radonic T, Barbe E, Bouman CS, de Boer HH, de Bree GJ, Bulle EB, Aronica EM, Florquin S, et al. Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe. 2020;1:e290–e299. doi: 10.1016/S2666-5247(20)30144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ouwendijk WJ, Raadsen MP, van Kampen JJ, Verdijk RM, von der Thusen JH, Guo L, Hoek RA, van den Akker JP, Endeman H, Langerak T, et al. High levels of neutrophil extracellular traps persist in the lower respiratory tract of critically ill patients with coronavirus disease 2019. J Infect Dis. 2021;223:1512–1521. doi: 10.1093/infdis/jiab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ledford H. Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature. 2020;582:469. doi: 10.1038/d41586-020-01824-5. [DOI] [PubMed] [Google Scholar]
- 9.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, et al. dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines [Internet] [accessed on 3 November 3, 2021]. Available at https://www.covid19treatmentguidelines.nih.gov/ [PubMed]
- 11.Plumb J, Gaffey K, Kane B, Malia-Milanes B, Shah R, Bentley A, Ray D, Singh D. Reduced glucocorticoid receptor expression and function in airway neutrophils. Int Immunopharmacol. 2012;12:26–33. doi: 10.1016/j.intimp.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Wang M, Gao P, Wu X, Chen Y, Feng Y, Yang Q, Xu Y, Zhao J, Xie J. Impaired anti-inflammatory action of glucocorticoid in neutrophil from patients with steroid-resistant asthma. Respir Res. 2016;17:153. doi: 10.1186/s12931-016-0462-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martineau AR, Forouhi NG. Vitamin D for COVID-19: a case to answer? Lancet Diabetes Endocrinol. 2020;8:735–736. doi: 10.1016/S2213-8587(20)30268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarohan AR. COVID-19: endogenous retinoic acid theory and retinoic acid depletion syndrome. Med Hypotheses. 2020;144:110250. doi: 10.1016/j.mehy.2020.110250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Midha IK, Kumar N, Kumar A, Madan T. Mega doses of retinol: a possible immunomodulation in Covid-19 illness in resource-limited settings. Rev Med Virol. 2020;2:e2204. doi: 10.1002/rmv.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jurk M, Heil F, Vollmer J, Schetter C, Krieg AM, Wagner H, Lipford G, Bauer S. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat Immunol. 2002;3:499. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- 17.Mol S, Hafkamp FM, Varela L, Simkhada N, Taanman-Kueter EW, Tas SW, Wauben MH, Groot Kormelink T, de Jong EC. Efficient neutrophil activation requires two simultaneous activating stimuli. Int J Mol Sci. 2021;22:10106. doi: 10.3390/ijms221810106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta S, Chan DW, Zaal KJ, Kaplan MJ. A high-throughput real-time imaging technique to quantify NETosis and distinguish mechanisms of cell death in human neutrophils. J Immunol. 2018;200:869–879. doi: 10.4049/jimmunol.1700905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parackova Z, Zentsova I, Bloomfield M, Vrabcova P, Smetanova J, Klocperk A, Mesežnikov G, Casas Mendez LF, Vymazal T, Sediva A. Disharmonic inflammatory signatures in COVID-19: augmented neutrophils’ but impaired monocytes’ and dendritic cells’ responsiveness. Cells. 2020;9:2206. doi: 10.3390/cells9102206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lacy P. Mechanisms of degranulation in neutrophils. Allergy Asthma Clin Immunol. 2006;2:98–108. doi: 10.1186/1710-1492-2-3-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borregaard N, Sørensen OE, Theilgaard-Mönch K. Neutrophil granules: a library of innate immunity proteins. Trends Immunol. 2007;28:340–345. doi: 10.1016/j.it.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Wu J, Newton R, Bahaie NS, Long C, Walcheck B. ADAM17 cleaves CD16b (FcγRIIIb) in human neutrophils. Biochim Biophys Acta. 2013;1833:680–685. doi: 10.1016/j.bbamcr.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Souwer Y, Groot Kormelink T, Taanman-Kueter EW, Muller FJ, van Capel TM, Varga DV, Bar-Ephraim YE, Teunissen MB, van Ham SM, Kuijpers TW, et al. Human TH17 cell development requires processing of dendritic cell-derived CXCL8 by neutrophil elastase. J Allergy Clin Immunol. 2018;141:2286–2289.e5. doi: 10.1016/j.jaci.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Smart SJ, Casale TB. TNF-alpha-induced transendothelial neutrophil migration is IL-8 dependent. Am J Physiol. 1994;266:L238–L245. doi: 10.1152/ajplung.1994.266.3.L238. [DOI] [PubMed] [Google Scholar]
- 26.Dai X, Yamasaki K, Shirakata Y, Sayama K, Hashimoto K. All-trans-retinoic acid induces interleukin-8 via the nuclear factor-kappaB and p38 mitogen-activated protein kinase pathways in normal human keratinocytes. J Invest Dermatol. 2004;123:1078–1085. doi: 10.1111/j.0022-202X.2004.23503.x. [DOI] [PubMed] [Google Scholar]
- 27.Mukaida N, Okamoto S, Ishikawa Y, Matsushima K. Molecular mechanism of interleukin-8 gene expression. J Leukoc Biol. 1994;56:554–558. [PubMed] [Google Scholar]
- 28.Vancurova I, Bellani P, Davidson D. Activation of nuclear factor-kappaB and its suppression by dexamethasone in polymorphonuclear leukocytes: newborn versus adult. Pediatr Res. 2001;49:257–262. doi: 10.1203/00006450-200102000-00021. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen GT, Green ER, Mecsas J. Neutrophils to the ROScue: Mechanisms of NADPH oxidase activation and bacterial resistance. Front Cell Infect Microbiol. 2017;7:373. doi: 10.3389/fcimb.2017.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laforge M, Elbim C, Frère C, Hémadi M, Massaad C, Nuss P, Benoliel JJ, Becker C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat Rev Immunol. 2020;20:515–516. doi: 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dandona P, Mohanty P, Hamouda W, Aljada A, Kumbkarni Y, Garg R. Effect of dexamethasone on reactive oxygen species generation by leukocytes and plasma interleukin-10 concentrations: a pharmacodynamic study. Clin Pharmacol Ther. 1999;66:58–65. doi: 10.1016/S0009-9236(99)70054-8. [DOI] [PubMed] [Google Scholar]
- 32.Shrestha S, Kim SY, Yun YJ, Kim JK, Lee JM, Shin M, Song DK, Hong CW. Retinoic acid induces hypersegmentation and enhances cytotoxicity of neutrophils against cancer cells. Immunol Lett. 2017;182:24–29. doi: 10.1016/j.imlet.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 34.Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, Rubin CJ, Zhao W, Olsen SH, Klinker M, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187:538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kruger P, Saffarzadeh M, Weber AN, Rieber N, Radsak M, von Bernuth H, Benarafa C, Roos D, Skokowa J, Hartl D. Neutrophils: between host defence, immune modulation, and tissue injury. PLoS Pathog. 2015;11:e1004651. doi: 10.1371/journal.ppat.1004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gillot C, Favresse J, Mullier F, Lecompte T, Dogné JM, Douxfils J. NETosis and the immune system in COVID-19: mechanisms and potential treatments. Front Pharmacol. 2021;12:708302. doi: 10.3389/fphar.2021.708302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wan T, Zhao Y, Fan F, Hu R, Jin X. Dexamethasone inhibits S. aureus-induced neutrophil extracellular pathogen-killing mechanism, possibly through toll-like receptor regulation. Front Immunol. 2017;8:60. doi: 10.3389/fimmu.2017.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lapponi MJ, Carestia A, Landoni VI, Rivadeneyra L, Etulain J, Negrotto S, Pozner RG, Schattner M. Regulation of neutrophil extracellular trap formation by anti-inflammatory drugs. J Pharmacol Exp Ther. 2013;345:430–437. doi: 10.1124/jpet.112.202879. [DOI] [PubMed] [Google Scholar]
- 39.Lewis HD, Liddle J, Coote JE, Atkinson SJ, Barker MD, Bax BD, Bicker KL, Bingham RP, Campbell M, Chen YH, et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat Chem Biol. 2015;11:189–191. doi: 10.1038/nchembio.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Makrygiannakis D, Revu S, Engström M, af Klint E, Nicholas AP, Pruijn GJ, Catrina AI. Local administration of glucocorticoids decreases synovial citrullination in rheumatoid arthritis. Arthritis Res Ther. 2012;14:R20. doi: 10.1186/ar3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song G, Shi L, Guo Y, Yu L, Wang L, Zhang X, Li L, Han Y, Ren X, Guo Q, et al. A novel PAD4/SOX4/PU.1 signaling pathway is involved in the committed differentiation of acute promyelocytic leukemia cells into granulocytic cells. Oncotarget. 2016;7:3144–3157. doi: 10.18632/oncotarget.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirsch D, Archer FE, Joshi-Kale M, Vetrano AM, Weinberger B. Decreased anti-inflammatory responses to vitamin D in neonatal neutrophils. Mediators Inflamm. 2011;2011:598345. doi: 10.1155/2011/598345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hafkamp FM, Groot Kormelink T, de Jong EC. Targeting DCs for tolerance induction: don’t lose sight of the neutrophils. Front Immunol. 2021;12:732992. doi: 10.3389/fimmu.2021.732992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Metzemaekers M, Cambier S, Blanter M, Vandooren J, de Carvalho AC, Malengier-Devlies B, Vanderbeke L, Jacobs C, Coenen S, Martens E, et al. Kinetics of peripheral blood neutrophils in severe coronavirus disease 2019. Clin Transl Immunology. 2021;10:e1271. doi: 10.1002/cti2.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Youn YJ, Lee YB, Kim SH, Jin HK, Bae JS, Hong CW. Nucleocapsid and spike proteins of SARS-CoV-2 drive neutrophil extracellular trap formation. Immune Netw. 2021;21:e16. doi: 10.4110/in.2021.21.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janke M, Poth J, Wimmenauer V, Giese T, Coch C, Barchet W, Schlee M, Hartmann G. Selective and direct activation of human neutrophils but not eosinophils by Toll-like receptor 8. J Allergy Clin Immunol. 2009;123:1026–1033. doi: 10.1016/j.jaci.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 47.Aarts CE, Downes K, Hoogendijk AJ, Sprenkeler EG, Gazendam RP, Favier R, Favier M, Tool AT, van Hamme JL, Kostadima MA, et al. Neutrophil specific granule and NETosis defects in gray platelet syndrome. Blood Adv. 2021;5:549–564. doi: 10.1182/bloodadvances.2020002442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jauregui-Amezaga A, Cabezón R, Ramírez-Morros A, España C, Rimola J, Bru C, Pinó-Donnay S, Gallego M, Masamunt MC, Ordás I, et al. Intraperitoneal administration of autologous tolerogenic dendritic cells for refractory Crohn’s disease: a phase I study. J Crohn’s Colitis. 2015;9:1071–1078. doi: 10.1093/ecco-jcc/jjv144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DEX dose-dependently inhibits neutrophil degranulation. Neutrophils were pretreated with DEX, VD3, RA or their respective controls medium, ethanol or DMSO and cultursed for 2 hours in the presence of R848 and TNF. (A) Secretory vesicle degranulation as measured by percentage of CD16-neutrophils is depicted, normalized to controls, mean ± SD. In the middle panel, data is normalized to medium control (35.2±21.0% CD16- neutrophils), indicated by the line at 1 and each donor is represented by a different symbol. Grey bars indicate VD3, while respective ethanol dilutions are shown in white bars. (B) Azurophilic degranulation as measured by percentage of CD63+ neutrophils is shown, normalized to controls, mean ± SD, with medium control 27.0±14.3% CD63+ neutrophils). Three independent experiments were performed.